Submitted:
17 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
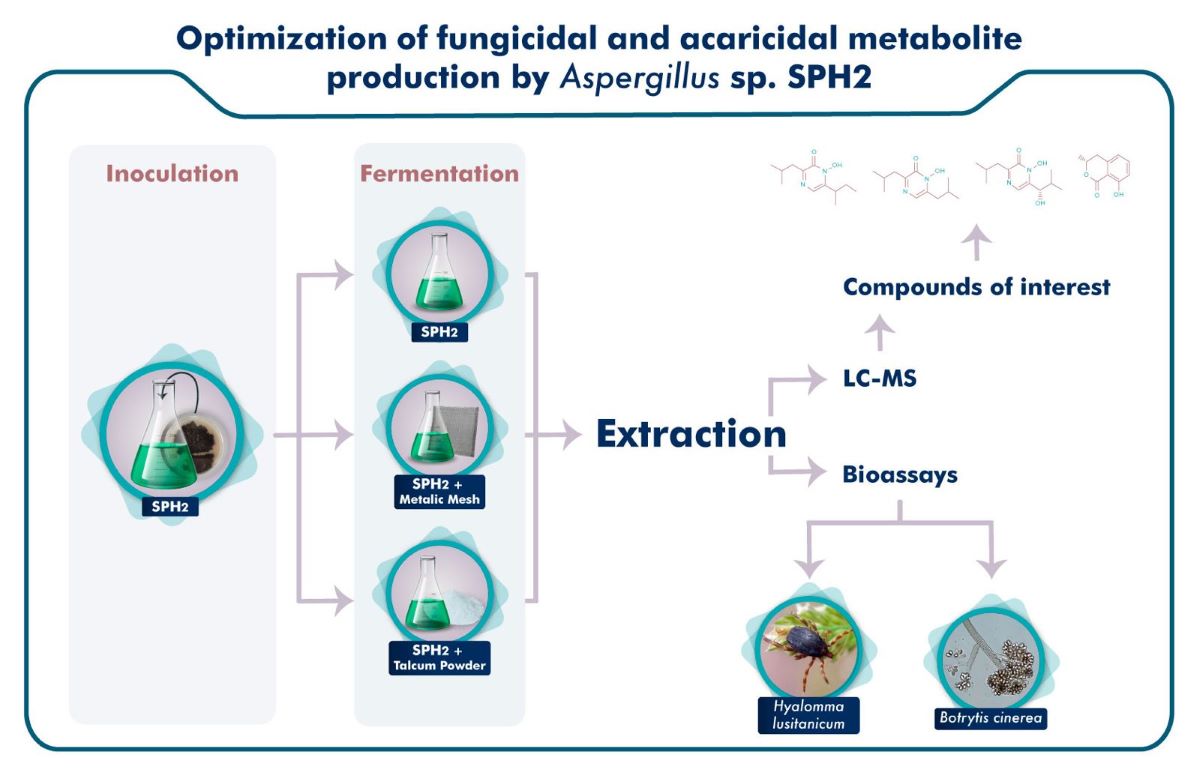
2.2. Cultivation of SPH2 for Extract Preparation
2.3. Modification of the Fermentation Conditions
2.4. Extract Preparation
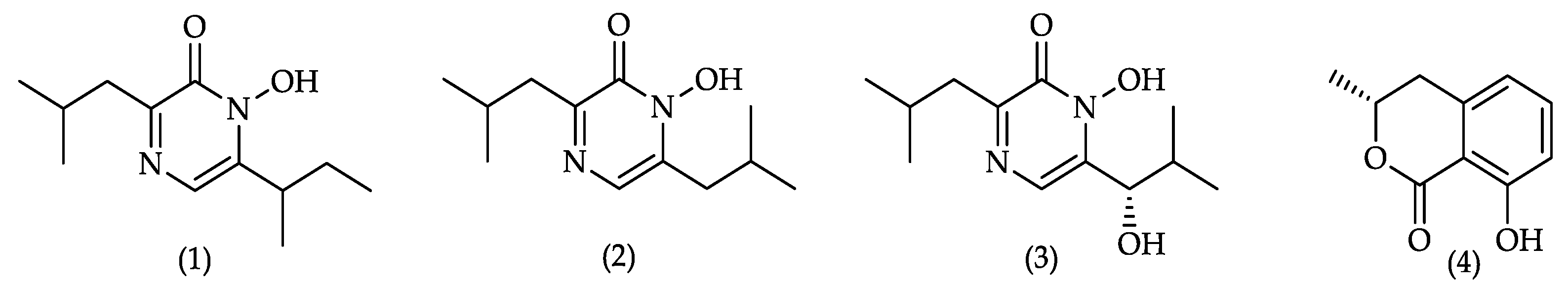
2.5. Compound Identification and Quantification
2.6. Bioassays
2.6.1. Antifungal activity
2.6.2. Ixodicidal activity
3. Results
3.1. Appearance and Coloring of SPH2 mycelium
3.3. Mycelium yield
3.4. Extract yield
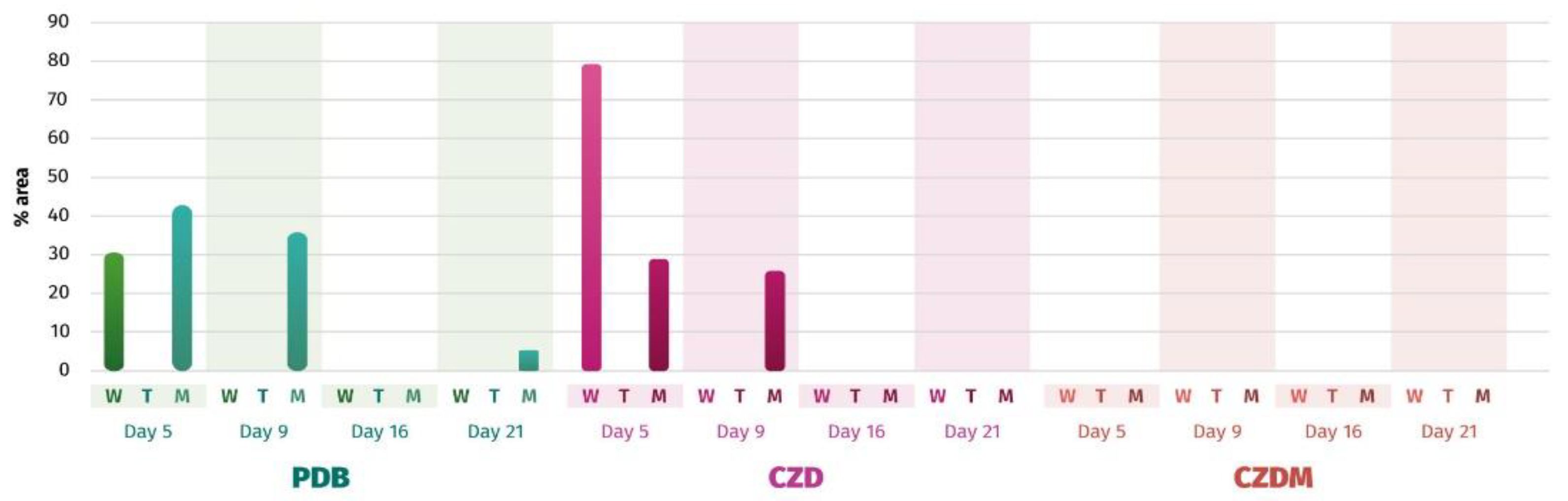
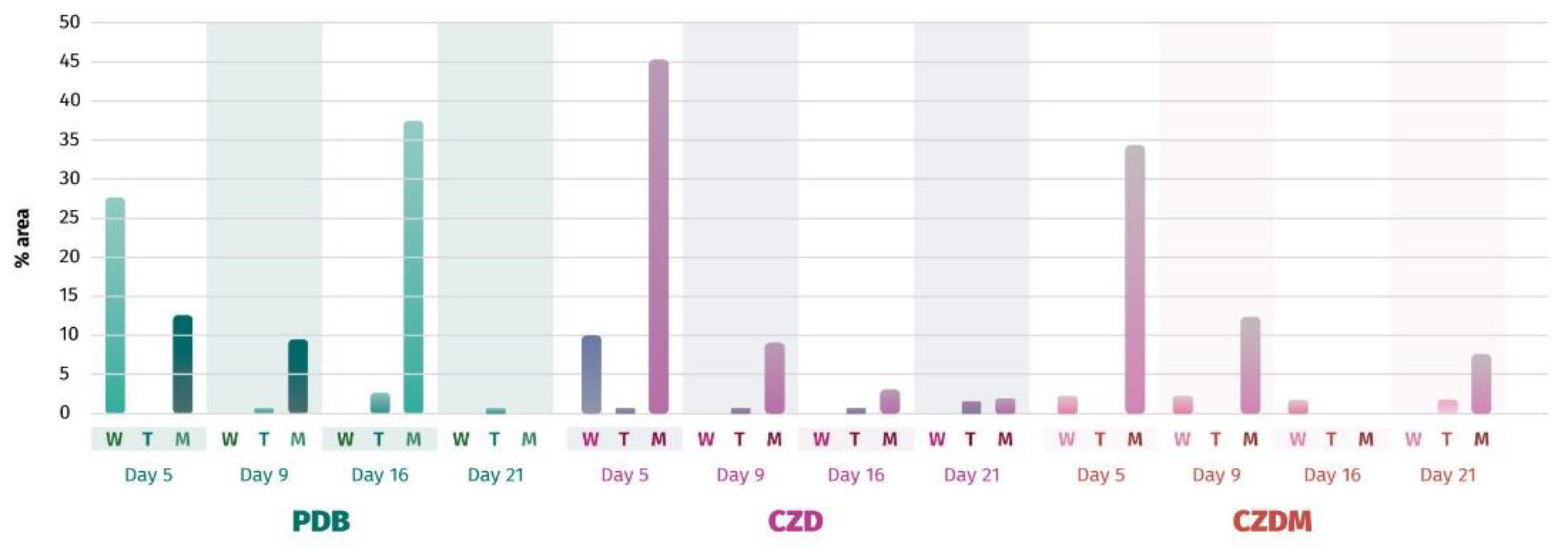
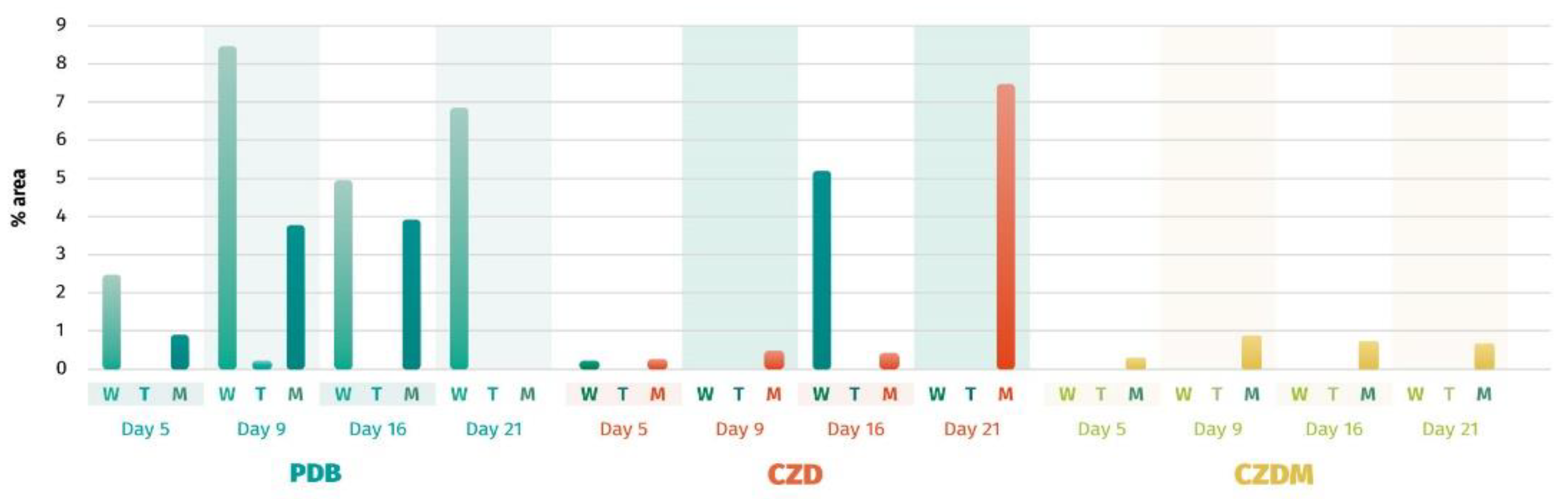
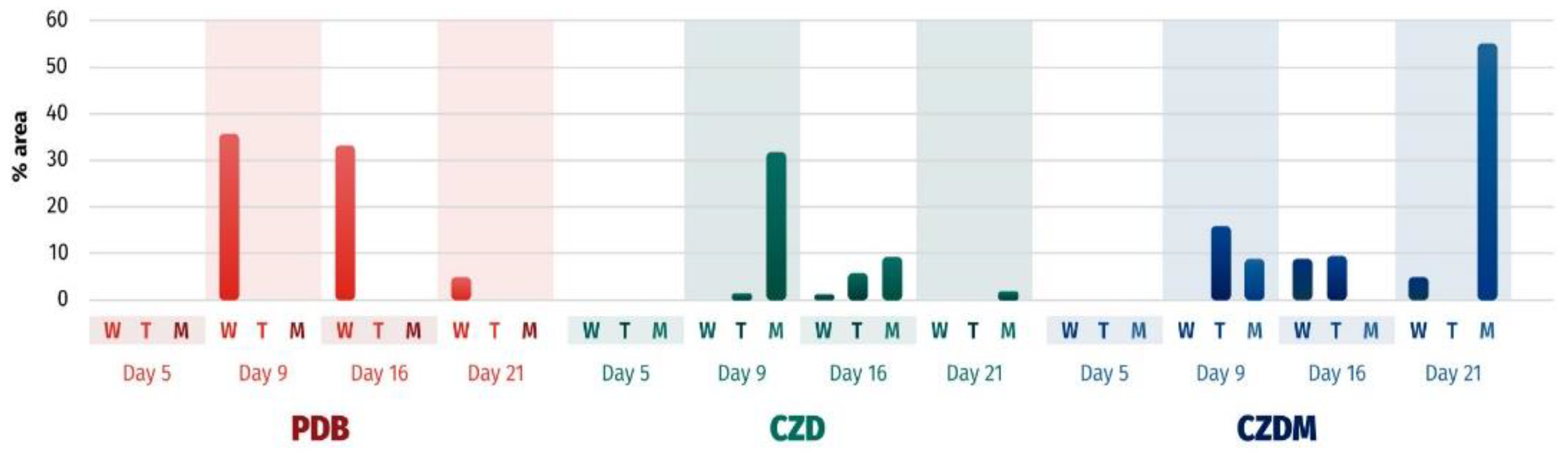
3.5. Secondary Metabolite Analysis
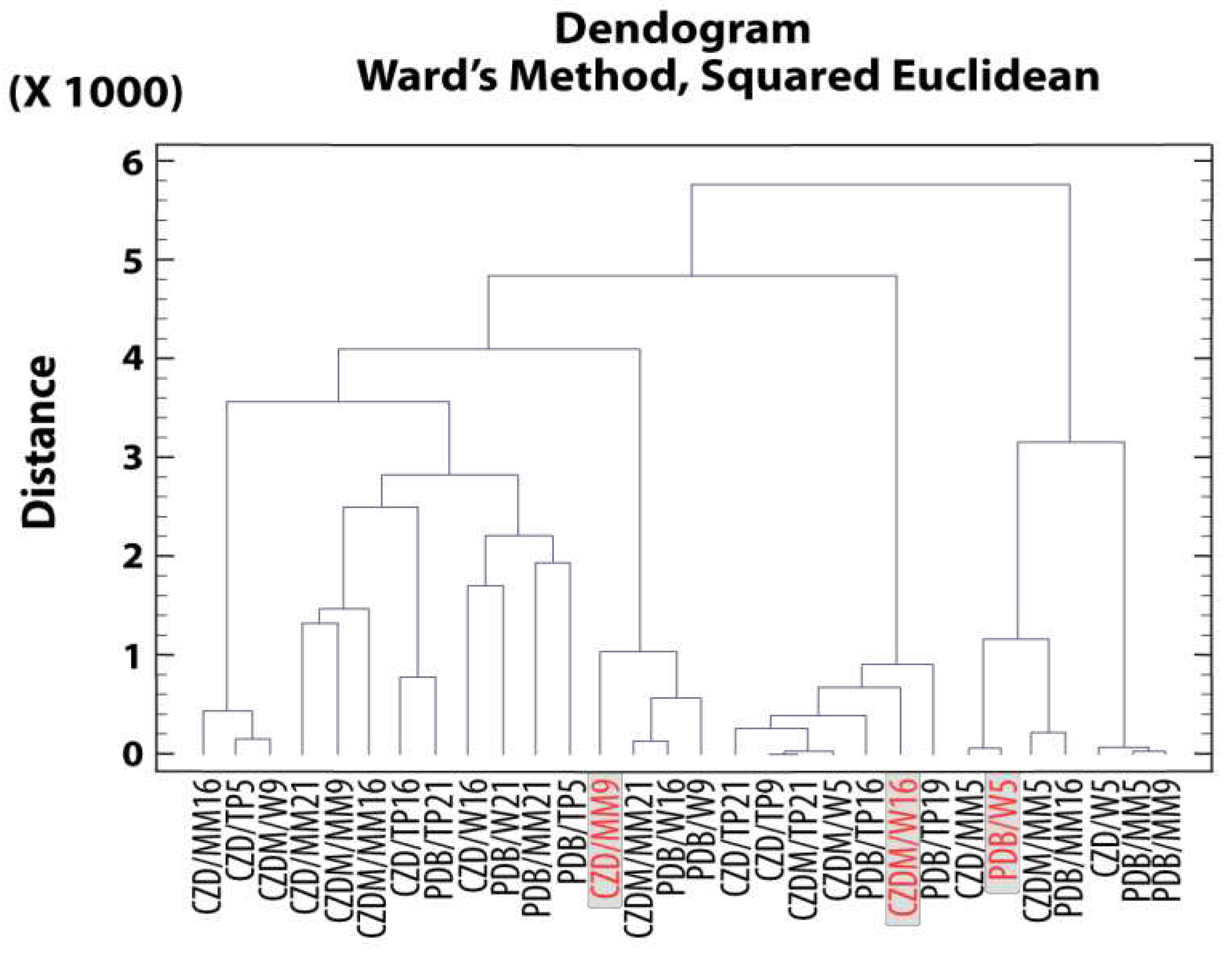
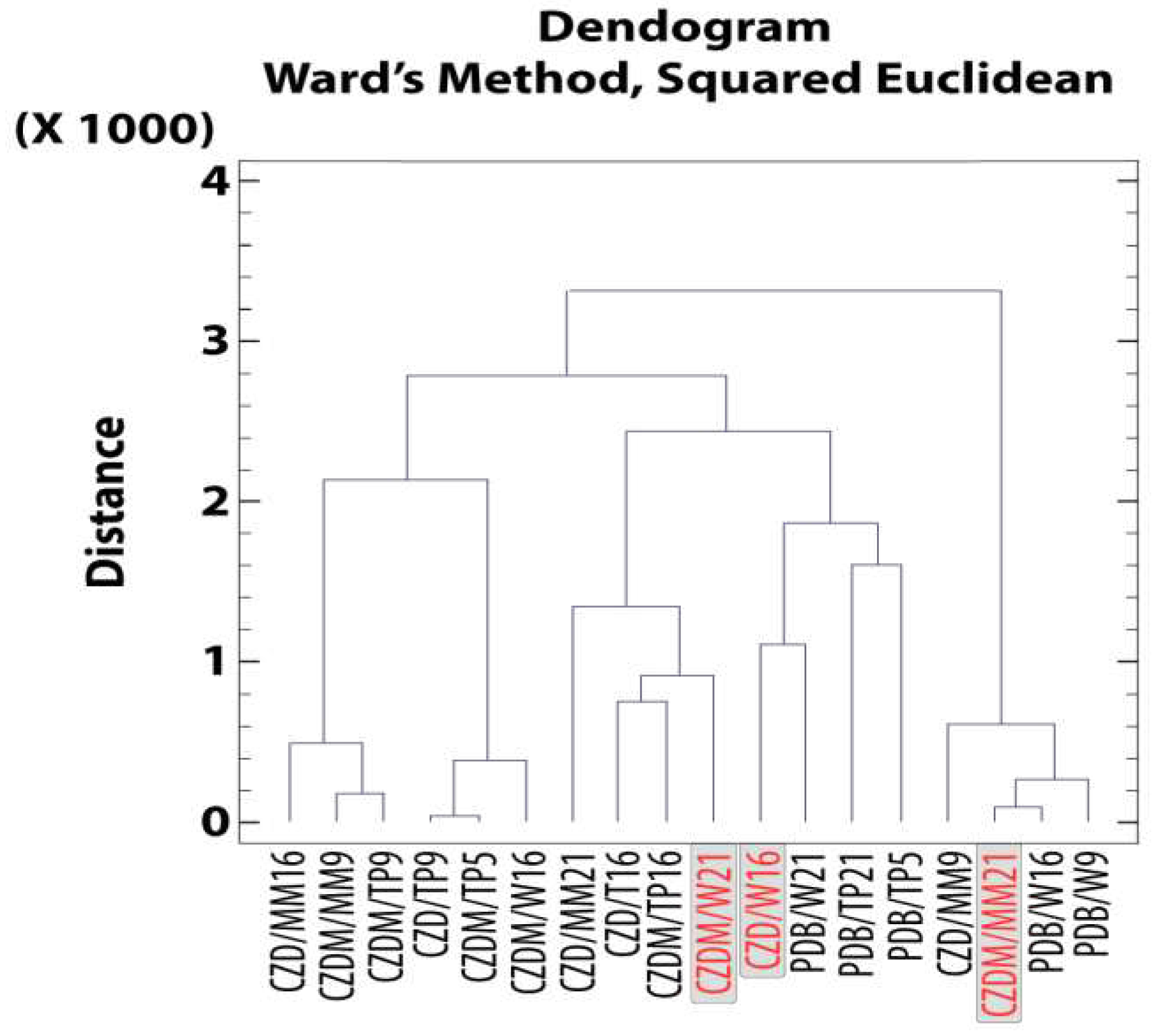
3.5.1. Cluster Analysis for Secondary Metabolites. Extract selection
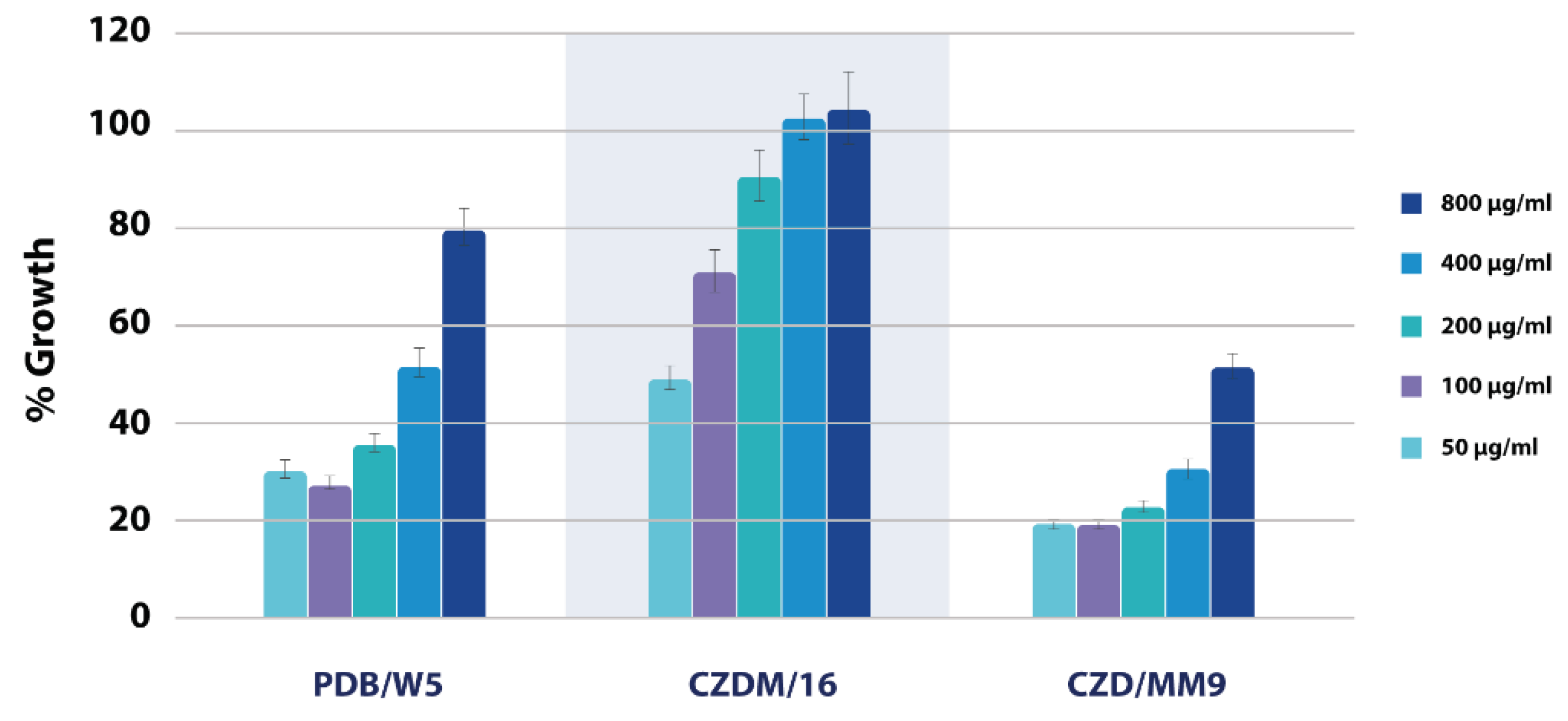
3.6. Bioassays
3.6.1. Spore germination inhibition bioassay
3.6.2. Ixodicidal bioassay
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological Control and Integrated Pest Management in Organic and Conventional Systems. Biological Control 2020, 140. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present Status and the Future Prospects. J Biofertil Biopestic 2015, 06. [Google Scholar] [CrossRef]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as Promising Alternatives to Chemical Pesticides: A Review of Their Current and Future Status. Online J Biol Sci 2020, 20. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current Status and Recent Developments in Biopesticide Use. Agriculture (Switzerland) 2018, 8. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Tate, R.; Abbott, G.; Young, L.; Viegelmann, C.; Schumacher, M.; Diederich, M.; Edrada-Ebel, R.A. Metabolomic Tools to Assess the Chemistry and Bioactivity of Endophytic Aspergillus Strain. Chem Biodivers 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Portero, A.G.; González-Coloma, A.; Reina, M.; Díaz, C.E. Plant-Defensive Sesquiterpenoids from Senecio Species with Biopesticide Potential. Phytochemistry Reviews 2012, 11. [Google Scholar] [CrossRef]
- Nordenstam, B. Canariothamnus B. Nord., a New Genus of the Compositae-Senecioneae, Endemic to the Canary Islands | Request PDF Available online: https://www.researchgate.net/publication/285661067_Canariothamnus_B_Nord_a_new_genus_of_the_Compositae-Senecioneae_endemic_to_the_Canary_Islands (accessed on 8 May 2022).
- Morales-Sánchez, V.; Díaz, C.E.; Trujillo, E.; Olmeda, S.A.; Valcarcel, F.; Muñoz, R.; Andrés, M.F.; González-Coloma, A. Bioactive Metabolites from the Endophytic Fungus Aspergillus Sp. SPH2. Journal of Fungi 2021, 7. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic Fungi as Biocontrol Agents: Elucidating Mechanisms in Disease Suppression. Plant Ecol Divers 2018, 11. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic Fungi: A Tool for Plant Growth Promotion and Sustainable Agriculture. Mycology 2022, 13. [Google Scholar] [CrossRef]
- Reveglia, P.; Masi, M.; Evidente, A. Melleins—Intriguing Natural Compounds. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- MacDonald, J.C. Biosynthesis of Compounds Similar to Aspergillic Acid. Can J Biochem 1970, 48. [Google Scholar] [CrossRef]
- Barrios-González, J. Solid-State Fermentation: Physiology of Solid Medium, Its Molecular Basis and Applications. Process Biochemistry 2012, 47. [Google Scholar] [CrossRef]
- Francis, F.; Druart, F.; di Mavungu, J.D.; de Boevre, M.; de Saeger, S.; Delvigne, F. Biofilm Mode of Cultivation Leads to an Improvement of the Entomotoxic Patterns of Two Aspergillus Species. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Boruta, T.; Bizukojć, M. Morphological Evolution of Various Fungal Species in the Presence and Absence of Aluminum Oxide Microparticles: Comparative and Quantitative Insights into Microparticle-Enhanced Cultivation (MPEC). Microbiologyopen 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Walisko, R.; Krull, R.; Schrader, J.; Wittmann, C. Microparticle Based Morphology Engineering of Filamentous Microorganisms for Industrial Bio-Production. Biotechnol Lett 2012, 34. [Google Scholar] [CrossRef]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of Filamentous Fungi in Submerged Culture: Problems and Possible Solutions. Crit Rev Biotechnol 2000, 20. [Google Scholar] [CrossRef] [PubMed]
- Antecka, A.; Bizukojc, M.; Ledakowicz, S. Modern Morphological Engineering Techniques for Improving Productivity of Filamentous Fungi in Submerged Cultures. World J Microbiol Biotechnol 2016, 32. [Google Scholar] [CrossRef]
- Parra Amin, J.E.; Cuca, L.E.; González-Coloma, A. Antifungal and Phytotoxic Activity of Benzoic Acid Derivatives from Inflorescences of Piper Cumanense. Nat Prod Res 2021, 35, 2763–2771. [Google Scholar] [PubMed]
- Singh, B. Engineering Fungal Morphology for Enhanced Production of Hydrolytic Enzymes by Aspergillus Oryzae SBS50 Using Microparticles. 3 Biotech 2018, 8, 283. [Google Scholar] [CrossRef]
- Gonciarz, J.; Bizukojc, M. Adding Talc Microparticles to Aspergillus Terreus ATCC 20542 Preculture Decreases Fungal Pellet Size and Improves Lovastatin Production. Eng Life Sci 2014, 14, 190–200. [Google Scholar] [CrossRef]
- Johansen, C.L.; Coolen, L.; Hunik, J.H. Influence of Morphology on Product Formation in Aspergillus Awamori during Submerged Fermentations. Biotechnol Prog 1998, 14. [Google Scholar] [CrossRef] [PubMed]
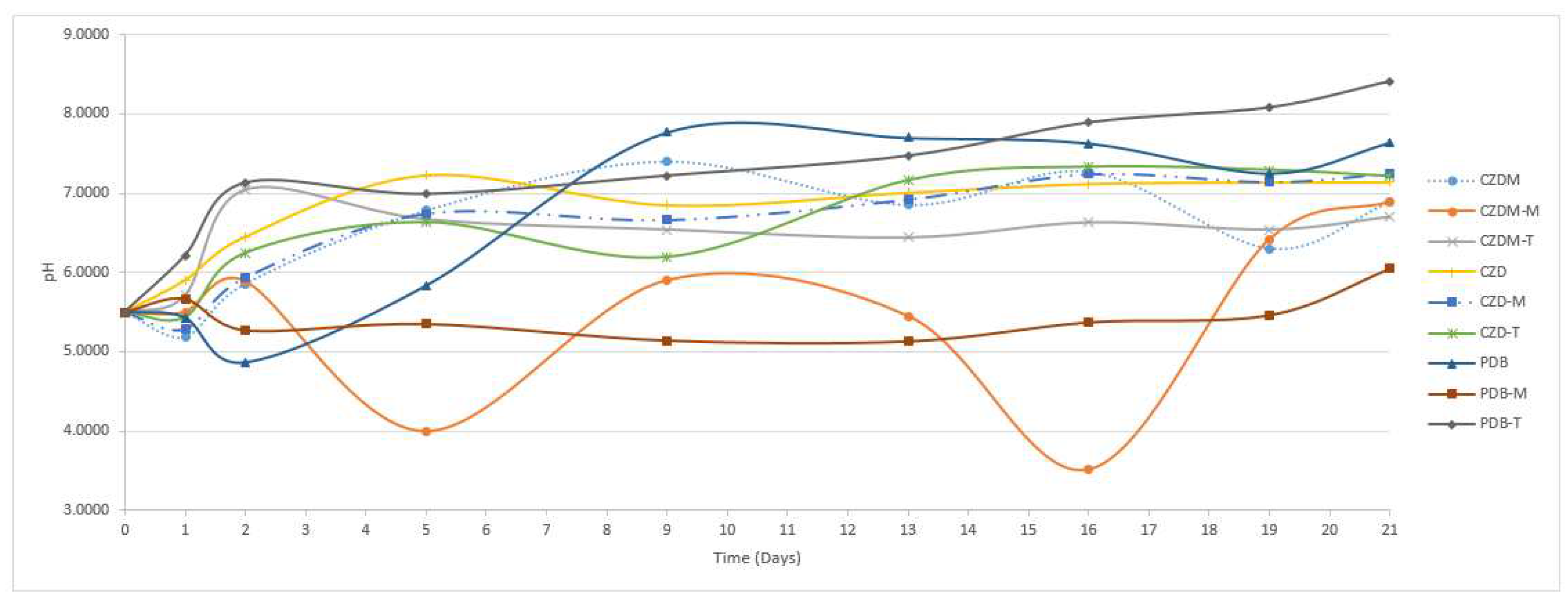
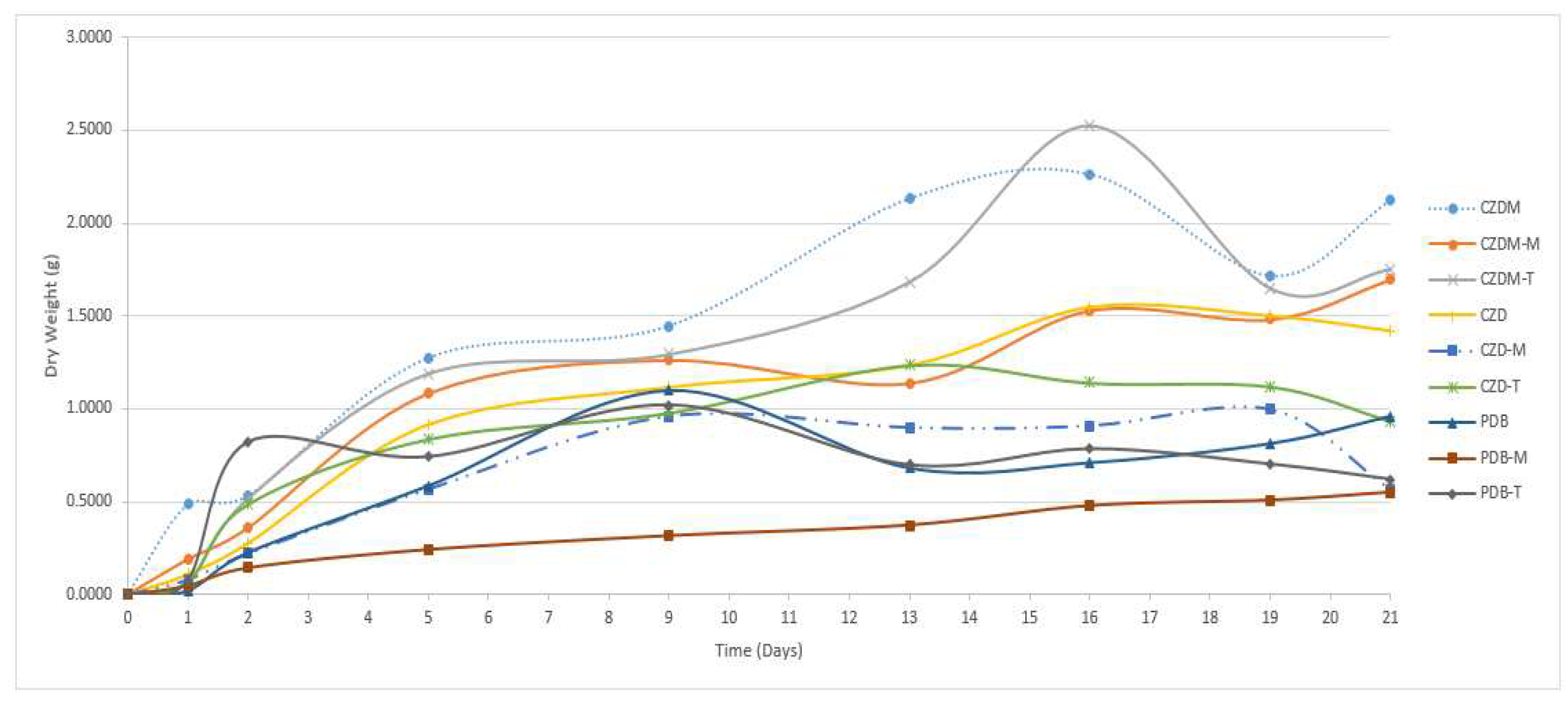
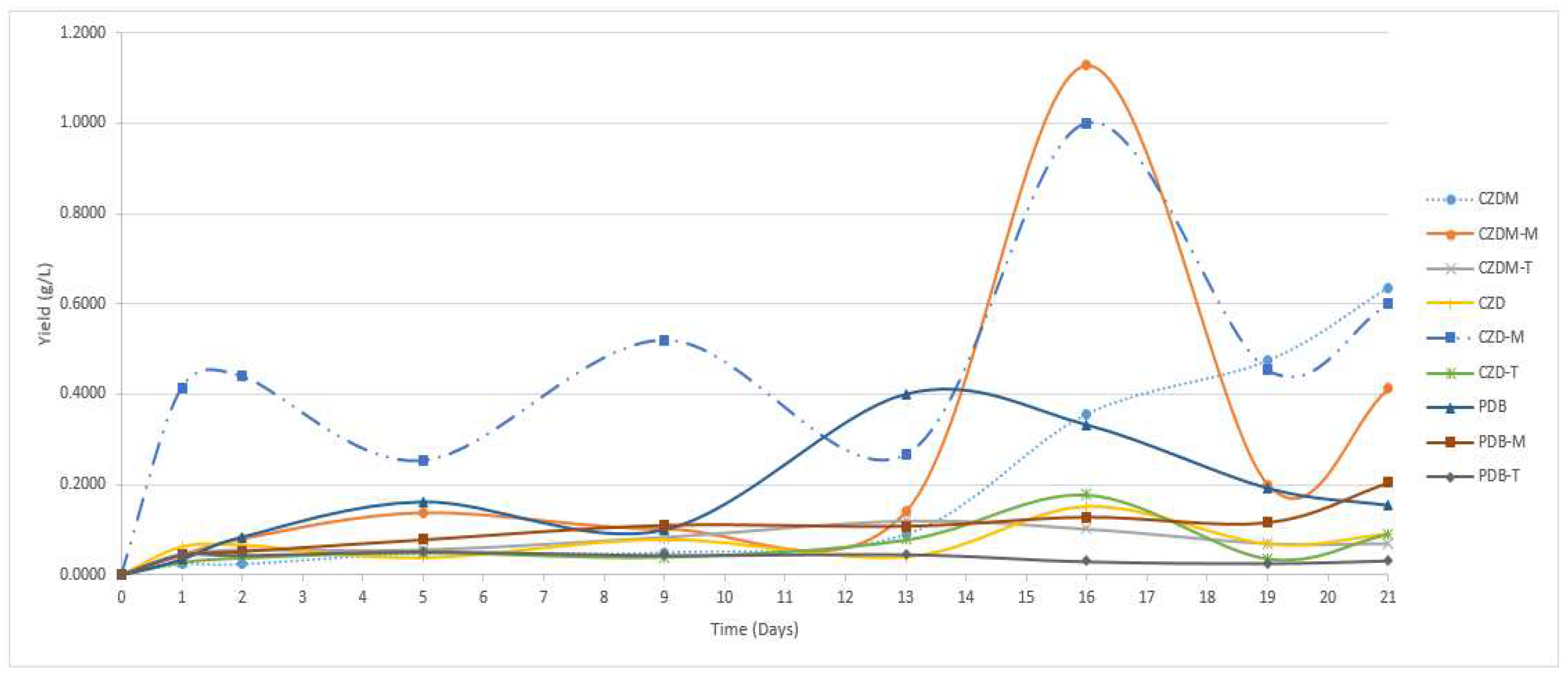
| Culture Media | Modification | Characteristics |
|---|---|---|
| PDB | None | Homogeneous pellets, reddish color |
| Talcum Powder | Pellet agglomeration, red-brown color | |
| Metallic Mesh | Mycelium on mesh, light brown color | |
| CZD | None | Homogeneous pellets, no change in color |
| Talcum Powder | Pellet agglomeration, light red color | |
| Metallic Mesh | Mycelium clumps on mesh, reddish color | |
| CZDM | None | Non-homogeneous pellet agglomeration, yellowish red |
| Talcum Powder | variable-sized pellets, light red color | |
| Metallic Mesh | pellet clustering on mesh, strong orange color |
| Extract | EC50 (μg/mg) | 95% Confidence Limits |
|---|---|---|
| CZD/MM9 | 27.17 | 14.84 – 49.86 |
| PDB/W5 | 149.75 | 116.49 – 192.38 |
| CZDM/W16 | >40 | >40 |
| SPH2 [8] | 22.00 | 19.00 – 26.00 |
| Extract | Mortality Rate (%) | LD50 (μg/mg) | 95% Confidence Limits |
|---|---|---|---|
| CZDM/MM21 | 100.00 | 5.00 | 4.4 - 5.66 |
| CZDM/W21 | 100.00 | 28.08 | 25.16 - 31.26 |
| CZD/W16 | 12.10 | >40 | >40 |
| Aspergillus spp. SPH2 [8] | 100.00 | 7.18 | 6.67 – 7.78 |
| Mellein (4) [8] | 100.00 | 0.48 | 0.44 – 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





