1. Introduction
Several clinical conditions including osteoporosis, Paget’s disease and other cancer-associated rarer bone diseases are characterised by bone loss and increased osteoclast activity [
1,
2] . Targeting of osteoclasts in skeletal pathobiology has thus been explored and has generated pharmacological agents capable of modulating osteoclastogenesis, resorption and ensuing bone loss. These agents, including bisphosphonates, selective estrogen receptor modulators and anti-RANKL antibodies, are effective at restricting osteoclastic bone resorption but nonetheless exert undesirable side effects such as impaired bone remodelling [
2]. For this reason, there remains demand for alternative approaches to control osteoclast formation and bone resorptive activity; antioxidant agents have been attributed with such anti-resorptive therapeutic potential [
3,
4].
Sulforaphane (4-methylsulfinylbutyl isothiocyanate, SFN) is a phytochemical produced from a biologically inactive precursor, glucoraphanin, during biting and chewing of certain plant sources [
5,
6,
7,
8,
9]; this precursor is found in cruciferous vegetables and many nutraceuticals [
10]. SFN is usually well tolerated and is known to exert potent antioxidant effects by modifying cytoplasmic KEAP1 conformation, releasing NRF2 for nuclear translocation and transcriptional activation of genes with antioxidant response elements (ARE) in their promoters [
11,
12,
13,
14,
15]. While cellular SFN uptake leads to an initial burst in ROS production, its rapid activation of the KEAP1-NRF2-ARE system induces an overall antioxidative or anti-inflammatory profile of cellular behaviours [
16,
17,
18]. SFN is, however, highly unstable, rapidly degraded by heat and light, and highly sensitive to oxygen and pH [
19]. SFN pharmacokinetics are therefore highly variable and inconsistent, making it difficult to ensure effective levels for sufficient duration for an attractive therapeutic, and its clinical advances have been restricted.
Technology for synthesising and concurrently stabilising SFN has therefore been developed to unlock its pharmaceutical potential. This has yielded a stable alpha-cyclodextrin: SFN complex, Sulforadex
TM (SFX-01), that is safe, well tolerated in humans, but rarely compared to SFN. SFX-01 is, nonetheless, known to recapitulate the vital targeting of NRF2 and STAT3 [
20] to inhibit lung metastasis by endocrine resistant stem-like cells in estrogen receptor (ER)-positive breast cancer [
20]. This clinical candidature of SFX-01 is reinforced by its efficacy in a mouse model of relapsing experimental autoimmune encephalomyelitis in which it reduces residual disability, decreases maximum severity of relapses and improves recovery [
21]. Beneficial effects of SFX-01 have also been observed in a preclinical human glioblastoma model [
22]. This has led to the examination of SFX-01 as a therapeutic in Phase 2 trials in ER-positive metastatic breast cancer and spontaneous subarachnoid haemorrhage patients [
23].
Few studies have, however, explored the effects of SFX-01 in skeletal pathophysiology. SFX-01 administration has been found to reduce levels of gait asymmetry that emerge spontaneously in a natural osteoarthritis model in STR/Ort mice [
24], in which such asymmetries are otherwise predictive of disease progression [
25]. This was accompanied by increases in trabecular bone mass and indices of bone strength, and higher procollagen type-I N-propeptide (PINP, bone formation) and, notably, lower type-I collagen cross-linked C-telopeptide (CTX-1, bone resorption) serum markers. It has also been shown that divergent basal bone mass in different mouse strains is linked both to in vitro osteoclastogenic potential and that this aligns with sensitivity to SFX-01 [
26]. These data suggest that SFX-01 represents an alternative approach to control osteoclast formation and resorptive activity.
There are extensive studies using SFN that support this notion. SFN inhibits RANKL-induced NF-KB activation in RAW 264.7 cells [
27] and restricts expression of the cell fusion molecules, DC-STAMP and OC-STAMP, by inducing STAT1 phosphorylation [
27]. This aligns with microarrays in RAW 264.7 cells and primary osteoclasts in which SFN lowered OSCAR, NFATc1 and TRAP, DC-STAMP and OC-STAMP mRNA levels whilst STAT1, a macrophage and osteoclast fusion regulator, was instead increased [
28]. Hyeon et al. (2013) explored links between SFN and NRF2, to find both more osteoclast multinucleation and resorption, with raised oxidative stress, in cells from NRF2 knockout mice, and SFN-related inhibition of osteoclast differentiation in cells from wildtype mice [
29], suggesting that SFN likely acts through NRF2 to suppress osteoclastogenesis. In vivo data showing elevated metaphyseal cancellous bone volume in both normal and ovariectomised mice treated with SFN indicate that SFN can indeed reduce the bone loss due to estrogen-deficiency [
30].
These data indicate that SFN and SFX-01 may have therapeutic value in skeletal diseases where bone resorption or accelerated remodelling is causal. It is therefore necessary to define whether SFX-01 also exerts antiresorptive actions and, if so, which osteoclastogenic stage(s) it targets and its mechanism(s) of action. This will verify its therapeutic potential and likely identify alternative antiresorptive candidates.
It is appropriate to also reflect on the methods used to study osteoclastogenesis. Initial advances involved cell isolation from fragmented neonatal bones and their deposition onto bone/dentine to allow for pit excavation [
31]. Long-term in vitro osteoclast formation now uses haematopoietic precursors from marrow, spleen or peripheral blood [
32,
33]; supplementation with, for example, PTH, M-CSF and RANKL with similar deposition onto bone/dentine. This has the advantage of allowing separate examination of key osteoclast formation, activation and resorption phases, impossible on plastic. Almost all studies reporting inhibitory SFN effects osteoclastogenesis have relied, however, on RAW 264.7 cell lines or primary cells seeded onto plastic, showing direct effects on differentiation, not resorption [
27,
28,
34]. One study elegantly extended this to study cells seeded on dentine, reporting that SFN reduces resorption pit number, not area of dentine resorbed per osteoclast, concluding that SFN strongly inhibited osteoclast formation at an early precursor differentiation stage [
27]. Here, using primary osteoclasts on dentine, we examine how SFX-01 influences each of the key osteoclast formation, activation and resorption phases, and explore whether, like SFN, it exerts its actions via the NRF2 and NF-KB pathways.
2. Materials and Methods
2.1. Animals
All the procedures conducted in the facility were in accordance with the Animals Act (Scientific Procedures) 1986 and approved by the Royal Veterinary College (RVC) Research Ethics Committee. Bone marrow (BM) cells were isolated from C57BL/6, CBA, and STR/Ort strains. Mice were housed at 21 ± 2°C with 12-h light/dark cycles with free access to food and water.
2.2. Osteoclast cell culture
Osteoclast precursors were isolated from the bone marrow of mouse long bones (6-8 weeks old) and cultured as described previously [
26] [
33]. Briefly, the resultant BM suspension was then centrifuged at 1500 rpm and resuspended in MEM supplemented with 10% foetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin, 100 nM prostaglandin E
2 (PGE
2) and 50 ng/ml M-CSF. The cell suspension was cultured for 24 h in 75 cm² flasks at 5% CO
2/95% atmospheric air. The next day (Day 1), the non-adherent cell suspension was removed, centrifuged, and resuspended in MEM containing 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin,100 nM PGE
2 and 200 ng/ml M-CSF as well as 5 ng/ml RANKL (R&D Systems Europe Ltd.)—referred to as S2MEM, see below. Cells were plated onto 5 mm diameter dentine discs (10
6 cells/disc) in 96-well plates and incubated overnight at 37°C/5% CO
2 to allow for attachment of osteoclast precursors. After 24 h (Day 2), the dentine discs were transferred to six-well plates (3–4 discs/well in 3 ml S2MEM) and maintained for 3 days. S2MEM was acidified to pH 6.9 (Day 5) by addition of HCl to activate osteoclast resorption for 48 h (Day 7).
To establish stages at which SFX-01 exerts actions on osteoclast formation, differentiation and resorption, cells were cultured in osteoclastogenic medium, with or without 2.5μΜ SFX-01 during very specifically defined and different time points; 2.5 μΜ was used as it has been previously showed to be the optimal concentration to inhibit osteoclastogenesis [
26]. Cells were exposed to SFX-01 (or vehicle) either: i) for the duration of post-plating culture from day 1-7 (during culture; DC; D1-D7); ii) at only the early stage (ES; D1-D2) for 24 hours after plating; iii) during only the differentiation stage and excluding the resorption phase (DS, between D2 and D5); iv) during only the later resorption phase (LS; D5-D7) (
Figure 1). All cultures were replenished with fresh control medium during the periods not supplemented with SFX-01 and in all cases, experimental protocols were terminated on day 7 of culture.
For some experiments, pharmacological inhibitors/activators of NRF2 and NF-KB were used initially alone for the duration of the culture (DC), at one of two different concentrations (1μM and 10μM). For NRF2 activation and inhibition, RA839 and Trigonelline (Trig) were used respectively; while for NF-KB activation and inhibition, Betulinic acid (BA) and SC-514 were used (all at 1μM or 10μM). Each compound was also added in conjugation with SFX-01 (2.5 μM) during the early stage (ES; D1-D2) of culture, for 24 hours after plating (at which time SFX-01 was found to be most effective).
All experiments used bone marrow cells derived from STR/Ort mice, with the sole exception of the experiment seeking to identify dose-dependent effects of pharmacological activators/ inhibitors of the NRF2 and NF-KB pathways in which CBA and C57BL/6 mice were used instead.
2.3. Analysis of osteoclast formation and bone resorption
Discs containing adherent cells/osteoclasts were fixed in 2% glutaraldehyde and stained to demonstrate tartrate-resistant acid phosphatase (TRAP) activity. Osteoclasts were defined as TRAP-positive cells containing two or more nuclei and/or clear evidence of resorption pit formation. Osteoclast number and the area resorbed on each disc were assessed ‘blind’ by transmitted light microscopy and reflective light microscopy and dot-counting morphometry using Image J, respectively [
33]
2.4. Immunocytochemistry
Osteoclasts grown in tissue culture 96-well plates for 6 days were treated with SFX01 as described above at specific, defined timepoints (ES, DS or DC), and then fixed with 4% paraformaldehyde in PBS for 20 minutes. Cells were washed twice with 0.05% PBS Tween 20 (PBS-T) and then exposed to 1% BSA in PBS-T for 30 minutes to block non-specific binding sites, prior to incubation for 2 hours with an anti-DCSTAMP antibody (clone 1A2 primary antibody, 1:500). Fluorescently tagged goat anti-mouse IgG was used as secondary antibody (1:1000) and added to for 1 hour at room temperature in the dark. For F-actin ring staining, phalloidiniFluor488 reagent was added for 30 min at room temperature. After washing with PBS, DAPI was used to stain nuclei. Cells were imaged using a DMIRB microscope to allow for simultaneous imaging of three fluorescent labels.
2.5. ELISA for 4-HNE
The levels of lipid peroxidation as a stable marker for oxidative stress were measured using the 4-hydroxynonenal (4-HNE) ELISA kit (ab238538, Abcam, USA) according to manufacturer’s instructions. BM cells extracted from STR/Ort mice were cultured for 6 days on plastic discs as described above and SFX-01 was added only during the early (ES) and/or differentiation stage (DS) stages along with appropriate untreated control group. The cultures were replenished with fresh control medium during the periods not supplemented with SFX-01. The absorbance was read immediately on the microplate reader at 450nm. The concentrations of the 4-HNE adduct were quantified by comparison to absorbance of a known 4-HNE-BSA standard curve.
2.4. Statistics
All statistical analysis was performed using GraphPad Prism 8. To show the difference between two groups, a two-tailed paired t-test was performed. Between three or more groups, a repeated measures one-way analysis of variance or a repeated measures mixed-effects model was performed with Fisher's LSD post hoc analysis. Each individual reported set of data represents n = 4–5 biological ‘experimental’ replicates using cells derived from different animals. Individual experiments comprised 6–8 technical replicates (number of discs) per group.
4. Discussion
These data show that SFX-01 preferentially, rapidly and irreversibly targets early osteoclastogenic stages by inhibiting the cell-cell fusion and cell-spreading required for multinucleation. Based on the use of pharmacological agents, it appears that SFX-01 appears to achieve these effects, akin to SFN, via the NRF2 pathway. These are the first data exploring the mechanism by which SFX-01 influences osteoclastogenesis.
Herein, SFX-01 can target all stages of osteoclastogenesis but exerts the most profound actions on early osteoclast precursor differentiation to exert lasting restriction. This aligns with previous findings with SFN showing that it similarly targets osteoclastogenesis and specifically decreases osteoclast formation rate, with less TRAP+ cells [
27,
28,
35] and expression of osteoclast differentiation-associated genes such as
NFATc1, NF-KB, c-Fos, DC-STAMP [
28]. These conclusions regarding SFN are based on the seeding of cells (RAW 264.7 or BMCs) onto plastic plates and thereby any possibility that SFN might directly influence resorption was negated. Only one study by Kim et al. (2005) describes assays using dentine to explore resorption, but no data for these assays are shown. To determine which osteoclastogenic stage was targeted by SFN, lymphocyte-free precursors were seeded on plastic plates and SFN was added at different timepoints to reveal that a minimum 3 days of exposure was required for inhibition [
27]. This aligns somewhat with the data herein, in which 7 days, 3 days or as little as 24 hour (1 day) of SFX-01 treatment was sufficient for effective inhibition.
Mononuclear osteoclasts promote cell–cell fusion by increasing the expression levels
of the fusogens
DC-STAMP or OC-STAMP, which ultimately leads to osteoclast formation and bone resorption [
36]. Additionally, it has been shown that Atp6v0d2-deficient mice exhibit impaired multinucleation emphasising its importance in cell fusion [
37]. SFN decreases osteoclast cell fusion genes such as
DC-STAMP, OC-STAMP, and Atp6v0d2 in sRANKL-induced multinucleated osteoclasts [
28,
35] and osteoclasts appear to be smaller in size with fewer nuclei. Also, the immunohistochemical data presented herein offer support for the notion that SFX-01 affects fusion/multinucleation, with some evidence indicating a decrease in the cellular levels of DC-STAMP and reduced labelling for polymerised actin.
There are many studies indicating that NRF2 plays an important role in bone cell regulation and homeostasis. Global NRF2 deletion increases RANKL expression and thereby increases number of osteoclasts and resorption [
29,
38,
39,
40]. Within this context, it is established that SFN activates NRF2, which via binding to the antioxidant response element in cognate genes serves to upregulate protective enzymes with known anti-inflammatory roles [
17]. There are several methods available for measuring ROS. Herein, we quantified oxidative stress by assessment of the lipid peroxidation marker, 4-HNE. The results indicated that exposure of cells to SFX-01 also decreased levels of oxidative stress in addition to lowering levels of osteoclastogenesis. This indicates an SFX-01-induced modulation of oxidation status and suggests that this occurs through NRF2 activation.
As a prelude to examining whether SFX-01 also achieves its effects on osteoclasts via the modulation of NFR2, our studies were initially focused on whether pharmacological NRF2 modulators, namely Trig and RA839, exerted direct effects on osteoclast formation and function. The alkaloid, Trig, has been used in many studies as an inhibitor of NRF2 in other cell types [
41,
42,
43]. On the other hand, RA839 has only recently been characterised as a non-covalent KEAP1 binding partner and selective activator of NRF2 [
44]; neither have been used in assays of osteoclastogenesis previously. Initially we used each at both 1 and 10μM and found that Trig (NRF2 inhibitor), did not modify osteoclastogenesis or resorption in BM precursors, but intriguingly that RA839 (NRF2 activator) significantly inhibited osteoclast formation and osteoclast resorption/cell when administered at 10μM. These data imply that direct NRF2 activation with RA839 abrogates all osteoclast maturation and resorptive phases extending to the activity of individual osteoclasts, but that inhibition of NRF2 (Trig) is insufficient to stimulate osteoclastogenesis or resorption, under these assay conditions. These data align to some extent with the findings that SFX-01 achieves its inhibition via activation of this NRF2 pathway.
To further explore interaction between SFX-01 and direct NRF2 pathway modulation, the effects of RA839 (10μΜ) were examined in the presence and absence of known inhibitory concentrations of SFX-01 (2.5μΜ). As previous data had defined early stage SFX-01 supplementation sufficient for inhibition, all compounds were added only for the initial 24 hours (ES). This confirmed that RA839 administration, in the absence of SFX-01, reduced osteoclast number and area resorbed/ osteoclast in BM precursors. Combined addition of SFX-01/RA839 to BM cultures revealed similar inhibition levels in osteoclastogenesis and resorption achieved by administration of SFX-01 alone, implying that the pharmacological NRF2 activator was rendered ineffective by the presence of SFX-01. These results suggest that SFX-01 likely exerts its action via activation of NRF2 to inhibit osteoclastogenesis and total resorptive function.
The results with Trig (NRF2 inhibitor) are more difficult to interpret. Whilst without any measurable effect even at 10μΜ on cells derived from C57BL/6 and CBA mice, Trig instead significantly inhibited both osteoclast formation and resorption/OC in BM cells derived from STR/Ort mice. These differences may be explained by different mouse strain-related sensitivities based upon bone mass [
26]. Nonetheless, there were no adjunct, additional, activities exerted by Trig over those exerted by SFX-01 alone in these studies, suggesting that Trig was ineffective when combined with SFX-01. The possibility that the NRF2-inhibitory functions of Trig may remain cryptic, unless combined with an activation of NRF2, in this case by SFX-01, would however require much more detailed pharmacological profiling. Likewise, it remains possible that Trig may not be very effective as an inhibitor of NRF2 under the conditions of these assays.
Treatment of BM cells with BA (NF-KB activator) revealed a concentration-dependent inhibition of osteoclast formation and resorption when added for the duration of the culture (DC). This aligns with prior studies that have found that BA suppresses osteoclast formation and acts as an inhibitor of bone resorption [
45,
46]. It nonetheless conflicts somewhat with the notion that NF-KB activation is required for OC formation; further studies into the actions of BA are required to resolve these apparent conflicts. Similar studies performed using SC-514, NF-KB inhibitor showed that it also restricted osteoclastogenesis without modifying resorption, even though one might predict based upon data from BA, that this NF-KB inhibitor would have opposing effects [
47] .
To explore interaction between the effects of SFX-01 and direct NF-KB pathway modulation, the effects of BA (10μΜ) were examined with and without SFX-01 (2.5μΜ) supplementation only for these initial 24 hours (ES). Treatment of mouse cells consistently indicated that BA more efficiently targets area resorbed/osteoclast than it does osteoclastogenesis compared with SFX-01. Treatment with SC-514 failed to exert statistical differences in osteoclast behaviour; albeit using a relatively small sample size.
It is important to emphasise that these studies explore the actions of stable SFX-01, with a strong focus upon the NRF2 and NF-KB pathways that are most strongly implicated in mediating the effects of its unstable counterpart SFN. It is important to stress that the NRF2 and NF-KB pathways are not the only potential targets for SFN in osteoclasts. Recent studies indicate that SFN may also inhibit osteoclastogenesis by suppressing the autophagic pathway, with fewer autophagosomes in RAW 264.7 cells and attenuation in autophagy markers [
34] . Simultaneous addition of rapamycin, an mTOR inhibitor that activates autophagy, was capable of reversing the inhibition of osteoclastogenesis induced by SFN [
34]. The possibility that SFX-01 may also achieve its effects via these mechanisms has not however been explored. It is also feasible that the chemical stabilisation of SFN, SFX01, may allow alternative mechanisms to be targeted. These would perhaps be best identified by some non-targeted screening; the studies described here serve to both verify a cellular, osteoclast precursor target for SFX-01 actions and also define timeframes over which exposure to SFX-01 could be explored. In conclusion, these studies show that SFX-01 preferentially and more effectively targets early stages of osteoclastogenesis rather than resorption and that it does so relatively rapidly and irreversibly. They also identify prominent roles for NRF2 activation over NF-KB pathway involvement in SFX-01-related inhibition of osteoclastogenesis.
Author Contributions
Conceptualization, P.L., I.O., and A.A.P; methodology, P.L., I.O., and A.A.P; software, P.L.; validation, P.L.; formal analysis, P.L.; investigation, P.L.; resources, A.A.P X.X.; data curation, P.L., and A.A.P; writing—original draft preparation, P.L. and A.A.P ; writing—review and editing, P.L., I.O., and A.A.P; visualization, P.L.; supervision, I.O., and A.A.P; project administration, A.A.P.; funding acquisition, A.A.P. All authors have read and agreed to the published version of the manuscript.
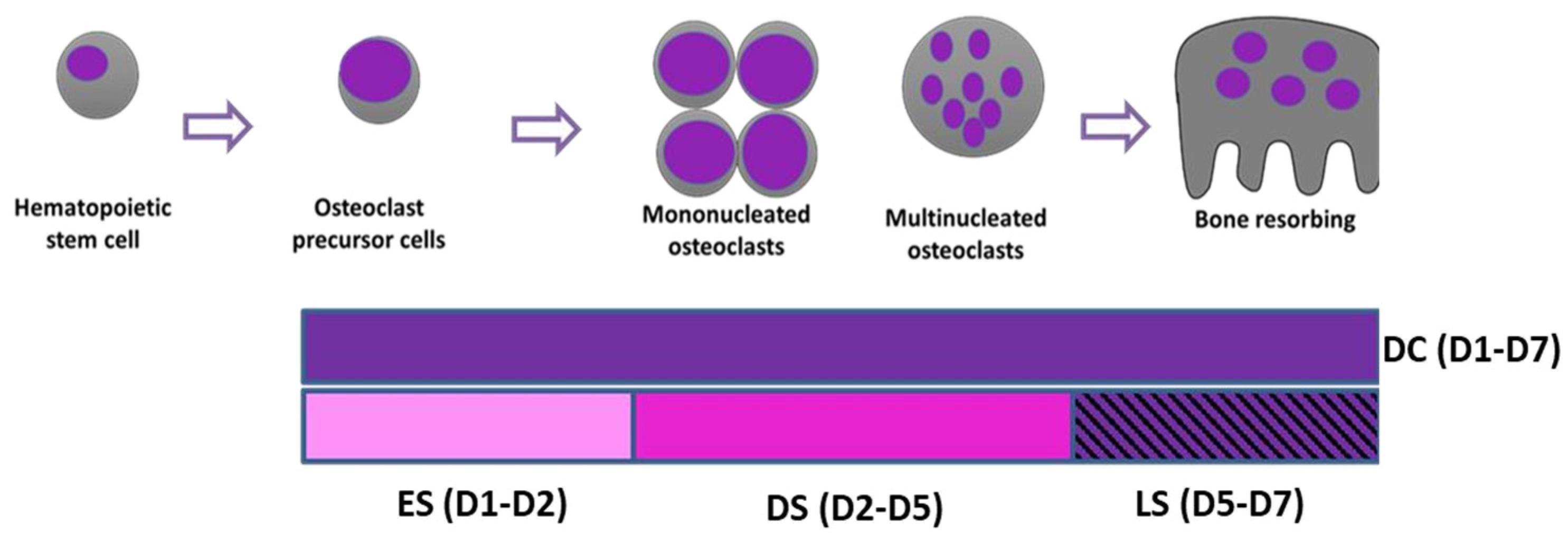
Figure 1.
Schematic illustration of dosing regimen to study the effects of SFX-01 on osteoclast differentiation and maturation. DC: treatment throughout (D1-D7). ES: treatment for first 24 hours (D1-D2). DS: treatment for differentiation stage (D2-D5). LS: SFX-01 for the resorption phase (D5-D7).
Figure 1.
Schematic illustration of dosing regimen to study the effects of SFX-01 on osteoclast differentiation and maturation. DC: treatment throughout (D1-D7). ES: treatment for first 24 hours (D1-D2). DS: treatment for differentiation stage (D2-D5). LS: SFX-01 for the resorption phase (D5-D7).
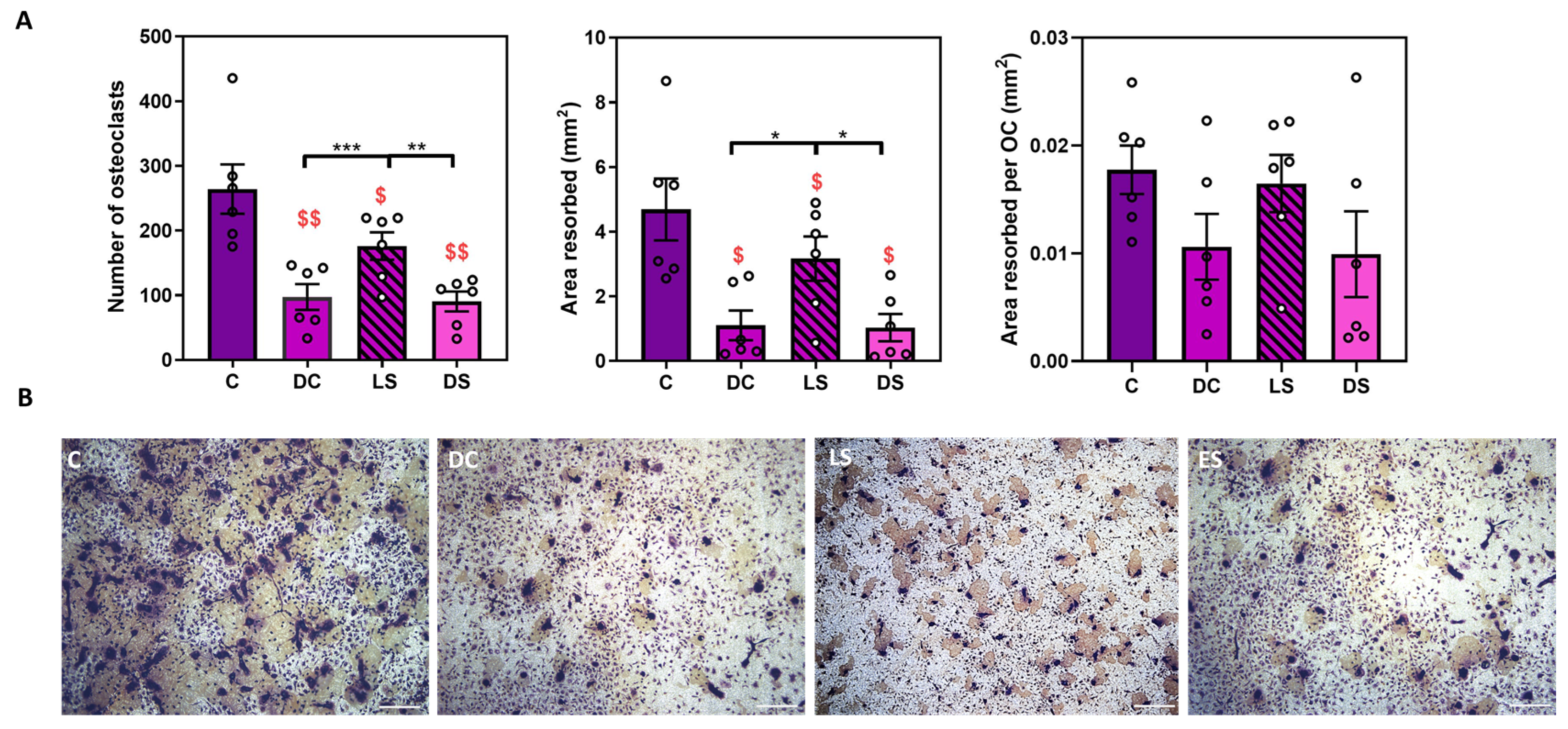
Figure 2.
SFX-01 targets osteoclast formation. A) 2.5μΜ SFX-01 was added at different time points during osteoclast culture, either throughout the culture (DC; D1-D7) or at later resorption stage (LS; D5-D7) or (differentiation stages (DS; D2-D5): A) TRAP+ osteoclast numbers (top left), area resorbed (middle), area resorbed/OC (top right, all mean ±SE) from STR/Ort mice. B Representative reflective light images of osteoclasts at different stages after SFX-01addition. $ and $$ denote p ≤ 0.05 and p ≤ 0.01 difference from control, respectively. *, ** and *** denote p ≤ 0.05, p ≤0.01 and p ≤0.001 differences within groups DC, LS, DS. C, respectively. Combined data from n=6 separate experiments, 6-8 replicates/condition/experiment.
Figure 2.
SFX-01 targets osteoclast formation. A) 2.5μΜ SFX-01 was added at different time points during osteoclast culture, either throughout the culture (DC; D1-D7) or at later resorption stage (LS; D5-D7) or (differentiation stages (DS; D2-D5): A) TRAP+ osteoclast numbers (top left), area resorbed (middle), area resorbed/OC (top right, all mean ±SE) from STR/Ort mice. B Representative reflective light images of osteoclasts at different stages after SFX-01addition. $ and $$ denote p ≤ 0.05 and p ≤ 0.01 difference from control, respectively. *, ** and *** denote p ≤ 0.05, p ≤0.01 and p ≤0.001 differences within groups DC, LS, DS. C, respectively. Combined data from n=6 separate experiments, 6-8 replicates/condition/experiment.
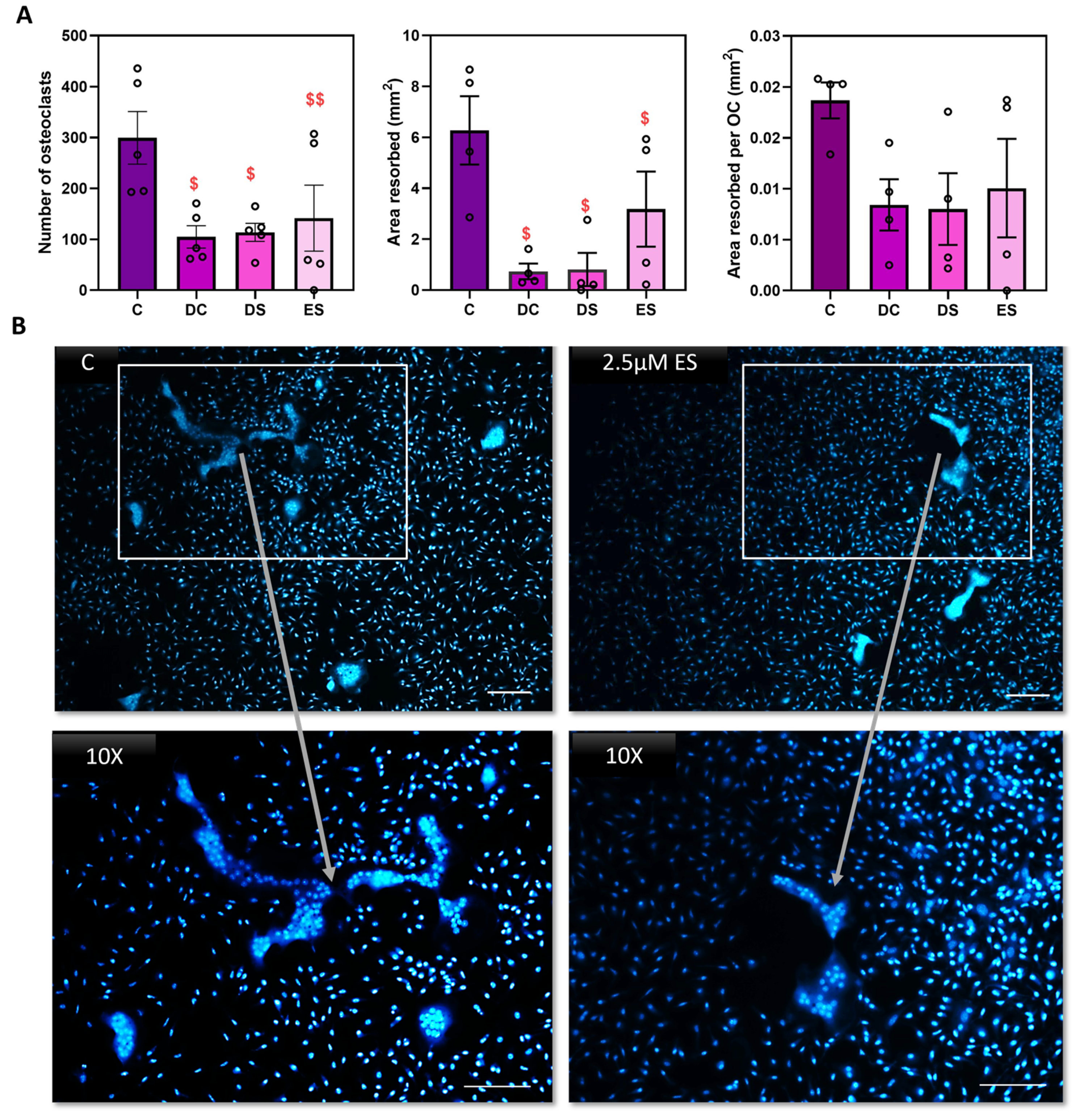
Figure 3.
24 hours exposure to SFX-01 is sufficient to inhibit osteoclastogenesis. (A) 2.5μΜ SFX-01 was added at defined timepoints during osteoclast culture either throughout the culture (DC; D1-D7) or at differentiation (DS; D2-D5), or only at early stage (ES; D1-D2) for 24 hours after plating on dentine and imaged and quantified conventionally: TRAP+ osteoclast numbers (top left), area resorbed (middle), area resorbed/OC (top right, all mean ±SE) from STR/Ort mice. $ and $$ denote p ≤ 0.05 and p ≤ 0.01 difference from control, respectively. Combined data from n=6 experiments, 6-8 replicates/condition. (B) 2.5μΜ SFX-01 was added during the early stage (ES) for 24 hours during osteoclast culture on plastic and imaged after DAPI nuclear staining to assess levels of multinucleation. SFX-01 reduces cell size and the number of nuclei comprising a single osteoclast.
Figure 3.
24 hours exposure to SFX-01 is sufficient to inhibit osteoclastogenesis. (A) 2.5μΜ SFX-01 was added at defined timepoints during osteoclast culture either throughout the culture (DC; D1-D7) or at differentiation (DS; D2-D5), or only at early stage (ES; D1-D2) for 24 hours after plating on dentine and imaged and quantified conventionally: TRAP+ osteoclast numbers (top left), area resorbed (middle), area resorbed/OC (top right, all mean ±SE) from STR/Ort mice. $ and $$ denote p ≤ 0.05 and p ≤ 0.01 difference from control, respectively. Combined data from n=6 experiments, 6-8 replicates/condition. (B) 2.5μΜ SFX-01 was added during the early stage (ES) for 24 hours during osteoclast culture on plastic and imaged after DAPI nuclear staining to assess levels of multinucleation. SFX-01 reduces cell size and the number of nuclei comprising a single osteoclast.
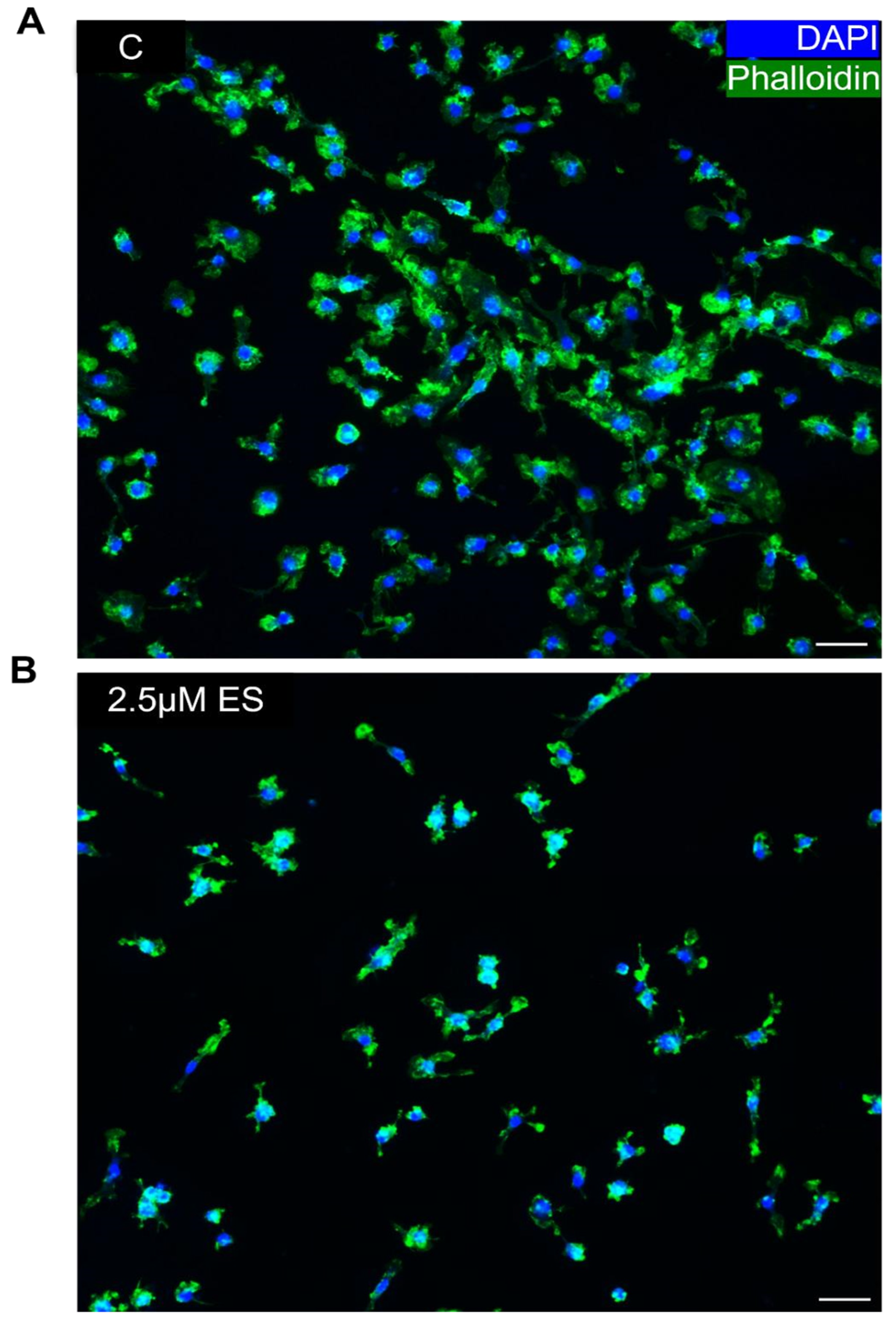
Figure 4.
Immunofluorescent labelling of mature OCs treated with SFX-01 at ES reveals modified polymerised actin organisation. Osteoclasts cultured for 6 days and then immunocytochemically labelled with phalloidin (for polymerised actin, green) and DAPI (for DNA, blue) and imaged by confocal microscopy indicating the difference in cytoskeletal architecture in untreated cells (A) and cells treated with SFX-01 (B).
Figure 4.
Immunofluorescent labelling of mature OCs treated with SFX-01 at ES reveals modified polymerised actin organisation. Osteoclasts cultured for 6 days and then immunocytochemically labelled with phalloidin (for polymerised actin, green) and DAPI (for DNA, blue) and imaged by confocal microscopy indicating the difference in cytoskeletal architecture in untreated cells (A) and cells treated with SFX-01 (B).
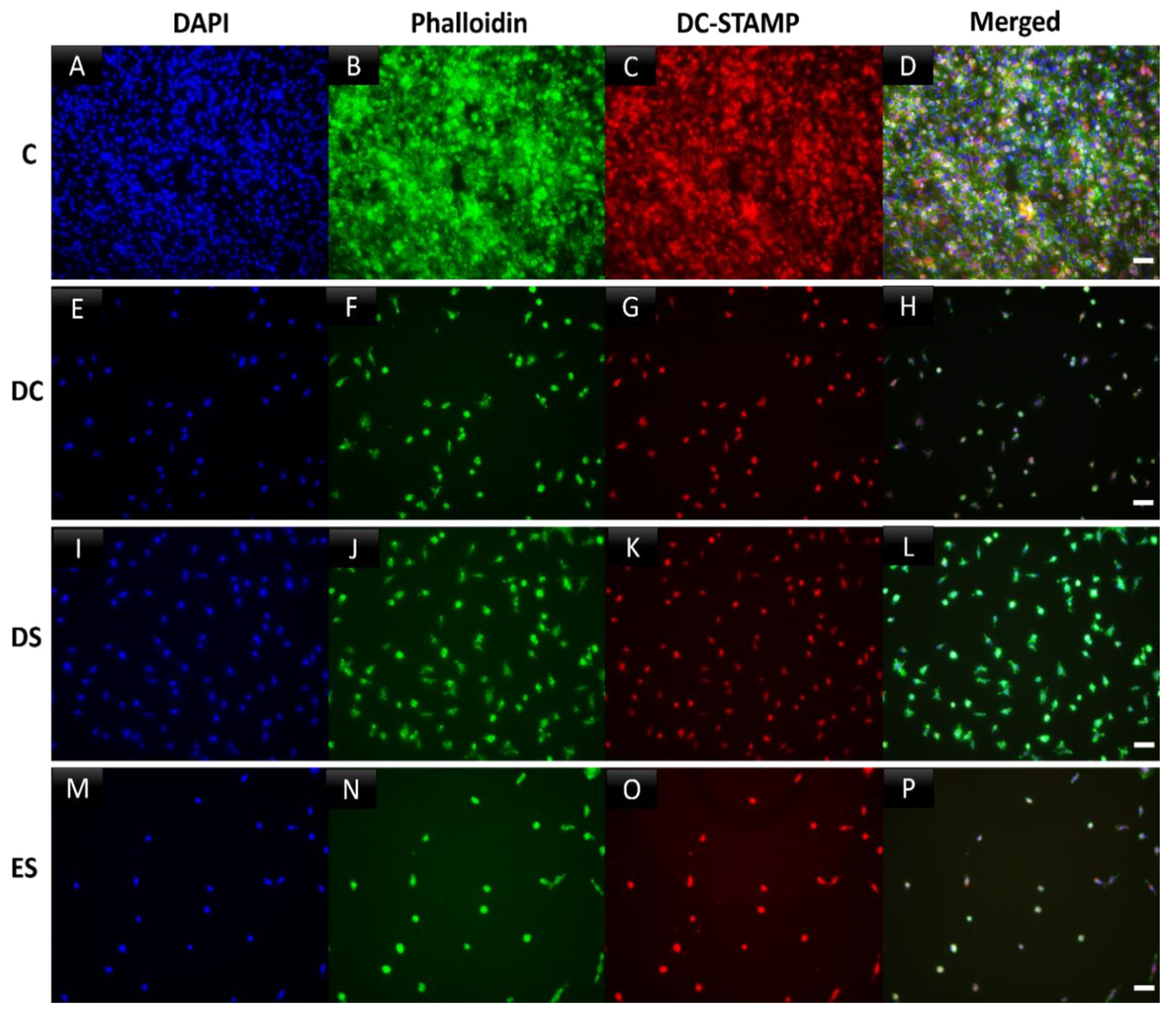
Figure 5.
Incubation with SFX-01 indicates inhibition of cell-cell fusion/multinucleation of osteoclasts grown on plastic. Osteoclasts cultured for 6 days and then immunocytochemically labelled for polymerized actin (phalloidin, green), DC-STAMP (red) and nuclear DNA (DAPI, blue). (AD) Control cultures showed positive labelling for cytoskeletal actin, cell spreading, cell fusion and multinucleation. These features were inhibited by the addition of SFX-01 at all timepoints, but particularly prominently at ES and DC (E-H, M-P). DS treatment likewise inhibited cell fusion (I-L) but less markedly.
Figure 5.
Incubation with SFX-01 indicates inhibition of cell-cell fusion/multinucleation of osteoclasts grown on plastic. Osteoclasts cultured for 6 days and then immunocytochemically labelled for polymerized actin (phalloidin, green), DC-STAMP (red) and nuclear DNA (DAPI, blue). (AD) Control cultures showed positive labelling for cytoskeletal actin, cell spreading, cell fusion and multinucleation. These features were inhibited by the addition of SFX-01 at all timepoints, but particularly prominently at ES and DC (E-H, M-P). DS treatment likewise inhibited cell fusion (I-L) but less markedly.
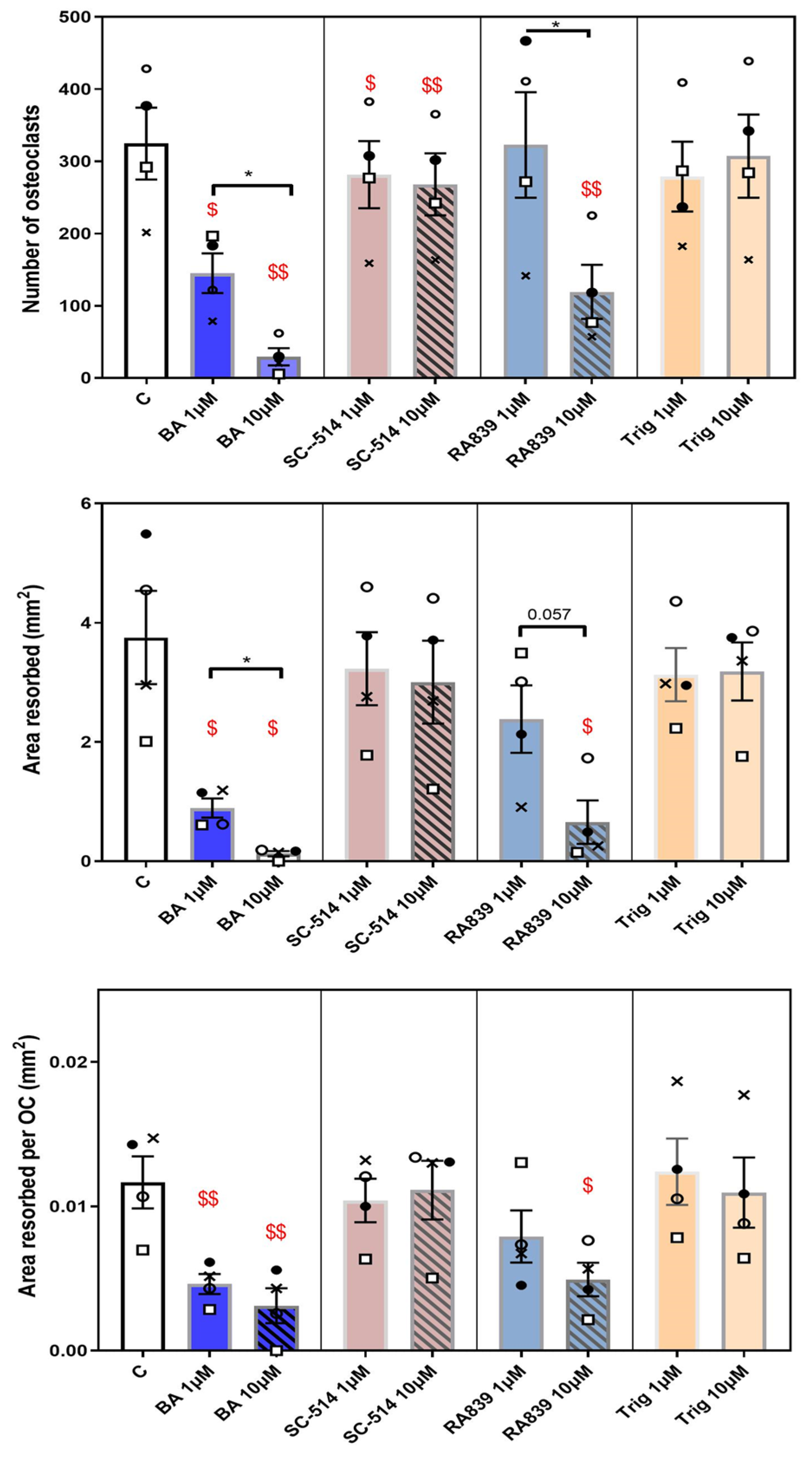
Figure 6.
Regulation of osteoclast behaviour by NRF2 and NF-KB modulators. RA839 (NRF2 activator) and BA (NF-KB activator) exert most marked inhibitory effects on osteoclast formation/resorption when added for duration of culture (7 days). TRAP+ osteoclast numbers, area resorbed, area resorbed/OC (mean± SEM). $ and $$ denote p ≤ 0.05 and p ≤ 0.01 difference from control. *denotes p ≤ 0.05 differences between 1μM and 10μΜ for each compound. Combined data from n=5 experiments, 6-8 replicates/condition. Open /closed circles and crosses denote BM cells from C57/Bl6 and squares CBA, respectively.
Figure 6.
Regulation of osteoclast behaviour by NRF2 and NF-KB modulators. RA839 (NRF2 activator) and BA (NF-KB activator) exert most marked inhibitory effects on osteoclast formation/resorption when added for duration of culture (7 days). TRAP+ osteoclast numbers, area resorbed, area resorbed/OC (mean± SEM). $ and $$ denote p ≤ 0.05 and p ≤ 0.01 difference from control. *denotes p ≤ 0.05 differences between 1μM and 10μΜ for each compound. Combined data from n=5 experiments, 6-8 replicates/condition. Open /closed circles and crosses denote BM cells from C57/Bl6 and squares CBA, respectively.
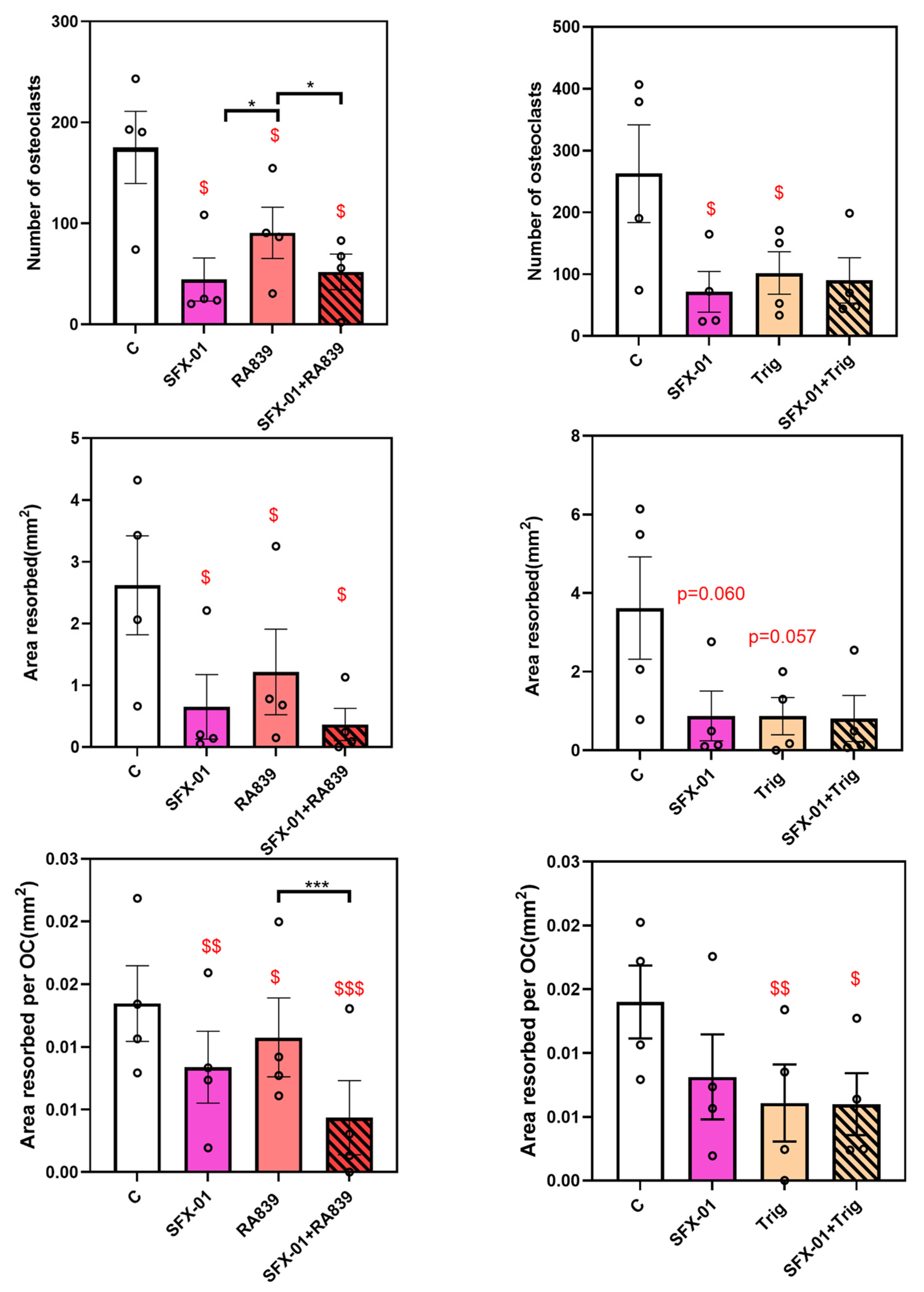
Figure 7.
Regulation of osteoclast behaviour by NRF2 and SFX-01. A) RA839 (NRF2 activator) inhibits osteoclast formation/resorption at early stage (ES; D1-D2) but there is no additional inhibition exerted when SFX-01 is co-administered; indicating that inhibition by SFX-01 is stronger than RA839. B) Trig (NRF2 inhibitor) also inhibits osteoclast formation/resorption at early stage (ES; D1-D2); no additional inhibition with SFX-01. TRAP+ osteoclast numbers (top), area resorbed (centre) and area resorbed/OC (bottom, mean± SE) from STR/Ort mice. $, $$ and $$$ denote p ≤ 0.05, p ≤ 0.01 and p≤ 0.001 from the control; * p ≤ 0.05, ***p<0.001 denote statistical difference between the different groups. Combined data from n=4 experiments, 6-8 replicates/condition.
Figure 7.
Regulation of osteoclast behaviour by NRF2 and SFX-01. A) RA839 (NRF2 activator) inhibits osteoclast formation/resorption at early stage (ES; D1-D2) but there is no additional inhibition exerted when SFX-01 is co-administered; indicating that inhibition by SFX-01 is stronger than RA839. B) Trig (NRF2 inhibitor) also inhibits osteoclast formation/resorption at early stage (ES; D1-D2); no additional inhibition with SFX-01. TRAP+ osteoclast numbers (top), area resorbed (centre) and area resorbed/OC (bottom, mean± SE) from STR/Ort mice. $, $$ and $$$ denote p ≤ 0.05, p ≤ 0.01 and p≤ 0.001 from the control; * p ≤ 0.05, ***p<0.001 denote statistical difference between the different groups. Combined data from n=4 experiments, 6-8 replicates/condition.
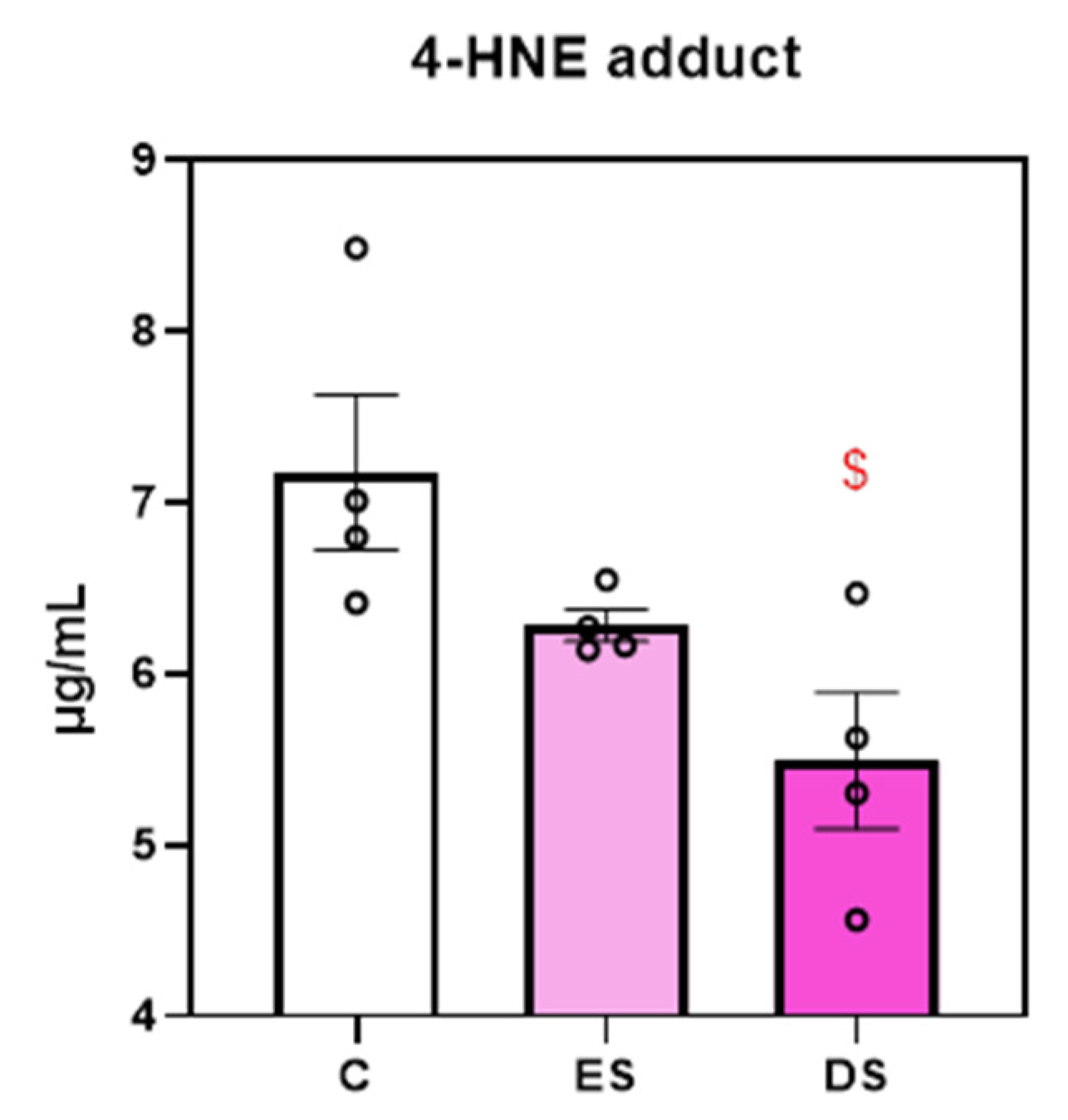
Figure 8.
Assay for the 4-HNE adduct indicates that cultures treated with SFX-01 (DS; D2-D5) expressed lower oxidative stress levels than the untreated cells.
Figure 8.
Assay for the 4-HNE adduct indicates that cultures treated with SFX-01 (DS; D2-D5) expressed lower oxidative stress levels than the untreated cells.
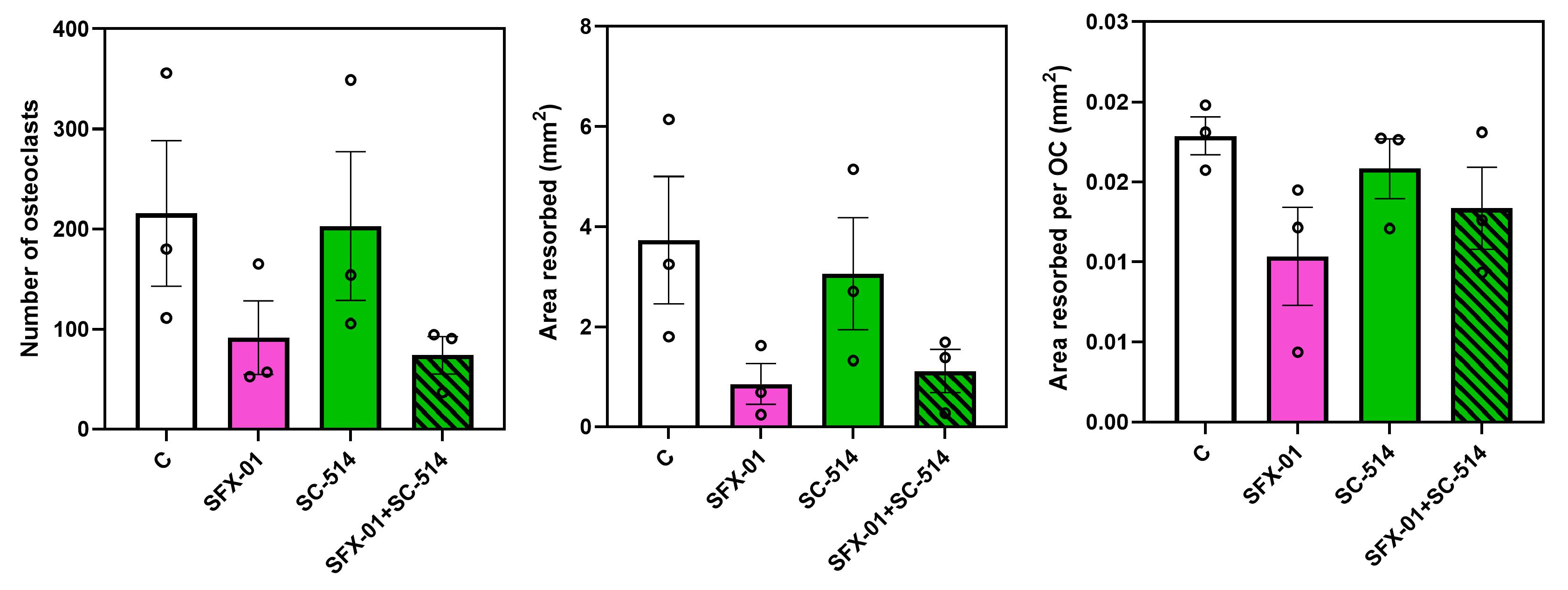
Figure 9.
Regulation of osteoclast behaviour by NF-KB and SFX-01. BA (NF-KB activator) inhibits osteoclast formation/resorption at early stage (ES; D1-D2); when supplemented with SFX-01 there was no additional inhibition greater than either with BA or SFX-01 alone. B) SC-514 (NF-KB inhibitor) did not modify osteoclast formation/resorption at early stage (ES; D1-D2) alone with SFX-01. TRAP+ osteoclast numbers (left), area resorbed (centre) and area resorbed/OC (right, mean± SE) from STR/Ort mice. $, $$ and $$$ denote p ≤ 0.05, p ≤ 0.01 and p≤ 0.001 from the control. * p ≤ 0.05, ***p<0.001 denote statistical difference between different groups.
Figure 9.
Regulation of osteoclast behaviour by NF-KB and SFX-01. BA (NF-KB activator) inhibits osteoclast formation/resorption at early stage (ES; D1-D2); when supplemented with SFX-01 there was no additional inhibition greater than either with BA or SFX-01 alone. B) SC-514 (NF-KB inhibitor) did not modify osteoclast formation/resorption at early stage (ES; D1-D2) alone with SFX-01. TRAP+ osteoclast numbers (left), area resorbed (centre) and area resorbed/OC (right, mean± SE) from STR/Ort mice. $, $$ and $$$ denote p ≤ 0.05, p ≤ 0.01 and p≤ 0.001 from the control. * p ≤ 0.05, ***p<0.001 denote statistical difference between different groups.