Submitted:
17 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
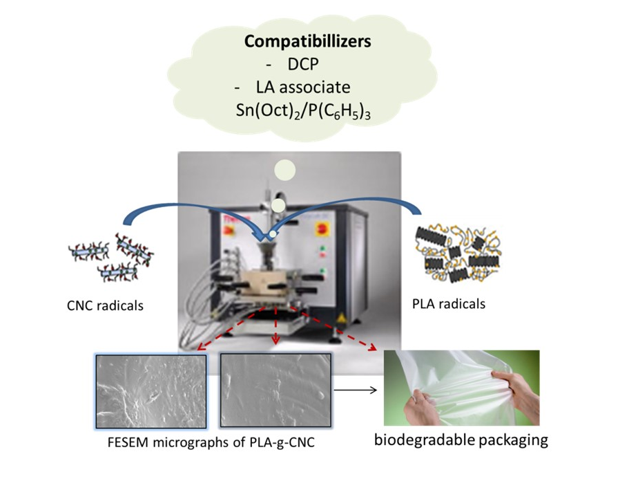
Keywords:
1. Introduction
2. Experimental
2.1. Chemical reagents
2.2. CNC preparation
2.3. Nanocomposite manufacturing
2.4. Characterization
3. Result and discursion
3.1. Composite processing
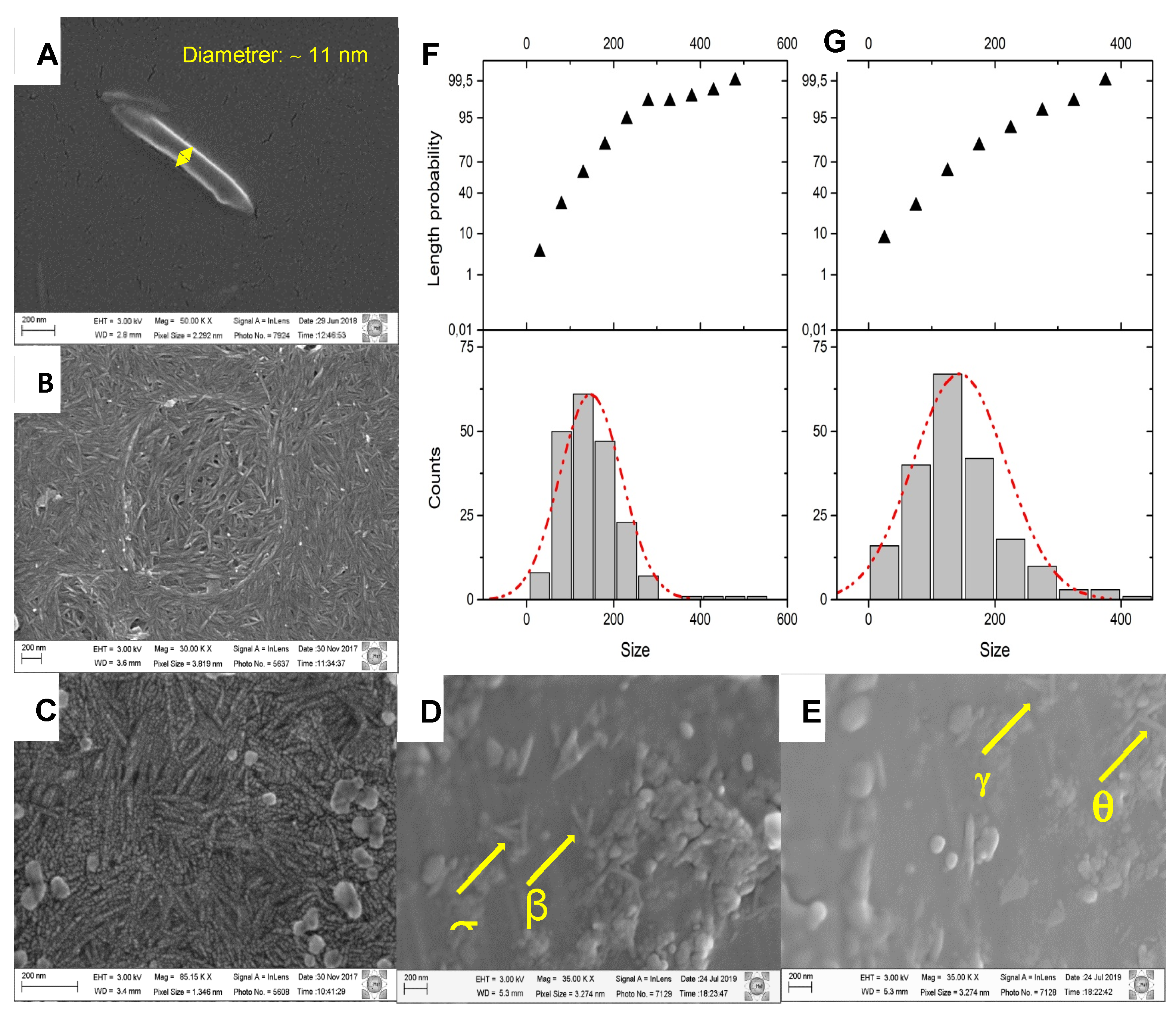
3.2. Field emission scanning electron microscopy (FESEM)
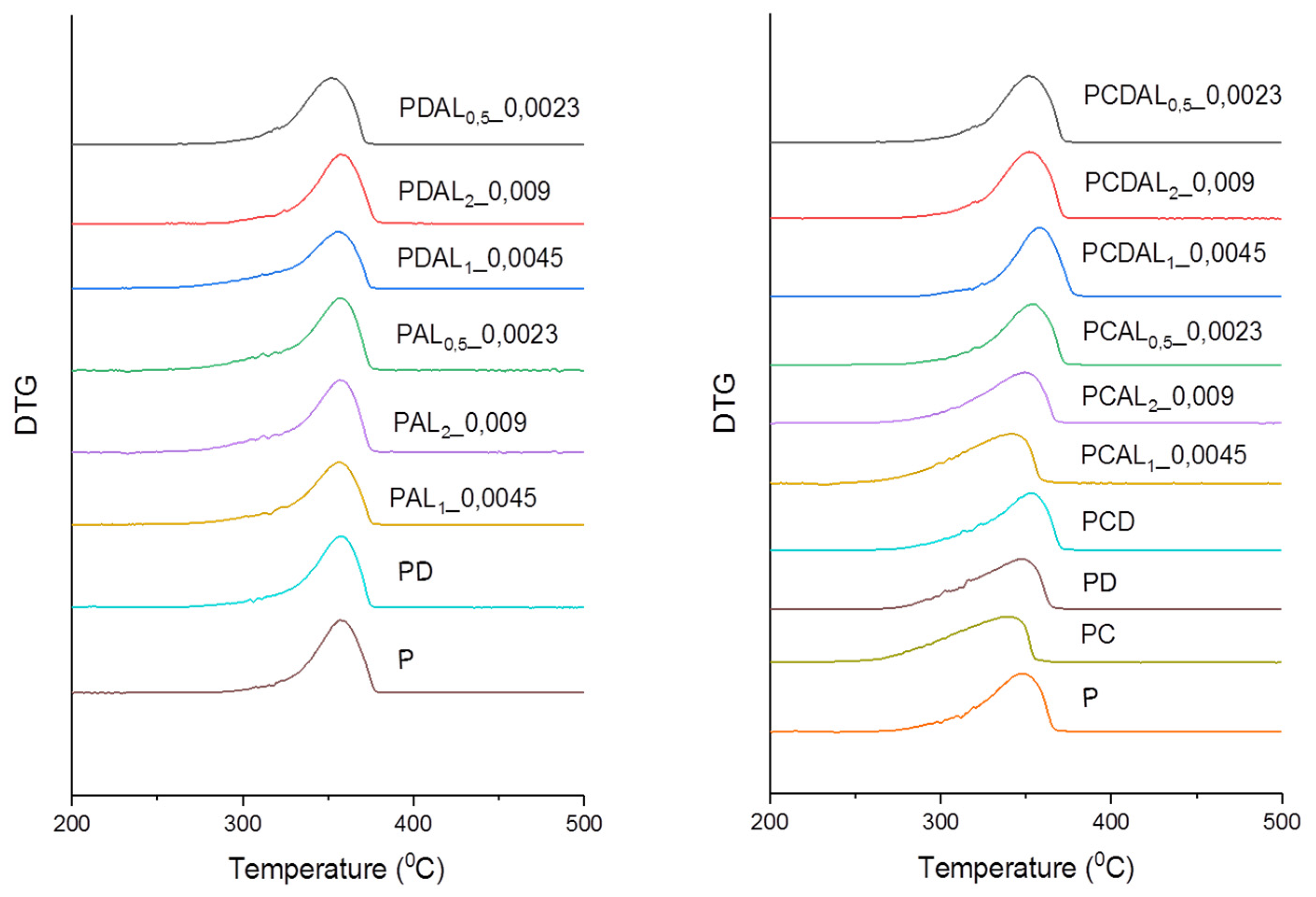
3.3. Thermogravimetric analysis (TGA)
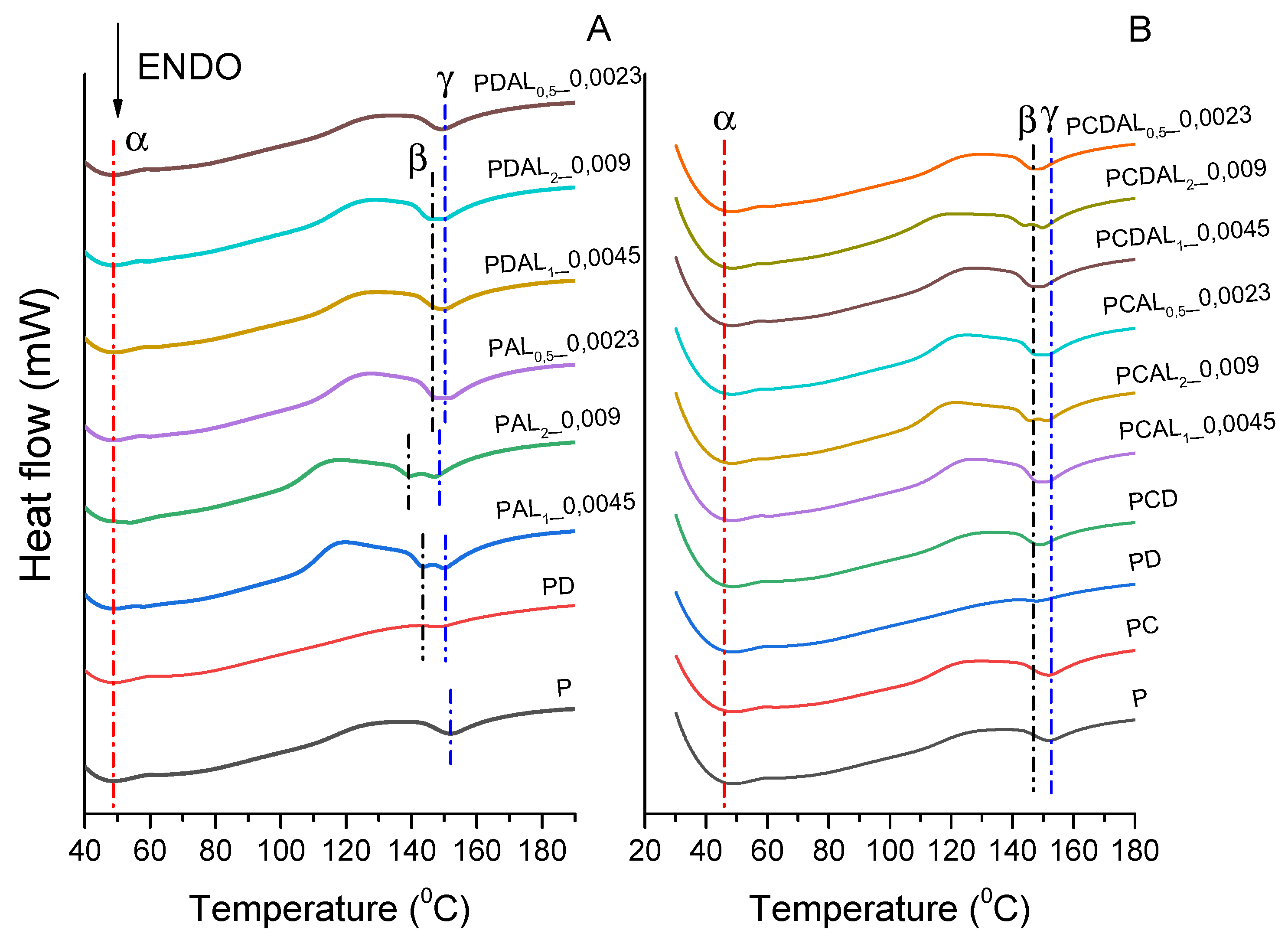
3.4. Differential scanning calorimetry (DSC)
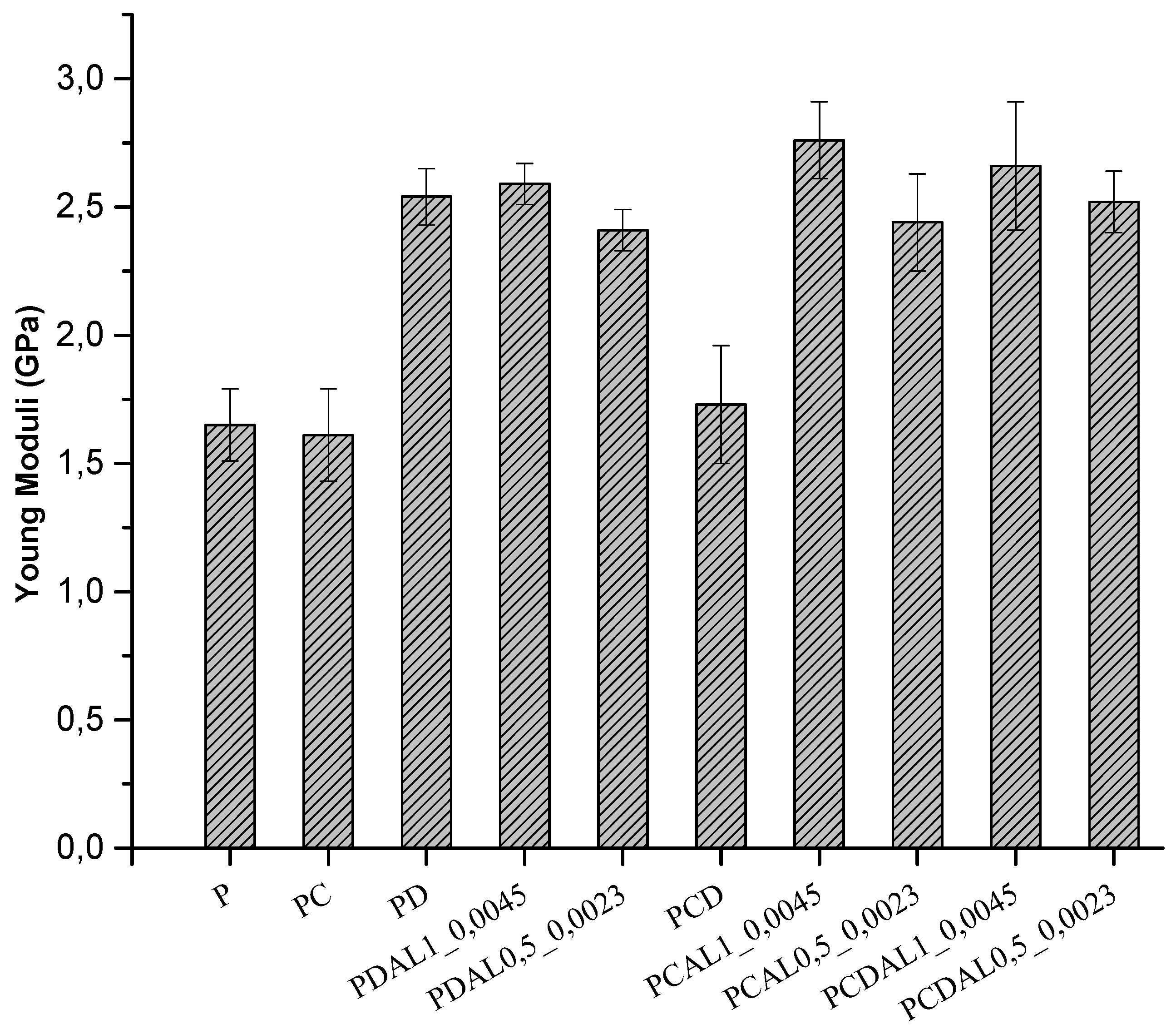
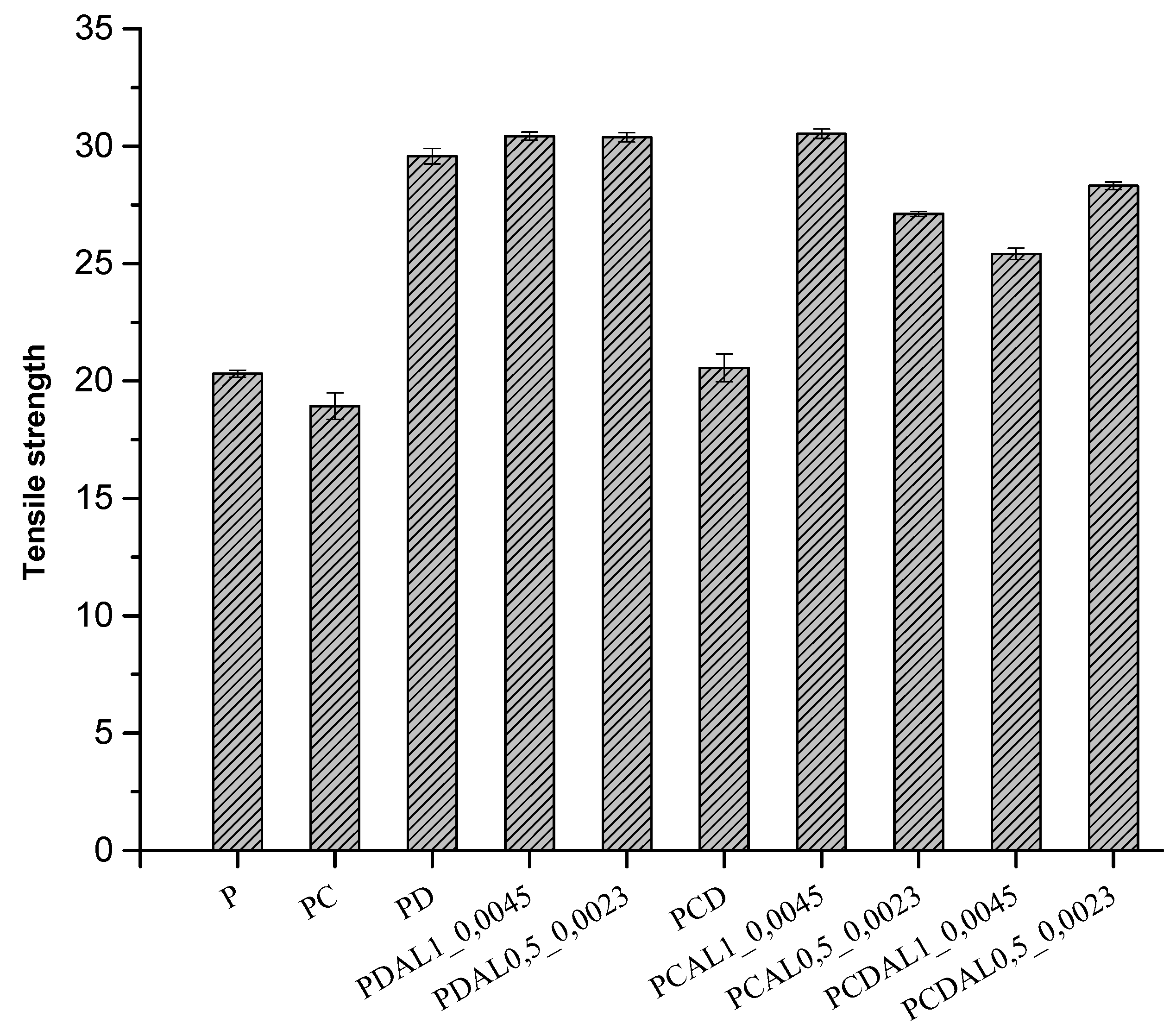
3.5. Mechanical characterization
4. Result and discursion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vatansever, E.; Arslan, D.; Nofar, M. Polylactide Cellulose-Based Nanocomposites. Int J Biol Macromol 2019, 137, 912–938. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime Prediction of Biodegradable Polymers. Prog Polym Sci 2017, 71, 144–189. [Google Scholar] [CrossRef]
- A Review on the Polymeric Laminates Reinforced with Natural Fibers Journal of Reinforced Plastics and Composites.Pdf.
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally Recyclable Polylactic Acid/Cellulose Nanocrystal Films through Reactive Extrusion Process. Polymer (United Kingdom) 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Sypaseuth, F.D.; Gallo, E.; Çiftci, S.; Schartel, B. Polylactic Acid Biocomposites: Approaches to a Completely Green Flame Retarded Polymer. E-Polymers 2017, 17, 449–462. [Google Scholar] [CrossRef]
- Zhou, Y.; Katsou, E.; Fan, M. International Journal of Biological Macromolecules Interfacial Structure and Property of Eco-Friendly Carboxymethyl Cellulose/Poly ( 3-Hydroxybutyrate- Co -3-Hydroxyvalerate) Biocomposites. Int J Biol Macromol 2021, 179, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Dickmann, M.; Tarter, S.; Egger, W.; Pegoretti, A.; Rigotti, D.; Brusa, R.S. Interface Nanocavities in Poly (Lactic Acid) Membranes with Dispersed Cellulose Nanofibrils : Their Role in the Gas Barrier Performances. Polymer (Guildf) 2020, 202, 122729. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular Imprinted Polymers for Separation Science: A Review of Reviews. J Sep Sci 2013, 36, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dutta, S.; Kwon, S.; Kwon, T.; Patil, S.S.; Kumari, N.; Jeevanandham, S.; Lee, I.S. Solid-State Reaction Synthesis of Nanoscale Materials: Strategies and Applications. Chem Rev 2022. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Pineda, A.; Lin, C.S.K.; Franchi, M.; Trotta, P.; Romero, A.A.; Luque, R. Natural Porous Agar Materials from Macroalgae. Carbohydr Polym 2013, 92, 1555–1560. [Google Scholar] [CrossRef]
- Lima, L.R.; Santos, D.B.; Santos, M.V.; Barud, H.S.; Henrique, M.A.; Pasquini, D.; Pecoraro, E.; Ribeiro, S.J.L. Cellulose Nanocrystals from Bacterial Cellulose Nanocristais de Celulose a Partir de Celulose Bacteriana. Quim Nova 2015, 38, 1140–1147. [Google Scholar] [CrossRef]
- De Jesus Silva, D.; D’Almeida, M.L.O. O Papel (Brazil). 2009, pp. 34–52.
- Gutiérrez, M.C.; Rosa, P.T.V.; De Paoli, M.-A.; Felisberti, M.I. Biocomposites Based on Cellulose Acetate and Short Curaua Fibers Treated with Supercritical CO2 Biocompósitos de Acetato de Celulose e Fibras Curtas de Curauá Tratadas Com CO2 Supercrítico. Polimeros 2012, 22, 295–302. [Google Scholar] [CrossRef]
- Carvalho, A. O Papel (Brazil). 2008, pp. 36–41.
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Effect of Cellulose Nanocrystal Polymorphs on Mechanical, Barrier and Thermal Properties of Poly(Lactic Acid) Based Bionanocomposites. RSC Adv 2015, 5, 60426–60440. [Google Scholar] [CrossRef]
- Dhar, P.; Gaur, S.S.; Soundararajan, N.; Gupta, A.; Bhasney, S.M.; Milli, M.; Kumar, A.; Katiyar, V. Reactive Extrusion of Polylactic Acid/Cellulose Nanocrystal Films for Food Packaging Applications: Influence of Filler Type on Thermomechanical, Rheological, and Barrier Properties. Ind Eng Chem Res 2017, 56, 4718–4735. [Google Scholar] [CrossRef]
- Bitinis, N.; Fortunati, E.; Verdejo, R.; Bras, J.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(Lactic Acid) Natural Rubber Cellulose Nanocrystal Bionanocomposites. Part II: Properties Evaluation. Carbohydr Polym 2013, 96, 621–627. [Google Scholar] [CrossRef]
- Shehzad, Q.; Liu, Z.; Zuo, M.; Wang, J. The Role of Polysaccharides in Improving the Functionality of Zein Coated Nanocarriers: Implications for Colloidal Stability under Environmental Stresses. Food Chem 2024, 431. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Shahi, N.C.; Lohani, U.C.; Pathania, S.; Malik, S.; Singh, S.; Amin, T. Cellulose Nanocrystals Reinforced Chitosan Titanium Dioxide Bionanocomposite with Enhanced Interfacial Compatibility: Fabrication, Characterization, and Application in Fresh-Cut Apple Slices. Int J Biol Macromol 2023, 253. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and Biotic Environmental Degradation of the Bioplastic Polymer Poly(Lactic Acid): A Review. Polym Degrad Stab 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose Nanomaterial Reinforced Polymer Nanocomposites. Curr Opin Colloid Interface Sci 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Yesilkir-Baydar, S. Characterization of Gelatin-Based Wound Dressing Biomaterials Containing Increasing Coconut Oil Concentrations. J Biomater Sci Polym Ed 2023. [Google Scholar] [CrossRef]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial Nanocomposite Films Made of Poly(Lactic Acid)-Cellulose Nanocrystals (PLA-CNC) in Food Applications: Part A-Effect of Nisin Release on the Inactivation of Listeria Monocytogenes in Ham. Cellulose 2014, 21, 1837–1850. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent Developments on Nanocellulose Reinforced Polymer Nanocomposites: A Review. Polymer (United Kingdom) 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Dhar, P.; Bhasney, S.M.; Kumar, A. Katiyar, Vimal. POLYMER. 2016, pp. 75–92.
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol Adv 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA Composites: From Production to Properties. Adv Drug Deliv Rev 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. Biopolymers - Applications and Trends; 2015; ISBN 9780323266987.
- Hartmann, M.H. High Molecular Weight Polylactic Acid Polymers BT - Biopolymers from Renewable Resources. 1998; 367–411. [Google Scholar] [CrossRef]
- Jeziórska, R. Effects of Bis(2-Oxazoline) Derivative as Compatibilizer and Reactive Extrusion Conditions on the Properties of Polyamide 6/Poly(Ethylene Terephthalate) Blends | Wpływ Pochodnej Bis(2-Oksazoliny) Jako Kompatybilizatora Oraz Warunków Wytłaczania Reaktywn. Polimery/Polymers 2006, 51, 351–358. [Google Scholar] [CrossRef]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent Polymers: A Review on the Characteristics and Applications of Synthetic, Polysaccharide-Based, Semi-Synthetic and ‘Smart’ Derivatives. Eur Polym J 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Review - Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol 2019, 1–18. [Google Scholar]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int J Polym Sci 2011, 1–35. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically Alloyed Nanocomposites. Prog Mater Sci 2013, 58, 383–502. [Google Scholar] [CrossRef]
- Ahmad, E.E.M.; Luyt, A.S. Morphology, Thermal, and Dynamic Mechanical Properties of Poly(Lactic Acid)/Sisal Whisker Nanocomposites. Polym Compos 2012, 33, 1025–1032. [Google Scholar] [CrossRef]
- Muñoz, P.A.R.; Bettini, S.H.P. Assessment of the Utilization of Different Peroxide Dispersion Media on the Controlled Degradation of Polypropylene. J Appl Polym Sci 2013, 127, 87–95. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.; Jérôme, R. New Developments on the Ring Opening Polymerisation of Polylactide. Ind Crops Prod 2000, 11, 265–275. [Google Scholar] [CrossRef]
- Raquez, J.M.; Narayan, R.; Dubois, P. Recent Advances in Reactive Extrusion Processing of Biodegradable Polymer-Based Compositions. Macromol Mater Eng 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Ahmad, E.E.M.; Luyt, A.S. Morphology, Thermal, and Dynamic Mechanical Properties of Poly(Lactic Acid)/Sisal Whisker Nanocomposites. Polym Compos 2012, 33, 1025–1032. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Goffin, A.; Raquez, J.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From Interfacial Ring-Opening Polymerization to Melt Processing of Cellulose Nanowhisker-Filled Polylactide-Based Nanocomposites. BIOMACROMOCULES 2011, 2456–2465. [Google Scholar] [CrossRef]
- Raquez, J.M.; Murena, Y.; Goffin, A.L.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-Modification of Cellulose Nanowhiskers and Their Use as Nanoreinforcers into Polylactide: A Sustainably-Integrated Approach. Compos Sci Technol 2012, 72, 544–549. [Google Scholar] [CrossRef]
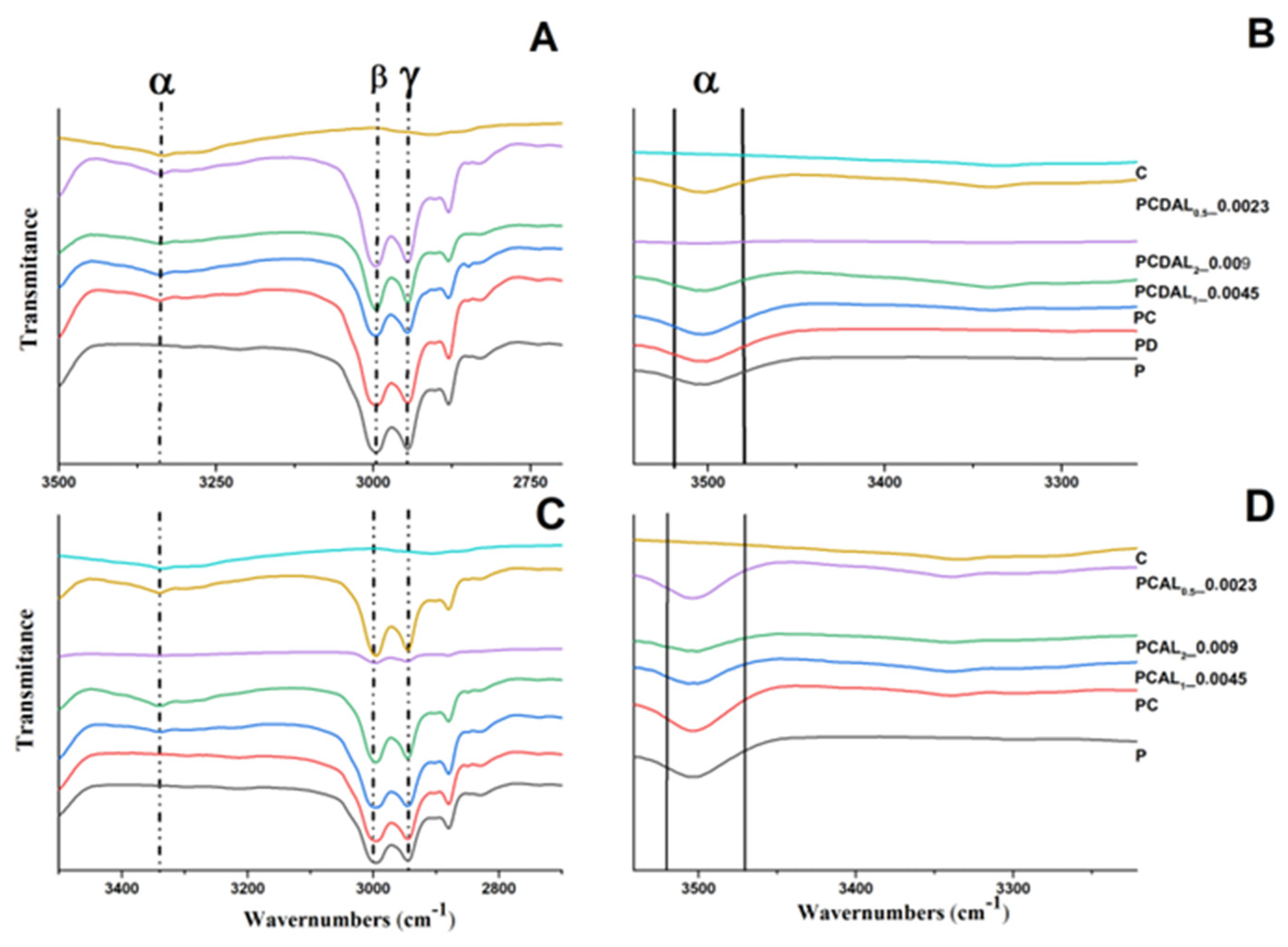
| ID | Sample | PLA (%) | LA (%) | CNC (%) | DCP (%) | P(C6H5)3 (%) | Sn(Oct)2 (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | P | PLA | 100 | [4,16] | |||||
| 1 | PC | PLA+CNC | 98 | 2.00 | [4,35] | ||||
| 2 | PD | PLA+DCP | 99.01 | 0.99 | [35,36] | ||||
| 3 | PAL1_0.0045 | PLA+AL1 | 98.99 | 1.00 | 0.0045 | 0.0045 | [37,38] | ||
| 4 | PAL2_0.009 | PLA+AL2 | 97.98 | 2.00 | 0.0090 | 0.0090 | [37,38] | ||
| 5 | PAL0.5_0.0023 | PLA+AL0.5 | 99.5 | 0.50 | 0.0023 | 0.0023 | [37,38] | ||
| 6 | PDAL1_0.0045 | PLA+DCP+AL1 | 98.01 | 1.00 | 0.98 | 0.0045 | 0.0045 | [4,37] | |
| 7 | PDAL2_0.009 | PLA+DCP+AL2 | 97.01 | 2.00 | 0.97 | 0.0090 | 0.0090 | [4,37] | |
| 8 | PDAL0.5_0.0023 | PLA+DCP+AL0.5 | 98.51 | 0.50 | 0.99 | 0.0023 | 0.0023 | [4,37] | |
| 9 | PCD | PLA+CNC+DCP | 97.03 | 2.00 | 0.97 | [35,36] | |||
| 10 | PCAL1_0.0045 | PLA+CNC+AL1 | 96.99 | 1.00 | 2.00 | 0.0045 | 0.0045 | [38] | |
| 11 | PCAL2_0.009 | PLA+CNC+AL2 | 95.98 | 2.00 | 2.00 | 0.96 | 0.0090 | 0.0090 | [38] |
| 12 | PCAL0.5_0.0023 | PLA+CNC+AL0.5 | 97.5 | 0.50 | 2.00 | 0.95 | 0.0023 | 0.0023 | [38] |
| 13 | PCDAL1_0.0045 | PLA+CNC+DCP+AL1 | 96.03 | 1.00 | 2.00 | 0.97 | 0.0045 | 0.0045 | [35,36] |
| 14 | PCDAL2_0.009 | PLA+CNC+DCP+AL2 | 95.03 | 2.00 | 2.00 | 0.0090 | 0.0090 | [35,36] | |
| 15 | PCDAL0.5_0.0023 | PLA+CNC+DCP+AL0.5 | 96.53 | 0.50 | 2.00 | 0.0023 | 0.0023 | [35,36] |
| ID | ΔHm (J/g) | Xc(%) | ONSET | Maximum temperature pick degradation (°C) (PMD) | |||
|---|---|---|---|---|---|---|---|
| T (°C) | Wt% | T1/2 | Wt% | ||||
| 0 | P | 21.43 | 23.02 | 319 | 93 | 353 | 29 |
| 1 | PC | 19.68 | 21.52 | 326 | 93 | 354 | 26 |
| 2 | PD | 5.52 | 5.99 | 336 | 93 | 360 | 29 |
| 3 | PAL1_0.0045 | 30.74 | 33.35 | 327 | 94 | 360 | 20 |
| 4 | PAL2_0.009 | 29.46 | 32.30 | 328 | 95 | 358 | 17 |
| 5 | PAL0.5_0.0023 | 29.21 | 31.54 | 331 | 95 | 357 | 19 |
| 6 | PDAL1_0.0045 | 23.55 | 25.81 | 329 | 94 | 358 | 26 |
| 7 | PDAL2_0.009 | 30.20 | 33.44 | 331 | 93 | 359 | 22 |
| 8 | PDAL0.5_0.0023 | 24.79 | 27.03 | 336 | 94 | 360 | 28 |
| 9 | PCD | 22.56 | 24.97 | 327 | 94 | 356 | 28 |
| 10 | PCAL1_0.0045 | 28.32 | 31.37 | 315 | 94 | 351 | 25 |
| 11 | PCAL2_0.009 | 28.78 | 32.22 | 302 | 94 | 344 | 16 |
| 12 | PCAL0.5_0.0023 | 28.23 | 31.10 | 321 | 93 | 353 | 25 |
| 13 | PCDAL1_0.0045 | 28.01 | 31.66 | 312 | 95 | 348 | 25 |
| 14 | PCDAL2_0.009 | 26.14 | 29.55 | 296 | 95 | 342 | 17 |
| 15 | PCDAL0.5_0.0023 | 26.53 | 29.52 | 319 | 95 | 349 | 26 |
| ID | Tm (oC) | Tc (oC) | Tg (oC) | Xc (%) | ||
|---|---|---|---|---|---|---|
| Tm1 (oC) | Tm2 (oC) | |||||
| 0 | P | 152 | - | 113 | 59 | 23.02 |
| 1 | PC | 151 | - | 128 | 58.8 | 21.52 |
| 2 | PD | 148 | - | 141 | 58.6 | 5.99 |
| 3 | PAL1_0.0045 | 144 | 150 | 119 | 55.3 | 33.35 |
| 4 | PAL2_0.009 | 140 | 147 | 117 | 56.7 | 32.30 |
| 5 | PAL0.5_0.0023 | 148 | 151 | 127 | 57 | 31.54 |
| 6 | PDAL1_0.0045 | 149 | - | 128 | 57.8 | 25.81 |
| 7 | PDAL2_0.009 | 146 | 150 | 129 | 56.5 | 33.44 |
| 8 | PDAL0.5_0.0023 | 149 | - | 134 | 58.1 | 27.03 |
| 9 | PCD | 149 | - | 133 | 58.4 | 24.97 |
| 10 | PCAL1_0.0045 | 149 | 151 | 127 | 57.5 | 31.37 |
| 11 | PCAL2_0.009 | 145 | 151 | 122 | 56.8 | 32.22 |
| 12 | PCAL0.5_0.0023 | 148 | 151 | 125 | 57.7 | 31.10 |
| 13 | PCDAL1_0.0045 | 147 | 149 | 128 | 57.4 | 31.66 |
| 14 | PCDAL2_0.009 | 144 | 150 | 121 | 57.1 | 29.55 |
| 15 | PCDAL0.5_0.0023 | 148 | - | 129 | 58 | 29.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





