Submitted:
16 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathogenetic Mechanisms of Coronary Microvascular Dysfunction
2.1. Microvascular Angina and Endothelial Dysfunction
3. Additional Risk Factors
3.1. Sex-Related Differences in Patients with Coronary Microvascular Dysfunction and Hypertension
3.2. Metabolic Syndrome
3.3. Diabetes Mellitus
3.4. Hypercholesterolemia
3.5. Obstructive Sleep Apnea
3.6. Smoking
4. Diagnostics of Coronary Microvascular Dysfunction in Patients with Hypertension
4.1. Non-Invasive Diagnostics
4.1.1. Echocardiography
4.1.2. Computerized Tomographic Angiography (CTA)
4.1.3. Single-Photon Emission Computed Tomography (SPECT)
4.1.4. Positron Emission Tomography (PET)
4.1.5. Cardiac Magnetic Resonance (CMR)
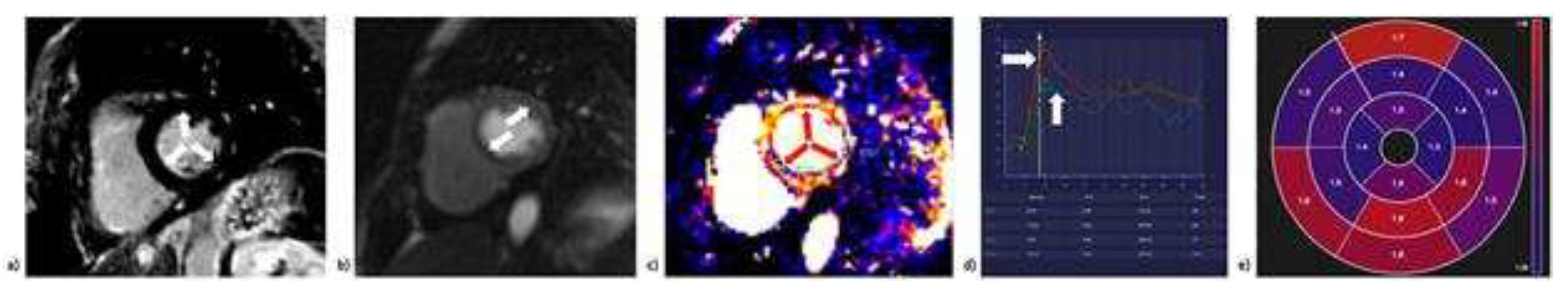
| Diagnostic modality | Parameter | Advantages | Disadvantages |
|---|---|---|---|
| Echocardiography | CFRV |
|
|
| CT coronary angiography and cardiac perfusion | MPR |
|
|
| PET | MPR, MBF |
|
|
| CMR | MBF, MPR, MPRI |
|
|
4.2. Invasive Diagnostics
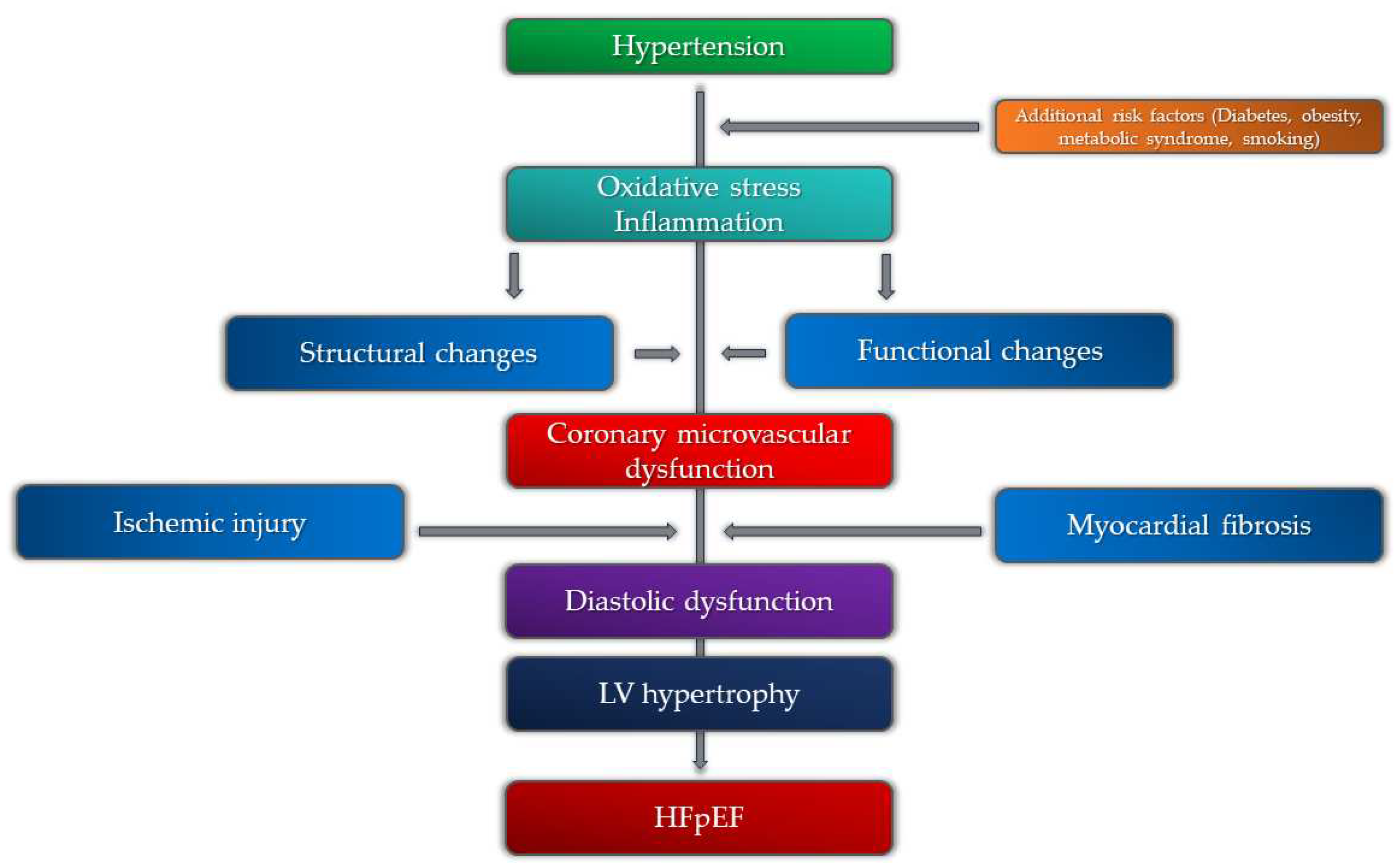
5. Coronary Microvascular Dysfunction, Hypertension, and HFpEF
6. Coronary Microvascular Dysfunction, Hypertension, and Atrial Fibrilation
7. Management of Coronary Microvascular Dysfunction in Patients with Hypertension
8. Prognosis
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen C, Wei J, AlBadri A, Zarrini P, Bairey Merz CN. Coronary Microvascular Dysfunction - Epidemiology, Pathogenesis, Prognosis, Diagnosis, Risk Factors and Therapy. Circ J. 2016, 81, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bradley C, Berry C. Definition and epidemiology of coronary microvascular disease. J Nucl Cardiol. 2022, 29, 1763–1775. [Google Scholar] [CrossRef]
- Vancheri F, Longo G, Vancheri S, Henein M. Coronary Microvascular Dysfunction. J Clin Med. 2020, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Godo S, Takahashi J, Yasuda S, Shimokawa H. Endothelium in Coronary Macrovascular and Microvascular Diseases. J Cardiovasc Pharmacol. 2021, 78 Suppl 6, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Labazi H, Trask AJ. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol Res. 2017, 123, 114–21. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson F, Nordestgaard BG, Tybjærg-Hansen A, Benn M. Impact of LDL Cholesterol on Microvascular Versus Macrovascular Disease: A Mendelian Randomization Study. J Am Coll Cardiol. 2019, 74, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Kibel A, Selthofer-Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, et al. Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res. 2017, 45, 1901–29. [Google Scholar] [CrossRef] [PubMed]
- Lee MP, Glynn RJ, Schneeweiss S, Lin KJ, Patorno E, Barberio J, et al. Risk Factors for Heart Failure with Preserved or Reduced Ejection Fraction Among Medicare Beneficiaries: Application of Competing Risks Analysis and Gradient Boosted Model. Clin Epidemiol. 2020, 12, 607–16. [Google Scholar] [CrossRef] [PubMed]
- Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013, 62, 263–71. [Google Scholar] [CrossRef]
- Crea F, Montone RA, Rinaldi R. Pathophysiology of Coronary Microvascular Dysfunction. Circ J. 2022, 86, 1319–28. [Google Scholar] [CrossRef]
- Masi S, Rizzoni D, Taddei S, Widmer RJ, Montezano AC, Lüscher TF, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J Am Coll Cardiol. 2019, 74, 2350–60. [Google Scholar] [CrossRef] [PubMed]
- Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res. 2018, 114, 954–64. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski J, Lennen RJ, Gray GA, Borthwick G, Boswell L, Baker AH, et al. Progression and regression of left ventricular hypertrophy and myocardial fibrosis in a mouse model of hypertension and concomitant cardiomyopathy. J Cardiovasc Magn Reson. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- de Moraes R, Tibirica E. Early Functional and Structural Microvascular Changes in Hypertension Related to Aging. Curr Hypertens Rev. 2017, 13, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hermann M, Flammer A, Lüscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006, 8 12 Suppl 4, 17–29. [Google Scholar] [CrossRef]
- da Silva GM, da Silva MC, Nascimento DVG, Lima Silva EM, Gouvêa FFF, de França Lopes LG, et al. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology (Basel). 2021, 10, 1041. [Google Scholar] [CrossRef]
- Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal. 2014, 20, 164–82. [Google Scholar] [CrossRef] [PubMed]
- Brandt MM, Cheng C, Merkus D, Duncker DJ, Sorop O. Mechanobiology of Microvascular Function and Structure in Health and Disease: Focus on the Coronary Circulation. Front Physiol 2021, 12, 771960. [Google Scholar] [CrossRef]
- Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015, 36, 3134–46. [Google Scholar] [CrossRef]
- Konst RE, Guzik TJ, Kaski JC, Maas AHEM, Elias-Smale SE. The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc Res. 2020, 116, 817–28. [Google Scholar] [CrossRef] [PubMed]
- Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, et al. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens. 2014, 32, 207–15. [Google Scholar] [CrossRef] [PubMed]
- Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010, 12, 448–55. [Google Scholar] [CrossRef] [PubMed]
- Goto K, Ohtsubo T, Kitazono T. Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels. Int J Mol Sci. 2018, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life (Basel). 2021, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: from pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. 2018, 8, 568–80. [Google Scholar] [CrossRef] [PubMed]
- Aribas E, Roeters van Lennep JE, Elias-Smale SE, Piek JJ, Roos M, Ahmadizar F, et al. Prevalence of microvascular angina among patients with stable symptoms in the absence of obstructive coronary artery disease: a systematic review. Cardiovasc Res. 2022, 118, 763–71. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, et al. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015, 8, 1433–41. [Google Scholar] [CrossRef] [PubMed]
- Loperena R, Harrison DG. Oxidative Stress and Hypertensive Diseases. Med Clin North Am. 2017, 101, 169–93. [Google Scholar] [CrossRef]
- Reynolds HR, Bairey Merz CN, Berry C, Samuel R, Saw J, Smilowitz NR, et al. Coronary Arterial Function and Disease in Women With No Obstructive Coronary Arteries. Circ Res. 2022, 130, 529–51. [Google Scholar] [CrossRef]
- Agarwal M, Shufelt C, Mehta PK, Gill E, Berman DS, Li D, et al. Cardiac risk factors and myocardial perfusion reserve in women with microvascular coronary dysfunction. Cardiovasc Diagn Ther. 2013, 3, 146–52. [Google Scholar] [CrossRef] [PubMed]
- Barnabas O, Wang H, Gao XM. Role of estrogen in angiogenesis in cardiovascular diseases. J Geriatr Cardiol. 2013, 10, 377–82. [Google Scholar] [CrossRef] [PubMed]
- Tunc E, Eve AA, Madak-Erdogan Z. Coronary Microvascular Dysfunction and Estrogen Receptor Signaling. Trends Endocrinol Metab. 2020, 31, 228–38. [Google Scholar] [CrossRef] [PubMed]
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010, 56, 1113–32. [Google Scholar] [CrossRef]
- Chilian W, Nystoriak MA, Sisakian H, Ohanyan V. Coronary microvascular disease during metabolic syndrome: What is known and unknown: Pathological consequences of redox imbalance for endothelial K+ channels. Int J Cardiol 2020, 321, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Sucato V, Madaudo C, Di Fazio L, Manno G, Vadalà G, Novo S, et al. Impact of Metabolic Syndrome on Coronary Microvascular Dysfunction: A Single Center Experience. Cardiology and Cardiovascular Medicine 2023, 145–50. [Google Scholar] [CrossRef]
- Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, et al. Coronary Microvascular Dysfunction in Diabetes Mellitus: Pathogenetic Mechanisms and Potential Therapeutic Options. Biomedicines. 2022, 10, 2274. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 2016, 90, 84–93. [Google Scholar] [CrossRef]
- Zhang W, Singh S, Liu L, Mohammed AQ, Yin G, Xu S, et al. Prognostic value of coronary microvascular dysfunction assessed by coronary angiography-derived index of microcirculatory resistance in diabetic patients with chronic coronary syndrome. Cardiovasc Diabetol. 2022, 21, 222. [Google Scholar] [CrossRef]
- Stapleton PA, Goodwill AG, James ME, Brock RW, Frisbee JC. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflamm (Lond) 2010, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Padró T, Vilahur G, Badimon L. Dyslipidemias and Microcirculation. Curr Pharm Des. 2018, 24, 2921–26. [Google Scholar] [CrossRef] [PubMed]
- Avtaar Singh SS, Nappi F. Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study. Biomedicines. 2022, 10, 3010. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic M, Popadic V, Klasnja S, Milic N, Rajovic N, Divac A, et al. Obstructive Sleep Apnea and Cardiovascular Risk: The Role of Dyslipidemia, Inflammation, and Obesity. Front Pharmacol 2022, 13, 898072. [Google Scholar] [CrossRef] [PubMed]
- Bozbas SS, Eroglu S, Ozyurek BA, Eyuboglu FO. Coronary flow reserve is impaired in patients with obstructive sleep apnea. Ann Thorac Med. 2017, 12, 272–77. [Google Scholar] [CrossRef] [PubMed]
- Michael Pittilo, R. Cigarette smoking, endothelial injury and cardiovascular disease. Int J Exp Pathol. 2000, 81, 219–30. [Google Scholar] [CrossRef] [PubMed]
- Gullu H, Caliskan M, Ciftci O, Erdogan D, Topcu S, Yildirim E, et al. Light cigarette smoking impairs coronary microvascular functions as severely as smoking regular cigarettes. Heart. 2007, 93, 1274–7. [Google Scholar] [CrossRef] [PubMed]
- Haig C, Carrick D, Carberry J, Mangion K, Maznyczka A, Wetherall K, et al. Current Smoking and Prognosis After Acute ST-Segment Elevation Myocardial Infarction: New Pathophysiological Insights. JACC Cardiovasc Imaging. 2019, 12, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Toya T, Nagatomo Y, Ikegami Y, Masaki N, Adachi T. Coronary microvascular dysfunction in heart failure patients. Front Cardiovasc Med 2023, 10, 1153994. [Google Scholar] [CrossRef]
- Fordyce CB, Newby DE, Douglas PS. Diagnostic Strategies for the Evaluation of Chest Pain: Clinical Implications From SCOT-HEART and PROMISE. J Am Coll Cardiol. 2016, 67, 843–52. [Google Scholar] [CrossRef]
- Carbone A, D'Andrea A, Sperlongano S, Tagliamonte E, Mandoli GE, Santoro C, et al. Echocardiographic assessment of coronary microvascular dysfunction: Basic concepts, technical aspects, and clinical settings. Echocardiography. 2021, 38, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Schroder J, Prescott E. Doppler Echocardiography Assessment of Coronary Microvascular Function in Patients With Angina and No Obstructive Coronary Artery Disease. Front Cardiovasc Med 2021, 8, 723542. [Google Scholar] [CrossRef] [PubMed]
- Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018, 39, 3439–50. [Google Scholar] [CrossRef] [PubMed]
- Völz S, Svedlund S, Andersson B, Li-Ming G, Rundqvist B. Coronary flow reserve in patients with resistant hypertension. Clin Res Cardiol. 2017, 106, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen TS, Christensen M, Løgstrup BB, Kronborg CJS, Knudsen UB. Reduced coronary flow velocity reserve in women with previous pre-eclampsia: link to increased cardiovascular disease risk. Ultrasound Obstet Gynecol. 2020, 55, 786–792. [Google Scholar] [CrossRef]
- Nieman K, Balla S. Dynamic CT myocardial perfusion imaging. J Cardiovasc Comput Tomogr. 2020, 14, 303–06. [Google Scholar] [CrossRef] [PubMed]
- Seitun S, Clemente A, De Lorenzi C, Benenati S, Chiappino D, Mantini C, et al. Cardiac CT perfusion and FFRCTA: pathophysiological features in ischemic heart disease. Cardiovasc Diagn Ther. 2020, 10, 1954–78. [Google Scholar] [CrossRef]
- Ihdayhid AR, Fairbairn TA, Gulsin GS, Tzimas G, Danehy E, Updegrove A, et al. Cardiac computed tomography-derived coronary artery volume to myocardial mass. J Cardiovasc Comput Tomogr. 2022, 16, 198–206. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael SE, van Rosendael AR, Kuneman JH, Patel MR, Nørgaard BL, Fairbairn TA, et al. Coronary Volume to Left Ventricular Mass Ratio in Patients With Hypertension. Am J Cardiol 2023, 199, 100–9. [Google Scholar] [CrossRef]
- Djaïleb L, Riou L, Piliero N, Carabelli A, Vautrin E, Broisat A, et al. SPECT myocardial ischemia in the absence of obstructive CAD: Contribution of the invasive assessment of microvascular dysfunction. J Nucl Cardiol. 2018, 25, 1017–22. [Google Scholar] [CrossRef]
- Mathew RC, Bourque JM, Salerno M, Kramer CM. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc Imaging. 2020, 13, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhou W, Brown JM, Bajaj NS, Chandra A, Divakaran S, Weber B, et al. Hypertensive coronary microvascular dysfunction: a subclinical marker of end organ damage and heart failure. Eur Heart J. 2020, 41, 2366–75. [Google Scholar] [CrossRef] [PubMed]
- Patel AR, Salerno M, Kwong RY, Singh A, Heydari B, Kramer CM. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J Am Coll Cardiol. 2021, 78, 1655–68. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic M, Klasnja S, Popovic M, Djuran P, Mrda D, Ivankovic T, et al. Cardiac Magnetic Resonance in Hypertensive Heart Disease: Time for a New Chapter. Diagnostics (Basel). 2022, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Liang L, Wang X, Yu Y, Zhang Y, Liu J, Chen M, et al. T1 Mapping and Extracellular Volume in Cardiomyopathy Showing Left Ventricular Hypertrophy: Differentiation Between Hypertrophic Cardiomyopathy and Hypertensive Heart Disease. Int J Gen Med 2022, 15, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- Engblom H, Xue H, Akil S, Carlsson M, Hindorf C, Oddstig J, et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: a comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J Cardiovasc Magn Reson. 2017, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Zorach B, Shaw PW, Bourque J, Kuruvilla S, Balfour PC Jr, Yang Y, et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson. 2018, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015, 8, 101161/CIRCIMAGING114002481 e002481. [Google Scholar] [CrossRef]
- Rahman H, Scannell CM, Demir OM, Ryan M, McConkey H, Ellis H, et al. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021, 14, 978–986. [Google Scholar] [CrossRef]
- Chang A, Kang N, Chung J, Gupta AR, Parwani P. Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR). Medicina (Kaunas). 2023, 59, 1570. [Google Scholar] [CrossRef]
- Scarsini R, Shanmuganathan M, De Maria GL, Borlotti A, Kotronias RA, Burrage MK, et al. Coronary Microvascular Dysfunction Assessed by Pressure Wire and CMR After STEMI Predicts Long-Term Outcomes. JACC Cardiovasc Imaging. 2021, 14, 1948–59. [Google Scholar] [CrossRef]
- Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, Aung N, et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation. 2020, 141, 1282–91. [Google Scholar] [CrossRef] [PubMed]
- Levelt E, Piechnik SK, Liu A, Wijesurendra RS, Mahmod M, Ariga R, et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J Cardiovasc Magn Reson. 2017, 19, 81. [Google Scholar] [CrossRef]
- Travieso A, Jeronimo-Baza A, Faria D, Shabbir A, Mejia-Rentería H, Escaned J. Invasive evaluation of coronary microvascular dysfunction. J Nucl Cardiol. 2022, 29, 2474–86. [Google Scholar] [CrossRef] [PubMed]
- Mangiacapra F, Viscusi MM, Verolino G, Paolucci L, Nusca A, Melfi R, et al. Invasive Assessment of Coronary Microvascular Function. J Clin Med. 2021, 11, 228. [Google Scholar] [CrossRef]
- Geng Y, Wu X, Liu H, Zheng D, Xia L. Index of microcirculatory resistance: state-of-the-art and potential applications in computational simulation of coronary artery disease. J Zhejiang Univ Sci B. 2022, 23, 123–40 English. [Google Scholar] [CrossRef]
- Fearon WF, Kobayashi Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ Cardiovasc Interv. 2017, 10, e005361. [Google Scholar] [CrossRef] [PubMed]
- Toya T, Nagatomo Y, Ikegami Y, Masaki N, Adachi T. Coronary microvascular dysfunction in heart failure patients. Front Cardiovasc Med 2023, 10, 1153994. [Google Scholar] [CrossRef]
- Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018, 314, H1033–H1042. [Google Scholar] [CrossRef]
- Lin X, Wu G, Wang S, Huang J. The prevalence of coronary microvascular dysfunction (CMD) in heart failure with preserved ejection fraction (HFpEF): a systematic review and meta-analysis. Heart Fail Rev 2023. [Google Scholar] [CrossRef]
- D'Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, et al. Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction. Front Physiol 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Paulus WJ, Zile MR. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure With Preserved Ejection Fraction Paradigm Revisited. Circ Res. 2021, 128, 1451–67. [Google Scholar] [CrossRef] [PubMed]
- Sagris M, Theofilis P, Antonopoulos AS, Oikonomou E, Paschaliori C, Galiatsatos N, et al. Inflammation in Coronary Microvascular Dysfunction. Int J Mol Sci. 2021, 22, 13471. [Google Scholar] [CrossRef] [PubMed]
- Kanagala P, Arnold JR, Singh A, Chan DCS, Cheng ASH, Khan JN, et al. Characterizing heart failure with preserved and reduced ejection fraction: An imaging and plasma biomarker approach. PLoS One. 2020, 15, e0232280. [Google Scholar] [CrossRef] [PubMed]
- Cornuault L, Rouault P, Duplàa C, Couffinhal T, Renault MA. Endothelial Dysfunction in Heart Failure With Preserved Ejection Fraction: What are the Experimental Proofs? Front Physiol 2022, 13, 906272. [Google Scholar] [CrossRef] [PubMed]
- Tam MC, Lee R, Cascino TM, Konerman MC, Hummel SL. Current Perspectives on Systemic Hypertension in Heart Failure with Preserved Ejection Fraction. Curr Hypertens Rep. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL, et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014, 7, 991–7. [Google Scholar] [CrossRef] [PubMed]
- Brann A, Miller J, Eshraghian E, Park JJ, Greenberg B. Global longitudinal strain predicts clinical outcomes in patients with heart failure with preserved ejection fraction. Global longitudinal strain predicts clinical outcomes in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2023. [CrossRef] [PubMed]
- Ma J, Chen Q, Ma S. Left atrial fibrosis in atrial fibrillation: Mechanisms, clinical evaluation and management. J Cell Mol Med. 2021, 25, 2764–75. [Google Scholar] [CrossRef]
- Fauchier L, Bisson A, Bodin A. Heart failure with preserved ejection fraction and atrial fibrillation: recent advances and open questions. BMC Med. 2023, 21, 54. [Google Scholar] [CrossRef]
- Gorter TM, van Veldhuisen DJ, Mulder BA, Artola Arita VA, van Empel VPM, Manintveld OC, et al. Prevalence and Incidence of Atrial Fibrillation in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: (Additive) Value of Implantable Loop Recorders. J Clin Med. 2023, 12, 3682. [Google Scholar] [CrossRef] [PubMed]
- Millan-Orge M, Torres-Peña JD, Arenas-Larriva A, Quintana-Navarro GM, Peña-Orihuela P, Alcala-Diaz JF, et al. Influence of dietary intervention on microvascular endothelial function in coronary patients and atherothrombotic risk of recurrence. Sci Rep. 2021, 11, 20301. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peña JD, Rangel-Zuñiga OA, Alcala-Diaz JF, Lopez-Miranda J, Delgado-Lista J. Mediterranean Diet and Endothelial Function: A Review of its Effects at Different Vascular Bed Levels. Nutrients 2020, 12, 2212. [Google Scholar] [CrossRef]
- Schindler TH, Valenta I. Coronary microvascular dysfunction and prognostication in diabetes mellitus. Eur Heart J Cardiovasc Imaging. 2023, 24, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Ong P, Athanasiadis A, Sechtem U. Pharmacotherapy for coronary microvascular dysfunction. Eur Heart J Cardiovasc Pharmacother. 2015, 1, 65–71. [Google Scholar] [CrossRef]
- Michelsen MM, Rask AB, Suhrs E, Raft KF, Høst N, Prescott E. Effect of ACE-inhibition on coronary microvascular function and symptoms in normotensive women with microvascular angina: A randomized placebo-controlled trial. PLoS One. 2018, 13, e0196962. [Google Scholar] [CrossRef]
- Soleymani M, Masoudkabir F, Shabani M, Vasheghani-Farahani A, Behnoush AH, Khalaji A. Updates on Pharmacologic Management of Microvascular Angina. Cardiovasc Ther 2022, 2022, 6080258. [Google Scholar] [CrossRef]
- Weber KT, Sun Y, Gerling IC, Guntaka RV. Regression of Established Cardiac Fibrosis in Hypertensive Heart Disease. Am J Hypertens. 2017, 30, 1049–1052. [Google Scholar] [CrossRef]
- Engholm M, Bertelsen JB, Mathiassen ON, Bøtker HE, Vase H, Peters CD, et al. Effects of renal denervation on coronary flow reserve and forearm dilation capacity in patients with treatment-resistant hypertension. A randomized, double-blinded, sham-controlled clinical trial. Int J Cardiol. 2018, 250, 29–34. [Google Scholar] [CrossRef]
- Ullrich H, Hammer P, Olschewski M, Münzel T, Escaned J, Gori T. Coronary Venous Pressure and Microvascular Hemodynamics in Patients With Microvascular Angina: A Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 979–83. [Google Scholar] [CrossRef]
- Kelshiker MA, Seligman H, Howard JP, Rahman H, Foley M, Nowbar AN, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022, 43, 1582–93. [Google Scholar] [CrossRef] [PubMed]
- Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med. 2014, 44, 344–57. [Google Scholar] [CrossRef] [PubMed]
- Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011, 124, 2215–24. [Google Scholar] [CrossRef]
- Zhou W, Lee JCY, Leung ST, Lai A, Lee TF, Chiang JB, et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2021, 14, 602–11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





