1. Introduction
The red palm weevil (RPW) Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) is an invasive pest in the Mediterranean basin and the Middle East. It is a severe pest of palms, particularly the genus Phoenix. As in several other countries of the Mediterranean basin, RPW is a significant problem in Israel (Soroker et al., 2005; Blumberg, 2008). The canary palm, Phoenix canariensis, is the most susceptible to RPW in the Mediterranean, but it also poses a significant threat to date palms (Phoenix dactylifera) (Red Palm Weevil, 2020). To date, attempts to limit the damage caused by RPW have relied mainly on the application of synthetic pesticides: pyrethroids as preventative treatments and neonicotinoids as reactive measures (Llácer et al., 2010; Dembilio et al., 2010a, 2015; Israeli Extension Service, 2017). Biological control of RPW is limited because this weevil has no natural enemies in its native habitat (Mazza et al., 2014; Ortega-García et al., 2017). Efforts to develop biological control management against RPW have focused on microbial control agents: entomopathogenic nematodes, e.g., Steinernema carpocapsae and Heterorhabditis bacteriophora (Dembilio et al., 2010a; Nurashikin-Khairuddin et al., 2022; Santhi et al., 2016; Santhi et al., 2015; Wakil et al., 2017; Yaacobi et al. 2023), and various genera and species of entomopathogenic fungi (EPF) (Cito et al., 2014; Dembilio et al., 2010b; El-Sufty et al., 2011; Gindin et al., 2006; Ortega-García et al., 2017; Sabbour and Abdel-Raheem, 2014; Sabbour and Solieman, 2014). In studies involving field trials, EPF was applied on the palm at the typical RPW infestation sites—the crowns of canary palms (Güerri-Agulló et al., 2011) and the trunks of date palms (El-Sufty et al., 2007). Other strategies for EPF application have been proposed, such as auto-contamination traps (Francardi et al., 2013) and attract-and-infect traps (Dembilio et al., 2018; El-Sufty et al., 2011). These strategies rely on the passive acquisition of conidia and their mechanical transmission among the adult RPW population.
The larval stages of RPW are cryptic, and they complete their life cycle inside the palm tissue. Mechanical transmission of conidia that is effective enough to infect the larvae and thus reduce palm infestation rates is an interesting strategy. The oviposition behaviour of the female weevil as an opportunity for conidial transmission toward the laying hole was recently examined by us. We recently demonstrated the mechanism governing this conidia transmission (Metveev et al., 2023).
The objectives of this study were to evaluate: (1) the virulence of commercial EPF products and laboratory preparations of EPF by evaluating their effect on RPW female survival, egg hatching, and larval survival in a microcosm designed to enable transmission of conidia into the egg-laying hole; and (2) the efficacy of the most promising EPF from objective 1 in protecting palms from RPW infestation under greenhouse conditions. This type of screening will enable further evaluation of the most promising EPF products in field trials that are now being conducted in Israeli date palm plantations.
2. Materials and Methods
2.1. Entomopathogenic fungi
Metarhizium brunneum 7 (Mb7) (Ment et al., 2020; Reingold et al., 2021) was routinely grown on Sabouraud dextrose agar (SDA; Difco) for 2 weeks at 28°C. Mb7 was produced by solid-state fermentation on rice to prepare conidial powder (Ment et al., 2020). Conidia were separated from the rice by sieving through a 12-mesh sieve, and harvested conidia were stored at 4°C in a sealed plastic box. For the Mb7 suspension, conidia were harvested by scraping the agar and putting the scrapings into glass tubes with sterile distilled water containing 0.01% (w/v) Triton X-100. The suspensions were vortexed and filtered through Miracloth (Calbiochem, La Jolla, CA), and spore concentrations were determined with a hemocytometer. Suspensions were adjusted to the required conidial concentrations in 0.01% Triton X-100, and the percentage of viable conidia was determined on SDA before each bioassay. Only conidial suspensions with at least 95% germination were used for bioassays.
The evaluated commercial products were two
Beauveria bassiana strains (
Table 1). To date, only Botanigard is registered and commercially available in Israel. Velifer® was under evaluation for registration process. Descriptions of the fungal species and strains used in the bioassays are presented in
Table 1. The afore described viability test was also performed for all of the products and strains examined in this study, and only EPF with at least 95% germination were used.
2.2. Insects
The RPW adults used in the bioassays were trapped by Picusan® traps (SANSAN PRODESING SL, Valencia) containing pheromone-kairomone lures (4-methyl-5-nonanol and 4-methyl-5-nonanone, ethyl acetate and sugar molasses), supplied by Biobee, Sde Eliyahu, Israel in northwestern Israel and along the Israeli coastline. The trapped insects were sexed for 7 days in a male: female ratio of 2:3. Adult sexing and maintenance were performed in groups of up to 50 adults in plastic boxes (20 cm wide × 40 cm long × 20 cm high). Each box had four 10-cm-diameter mesh-covered holes for ventilation. The insects were fed on fresh sugarcane. Boxes were kept in environmental chambers at 25°C and 70% RH with a 10 h:14 h dark: light regime.
2.3. Laboratory Bioassay
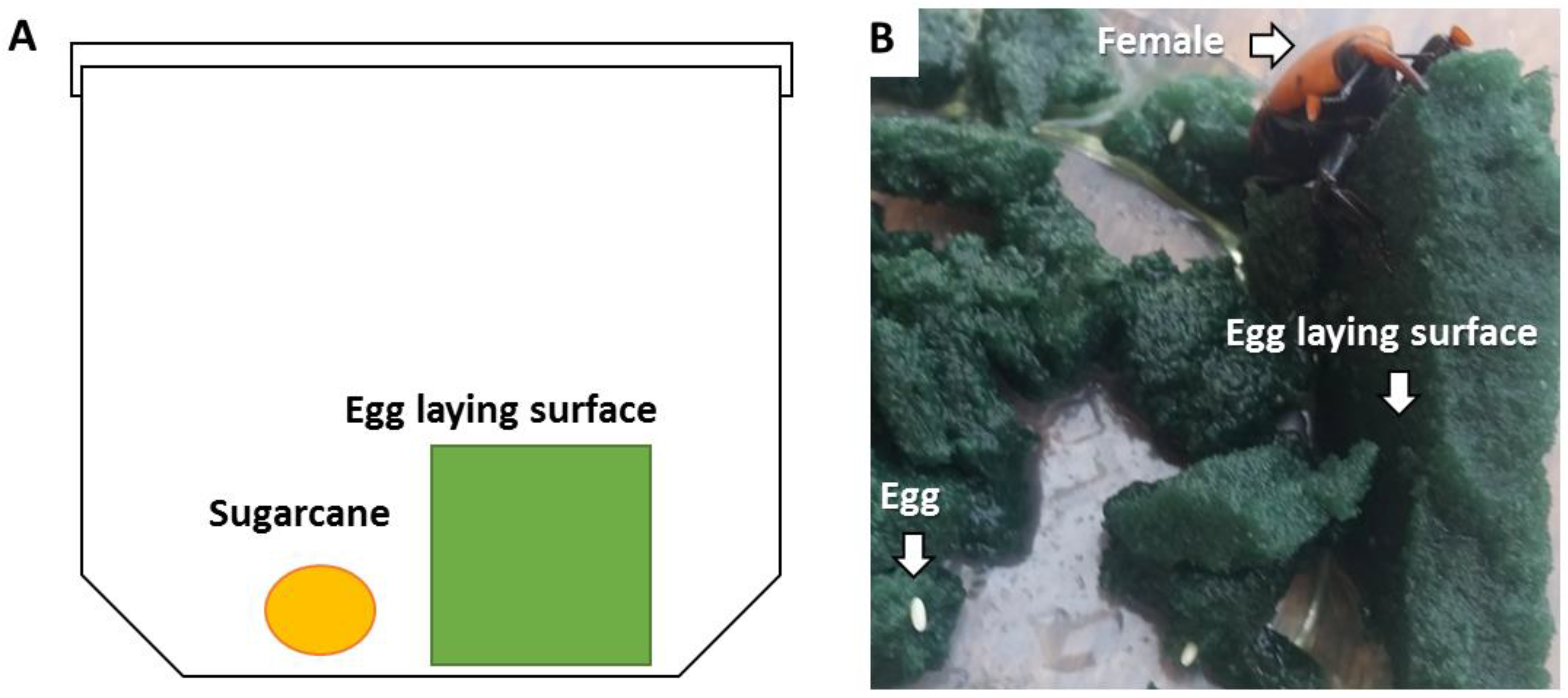
This microcosm bioassay was established in a previous study (Metveev et al., 2023). In brief, mated females were transferred individually to 500-ml plastic boxes with ventilated caps for 6 days. A piece of moist styrofoam (green styrofoam, 5 cm wide × 5 cm long × 1 cm high) and a piece of sugarcane (1 cm3, to serve as a food source) were put into each box (
Figure 1A). For the control treatment, pieces of foam were soaked in a glass cup with 100 ml distilled water for 5 s. For the EPF-suspension treatments, pieces of foam were soaked in a glass cup with 100 ml suspension of 108 conidia/ml for 5 s. For the EPF-rice granule treatments, pieces of foam were rubbed on all sides with 0.5 g of rice granules, an equivalent of 5×107 conidia. Boxes were kept in an environmental chamber at 25°C and 70% RH, with a 10 h:14 h dark: light regime for up to 2 weeks. Five days after the start of the experiment, females were removed from the boxes. Each female was placed in a clean box with sugarcane as food to monitor its mortality. Eggs were counted by examining the foam for oviposition holes made by the females. The pieces of foam were cut gently by hand, and eggs were counted for each replicate (
Figure 1B). During the experimental period, egg hatching was monitored for up to 13 days, and the larvae that hatched in the foam were counted. Dead larvae or unhatched eggs were incubated in the dark at 25°C in a 55-mm Petri dish lined with moist filter paper to confirm mycosis. Each treatment consisted of 10 RPW females. This experiment was repeated four to five times.
2.4. Greenhouse Experiment
A total of 15 young Washingtonia robusta palms, 2 m in height, were placed in a climate-controlled greenhouse. Palms were watered daily, and dry leaves were pruned a week before the beginning of the experiment. At the start of the experiment, the palms were covered with 50-mesh nets to prevent the weevils' movement between palms. The palms of the control group remained untreated. For dry conidial application, 100 g of sporulated rice granules were applied by hand to each palm to achieve complete coverage. For conidial suspension application, 500 ml of a 2.5 × 108 conidia/ml suspension was sprayed manually to complete coverage of the palm. Two hours after application, 6 females and 5 males per palm were introduced into the nets. Weevils were monitored every 2 weeks. Dead weevils were removed, surface-sterilized, and incubated to assess rates of mycosis as described previously (Reingold et al., 2021). New weevils were introduced instead of the dead ones to maintain the same number of total weevils throughout the experimental period. Palm-infestation rate was assessed 60 days after treatment by dissection; the leaves were cut off with garden tools to reveal larvae and pupae. Temperature in the greenhouse was 28 ± 2 °C and RH ranged between 60-80%.
2.5. Data Analysis
All statistical analyses were performed using JMP® Version 16 software (SAS Institute Inc., Cary, NC). Results are presented as mean ± SE of replicate analyses and either represent or include at least three independent experiments. Means of replicates were subjected to statistical analysis and considered significant when P ≤ 0.05.
For the laboratory experiment dataset, female mortality on day 7 post-inoculation and egg hatch on day 10 post-inoculation were analyzed. The square roots of the proportion data were arcsine-transformed and then subjected to analysis of variance (ANOVA) to examine the effects of the different treatments. If the effect was found to be significant (P < 0.05), Tukey–Kramer HSD test was used for comparisons among means.
For the greenhouse experimental dataset, adult mortality and the total number of larvae and pupae were analyzed by ANOVA, followed by Tukey–Kramer HSD test for comparisons among means.
3. Results
3.1. Laboratory Experiments
The efficacy of the
B. bassiana-based products and laboratory strain of
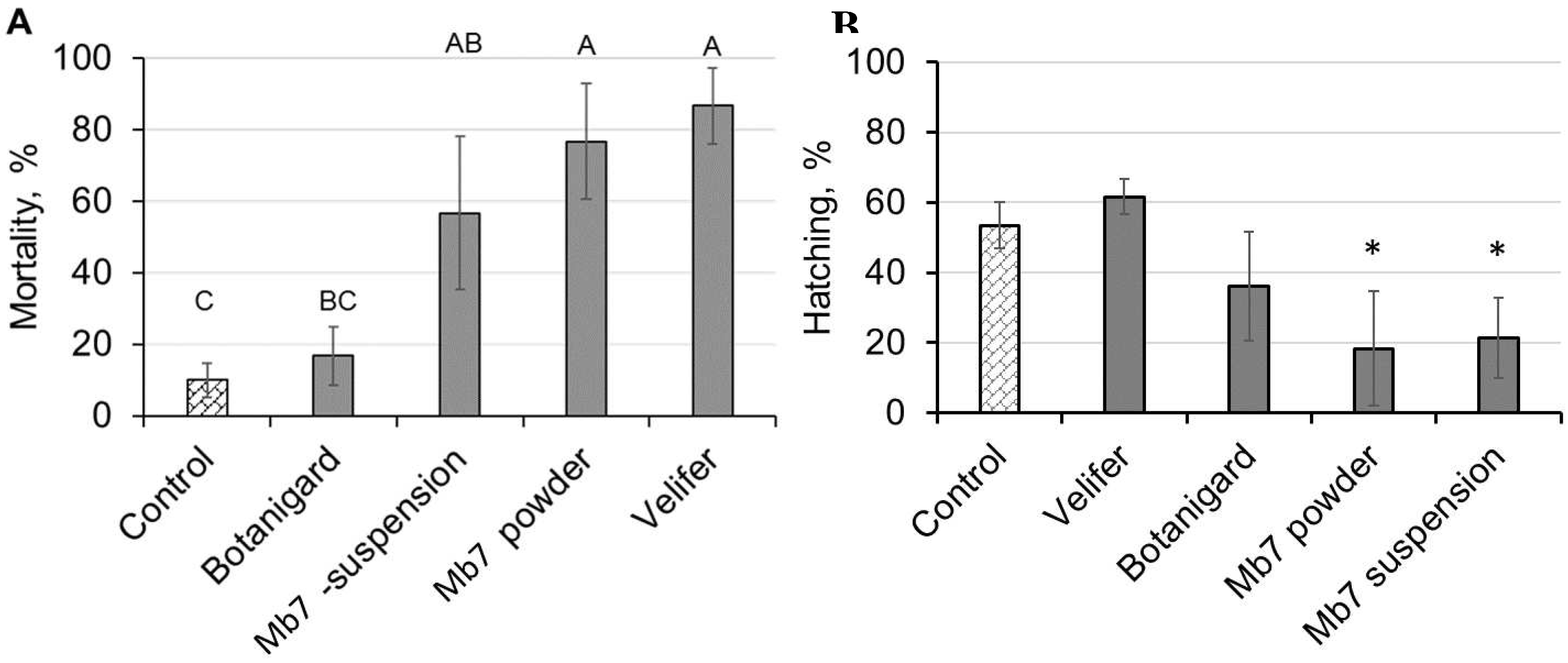
M. brunneum (applied in a suspension or as sporulated rice) for the preventative control of RPW was evaluated using the experimental microcosm described in section 2.3. Seven days after RPW females were put in the oviposition boxes, three of the four EPF treatments had caused significant female mortality (
Figure 2A; ANOVA: P < 0.0006, DF = 4, F = 11.6). Botanigard (
B. bassiana-GHA) treatment resulted in female mortality similar to that observed in the control group. Ten days after females were put in the oviposition boxes, egg-hatching rates were only significantly lower for the Mb7 suspension and powder compared to the control (
Figure 2B). The hatching rates for those treatments were 21% and 18%, respectively (
Figure 2B; ANOVA: P < 0.0001, DF = 6, F = 24.54). Sporulation on eggs and larvae was observed in all treatments but with variations that did not allow statistical analysis. All non-hatched eggs in the Mb7 treatments exhibited signs of mycosis and sporulation.
3.2. Greenhouse Experiments
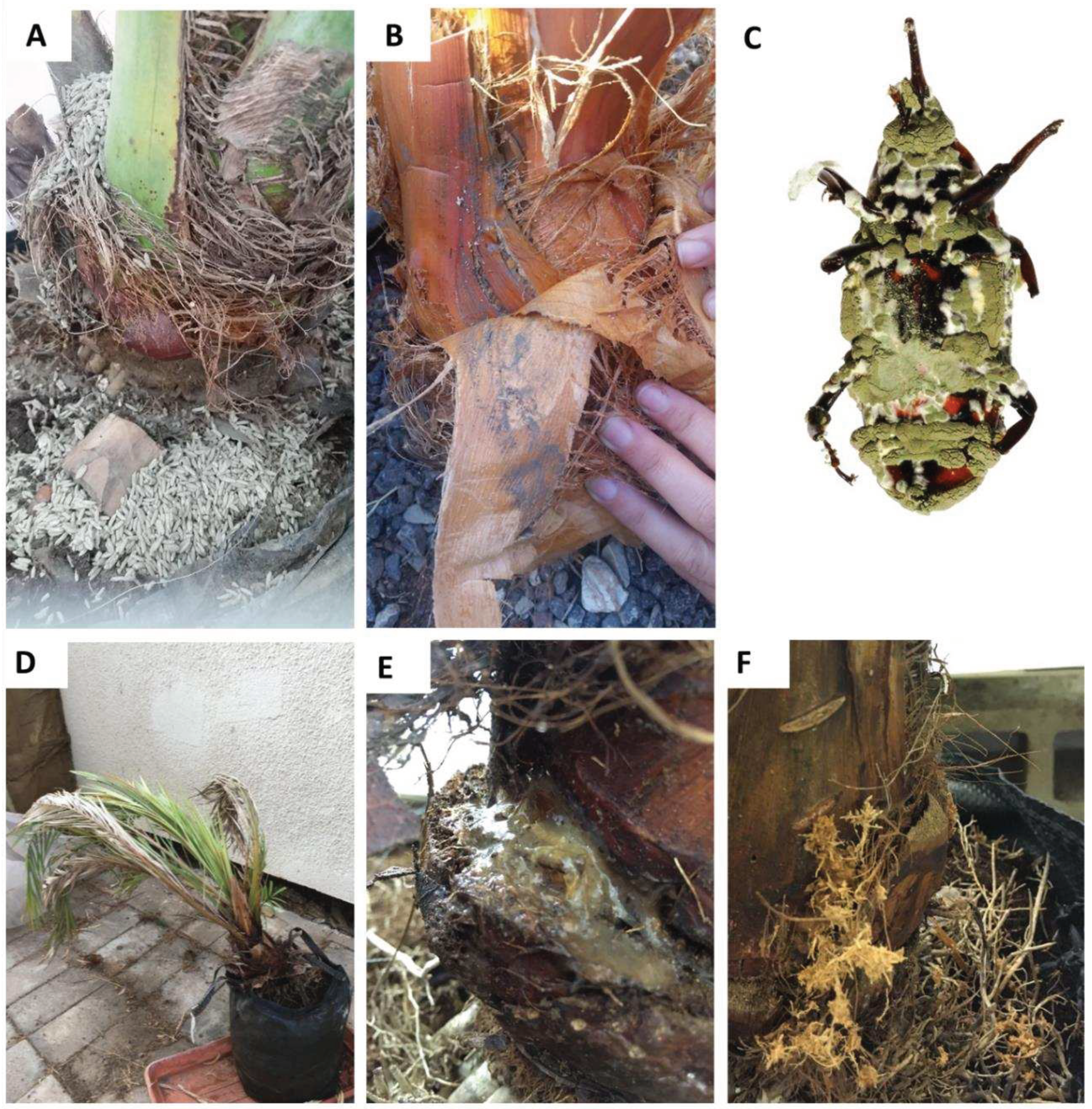
The greenhouse experiment evaluated the virulence of Mb7 applied as either sporulated rice granules or conidial suspension; these were chosen because they exhibited the highest virulence toward eggs in the laboratory experiments. During the greenhouse experiment, conidia were visible on the fibrous threads and dried petioles (
Figure 3A, B); dead weevils were collected, but the level of mortality was low, ranging from 6.25–10.5% (ANOVA: DF = 2, F = 0.29, P = 0.755;
Table 2). Some adult cadavers were mycosed with
M. brunneum (
Figure 3C). Due to the scant mycosis that was exhibited by
M. brunneum, we decided to dissect the palms 60 days into experiment, when all palms in the control group exhibited clear signs of infestation: leaf collapse (
Figure 3D), secretions (
Figure 3E), and signs of boring (
Figure 3F).
Secretion and boring signs were evaluated before dissection. All palms in the control group exhibited secretions, and 75% exhibited signs of boring (
Table 2). There were no signs of boring in any of the Mb7-treated palms, and secretions were only observed in 25% of the palms treated with Mb7 suspension (
Table 2). The control group had the highest average number of larvae per palm compared to both Mb7 treatments. However, the difference was only significant for the sporulated rice treatment (ANOVA: DF = 2,
F = 5.43,
P = 0.0283). The total number of pupae per palm was not significantly different between treatments (ANOVA: DF = 2,
F = 0.648,
P = 0.545;
Table 2).
4. Discussion
The use of EPF as microbial control agent against RPW has been suggested in several studies (reviewed by Ortega-García et al., 2017). There are a variety of strategies for applying EPF products against RPW, including both inundative and inoculative applications, involving direct application of the EPF products to palm trees (Güerri-Agulló et al., 2011) or attract-and-infect traps (Dembilio et al., 2018; El-Sufty et al., 2011). The common principle underlying these strategies is the attraction of adult RPW to a specific site where the EPF is applied. The adult weevils that come into contact with the EPF conidia then disseminate those conidia among the RPW population. Vectoring of the conidia can result in infection of RPW progeny via egg contamination during the oviposition process, as in Metveev et al. (2023), who demonstrated the mechanical transfer of conidia between females and progeny. In that study, transmission resulted in inoculation of the egg-laying hole, reducing egg hatch and larval survival.
Here we report on utilizing the bioassay procedure to evaluate the efficacy of commercial products and lab preparations of EPF for RPW prevention by their transfer from the oviposition site to RPW eggs and larvae. In a previous study by Gindin et al. (2006), adult mortality following inoculation with M. anisopliae-Ru reached 84.6–100% within 2 to 5 weeks of inoculation. That strain was not included in the current study. Here, adult mortality was significant following exposure to Mb7 as conidia suspension or powder and Velifer® (B. bassiana- PPRI 5339) but insignificant following exposure to Botanigard (B. bassiana- GHA). These differences in adult mortality can be attributed to the differences in the fungal strains examined and the formulations.
Based on the conclusions from the lab bioassay, we examined the potential of M. brunneum as a preventative treatment for RPW infestation under greenhouse conditions. The palm trees were infested with adult RPW as described by Dembilio et al. (2018). All of the palm trees from the control group showed signs of infestation—secretions and wilted dry leaves. Sporulated rice was superior to a conidial-suspension spray for preventing infestation by RPW. Although we were not able to examine the mechanical transmission of conidia toward eggs and larvae, we suggest that the positive results observed in the greenhouse experiment were due to the transfer of conidia from the treated surface of the palm to the oviposition site when the females laid their eggs. The acquisition of conidia by individual weevils, as described in Metveev et al. (2023) and (Dembilio et al., 2018), could not be confirmed in our greenhouse experiment because not all of the released adult RPW could be recovered, mortality rates were low, and some adult cadavers were mycosed probably with the applied fungus. High efficacy of the sporulated-rice formulation has been observed in field trials involving RPW (Güerri-Agulló et al., 2011; Ricaño et al. 2013) and the black palm weevil Rhynchophorus bilineatus (Prior and Arura, 1985), indicating the potential of this method for reducing RPW infestation.
The bioassay developed in this study was used to test the effects of different strains and commercial formulations of EPF on female RPW and their progeny and to assess the commercial potential of the preventative use of EPF. The results were in accordance with those of Hajjar et al. (2015), who reported a median lethal time (LT50) of 4.15 days for the B. bassiana product referred to as Velifer® in this work for adult RPW at a concentration of 108 conidia/ml. In our study, that same concentration of Velifer® resulted in >80% female mortality 7 days after inoculation. Female mortality depended on the product used but probably not on the formulation; Mb7, as a suspension or powder, caused similar mortality rates of females and similar egg-hatching rates. Of the three strains and four formulations examined, only the M. brunneum laboratory preparations significantly reduced egg hatching. This finding is in accordance with Gindin et al. (2006), who found that M. anisopliae strains are more virulent toward larvae than B. bassiana strains. However, the differences between the various products, in terms of their formulations, may influence the horizontal transfer of conidia and, consequently, the observed efficacy of the different treatments.
The results of the current study encourage further research to develop EPF applications as a preventative measure for the control of RPW on palm trees. Questions for further investigations include: How long can conidia persist under field conditions? Would EPF treatments be more cost-effective if they were focused on oviposition sites? Even in a situation in which EPF would account for a significant share of the total pesticide applied to palm trees, other issues, such as early detection of infestations and reactive treatments for infested palms, still need to be explored.
5. Conclusions
Examination of the efficacy of the proposed preventative strategy on palms on which adult RPW were released revealed evident inhibition of palm infestation by RPW larvae. The efficacy of the different EPF products for reduced egg hatching and female mortality varied widely. Of the examined products, a dry formulation of M. brunneum is the most promising candidate for field evaluation of RPW prevention, but such a product is not available in the Israeli market. Overall, our results suggest that EPF-based products can serve as a valuable and safe preventative means that can be incorporated into a broader management strategy toward RPW.
Acknowledgments
We thank Dr. Victoria Soroker for providing palms for the experiments, Ami Haberman for providing weevils, and Dr. Alex Protasov for the photographs. We greatly appreciate the administrative efforts of Amnon Greenberg. This study was funded by the Chief Scientist of the Israeli Ministry of Agriculture and the Plant Board (project number 20-02-0091, “Study the effects of application of entomopathogenic fungi on the red palm weevil in the laboratory and in field assays,” grant to D.M.).
References
- Blumberg, D., 2008. Review: Date palm arthropod pests and their management in Israel. Phytoparasitica 36, 411–418. [CrossRef]
- Cito, A., Mazza, G., Strangi, A., Benvenuti, C., Barzanti, G.P., Dreassi, E., Turchetti, T., Francardi, V., Roversi, P.F., 2014. Characterization and comparison of Metarhizium strains isolated from Rhynchophorus ferrugineus. FEMS Microbiol. Lett. 355, 108–115. [CrossRef]
- Dembilio, Ó., Llácer, E., Martínez de Altube, M. del M., Jacas, J.A., 2010a. Field efficacy of imidacloprid and Steinernema carpocapsae in a chitosan formulation against the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Phoenix canariensis. Pest Manag. Sci. 66, 365–370. [CrossRef]
- Dembilio, Ó., Moya, P., Vacas, S., Ortega-García, L., Quesada-Moraga, E., Jaques, J.A., Navarro-Llopis, V., 2018. Development of an attract-and-infect system to control Rhynchophorus ferrugineus with the entomopathogenic fungus Beauveria bassiana. Pest Manag. Sci. 74, 1861–1869. [CrossRef]
- Dembilio, Ó., Quesada-Moraga, E., Santiago-Álvarez, C., Jacas, J.A., 2010b. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the Red Palm Weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol. 104, 214–221. [CrossRef]
- Dembilio, Ó., Riba, J.M., Gamón, M., Jacas, J.A., 2015. Mobility and efficacy of abamectin and imidacloprid against Rhynchophorus ferrugineus in Phoenix canariensis by different application methods. Pest Manag. Sci. 71, 1091–1098. [CrossRef]
- El-Sufty, R., Al-Awash, S.A., Al Amiri, A.M., Shahdad, A.S., Al Bathra, A.H., Musa, S.A., 2007. Biological control of red palm weevil, Rhynchophorus ferrugineus (Col.: Curculionidae) by the entomopathogenic fungus Beauveria bassiana in United Arab Emirates. Acta Hortic. 736, 399–404. [CrossRef]
- El-Sufty, R., Al Bgham, S., Al-Awash, S., Shahdad, A., Al Bathra, A., 2011. A trap for auto-dissemination of the entomopathogenic fungus Beauveria bassiana by red palm weevil adults in date palm plantations. Egypt. J. Biol. Pest Control 21, 271–276.
- Francardi, V., Benvenuti, C., Barzanti, G.P., Roversi, P.F., 2013. Autocontamination trap with entomopathogenic fungi: a possible strategy in the control of Rhynchophorus ferrugineus (Olivier) (Coleoptera Curculionidae). Redia 96, 57–67.
- Gindin, G., Levski, S., Glazer, I., Soroker, V., 2006. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica 34, 370–379. [CrossRef]
- Güerri-Agulló, B., López-Follana, R., Asensio, L., Barranco, P., Lopez-Llorca. L.V., 2011. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. Fla. Entomol. 94, 737–747. [CrossRef]
- Hajjar, M.J., Ajlan, A.M., Al-Ahmad, M.H., 2015. New approach of Beauveria bassiana to control the red palm weevil (Coleoptera: Curculionidae) by trapping technique. J. Econ. Entomol. 108, 425–432. [CrossRef]
- Israeli Extension Service, 2017. Management of the red palm weevil, professional information. Retrieved from: https://www.moag.gov.il/shaham/ProfessionalInformation/Pages/meniat_vadbarat_chedkonit_hadekel_june_2017.aspx.
- Llácer, E., Dembilio, O., Jacas, J.A., 2010. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Econ. Entomol. 103, 402–408. [CrossRef]
- Mazza, G., Francardi, V., Simoni, S., Benvenuti, C., Cervo, R., Faleiro, J.R., Llácer, E., Longo, S., Nannelli, R., Tarasco, E., Roversi, P.F., 2014. An overview on the natural enemies of Rhynchophorus palm weevils, with focus on R. ferrugineus. Biol. Control 77, 83–92. [CrossRef]
- Ment, D., Raman, S., Gal, S., Ezra, D., Palevsky, E., 2020. Interactions of Metarhizium brunneum-7 with phytophagous mites following different application strategies. Insects 11, 330. [CrossRef]
- Metveev, S.; Reingold, V.; Yossef, E.; Levy, N.; Kottakota, C.; Mechrez, G.; Protasov, A.; Belausov, E.; Birnbaum, N.; Davidovitz, M.; Ment D. The dissemination of Metarhizium brunneum conidia by females of the red palm weevil, Rhynchophorus ferrugineus, from treated surface to laying hole reduces egg hatch and larval survival, suggesting a new mechanism for prevention practic-es. J. Fungi 2023, 7, x. [CrossRef]
- Nurashikin-Khairuddin, W., Abdul-Hamid, S.N.A., Mansor, M.S., Bharudin, I., Othman, Z., Jalinas, J., 2022. A review of entomopathogenic nematodes as a biological control agent for red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects 13, 245. [CrossRef]
- Ortega-García, L., Tabone, E., Beaudoin-Ollivier, L., Ment, D., Buradino, M., Jaques, J.A., Garrido-Jurado, I., Dembilio, O., Moraga, E.Q., 2017. Natural enemies of Rhynchophorus ferrugineus and Paysandisia archon, in: Soroker, V., Colazza, S. (Eds.), Handbook of Major Palm Pests. John Wiley & Sons, pp. 171–186. [CrossRef]
- Prior, C., & Arura, M. (1985). The infectivity of Metarhizium anisopliae to two insect pests of coconuts. Journal of Invertebrate Pathology, 45(2), 187-194. [CrossRef]
- Red Palm Weevil: guidelines on management practices, 2020. FAO. [CrossRef]
- Reingold, V., Kottakota, C., Birnbaum, N., Goldenberg, M., Lebedev, G., Ghanim, M., Ment, D., 2021. Intraspecies variation of Metarhizium brunneum against the green peach aphid, Myzus persicae, provides insight into the complexity of disease progression. Pest Manag. Sci. 77, 2557–2567. [CrossRef]
- Ricaño, J., Güerri-Agulló, B., Serna-Sarriás, M.J., Rubio-Llorca, G., Asensio, L., Barranco, P., Lopez-Llorca, L.V., 2013. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Fla. Entomol. 96, 1311–1324. [CrossRef]
- Sabbour, M.M., Abdel-Raheem, M.A., 2014. Evaluations of Isaria fumosorosea isolates against the Red Palm Weevil Rhynchophorus ferrugineus under laboratory and field conditions. Curr. Sci. Int. 3, 179–185. [CrossRef]
- Sabbour, M.M., Solieman, N.Y., 2014. Preliminary investigations into the biological control of Red Palm Weevil Rhynchophorus ferrugineus by using three isolates of the fungus Lecanicillium (Verticillium) lecanii in Egypt. Int. J. Sci. Res. 3, 2060–2066.
- Santhi, V.S., Ment, D., Salame, L., Soroker, V., Glazer, I., 2016. Genetic improvement of host-seeking ability in the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora toward the Red Palm Weevil Rhynchophorus ferrugineus. Biol. Control 100, 29–36. [CrossRef]
- Santhi, V.S., Salame, L., Nakache, Y., Koltai, H., Soroker, V., Glazer, I., 2015. Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus). Biol. Control 83, 75–81. [CrossRef]
- Soroker, V., Blumberg, D., Haberman, A., Hamburger-Rishard, M., Reneh, S., Talebaev, S., Anshelevich, L., Harari, A.R., 2005. Current status of red palm weevil infestation in date palm plantations in Israel. Phytoparasitica 33, 97–106. [CrossRef]
- Wakil, W., Yasin, M., Shapiro-Ilan, D., 2017. Effects of single and combined applications of entomopathogenic fungi and nematodes against Rhynchophorus ferrugineus (Olivier). Sci. Rep. 7, 5971. [CrossRef]
- Yaacobi, G., Salame, L., and Glazer, I. 2023. Persistence of the entomopathogenic nematode Steinernema carpocapsae on red palm weevil-infested date palm trees in an arid environment. Nematology 25, 6, 669-675, Available From: Bril. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).