1. Introduction
Lignocellulosic wastes continue to pose environmental pollution problems in the agriculturally based ecosystems [
1]. The management of such waste, includes burning, adds to the environmental pollution. Despite the efficacy of the currently used pre-treatments methods, their limitations raise the search for a new strategy with an improved efficacy and safety outline [
2]. Lignocellulosic materials are composed of cellulose, hemicellulose, and lignin. Cellulose and hemicellulose are known to have sugar polymers, which are important in most industries as raw materials in producing commercial goods [
3]. But lignin is found to be a hindrance during conversion of lignocellulose materials, since it is an intricate compound that is hard to degrade and causes serious problems to the environment [
4].
Lignin peroxidase, an oxidoreductase enzyme has recently attracted attention based on their high redox potential to oxidized compounds with resistance to degradation [
5]. These characteristics have motivated the use of lignin peroxidase in various textiles and pharmaceutical industry [
6,
7,
8,
9,
10]. However, the application of lignin peroxidase for industrial use are hampered by high cost of production and poor yield [
11].
Bacteria are classified as ‘’workhorse industrial microorganism’’ based on their enzymes production capability, safety, and exponential growth rate [
12]. Moreover, various bacterium has been reported in production of extracellular enzymes [
13,
14,
15]. Previously, some bacteria strains have been reported in the production of pectinolytic and cellulolytic enzyme [
16,
17,
18]. Bacterial strains (including
Streptomyces viridosporus, strains of
Nocardia and
Pseudomonas and
Raoultella ornithinolytica and
Ensifer adhaerens) capable of producing lignin modifying enzymes like Lignin Peroxidase, do have the potential to be exploited in the degradation of lignin [
19,
20,
21]. In our laboratory, six bacterial strains [
P.
aeruginosa (MF144461.1),
E.
coli (LR025096.1),
P.
aeruginosa (KR136350.1),
P.
aeruginosa (CP031449.2),
E.
xiangfangensis (MH304301.1) and
E.
kobei (CP032897.1)], exhibiting the potential of producing ligninolytic enzymes (lignin peroxidase) and degrading kraft lignin, have been isolated and characterized [
22]. However, low enzyme yield produced by the bacteria culture is a limitation for its application and commercialization.
Previous studies have shown that culture conditions can enhance various enzyme production [
23] The growth and enzyme production efficiency of an organism is contingent to the components, incubation temperature, incubation time and pH of the production medium [
9]. Recently, research have been geared toward optimization of the culture of some microorganism in other to promote the production of ligninolytic enzymes, including lignin peroxidase [
24]. Nonetheless, there is still paucity of successful large-scale production of lignin peroxidase from bacterial strain [
25]. Hence, this study is aimed at determining the culture condition including pH, temperature and concentration to optimize the production of lignin peroxidase by
Escherichia coli LR0250096.1 and
Pseudomonas aeruginosa CP031449.2. The results of such a study could be essential in the industrial application of the bacteria as an alternative approach to the reduction of lignocellulosic wastes.
2. Materials and Methods
2.1. Chemicals
The chemicals and reagents in this study were of analytical grades, unless otherwise stated. Nutrient both, MSM-L-glucose, nutrient agar, and salts were purchased from Merck, Darmstadt, Germany. The kraft lignin and Citrate phosphate buffer were supplied by Sigma Aldrich. St Louis, MO, USA. Bacteriological agar was bought from Lasec (SA).
2.2. Sample collections
The sample (soil, sawdust, and cow dung) were collected from Richard Bay, Kwazulu-Natal Province, South Africa (GPS coordinates; LA: -28.936164 and LO: 31.763771). The samples were stored in sterile container at 4 ºC until required for use. Ethical clearance (UZREC 171110-030 PGM 2018/575) was obtained from Research Committee, University of Zululand for the use of bacteria for research purpose.
2.3. Bacteria isolation
The bacteria were isolated from collected samples using the method of Sasikumar
et al. [
26], describe in detail elsewhere [
22]. Briefly, 1 g/L Kraft lignin was added to the minimal salts medium (MSM) with 5 g/L NH
4NO
3, 0.1 g/L yeast extract, 4.55 g/L K
2HPO
4, 0, 0.5 g/L MgSO
4 and 5.3 g/L KH
2PO
4 to produce MSM-L broth. The mixture was autoclaved for 15 minutes at 121 °C. Afterward, enrichment culture was made by adding 5 g of each sample respectively to 95 mL MSM-L broth in a 250 mL Erlenmeyer flask. The enriched culture was incubated in an orbital shaker (30 °C, 140 rpm, 168 hr). The enriched sample (1 mL) was mixed with sterilized NaCl (9 mL ,0.9%) at 25 °C. Nystatin (50 mg/L) was added to MSM-L agar plate to prevent fungal development. The agar plates were then incubated for 168 hr at 30 °C. The cultures were purified using streaking procedure, obtained single colonies were kept in glycerol (20%) at −80 °C until required.
2.4. Identification of isolated bacterium using 16S rDNA
The confirmation of isolated bacterial have been described in detail our previous study [
22] using PCR by amplifying the 16S rDNA and analyzing the sequenced products through BLAST Search (NCBI). Briefly, genomic DNA of the isolates was extracted from the cultures using the quick-DNA
TM Fugal/Bacterial Miniprep Kit (Zymo Research, Catalogue No. D6005). The extracted fragments were sequenced in the forward and reverse direction (Nimagen, BrilliantDye
TMTerminator Cycle Sequencing Kit V3.1, BRD3-1000) and purified (Zymo Research, ZR-96 DNA Sequencing Clean-up Kit
TM, Catalogue No. D4050). The purified fragments were then analysed using ABI 3500xI Genetic Analyzer. GLC Bio Main Workbench v7.6 was used to analyse the abI files generated by the ABI 3500XL Genetic Analyzer and the results were obtained by a BLAST search (NCBI).
The identified bacteria in our previous study were Escherichia coli LR0250096.1, Pseudomonas aeruginosa CP031449.2, Pseudomonas aeruginosa KR136350.1, Enterobacter xiangfangensis MH304301.1, Enterobacter kobei CP032897.1 and Pseudomonas aeruginosa KT799669.1 [
22].
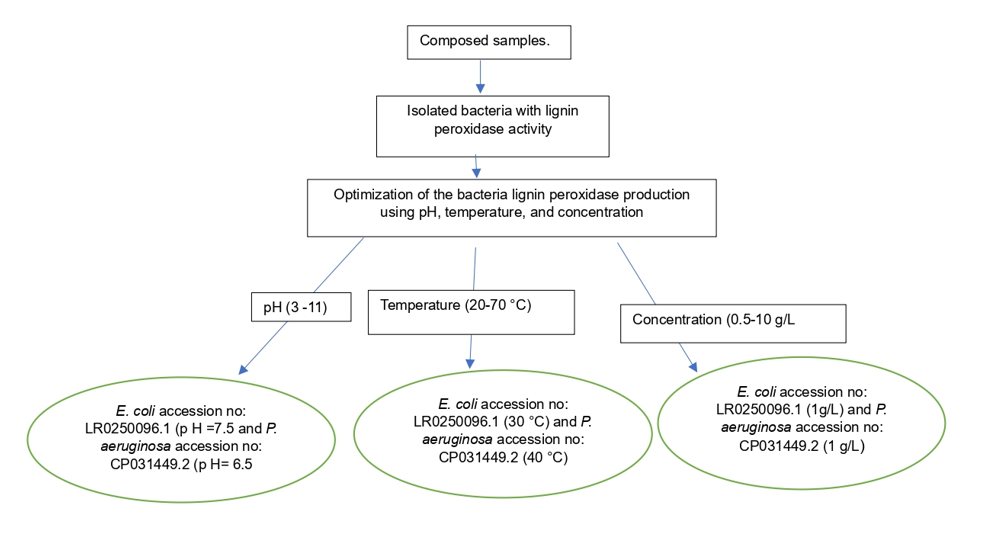
2.6. Optimization of physical parameters for enzyme production
Lignin peroxidase production by the isolates was optimized by adjusting various culture parameters including pH, temperature and concentration using the modified method described by [
27].
2.6.1. Optimization of the culture pH
Escherichia coli LR0250096.1, Pseudomonas aeruginosa CP031449.2 and the other microbial colonies, indicative of lignin degradation, were selected for the optimization of growth and enzyme production. Cultures were activated in nutrient broth and incubated at 30 °C for 48 hr. A 2 % standard inoculum of the batch was prepared in saline (O.D. 620 nm≈ 1.0). Two millilitres of the inoculum were inoculated into an autoclaved 1 L Erlenmeyer flask containing MSM-L-glucose (4.55 g/L K2HPO4, 0.53 g/L KH2PO4, 0.5 g/L MgSO4, 5 g/L NH4NO3, 1 g/L kraft lignin, 0.1 g/L yeast extract, 1% (w/v) glucose), and then incubated at different pH ranges from 3.0 – 11.0 on an orbital shaker (Infors HT Ecotron; Infors HT, Switzerland; 120 rpm for 168 hr at 30 °C).
2.6.2. Optimize the culture temperature.
Only the isolates with best pH condition were selected for temperature optimization studies. The experiments above were chosen to grow the organism at different temperature ranging from 20 °C – 70 °C on the orbital shaker. whereas the culture condition for pH and concentration selected were 7.5 and 2 g/l respectively.
2.6.2. Optimize the culture concentration.
Isolates with appreciable enzymatic activity in the above optimized culture temperature experiment were selected for culture concentration studies. The experiment describe above was adhered to, expect adjusting the concentration range (0.5, 1, 5, and 10 g/L). The pH and temperature parameters used in this study were 7.5 and 30 ºC respectively.
2.7. Optimized culture parameters for crude extract production
The optimal culture parameters (pH, temperature, lignin concentration) were used to produce the enzyme complex, using MSM-L-glucose medium in a submerged batch fermentation (1 L conical flasks). The production was done in duplicate for each isolate that showed high enzyme activity and bacterial growth. After 7 days of culture growth, cultures were filtered, and the filtrate centrifuged at 15000 rpm for 5 minutes. The supernatant was then freeze-dried (VirTrisbencbtop K: 6KBTEL-85). The resulting dry material was stored in the fridge at 4 °C and used as source of crude enzyme for subsequent experiments [
27].
2.8. Determination of bacterial growth
Bacterial growth was measured as absorbance at 620 nm using Biotek plate reader and after centrifugation (15000 rpm, 10 minutes, 4 °C). The peroxidase activity of the supernatant was measured by the rate of hydrogen peroxide-dependent oxidation of pyrogallol to purpurogallin [
28]. Pyrogallol (310 μL ;5% w/v) in 100 mM potassium phosphate buffer (pH 6) was added to of culture supernatant (25 μL). The reaction was then stimulated by adding 15 μL of 0.5% v/v of H
2O
2 (30% w/w)
2.9. Determination of protein concentration of produced enzyme
The protein concentration was determined using Bradford method [
29]. Bradford reagent consisting of 100 mg of Coomassie Brilliant Blue G-250; 5ml of ethanol (95 %); 100 ml of phosphoric acid (85 %) were diluted to one litre after dissolving the dye. Standard consisting range of 5 to 10 microgram of protein (albumin) were prepared in 100 microlitre volume. The Bradford reagent (5 ml) was then added and incubated for 5 minutes. The absorbance was read at 595 nm using Biotek plate reader. The results were used to draw the standard curve of absorbance against microgram protein. The crude enzymes obtained using the optimized parameter were weight and their protein concentration determined using the standard curve.
2.6. Data Analysis
The experiments were triplicated and expressed as mean ± standard deviation (SD). Comparison analysis by Dunn’s post hoc and one way ANOVA was carried by using GraphPad prism (Version 6). The results were considered statistically significant at P < 0.05.
3. Results
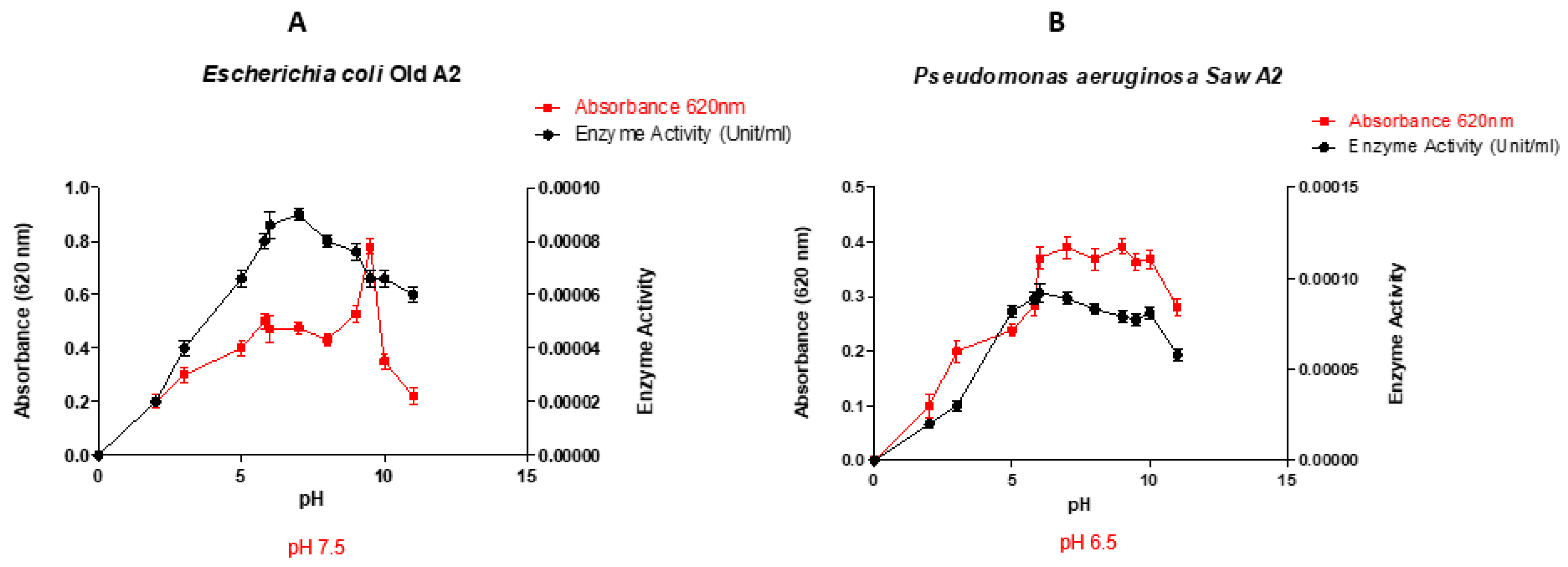
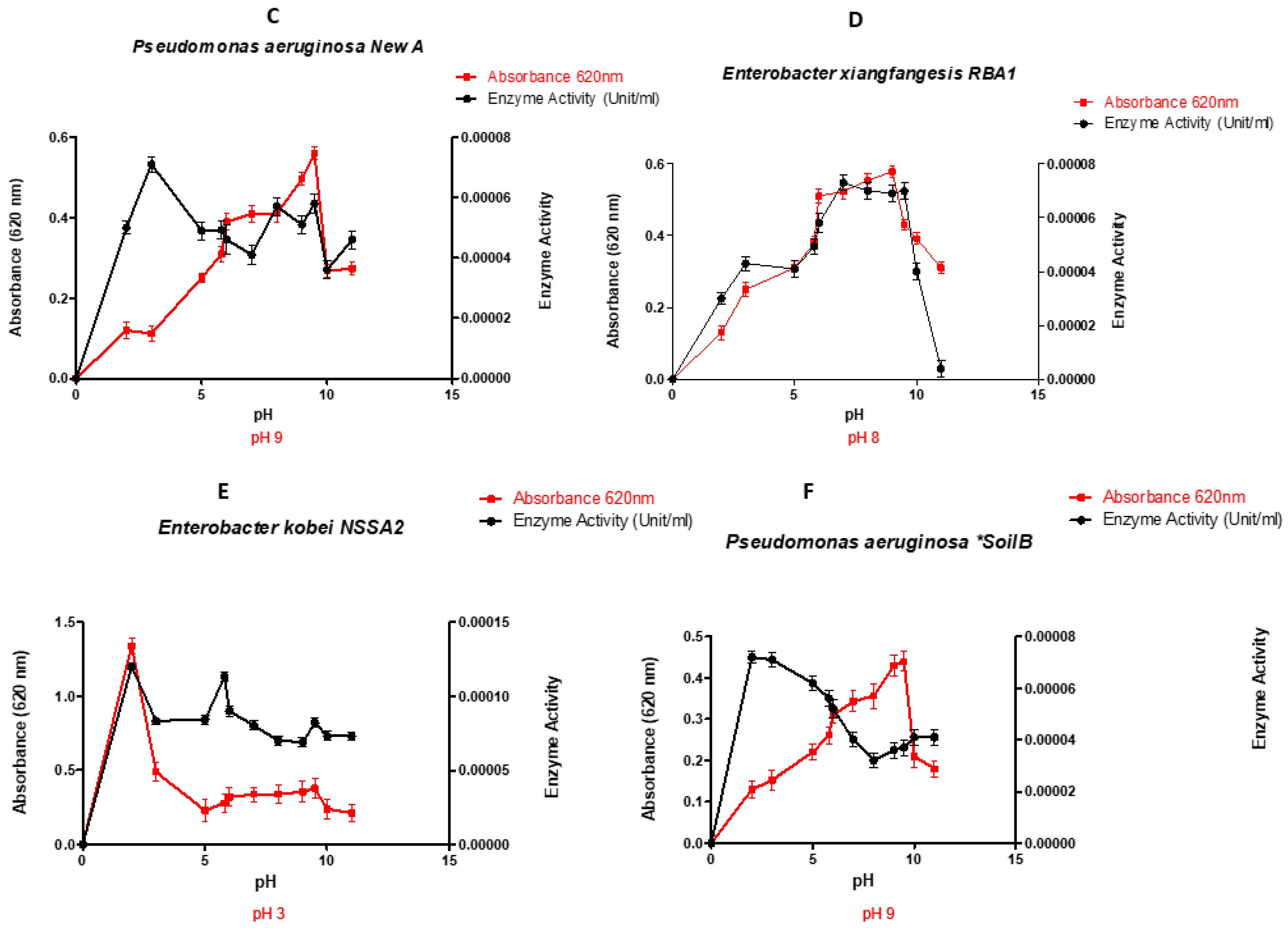
3.1. Effect of pH, on growth and production of lignin peroxidase
Figure 1 depicts the performance of the studied bacterial strain grown at different pH. In most cases, enzyme production was linked to bacterial growth, and they followed the expected bell-shaped profile, each bacterium peaking at its optimum pH. The observed optima pH for lignin peroxidase production were
Escherichia coli LR025096.1 pH 7.5;
Pseudomonas aeruginosa CP031449.2 pH 6.5;
Pseudomonas aeruginosa KR136350.1 pH 9;
Enterobacter xiangfangensis MH304301.1 pH 8;
Enterobacter kobei CP032897.1 pH 3 and
Pseudomonas aeruginosa KT799669.1 pH 9. However,
E. coli LR025096.1 and
P. aeruginosa CP031449.2 showed the highest enzymatic activity (activity ≥0.8 x10
-2 U/L). In addition,
Pseudomonas aeruginosa KR136350.1 and
Enterobacter xiangfangensis MH304301.1 displayed moderate enzymatic activity. Hence, four isolates including
P. aeruginosa CP031449.2,
E. coli LR025096.1,
Pseudomonas aeruginosa KR136350.1 and
Enterobacter xiangfangensis MH304301.1 with appreciable enzymatic activities were selected for optimum temperature study.
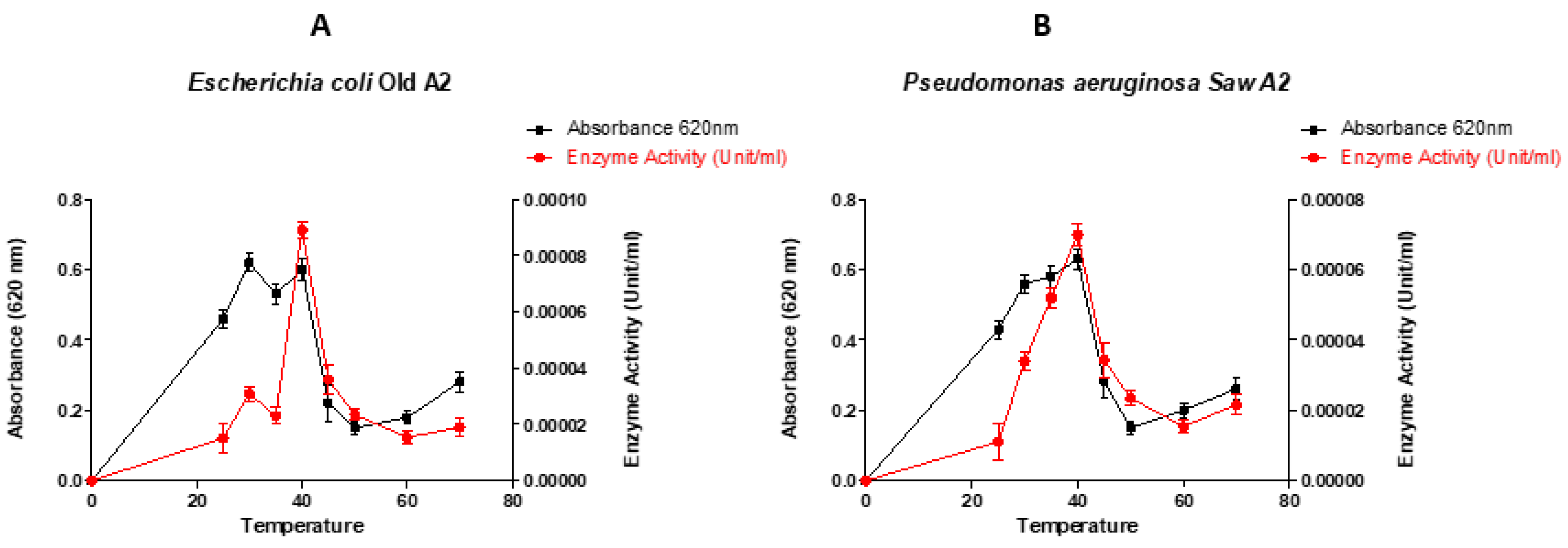
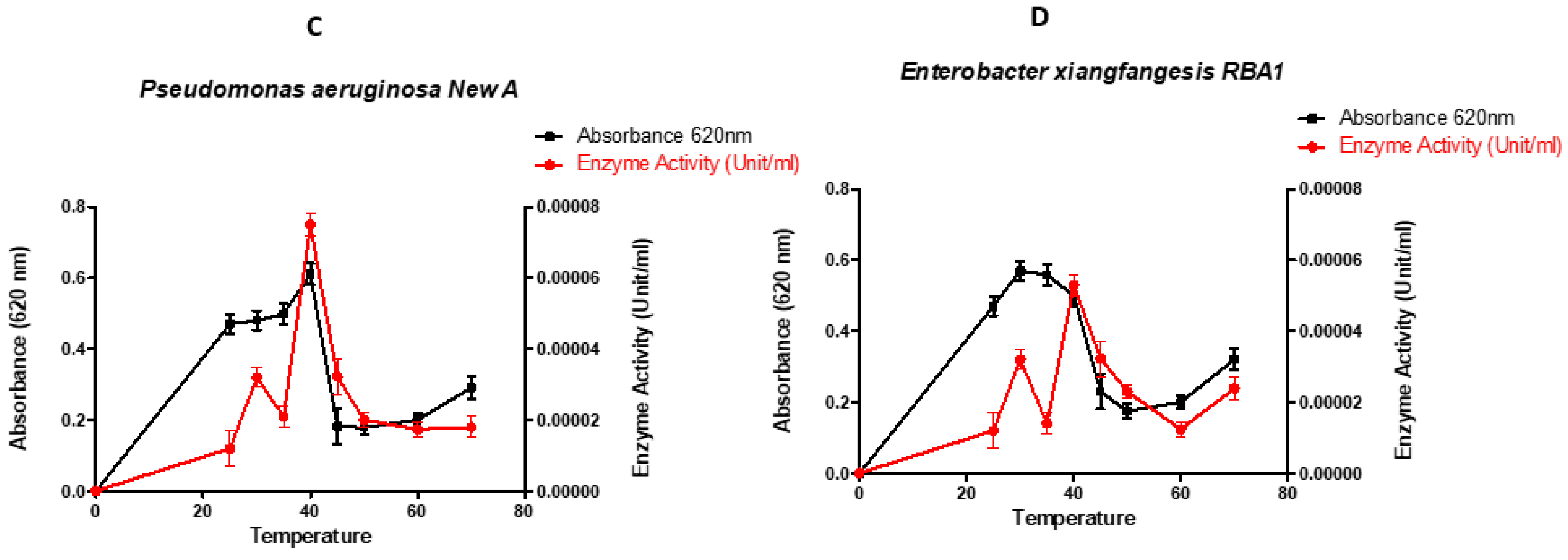
3.2. Effect of temperatures on lignin peroxidase production.
Optimization of temperature to produce lignin peroxidase, ranging from 20 °C to 70 °C (
Figure 2). The results reveal that the optimum temperature of the selected strains was 30 °C for
Escherichia coli LR025096.1, 40 °C for
Pseudomonas aeruginosa CP031449.2, 40 °C for
Pseudomonas aeruginosa KR136350.1 and 30 °C for
Enterobacter xiangfangensis MH304301.1.
Escherichia coli LR025096.1 and
Pseudomonas aeruginosa CP031449.2 showed highest enzymatic activity, an indicator of their lignin peroxidase efficacy. Meanwhile,
Pseudomonas aeruginosa KR136350.1 and
Enterobacter xiangfangensis MH304301.1 showed poor enzymatic activity. Therefore,
Escherichia coli LR025096.1 and
Pseudomonas aeruginosa CP031449.2 were selected for concentration optimum study.
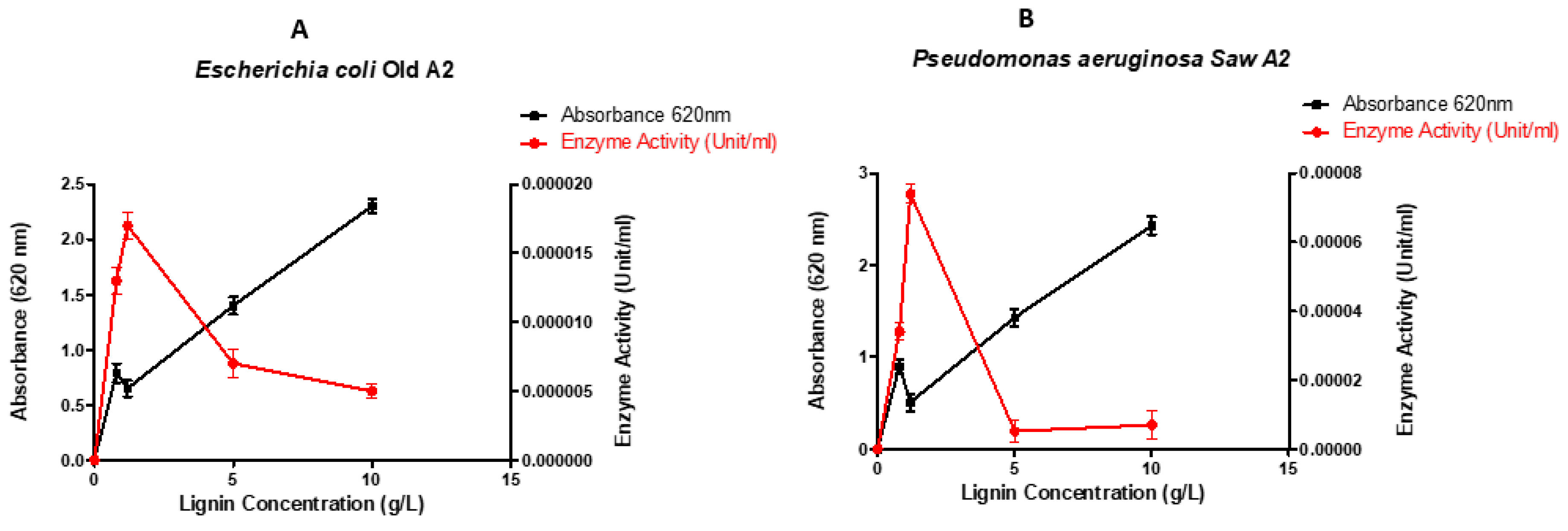
3.3. Optimization of lignin concentrations for lignin peroxidase production.
The concentration of the carbon source of the medium (lignin) was varied from 0.1 g/L to 10 g/L (
Figure 3). The results indicate that even though the growth of the organisms increased with increase in lignin concentration, lignin peroxidase activity decreased with lignin concentrations higher than 1 g/L. Therefore, 1 g/L of lignin concentration showed maximum lignin peroxidase activity of 1.7x10
-1 U/L for
Escherichia coli old A
2 LR025096.1 and 7.3x10
-1 U/L for
Pseudomonas aeruginosa Saw A2 CP031449.2.
3.4. Production of crude lignin peroxidase by E. coli and P. aeruginosa
The optimum pH, temperature and lignin concentrations were selected to separately culture the best performing ligninolytic bacteria (
Escherichia coli LR025096.1 and
Pseudomonas aeruginosa CP031449.2) to produce lignin peroxidase. The 7 days’ cultures were centrifuged and the supernatant freeze dried.
Table 1 showed the amount of crude extract obtained and protein content of the crude LiP preparation. The result revealed that the
E. coil showed higher non-significant (
P < 0.05) amount of crude extract than
P. aeruginosa. Likewise, the
E. coil showed significant (
P > 0.05) higher protein content in comparison with
P.
aeruginosa.
4. Discussion
Degradation of lignocellulosic material or lignin wastes, in most agriculturally based industries, are crucial to produce valuable products and prevention of lignocellulosic wastes or rigid lignin wastes [
30]. Isolation of ligninolytic bacteria and optimizing culture conditions, to improve produce ligninolytic enzymes during fermentation of lignocellulosic materials or lignin wastes to a usable product, is considered an attractive and alternative approach to reduce environmental waste challenges in most industries [
31]. Therefore, it is significant to explore lignin peroxidase source with high production capability.
The pH, temperature, carbon source, etc. are important culture conditions which determine bacterial growth and production of ligninolytic enzymes. Every bacterial species requires its own specific growth parameters which are largely determined by the required optimal culture conditions of its growth and enzyme activity [
32]. The lignin peroxidase production in this study was monitored by measuring the enzyme activity from the bacteria. Generally, changes in pH, temperature and carbon source for bacterial strains either badly affects or promotes both bacterial growth and enzyme activity [
33]. Therefore, the optimum culture conditions are most beneficial for the bacterial growth and high active enzyme yields. Inadequate optimum culture conditions can promote changes in the folding of enzymes or alter functional groups, leading to denaturation and terminating the enzyme activity [
34,
35].
The optimal pH values obtained in this study (
Figure 1) indicated that, except for
Pseudomonas aeruginosa CP031449.2 that showed maximum activity at pH 6.5, all the other selected isolates exhibited optimal activity in the alkaline pH range (pH 7.5-9). Previous study of Falade
et al. [
36] has revealed that the
Ensifer adhaerens NWODO-2 produces peroxidase at the optimum neutral pH (pH 7). while Zanirun
et al. [
37] has determined the acidic optimum pH of 3.5 that significantly influences the production of lignin peroxidase by
Pycnoporus sp. These finding also concur the work of Nour EL-Dein et al [
38] and Rob et al, [
39] in which optimum peroxidase activity by Streptomyces
sp. K37 and Streptomyces avermitilis UAH30 was obtained at pH 7.5 and 7 respectively. This indicate that pH plays crucial role in peroxidase production, and specific to bacteria strain [
40]. It is noteworthy that insight into optimum pH for large scale peroxidase production by selected bacteria are beneficial, reducing time and cost of peroxidase production [
41].
Bacteria growths are influenced by certain limit temperature. This characteristic enhances the macromolecular composition, intracellular metabolite, enzyme production and general growth rate [
24]. Hence, it is expedient to ascertain the temperature that support a particular bacteria strain to optimum enzyme production. In this study, for all strains cultured at their optimal pH, maximum bacterial growth and enzyme production occurred at about 40 °C (
Figure 2). At temperatures higher than 45 °C, a decrease in bacterial growth and lignin peroxidase production was observed. The observed diminish in enzyme activity above 45 °C was an indication of poor metabolic activities in the bacteria as well as onset of protein denature [
33]. This may subsequently hampered bacteria growth and enzyme production [
42,
43]. Based on the classification of bacteria by their growth temperature, it has been clear that all the selected bacterial strains (
Figure 2) were mesophilic organisms since they were able to live and thrive at moderate temperatures between 30 °C – 40 °C. These finding agrees with the work of Falade et al., [
36] that optimal peroxidase production by Ensifer adhaerens NWODO-2 isolates was achieved at mesophilic temperature. Similarly, temperature range of 37 °C – 40 °C have previously been reported in other bacterial species for optimum peroxidase production [
38,
44]. This infer that incubating temperature for optimising lignin peroxidase production are bacteria strain dependent [
45].
Carbon sources, like glucose, cellulose, and other glucose mixtures, play a vital role in improving the activity and the production of lignin peroxidase [
46]. This study reported that the isolated bacteria were able to degrade and utilize lignin for growth (
Figure 3). However, there seems to be substrate inhibition of the enzyme production as the amount of enzyme produced decreases with an increase in lignin concentration. It is also most likely that enough LiP is produced to hydrolyse just enough lignin (as carbon source) for growth. This finding concurs the report of Musengi et al., [
44] in which high concentration of veratryl alcohol in streptomyces sp. BSII#1 decreased lignin peroxidase production.
In a laboratory work that involve enzyme/protein extraction, determining the protein concentration is an important stage and it can assist in comparing the outcomes from one enzyme/protein to another and from one trial to the next. It is apparent (
Table 1) that the two bacteria studied (
Escherichia coli LR025096.1 and
Pseudomonas aeruginosa CP031449.2) could be cultivated to produce crude preparations that are active as Lignin Peroxidase.
5. Conclusion
Escherichia coli LR025096.1 and Pseudomonas aeruginosa CP031449.2 exhibited the potential to grow on kraft lignin and produce crude proteins that show lignin peroxidase activity when culture conditions including pH, temperature and concentration are optimized. These indicate proficiency of bacteria strain in biotechnology, especially in large scale production of lignin peroxidase. For further study, purification of crude enzyme and SDS page need to be investigated.
Author Contributions
Conceptualization, A.R.O and M.A.R.; methodology, A.R.O. F.O.O., S.L.D and M.A.R.; formal analysis, A.R.O., F.O.O and M.A.R.; investigation, S.L.D., F.O.O, M.A.R and A.R.O.; resources, A.R.O., S.L.D and F.O.O.; Data curation, F.O.O., S.L.D and A.O.I.; Writing- original draft preparation, S.L.D., A.R.O., M.A.R., A.O.L and F.O.O.; Writing – review and editing, S.L.D., F.O.O., M.A.R and A.R.O.; visualization, S.L.D., A.R.O and F.O.O.; supervision, A.R.O., M.A.R and F.O.O.; project administration, F.O.O, A.R.O and M.A.R.; Funding acquisition, S.L.D.;. All the authors have read and supported the published version of the manuscript.
Funding
The National Research Foundation (SA) sponsored the studies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Data Availability Statement
The datasets from this study are available on request to the corresponding authors.
Acknowledgments
The authors appreciate the support of Research Office of University of Zululand for their financial support. of SLD.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Petre, M.; Pătrulescu, F.; Teodorescu, R.I. Controlled cultivation of mushrooms on winery and vineyard wastes. In Mushroom Biotechnology; Academic Press: New York, NY, USA, 2016; pp. 31–47. [Google Scholar]
- Lee, S.; Kang, M.; Bae, J.H.; Sohn, J.H.; Sung, B.H. Bacterial Valorization of Lignin in Biorefinery Processes. Front. Bioeng. Biotechnol. 2019, 7, 2019. [Google Scholar]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. Biotechnology 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Falade, A.O.; Nwodo, U.U.; Iweriebor, B.C.; Green, E.; Mabinya, L.V.; Okoh, A.I. Lignin peroxidase functionalities and prospective applications. Microbiol. Open 2017, 6, e00394. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.B.; Wang, B.Q.; Wu, A.G.; Cheng, G.J.; Li, Z.; Dong, S.J. A method to construct a third-generation horseradish peroxidase biosensor; self-assembling gold nanoparticles to three-dimensional sol-gel network. Analytical Chemistry 2002, 74, 2217–2223. [Google Scholar] [CrossRef]
- Agostini, E.; Hernandez-Ruiz, J.; Arnao, M.B.; Milrand, S.R.; Tigier, H.A.; Acosta, M.A. Peroxidase isoenzyme secreted by turnip (Brassica napus) hairy-root culture inactivation by hydrogen peroxide and application in diagnostic kits. Biotechnol. Appl. Biochem. 2002, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, D.C.; Phugare, S.S.; Shedbalkar, U.U.; Adhar, J.P. Purification and characterization of a bacterial peroxidase from the isolated strain Pseudomonas sp. SUK1 and its application for textile dye decolourization. Ann. Microbiol. 2011, 61, 483–491. [Google Scholar]
- Draelos, Z.D. A split-face evaluation of a novel pigment-lightening agent compared with no treatment and hydroquinone. J. Am. Acad. Dermatol. 2015, 72, 105–107. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Lu-Chau, T.A.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Continuous removal of endocrine disruptors by versatile peroxidase using a two-stage system. Biotechnol. Prog. 2015, 31, 908–916. [Google Scholar] [CrossRef]
- Torres, E.; Bustos-Jaimes, I.; Le Borgne, S. Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl. Catal. B Environ. 2003, 46, 1–15. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, N.; Demirkan, E. . Production of protease by Bacillus sp. N-40 isolated from soil and its enzymatic properties. J. Biol. Environ. Sci. 2011, 5, 95–103. [Google Scholar]
- Barros, F.F.C.; Simiqueli, A.P.R.; de Andrade, C.J.; Pastore, G.M. Production of enzymes from agro industrial wastes by biosurfactant-producing strains of Bacillus subtilis. Biotechnol. Research. Int. 2013, 2013, 103960. [Google Scholar] [CrossRef] [PubMed]
- Pant, G.; Prakash, A.; Pavani, J.V.P.; Bera, S.; Deviram, G.V.N.S.; Kumar, A.; Panchpuri, M.; Prasuna, R.G. Production, optimization and partial purification of protease from Bacillus subtilis. J. Taibah Univ. Sci. 2015, 9, 50–55. [Google Scholar] [CrossRef]
- Soares, M.M.C.N.; Da Silva, R.; Carmona, E.C.; Gomes, E. Pectinolytic enzyme production by Bacillus species and their potential application on juice extraction. World J. Microbiol. Biotechnol. 2001, 17, 79–82. [Google Scholar] [CrossRef]
- Dias, P.V.S.; Ramos, K.O.; Padilha, I.Q.M.; Araújo, D.A.M.; Santos, S.F.M.; Silva, F.L.H. Optimization of cellulase production by Bacillus sp. isolated from sugarcane cultivated soil. Chem. Eng. Trans. 2014, 38, 227–282. [Google Scholar]
- Padilha, I.Q.M.; Carvalho, L.C.T.; Dias, P.V.S.; Grisi, T.C.S.L.; Honorato da Silva, F.L.S.; Santos, F.M.; Araújo, D.A.M. Production and characterization of thermophilic carboxymethyl cellulase synthesized by Bacillus sp. growing on sugarcane bagasse in submerged fermentation. Braz. J. Chem. Eng. 2015, 32, 35–42. [Google Scholar] [CrossRef]
- Zimmermann, W. Degradation of lignin by bacteria. J. Biotechnol. 1990, 13, 119–130. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef]
- Falade, A.O.; Eyisi, O.A.; Mabinya, L.V.; Nwodo, U.U.; Okoh, A.I. Peroxidase production and ligninolytic potentials of freshwater bacteria. Raoultella ornithinolytica and Ensifer adhaerens. Biotechnol. Rep. 2017, 16, 12–17. [Google Scholar] [CrossRef]
- Dube, S.L.; Osunsanmi, F.O.; Ngcobo, B.P.; Mkhwanazi, L.B.; Jobe, Z.Z.; Aruleba, R.T.; Mosa, R.A.; Opoku, A.R. Isolation and Characterization of Potential Lignin Peroxidase-Producing Bacteria from Compost Samples at Richards Bay (South Africa). Pol. J. Microbiol. 2023, 72, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second generation bioethanol production: concepts and recent developments. Biotechnology 2014, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Falade, A.O.; Mabinya, L.V.; Okoh, A.I.; Nwodo, U.U. Studies on peroxidase production and detection of Sporotrichum thermophile-like catalase-peroxidase gene in a Bacillus species isolated from Hogsback Forest reserve, South Africa. Heliyon 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Wi, S.G.; Singh, A.P.; Kim, Y.S. Micromorphological characteristics of decayed wood and laccase produced by the brown-rot fungus Coniophora puteana. J. Wood Sci. 2004, 50, 281–284. [Google Scholar] [CrossRef]
- Sasikumar, V.; Priya, V.; Shiv, S.C.; Sathish, S.D. Isolation and preliminary screening of lignin degrading microbes. J. Acad.. Ind. Res. 2014, 3, 291–294. [Google Scholar]
- Lambertz, C.; Ece, S.; Fischer, R.; Commandeur, U. Progress and obstacles in the production and application of recombinant lignin-degrading peroxidases. Bioengineered 2016, 7, 145–54. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.R. Production and purification of lignin peroxidase from Bacillus megaterium and its application in bioremediation. CIBTech J. Microbiol. 2014, 3, 22–28. [Google Scholar]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pothiraj, C.; Kanmani, P.; Balaji, P. Bioconversion of lignocellulose materials. Mycobiology 2006, 34, 159–165. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; Setty, Y.P. Extraction of ligninolytic enzymes from novel Klebsiella pneumoniae strains and its application in wastewater treatment. Appl. Water Sci. 2018, 8, 111–117. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska-Tkaczyk, K. Growth conditions, physiological properties, and selection of optimal parameters of biodegradation of anticancer drug daunomycin in industrial effluents by Bjerkandera adusta CCBAS930. Int. Microbiol. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dutta, H.; Dutta, A. The microbial aspect of climate change. Energy Ecol. Environ. 2016, 1, 209–232. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Moreira Neto, S.L.; Matheus, D.R.; Machado, K.M.G. Influence of pH on the growth, laccase activity and RBBR decolorization by tropical basidiomycetes. Braz. Arch. Biol. Technol. 2009, 52, 1075–1082. [Google Scholar] [CrossRef]
- Falade, A.; Jaouani, A.; Mabinya, L.; Okoh, A.; Nwodo, U. Exoproduction and molecular characterization of peroxidase from Ensifer adhaerens. Appl. Sci. 2019, 9, 3121. [Google Scholar] [CrossRef]
- Zanirun, Z.; Abd-Aziz, S.; Ling, F.H.; Hassan, M.A. Optimisation of lignin peroxidase production using locally isolated Pycnoporus sp. through factorial design. Biotechnology (Faisalabad), 2009, 8, 296–305. [Google Scholar] [CrossRef]
- Nour El-Dein, M.M.; Shereif, A.E.A.; Mansour, F.A.; Abou-Dobara, M.I.; Ball, A.S. . Optimization of xylanase and peroxidase production from Streptomyces sp. K37. J. Biosci. Biotechnol. 2014, 3, 29–42. [Google Scholar]
- Rob, A.; Hernandez, M.; Ball, A.S.; Tuncer, M.; Arias, M.E.; Wilson, M.T. Production and partial characterization of extracellular peroxidase produced by Streptomyces avermitilis UAH30. Appl. Biochem. Biotechnol. 1997, 62, 159–174. [Google Scholar] [CrossRef]
- Niladevi, K.N.; Prema, P. Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour. Technol. 2008, 99, 4583–4589. [Google Scholar] [CrossRef]
- Xia, S.Q.; Zhang, Z.Q.; Wang, X.J.; Yang, A.M.; Chen, L.; Zhao, J.; Leonard, D.; Jaffrezic-Renault, N. Production and characterization of a bioffloculant by Proteus mirabilis TJ-1. Bioresources and Technology 2008, 99, 6520–6527. [Google Scholar] [CrossRef]
- Ray, A.K.; Bairagi, A.; Ghosh, K.S.; Sen, S.K. Optimization of fermentation conditions for cellulase production by Bacillus subtilis CY5 and Bacillus circulans TP3 isolated from fish gut. Acta Ichthyol. Piscat. 2007, 3, 47–53. [Google Scholar] [CrossRef]
- Tandon, D.; Sharma, N. Isolation of potential novel cellulolytic and xylanolytic bacteria and optimization of their cultural conditions for enhanced production of cellulase and xylanase. J. Agro Aliment. Process Technol. 2014, 20, 231–240. [Google Scholar]
- Musengi, A.; Khan, N.; Le Roes-Hill, M.; Pletschke, B.I.; Burton, S.G. Increasing the scale of peroxidase production by Streptomyces sp. strain BSII#1. J. Appl. Microbiol. 2014, 116, 554–562. [Google Scholar] [PubMed]
- Gautam, S.P. , Bundela, P.S., Pandey, A.K., Khan, J., Awasthi, M.K., et al., 2011. Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol. Res. Int. 2011, 1–8. [Google Scholar] [CrossRef]
- Singh, A.D.; Kumar, S.R. Ligninolytic fungal laccase and their biotechnological applications. Appl. Biochem. Biotechnol. 2010, 160, 1760–1788. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).