Submitted:
16 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Preparation of phenolic extracts
2.3.1. MAE
2.3.2. ASE
2.4. Determination of total phenolic content (TPC)
2.5. Determination of the individual phenolic content
2.6. Determination of antioxidant properties
2.6.1. ABTS
2.6.2. DPPH
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.7. Statistical analysis
3. Results and Discussion
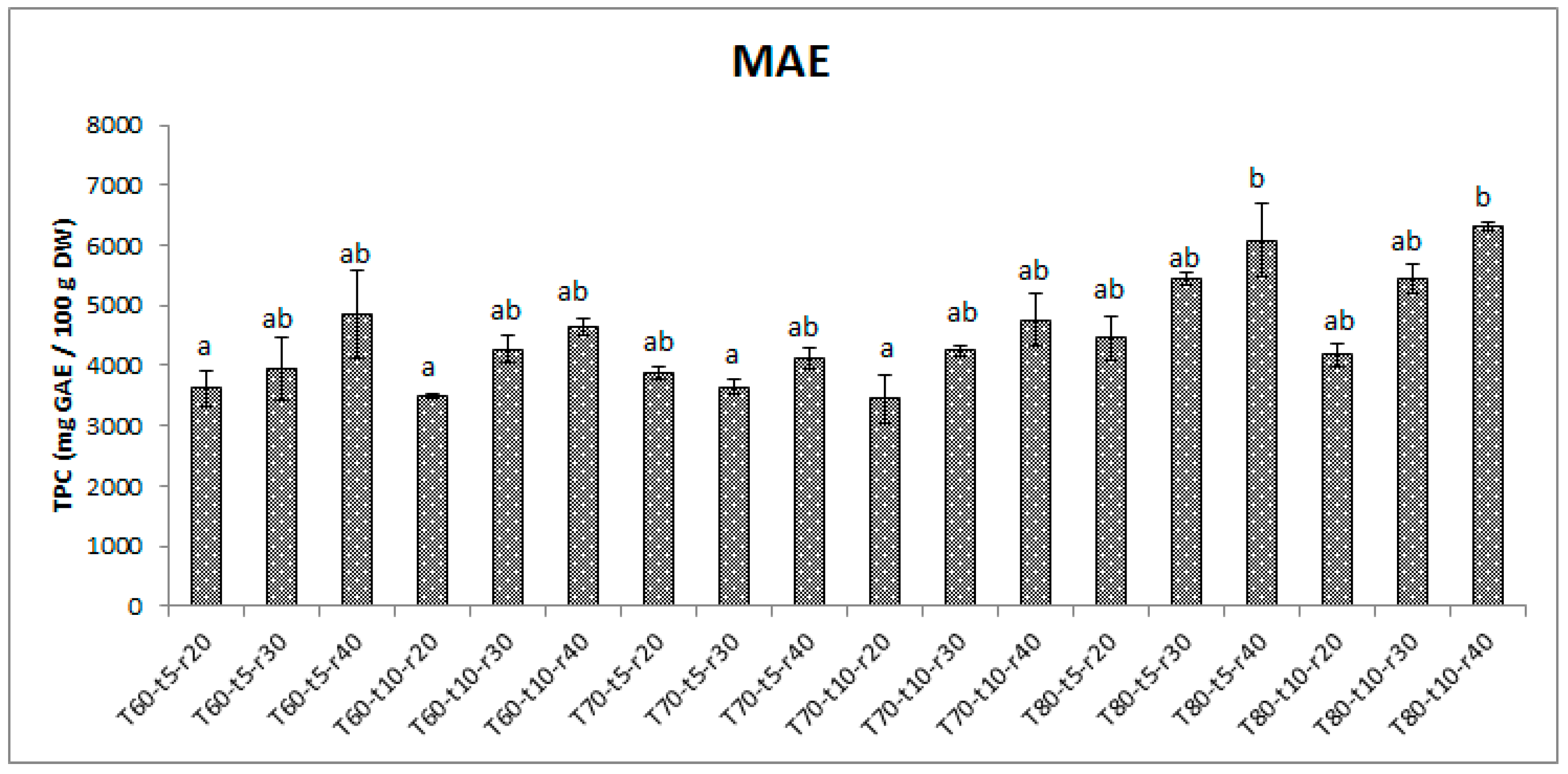
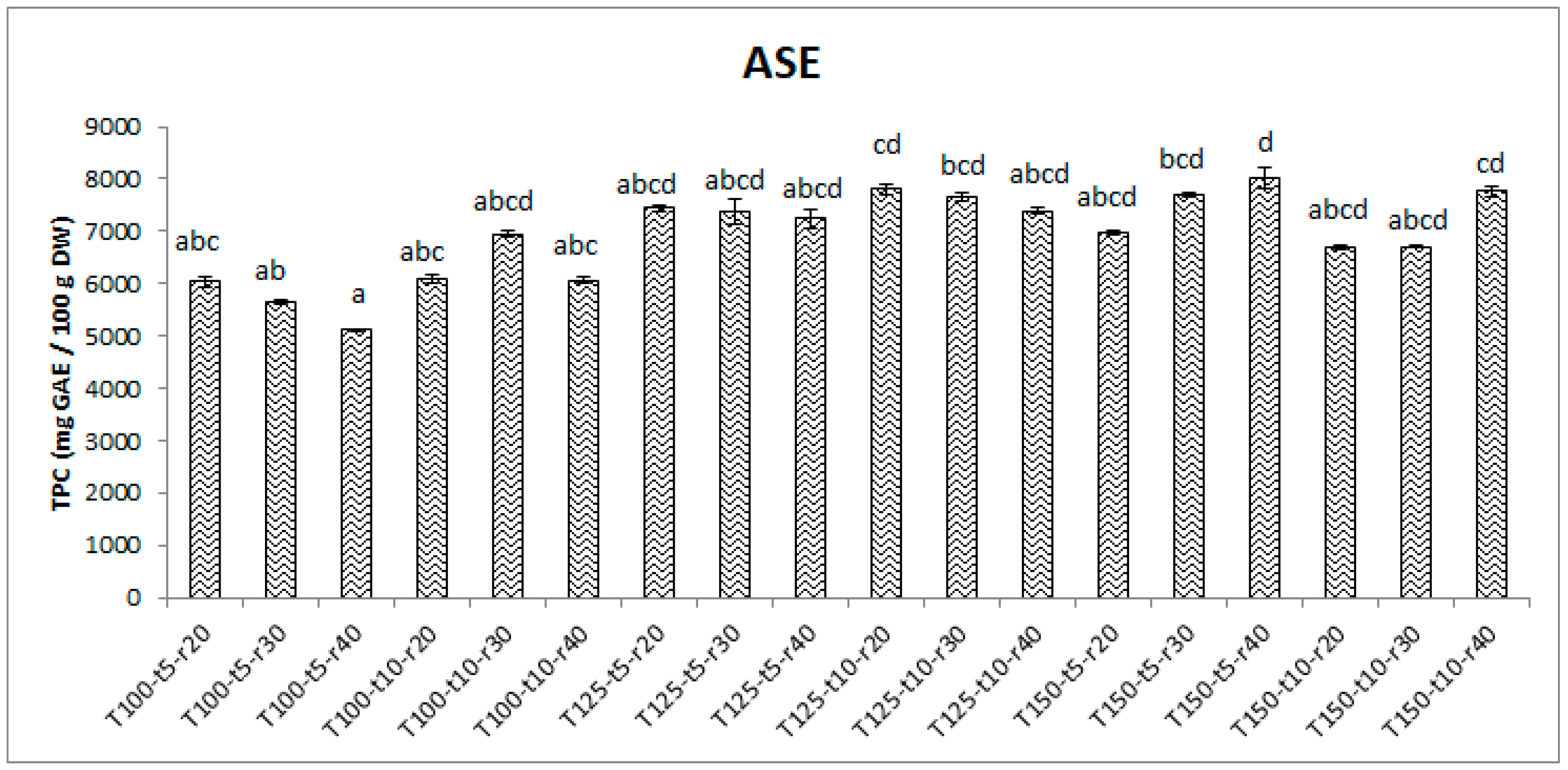
3.1. Effects of extraction parameters on yield of phenolics
3.2. Effects of extraction methods on phenolic profile
3.3. Effects of extraction parameters on antioxidant properties
3.3.1. ABTS
3.3.2. DPPH
3.3.3. FRAP
3.4. Correlation between the contents of phenolics and their different antioxidant properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dias, M.I.; Barros, L.; Fernandes, I.P.; Ruphuy, G.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. A bioactive formulation based on Fragaria vesca L. vegetative parts: Chemical characterisation and application in κ-carrageenan gelatin. Journal of Functional Foods 2015, 16, 243–255. [Google Scholar]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. Journal of Ethnopharmacology 2014, 158, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liberal, J.; Costa, G.; Carmo, A.; Vitorino, R.; Marques, C.; Domingues, M.R.; Domingues, P.; Gonçalves, A.C.; Alves, R.; Sarmento-Ribeiro, A.B.; Girão, H.; Cruz, M.T.; Batista, M.T. Chemical characterization and cytotoxic potential of an ellagitannin-enriched fraction from Fragaria vesca leaves. Arabian Journal of Chemistry 2019, 12, 3652–3666. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomedicine 2009, 16, 462–462. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, J.; Simões, D.M.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular effects of Fragaria vesca L. in human arteries. Natural Product Research 2023. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.B.; Turker, A.U. Effects of regeneration enhancers on micropropagation of Fragaria vesca L. and phenolic content comparison of field-grown and in vitro-grown plant materials by liquid chromatography-electrospray tandem mass spectrometry (LC–ESI-MS/MS). Scientia Horticulturae 2014, 169, 169–178. [Google Scholar]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Enhancement of nutritional and bioactive compounds by in vitro culture of wild Fragaria vesca L. vegetative parts. Food Chemistry 2017, 235, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Yeasmen, N.; Orsat, V. Green extraction and characterization of leaves phenolic compounds: a comprehensive review. Critical Reviews in Food Science and Nutrition 2023, 63, 5155–5193. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chemistry 2022, 378, 131918. [Google Scholar] [CrossRef]
- AOAC. In Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting; Association of Official Analytical Chemists: Rockville, MD, USA, 1990; Vol. 15.
- Shortle, E.; O'Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Science 2014, 98, 828–834. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. Journal of Food Science 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender differences in antioxidant capacity of rat tissues determined by 2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2005, 140, 47–52. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Tade, M.O.; Ali, H.A. Thermodynamics and kinetic studies for the microwave-enhanced extraction of phenolics from Phyllanthus niruri leaves. Chemical Engineering Communications 2022, 1–9. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Applied Sciences 2022, 12. [Google Scholar] [CrossRef]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical analysis of research trends and issues in microwave assisted extraction of phenolics: Have we really done enough. TrAC Trends in Analytical Chemistry 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. Journal of Agricultural and Food Chemistry 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.P.; Nguyen, N.T.U.; Le, V.H.; Phan, T.H.; Nguyen, T.H.Y.; Nguyen, D.Q. Optimizing ultrasonic-assisted and microwave-assisted extraction processes to recover phenolics and flavonoids from passion fruit peels. ACS Omega 2023. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic compounds with antioxidant activity from strawberry leaves: a study on microwave-assisted extraction optimization. Preparative Biochemistry & Biotechnology 2020, 50, 874–882. [Google Scholar]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chemistry 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Alhallaf, W.; Bishop, K.; Perkins, L.B. Optimization of accelerated solvent extraction of phenolic compounds from chaga using Response Surface Methodology. Food Analytical Methods 2022, 15, 2777–2790. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Analytical Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Doi, S.; Higashino, H.; Karatsu, A.; Masuda, A.; Masuda, T. Identification of polyphenol and reductone antioxidants in the caramelization product of N-acetylglucosamine. ACS Food Science & Technology 2022, 2, 1135–1140. [Google Scholar]
- Terpinc, P.; Polak, T.; Šegatin, N.; Hanzlowsky, A.; Ulrih, N.P.; Abramovič, H. Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acids. Food Chemistry 2011, 128, 62–69. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of phenolic antioxidants extraction from Fucus vesiculosus by pressurized liquid extraction. Journal of Applied Phycology 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Current Research in Food Science 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Luthria, D.L. Optimization of extraction of phenolic acids from a vegetable waste product using a pressurized liquid extractor. Journal of Functional Foods 2012, 4, 842–850. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar]
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Polyphenolic Characterization and Antioxidant Capacity of Laurus nobilis L. Leaf Extracts Obtained by Green and Conventional Extraction Techniques. Processes 2021, 9, 1840. [Google Scholar]
- Carev, I.; Maravić, A.; Ilić, N.; Čikeš Čulić, V.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes 2022, 10, 510. [Google Scholar]
- Bursać Kovačević, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chemistry 2016, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyl̷o, A.; Gorzelany, J.; Kapusta, I. Identification and Characterization of Low Molecular Weight Polyphenols in Berry Leaf Extracts by HPLC-DAD and LC-ESI/MS. Journal of Agricultural and Food Chemistry 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Hanhineva, K.; Lehtonen, M.; McDougall, G.J.; Stewart, D.; Karjalainen, R.O. Non-targeted metabolite profiling highlights the potential of strawberry leaves as a resource for specific bioactive compounds. Journal of the Science of Food and Agriculture 2017, 97, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibáñez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Elez Garofulić, I.; Balbino, S.; Pedisić, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). Journal of Agricultural and Food Chemistry 2012, 60, 10920–10929. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, L.S.; Borah, P.K.; Deka, S.C. Optimization of microwave assisted extraction of antioxidant extract from Garcinia pedunculata Robx. Separation Science and Technology 2015, 50, 1814–1822. [Google Scholar] [CrossRef]
- Cha, K.H.; Kang, S.W.; Kim, C.Y.; Um, B.H.; Na, Y.R.; Pan, C.-H. Effect of pressurized liquids on extraction of antioxidants from Chlorella vulgaris. Journal of Agricultural and Food Chemistry 2010, 58, 4756–4761. [Google Scholar] [CrossRef]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the antiradical properties of phenolic acids. International Journal of Molecular Sciences 2014, 15, 16351–16380. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and Standardization of <i>In Vitro</i> Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. Journal of Chemistry 2018, 2018, 4608405. [Google Scholar]
- Elez Garofulić, I.; Dragović-Uzelac, V.; Režek Jambrak, A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). Journal of Food Engineering 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Tang, Y.-Z.; Liu, Z.-Q. Free-radical-scavenging effect of carbazole derivatives on DPPH and ABTS Radicals. Journal of the American Oil Chemists' Society 2007, 84, 1095–1100. [Google Scholar] [CrossRef]
- Marković, S.; Tošović, J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chemistry 2016, 210, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays— A practical approach. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Anouar, E.; Marzouk, M.; Taie, H.A.A.; Ahudhaif, A.; Al-Salahi, R. DFT study and radical scavenging activity of 2-phenoxypyridotriazolo pyrimidines by DPPH, ABTS, FRAP and reducing power capacity. Chemical Papers 2020, 74, 2893–2899. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. Journal of Agricultural and Food Chemistry 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. Journal of the American Oil Chemists' Society 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Maximising recovery of phenolic compounds and antioxidant properties from banana peel using microwave assisted extraction and water. Journal of Food Science and Technology 2019, 56, 1360–1370. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids – ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25. [Google Scholar] [CrossRef]

| No. | Compound name | RT (min) | m/z | m/z (prod.) | MAE mg/100 g |
ASE mg/100 g |
|---|---|---|---|---|---|---|
| PHENOLIC ACIDS | ||||||
| 1 | ferulic acid* | 1.937 | 193 | 178 | 5.13 ± 015 a | 6.07 ± 0.17 b |
| 2 | 3-p-caffeoylquinic acid | 2.906 | 337 | 163 | 3.64 ± 0.10 b | 2.90 ± 0.08 a |
| 3 | rosmarinic acid* | 3.138 | 359.1 | 161 | 16.74 ± 0.47 a | 16.82± 0.48 a |
| 5 | chlorogenic acid* | 4.615 | 353 | 191 | 43.57 ± 1.23 a | 40.06 ± 1.13 a |
| 8 | 3,5-di-caffeoylquinic acid | 5.573 | 515 | 173 | 0.83 ± 0.02 b | 0.76 ± 0.02 a |
| 9 | 4,5-di-caffeoylquinic acid | 5.573 | 515 | 353 | 1.12 ± 0.03 a | 1.13± 0.03 a |
| 12 | syringic acid* | 6.354 | 197 | 182 | 7.39 ± 0.21 a | 19.42± 0.55 b |
| 13 | caffeic acid* | 6.368 | 179 | 135 | 8.44 ± 0.24 b | 6.91± 0.20 a |
| 17 | 4-O-caffeoylquinic acid | 7.821 | 324 | 173 | 0.15 ± 0.00 b | 0.07± 0.00 a |
| 25 | 5-O-galloylquinic acid | 9.775 | 343 | 191 | 61.38 ± 1.74 a | 62.22± 1.76 a |
| 28 | 3-O-ferruylquinic acid | 11.238 | 367 | 193 | 1.77 ± 0.05 a | 1.42± 0.04 a |
| 39 | gallic acid* | 11.528 | 169 | 125 | 156.96 ± 4.44 b | 39.84± 1.13 a |
| 40 | p-hydroxybenzoic acid | 11.538 | 137 | 93 | 172.41 ± 4.88 b | 141.78 ± 4.01 a |
| 53 | 3,5-Digalloylquinic acid | 11.968 | 495 | 343 | 5.19 ± 0.15 a | 6.50 ± 0.18 b |
| PROANTHOCYANIDINS | ||||||
| 4 | procyanidin trimer | 3.438 | 865 | 575 | 1.84 ± 0.05 a | 43.71± 1.24 b |
| 10 | procyanidin B2* | 5.815 | 577 | 289 | 0.92 ± 0.03 a | 21.84± 0.62 b |
| 30 | procyanidin B1 | 11.351 | 579 | 291 | 44.73 ± 1.27 a | 332.26 ± 9.40 b |
| FLAVONOLS | ||||||
| 6 | isorhamnetin-3-rhamnoside | 5.178 | 625 | 317 | 1.13 ± 0.03 a | 1.72 ± 0.05 b |
| 7 | isorhamnetin-3-hexoside | 5.232 | 479 | 317 | 7.84 ± 0.22 b | 2.06 ± 0.06 a |
| 11 | kaempferol-3-O-hexoside | 6.252 | 449 | 287 | 5.36 ± 0.15 a | 5.69 ± 0.16 a |
| 15 | myricetin* | 7.258 | 319 | 273 | 48.33 ± 1.37 b | 33.19 ± 0.94 a |
| 16 | quercetin-3-glucuronide | 7.442 | 479 | 303 | 129.31 ± 3.66 a | 268.97 ± 7.61 b |
| 18 | kaempferol-3-glucuronide | 8.192 | 463 | 287 | 39.79 ± 1.13 a | 70.47 ± 1.99 b |
| 19 | quercetin-3-rhamnoside | 8.213 | 449 | 303 | 2.30 ± 0.06 a | 3.97 ± 0.11 b |
| 21 | kaempferol-3-O-deoxyhexoside | 8.475 | 433 | 286 | 1.40 ± 0.04 a | 1.93 ± 0.05 a |
| 23 | quercetin-3-pentoside | 9.700 | 435 | 303 | 1.68 ± 0.05 a | 11.81 ± 0.33 b |
| 26 | myricetin-3-O-rhamnoside | 9.905 | 465 | 319 | 6.13 ± 0.17 a | 43.71 ± 1.24 b |
| 27 | kaempferol-3-O-pentoside | 10.689 | 419 | 287 | 3.08 ± 0.09 b | 0.91 ± 0.03 a |
| 29 | kaempferol-pentosyl-hexoside | 11.344 | 581 | 287 | 0.25 ± 0.01 a | 0.77 ± 0.02 b |
| 31 | quercetin-acetyl-hexoside | 11.357 | 507 | 303 | 1.41 ± 0.04 a | 2.55 ± 0.07 b |
| 32 | kaempferol-acetyl-hexoside | 11.361 | 491 | 287 | 1.22 ± 0.03 b | 0.34 ± 0.01 a |
| 33 | isorhamnetin-3-O-glucoside | 11.364 | 483 | 317 | 1.41 ± 0.04 b | 0.83 ± 0.02 a |
| 34 | myricetin-3-O-galactoside | 11.368 | 481 | 319 | 68.99 ± 1.95 b | 52.33 ± 1.48 a |
| 35 | quercetin-3-glucoside* | 11.381 | 465 | 303.1 | 11.45 ± 0.32 a | 37.48 ± 1.06 b |
| 37 | myricetin-3-O-arabinoside | 11.395 | 451 | 319 | 8.37 ± 0.24 b | 4.36 ± 0.12 a |
| 41 | quercetin-acetyl-rutinoside | 11.552 | 653 | 303 | 0.28 ± 0.01 a | 1.29 ± 0.04 b |
| 42 | kaempferol-acetyl-rutinoside | 11.556 | 637 | 287 | 0.12 ± 0.00 a | 0.54 ± 0.01 b |
| 43 | quercetin-3-O-dihexoside | 11.559 | 627 | 303 | 2.74 ± 0.08 b | 1.97 ± 0.06 a |
| 44 | rutin* | 11.566 | 611 | 303 | 44.31 ± 1.25 a | 116.04 ± 3.28 b |
| 45 | isorhamnetin-pentosylhexoside | 11.566 | 611 | 317 | 1.23 ± 0.03 b | 0.43 ± 0.01 a |
| 46 | quercetin-3-O-vicianoside | 11.576 | 597 | 434 | 2.77 ± 0.08 b | 1.82 ± 0.05 a |
| 47 | kaempferol-3-rutinoside* | 11.586 | 595 | 287 | 3.56 ± 0.10 a | 7.54 ± 0.21 b |
| 49 | quercetin | 11.681 | 303 | 303 | 336.35 ± 9.51 a | 472.63 ± 13.34 b |
| 50 | kaempferol | 11.698 | 287 | 287 | 296.57 ± 8.39 a | 298.96 ± 8.46 a |
| 51 | quercetin-pentosylhexoside | 11.825 | 597 | 303 | 1.42 ± 0.04 a | 2.67 ± 0.08 b |
| FLAVAN-3-OLS | ||||||
| 24 | epicatechin | 9.727 | 291 | 139 | 45.42 ± 1.28 a | 100.29 ± 2.84 b |
| 36 | epigallocatechin gallate* | 11.388 | 459 | 289 | 5.41 ± 0.15 a | 5.48 ± 0.15 a |
| 54 | epicatechin gallate* | 12.149 | 442.9 | 273 | 3.53 ± 0.10 b | 2.26 ± 0.06 a |
| FLAVONES | ||||||
| 14 | luteolin-6-C-glucoside | 6.978 | 449 | 359 | 2.73 ± 0.08 b | 0.80 ± 0.02 a |
| 20 | luteolin * | 8.264 | 287 | 153 | 9.82 ± 0.28 a | 17.75 ± 0.50 b |
| 22 | apigenin* | 8.758 | 271 | 153 | 0.34 ± 0.01 a | 0.56 ± 0.02 b |
| 38 | apigenin pentoside | 11.429 | 403 | 271 | 0.399 ± 0.01 a | 0.55 ± 0.02 b |
| 48 | apigenin-6-C-(O-deoxyhexosyl)-hexoside | 11.593 | 579 | 459 | 0.24 ± 0.01 a | 0.57 ± 0.02 b |
| 52 | luteolin-7-O-rutinoside | 11.828 | 595 | 287 | 3.45 ± 0.109 a | 7.51 ± 0.21 b |
| TOTAL PHENOLICS | 1632.32 ± 26.17 a | 2326.42 ± 65.80 b | ||||
| MAE | T (°C) |
t (min) |
R (mL/g) |
ABTS (mmol TE/g) |
DPPH (mmol TE/g) |
FRAP (mmol TE/g) |
|---|---|---|---|---|---|---|
| T60-t5-r20 | 60 | 5 | 20 | 350 ± 12 ab | 444 ± 5 ab | 458 ± 29 abcd |
| T60-t5-r30 | 60 | 5 | 30 | 368 ± 51 abc | 539 ± 33 abc | 536 ± 63 abcd |
| T60-t5-r40 | 60 | 5 | 40 | 433 ± 12 abc | 661 ± 32 bc | 604 ± 79 abcd |
| T60-t10-r20 | 60 | 10 | 20 | 312 ± 8 a | 434 ± 14 ab | 429 ± 12 abc |
| T60-t10-r30 | 60 | 10 | 30 | 466 ± 5 abc | 557 ± 20 abc | 515 ± 49 abcd |
| T60-t10-r40 | 60 | 10 | 40 | 466 ± 42 abc | 669 ± 14 bc | 541 ± 25 abcd |
| T70-t5-r20 | 70 | 5 | 20 | 392 ± 77 abc | 477 ± 28 abc | 427 ± 31 ab |
| T70-t5-r30 | 70 | 5 | 30 | 371 ± 24 abc | 545 ± 1 abc | 360 ± 20 a |
| T70-t5-r40 | 70 | 5 | 40 | 442 ± 47 abc | 711 ± 20 c | 510 ± 73 abcd |
| T70-t10-r20 | 70 | 10 | 20 | 347 ± 19 ab | 470 ± 12 abc | 402 ± 36 ab |
| T70-t10-r30 | 70 | 10 | 30 | 444 ± 43 abc | 617 ± 8 abc | 515 ± 86 abcd |
| T70-t10-r40 | 70 | 10 | 40 | 440 ± 12 abc | 697 ± 3 c | 442 ± 12 abcd |
| T80-t5-r20 | 80 | 5 | 20 | 471 ±31 abc | 352 ± 2 a | 1834 ± 76 abcd |
| T80-t5-r30 | 80 | 5 | 30 | 517 ± 28 bc | 475 ± 7 abc | 2118 ± 34 bcd |
| T80-t5-r40 | 80 | 5 | 40 | 681 ± 11 c | 583 ± 45 abc | 2389 ± 175 cd |
| T80-t10-r20 | 80 | 10 | 20 | 413 ± 29 abc | 345 ± 23 a | 1666 ± 55 abcd |
| T80-t10-r30 | 80 | 10 | 30 | 584 ± 32 bc | 457 ± 1 abc | 2082 ± 143 bcd |
| T80-t10-r40 | 80 | 10 | 40 | 683 ± 73 c | 580 ± 3 abc | 2461 ± 89 d |
| ASE | T (°C) |
t (min) | R (mL/g) |
ABTS (mmol TE/g) |
DPPH (mmol TE/g) |
FRAP (mmol TE/g) |
|---|---|---|---|---|---|---|
| T100-t5-r20 | 100 | 5 | 20 | 503 ± 13 abcd | 514 ± 3 ab | 758±26 a |
| T100-t5-r30 | 100 | 5 | 30 | 483 ± 16 abc | 688 ± 2 abcd | 809±5 abc |
| T100-t5-r40 | 100 | 5 | 40 | 509 ± 35 abcd | 717 ± 8 abcd | 742±69 a |
| T100-t10-r20 | 100 | 10 | 20 | 508 ± 7 abcd | 515 ± 3 abc | 757±8 a |
| T100-t10-r30 | 100 | 10 | 30 | 575 ± 8 abcd | 734 ± 1 abcd | 953±21 abc |
| T100-t10-r40 | 100 | 10 | 40 | 496 ± 10 abcd | 757 ± 4 abcd | 1057±37 c |
| T125-t5-r20 | 125 | 5 | 20 | 581 ± 4 abcd | 514 ± 4 ab | 860±14 abc |
| T125-t5-r30 | 125 | 5 | 30 | 602 ± 23 bcd | 742 ± 4 abcd | 1045±10 bc |
| T125-t5-r40 | 125 | 5 | 40 | 627 ± 14 cd | 839 ± 5 abcd | 786±19 ab |
| T125-t10-r20 | 125 | 10 | 20 | 581 ± 5 abcd | 514 ± 0 ab | 966±3 abc |
| T125-t10-r30 | 125 | 10 | 30 | 595 ± 8 bcd | 756 ± 4 abcd | 853±18 abc |
| T125-t10-r40 | 125 | 10 | 40 | 605 ± 13 cd | 843 ± 2 bcd | 896±23 abc |
| T150-t5-r20 | 150 | 5 | 20 | 540 ± 6 abcd | 512 ± 2 a | 865±19 abc |
| T150-t5-r30 | 150 | 5 | 30 | 595 ± 7 bcd | 754 ± 3 abcd | 959±15 abc |
| T150-t5-r40 | 150 | 5 | 40 | 595 ± 4 bcd | 903 ± 3 d | 1086±15 c |
| T150-t10-r20 | 150 | 10 | 20 | 450 ± 5 ab | 514 ± 1 abc | 893±9 abc |
| T150-t10-r30 | 150 | 10 | 30 | 442 ± 7 a | 706 ± 3 abcd | 928±13 abc |
| T150-t10-r40 | 150 | 10 | 40 | 504 ± 9 abcd | 889 ± 3 cd | 911±14 abc |
| TPC | ABTS | DPPH | FRAP | |
|---|---|---|---|---|
| TPC | 0.894** | 0.261* | 0.790** | |
| ABTS | 0.627** | 0.21 | 0.798** | |
| DPPH | 0.311** | 0.340** | -0.297* | |
| FRAP | 0.524** | 0.21 | 0.373** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





