1. Introduction
One of the current issues on atopic dermatitis (AD) is to divide this complicated disease into subtypes [
1,
2,
3,
4]. Since AD is a heterogeneous condition, it is a requirement to comprehend the pathogenesis of AD by virtue of subtyping. Moreover, to optimize management of allergic diseases, personalized therapeutic approach is essential [
5]. Categorization of AD may lead to precision medical approach.
Although phenotypes have been historically used for subtyping of AD [
1], current concept of endotypes may be of greater clinical importance [
1,
2,
3,
4]. Therefore, physicians are willing to establish personalized therapeutic approach to AD ideally based on the endotype. Since a number of new effective drugs have been marketed [
6,
7] and clinical trials of novel therapies are ongoing, information on the endotypic properties in individual patients is required for choice of medicine [
2]. However, endotypes may not necessarily be familiar to clinicians because of difficult accessibility to endotypes in clinical settings. Physicians usually see AD patients consciously or even unconsciously by using classical subtypes. To better understand endotypes, therefore, attempts to adjust individual endotypes to classical subtypes are needed.
2. Methodology of AD Subtyping
The phenotype is the first methodology for AD subtyping and can be easily recognized in daily outpatient care without any specific examination [
1]. However, since AD patients usually have many common skin manifestations, there is a limitation to subdivide this complicated disease into defined groups only with the use of phenotype.
Endotypes represent pathogenetic mechanisms, and various viewpoints have been proposed for endotyping of AD [
1,
4,
5]. First, skin barrier condition contributes to endotypes. The extent of barrier impairment, in particular, presence or absence of filaggrin (FLG) gene mutation [
8,
9] and ceramide variation [
10] are representative elements. Another critical viewpoint for endotyping is the immune condition. AD usually shows type 2-shifted immunological condition [
11], but some patients have additional involvement of type 1 and/or type 3 (Th17/Tc17 and Th22/Tc22) immune cells [
1,
2,
3,
4]. In relation to the skewing immune condition, the type of antigens, to which patients are exposed, is involved in the endotyping. Protein allergens and metal/hapten antigens induce the expression of interleukin (IL)-4/IL-13 and interferon (IFN)-g/IL-22/IL-17, respectively [
1,
2,
3,
4].
As other methods to subdivide AD, genotypes, regiotypes and theratypes have been expected [
5]. However, these types are not independent and may be overlapped with the other types.
3. Phenotypes of AD
Main clinical manifestations of AD include infantile eczema, goose flesh-like skin, eczema on creases, lick dermatitis, dirty neck, Hertoghe sign, Dennie-Morgan folds, red face, and dirty neck [
1]. It is difficult for even dermatologists to use these skin lesions for categorization into subtypes because most of them are the phenotype of common extrinsic AD [
1,
12]. Among them, only Dennie-Morgan fold is exceptional, as its frequency was reported to be higher in intrinsic AD than in extrinsic AD [
13].
Notably, palmar and plantar hyperlinearity and ichthyosis vulgaris are frequently seen in extrinsic AD patients and are closely associated with
FLG mutations and subsequent barrier abnormality [
1,
12]. Thus, these phenotypic signs are not only of endotypes, but also of genotypes. As less frequent phenotypic skin lesions, there have been reported lichen amyloidosus, vitiligo or leukoderma [
14], chondrodermatitis of the auricle [
15], and angiohistiocytoid papules [
16]. Since these lesions arise in patients with high serum IgE levels and eosinophilia, they are estimated to be the manifestations of type 2-predominant extrinsic AD [
14,
15,
16]. Again, we cannot use even these intriguing lesions for elaborate subtyping of AD.
When we refer to the historical diagnostic standard of AD by Hanifin and Rajka [
17], we can find that elements of both phenotype and endotype are blended in the list. The phenotypic signs include xerosis, ichthyosis, palmar hyperlinearity, hand or foot dermatitis, nipple eczema, cheilitis, recurrent conjunctivitis, Dennie-Morgan folds, keratoconus, anterior subcapsular cataracts, orbital darkening, facial pallor/facial erythema, and pityriasis alba. Meanwhile, the endotypic elements include immediate (type 1) skin test reaction, elevated serum IgE, impaired cell mediated immunity, food intolerance, clinical course influenced by environmental and emotional factors. Thus, phenotypes and endotypes were historically intermingled and some of them are difficult to be distinguished from each other.
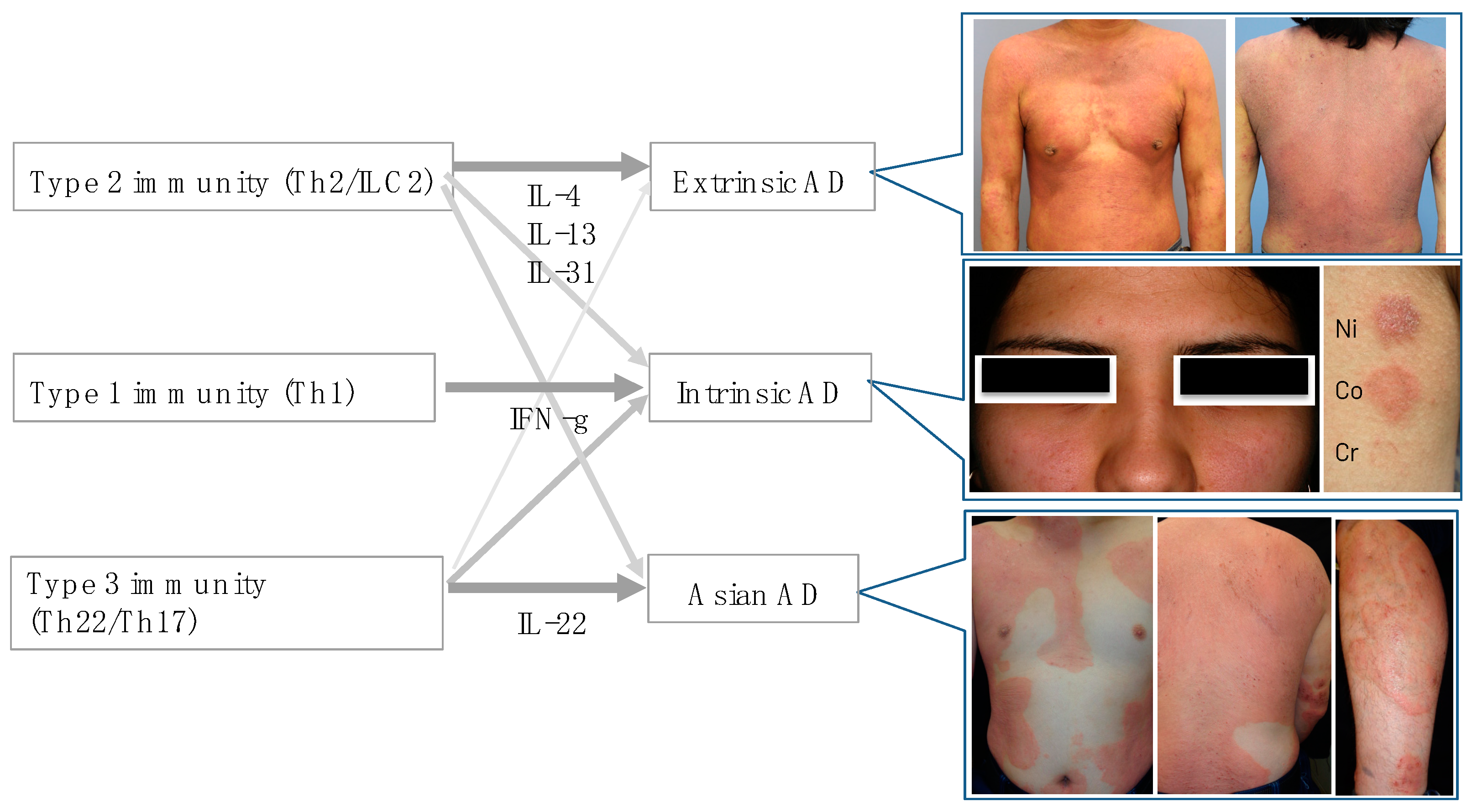
4. Classical AD Subtypes and Cytokine Skewing
In the era when the idea of endotypes had not yet been apparent, AD was categorized into extrinsic and intrinsic types mainly by serum levels of IgE [
12], into European American and Asian subtypes by ethnicity [
18,
19], or into pediatric and adult subtypes by age bracket [
1,
3] (
Table 1). It is notable that different immune conditions operate between the counterparts in these three representative classical subtypes (
Table 1). In “extrinsic”, “European American”, and “pediatric” subtypes, the Th2 (type 2) inflammation is dominant. On the other hand, “intrinsic”, “Asian”, and “adult” subtypes are characterized by various extents of Th1 (type 1)-, Th22/Th17 (type 3)-, and/or IL-19A-skewing condition [
1,
2,
3,
4,
18,
19,
20]. A current issue is how these classical subtypes correspond to endotypes. Alternatively, some investigators might feel that endotypes are not necessarily relevant to classical subtypes. In any case, attempts to adjust each classical subtype to endotype are prerequisite to better understand endotypes.
4.1. Extrinsic and Intrinsic AD
We first describe the serum IgE level-high extrinsic and IgE-normal intrinsic types, which were historically called mixed and pure AD, allergic and non-allergic AD, or classical AD and atopiform dermatitis, respectively [
12]. Extrinsic and intrinsic AD began to be adapted in the late 1980’s. While extrinsic AD is the common type with high prevalence, the incidence of intrinsic AD is approximately 20% or less with female predominance [
12]. The similar frequency rates of the two subtypes have been reported in European and Asian countries, including Germany, Hungary, Netherland, Korea, and Japan [
12]. The skin barrier is perturbed in the extrinsic AD [
12], and FLG mutation represents a typical cause of barrier impairment [
22]. Thus, allergic conditions may be preceded by skin barrier abnormalities, which allows protein antigens to penetrate through the disrupted stratum corneum barrier in extrinsic AD. Protein allergens generally induce and evoke Th2 cells, which produce IL-4 and IL-5, thereby elevating IgE and eosinophils.
On the other hand, intrinsic AD is immunologically characterized by the higher expression of IFN-γ, IL-22/IL-17 [
22,
23], although type 2 cytokines, such as IL-4, are also overexpressed because of the background of AD. Non-protein antigens, such as metals (nickel, cobalt, and chromium) and haptens, may induce the Th1/Th17/Th22 (type 1 and type 3) responses and evoke eczematous dermatitis [
24]. Higher levels of nickel concentration were found in peripheral blood and sweat from intrinsic AD than those from extrinsic AD [
24,
25]. Some intrinsic AD patients intake high nickel-containing foods, such as coffee and chocolate [
25]. Since protein allergens are not the primary antigen in intrinsic AD, serum total IgE or IgE specific for mite antigens is not elevated. We use tentative criteria as follows: intrinsic AD is defined as serum IgE levels ≤200kU/L or 200<IgE≤400 plus class 0 or 1 of IgE specific to Dermatophagoides pteronyssinus or Dermatophagoides farinae, and extrinsic AD is defined as 400<IgE levels or 200<IgE≤400 plus class 2 or more of the specific IgE [
24].
Although the exact mechanisms underlying intrinsic AD remain unclear, we focused on suprabasin deficiency as one of the causes. The expression of suprabasin was decreased in the stratum corneum of AD patients compared with healthy subjects by proteome analysis [
26]. The serum suprabasin in intrinsic AD was significantly lower than that in control and tended to be lower than in extrinsic AD [
27]. In 3-dimentionally constructed skin, suprabasin deficiency induced apoptosis of epidermal keratinocytes [
27]. Suprabasin is expressed in the upper digestive tract where nickel is absorbed. In suprabasin-knock-out mice, the impaired expression of suprabasin increased absorption of orally administered nickel and elevated serum nickel. In those nickel-loaded, suprabasin-deficient mice, contact hypersensitivity to nickel was enhanced [
28]. These findings suggest that suprabasin deficiency is one of the causes of intrinsic AD. However, there may be other mechanisms underlying intrinsic AD.
4.2. European American and Asian AD
Different T-cell activation profiles have been found between Western and Asian populations [
18,
19]. In addition to type 2, there is the co-existence of multiple cytokine axes of type 1 and type 3 in Asian types of AD [
3]. Accordingly, while Th17 frequencies were elevated in both peripheral blood and lesional skin of Japanese patients [
20], Th17 axis activation was not seen in European American extrinsic patients [
18]. Thus, Asian AD patients are characterized by a unique blended immune dysregulation between AD and psoriasis [
2,
3,
18]. Nevertheless, increased levels of Th2 cytokines and related chemokines were similarly seen in European American and Asian patients, correlating with AD severity and IgE levels [
18,
19]. A study comparing lesional and nonlesional skin of European American and Asian (Japanese and Koreans) patients showed prominent epidermal hyperplasia and marked parakeratosis in Asian subjects but relatively preserved barrier proteins, FLG and loricrin [
18,
19]. In accordance with these histological findings, Asian type of AD occasionally shows skin lesions resembling psoriasis (
Figure 1). IL-19 is induced by IL-4, IL-13 and IL-17 and it augments IL-17 effects on keratinocytes. Levels of IL-19 were significantly greater in AD lesions of Asian patients [
18,
19]. Chinese AD patients share the consistent Th17/Th2 or blended AD-psoriasis type with Japanese and Korean patients [
29].
In another ethnic study, African American and European American patients were compared. Loss-of-function mutations of FLG are not prevalent in African American patients with AD, but FLG variations were associated with AD persistence in these patients [
30]. Immunologically, Th1/Th17 attenuation and Th2/Th22 skewing were seen in these patients [
31].
4.3. Pediatric and Adult AD
In infancy, seborrheic dermatitis is a common eruption on the face and scalp. In young children, dry skin is overt as represented by goose flesh-like skin, and eczema occurs on the antecubital and popliteal fossae. In adults, lichenified lesions are prominent, and patients may develop red face, exhibiting persistent dark reddish erythema on the face, and dirty neck, showing poikilodermatous reticulate lesions on the neck. These skin lesions may be associated with infiltrating T-cell populations, epidermal changes, and dermal remodeling [
1].
In peripheral blood lymphocytes, the frequencies of cutaneous lymphocyte-associated antigen (CLA)
+ Th1 cells were significantly lower in AD infants than older patients, but the frequencies of CLA
+ Th2 cells were similarly expanded across all AD age groups [
32]. After infancy, CLA
- Th2 frequencies were increased in all age groups, suggesting systemic immune activation with disease chronicity. IL-22 frequencies serially increased from normal levels in infants to highly significant levels in adolescents and adults [
32]. Thus, in adults, the type 3 and type 1 pathways are involved, and a weakened epidermal barrier is characteristic. In contrast, pediatric patients exhibit less type 1 activation, and defects in epidermal lipid metabolism contribute to their barrier defect [
4].
5. Viewpoints to Classify AD Endotypes
Endotypes of AD can be stratified from different factors, including cytokine patterns, allergen properties, epidermal barrier condition, ceramide variation, involvement of innate immunity, and serum biomarkers [
1,
2,
3,
4]. These factors are mostly viewpoints, but methodologies are intermingled (
Table 2). Thus, endotypes are not as simply understandable as phenotypes. Recent technologies further provide new methodologies for endotyping AD [
33,
34].
It should be noted that these viewpoints are not independent, but rather confounding each other. For example, the expression of individual cytokines is deeply influenced by types of allergens [
1,
2,
3,
4,
24]. Penetration of allergens through the epidermis depends on epidermal barrier condition, including
stratum corneum barrier constituents and intracellular lipids [
8,
10,
21]. Innate immunity and microbiome are closely associated with the other factors [
35]. Finally, new methodologies may propose novel endotypes and affects the importance of individual viewpoints [
33,
34].
5.1. Cytokines
Cytokines are one of the most frequently used viewpoints for endotyping AD, and their expression levels can be measured at the protein or mRNA levels in the blood and skin specimens from the patients [
1,
2,
3,
4,
22,
23]. The cytokine-based endotypes can be categorized into type 2 cytokine (IL-4, IL-13, IL-5 and IL-31)-high, type 1 cytokine (IFN-g)-high, and/or type 3 cytokine (IL-22 and IL-17)-high, or mixed subtypes. The classical subtypes are largely related to these cytokine-based endotypes (
Figure 1).
Along with type 2, co-existence of multiple cytokine axes of type 3 and type 1 types is seen especially in intrinsic and Asian types of AD. Intrinsic AD shows similar Th2 and higher Th1 and Th17 immune activation compared with extrinsic AD [
22,
23]. The Asian AD phenotype combines features of AD and psoriasis with increased Th17 polarization [
18,
19,
20]. The frequencies of CLA
+ Th1 were significantly lower in AD infants than older patients, but CLA
+ Th2 were similarly expanded across all age groups [
32]. Thus, type 2 dominant inflammatory state, generally known to be an essential characteristic of AD, is seen in “extrinsic”, “European American”, and “pediatric” subtypes. Taken together, type 3 and type 1 cytokines are additionally accompanied with type 2 axis in “Asian”, “intrinsic”, and adult” types (
Figure 1).
5.2. Allergens and Epidermal Barrier
Endotyping based on allergens is closely associated with epidermal barrier-based endotyping. In the epidermal barrier-disrupted skin, protein antigens or allergens can penetrate through the skin and induce type 2 inflammation with Th2 cells and innate lymphoid cell type 2 (ILC2) [
11]. The barrier damage stimulates epidermal keratinocytes to produce alarmin IL-33, IL-25 and thymic stromal lymphopoietin (TSLP), thereby activating type 2 reaction and promoting IL-4 production [
36]. This sequential event provides the mechanism of extrinsic AD and European American AD. On the other hand, relatively preserved epidermal barrier may allow metals and haptens to penetrate the epidermis, as typically seen in intrinsic AD [
24].
Ceramides are involved in skin barrier function [
10]. It is assumed that ceramides participate in AD endotypes, but exact contribution of ceramide variation to endotyping will be investigated in future.
5.3. Innate Immunity
IL-33 is an inflammatory cytokine that is over-expressed in epidermal keratinocytes of patients with AD [
37]. IL-33 transgenic mice, which express IL-33 specifically in keratinocytes, spontaneously develop AD-like eczema, suggesting that IL-33 is sufficient for the development of AD [
38]. IL-33 stimulates various cells, including ILC2, to produce type 2 cytokines, such as IL-5 and IL-13, and IL-33-stimulated basophils activate ILC2
via IL-4 [
39]. Moreover, the mechanism of IL-4 production by ILC2 has recently been investigated and was elaborated with other cytokines [
40]. This raises a possibility that the IL-4 production by ILC2 varies among patients, leading to endotyping.
The roles of IL-1 family members, IL-36 and IL-38, are postulated in the pathogenesis of AD [
41]. There have been scarce studies how the production of these cytokines varies in AD individuals. Future studies are necessary to elucidate this issue.
5.4. Serum Biomarkers
We mention a representative methodology for endotyping. Biomarkers are valuable parameters for precision medicine as they provide information on the disease endotypes, clusters, precision diagnoses, identification of therapeutic targets, and monitoring of treatment efficacies.
Thijs JL et al conducted two cohort studies to investigate AD endotypes by using serum biomarkers [
33,
34]. In both studies, they found four clusters in the endotype, and three of them were virtually identical. In the latest analysis based on 143 serum biomarkers from 146 patients with severe AD, cluster A (33.6%) could be distinguished from the other clusters as being a “skin-homing chemokines/IL-1R1-dominant” cluster, whereas cluster B (18.5%) was a “Th1/Th2/Th17-dominant” cluster, cluster C (18.5%) was a “Th2/Th22/PARC-dominant” cluster, and cluster D (29.5%) was a “Th2/eosinophil-inferior” cluster [
34]. Their findings indicate that, in addition to Th2, Th1 and Th17/Th22 are involved in some subgroups of AD, and that even Th2/eosinophil-inferior type exists. We speculate that cluster A corresponds to a group with high levels of severity-associated chemokines, cluster B to heavily inflammatory AD, cluster C to AD with chronic lesions or Asian type, and cluster D to intrinsic AD. However, endotyping using serum biomarkers may be fundamentally different from the conventional subtyping, and it may be difficult for the endotypes to comprehensively correspond to the classical subtypes.
In their study, the top 10 biomarkers include IL-37, IL-1ra, XCL-1, eotaxin/CCL11, IL-1ß, IL-26, LIGHT/TNFSF14, IL-1r1, EGF and TSLP [
34]. The authors were surprised at the fact that none of these markers are Th2-related cytokines, but they consisted of IL-1-, IL-10-, and epithelium-related markers. Their study might indicate that individualized treatment options should be based not on clinical phenotypes of AD but instead on biomarker-based endotypes.
6. Possible Therapeutic Application of Endotypes
Endotypes provide important information on individualized treatment options. When an AD patient belonging to type 2-dominant endotypes, anti-IL-4Ra antibody dupilumab, blocking both IL-4R type 1 (for IL-4) and type 2 (for IL-4 and IL-13) [
42,
43] and neutralizing antibodies against IL-13 activity, tralokinumab [
44] and lebrikizumab [
45], are effective. These treatments also theoretically exert a beneficial effect on extrinsic AD and European American AD. Meanwhile, they might be less sufficient efficacious for Th2/eosinophil-inferior endotype or intrinsic AD. However, clinical trials (Chronos) showed that the therapeutic effect of dupilumab on AD did not depend on the serum levels of IgE or TARC (data from clinical trials; Dupiumab Common Technical Document Part 2 – Clinical Overview 2.7.3 Clinical Effectiveness). It would be interesting if this finding could be confirmed in the real world. Besides, anti-IL-31Ra antibody nemolizumab is another modality and decreases pruritus of AD [
46].
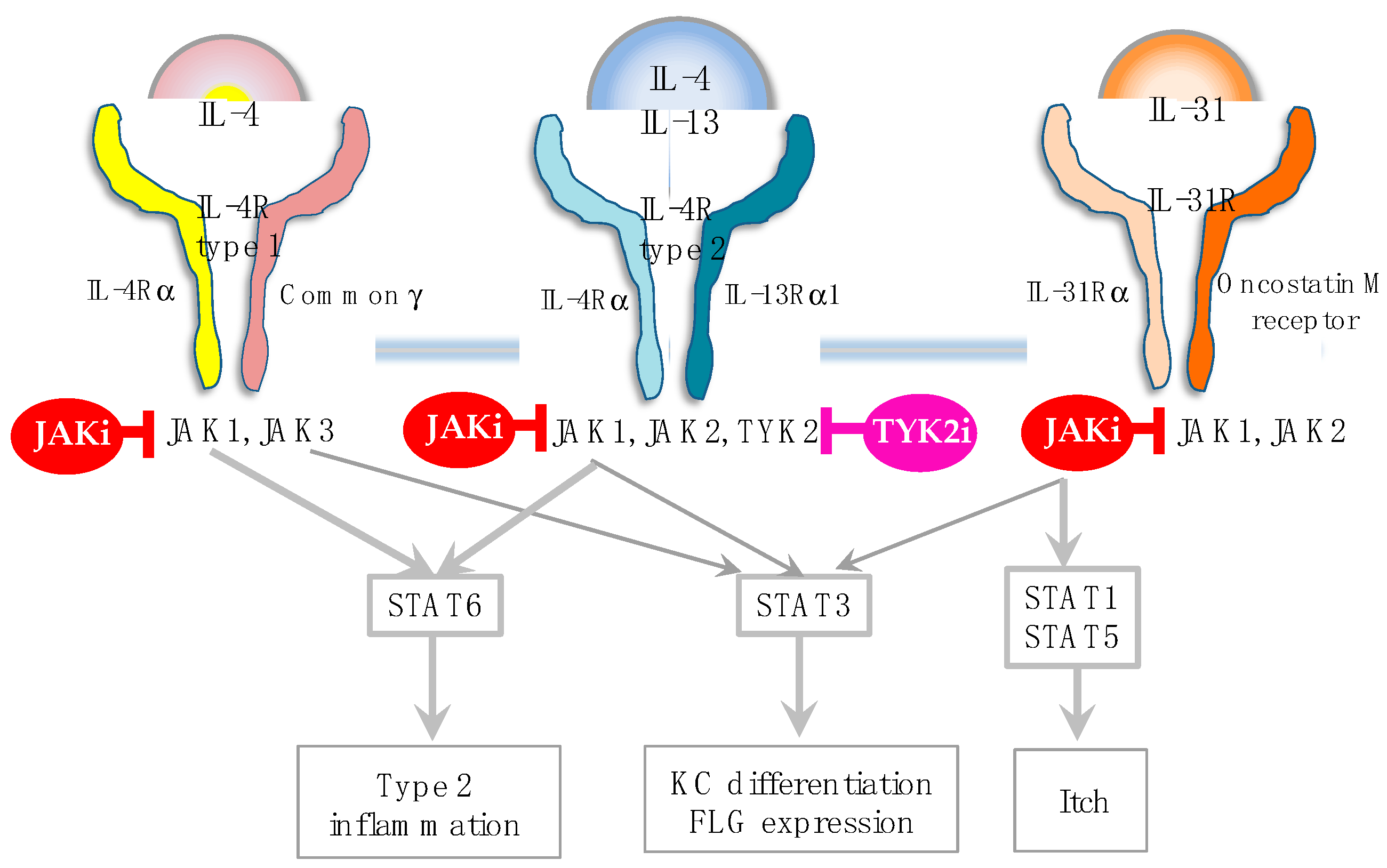
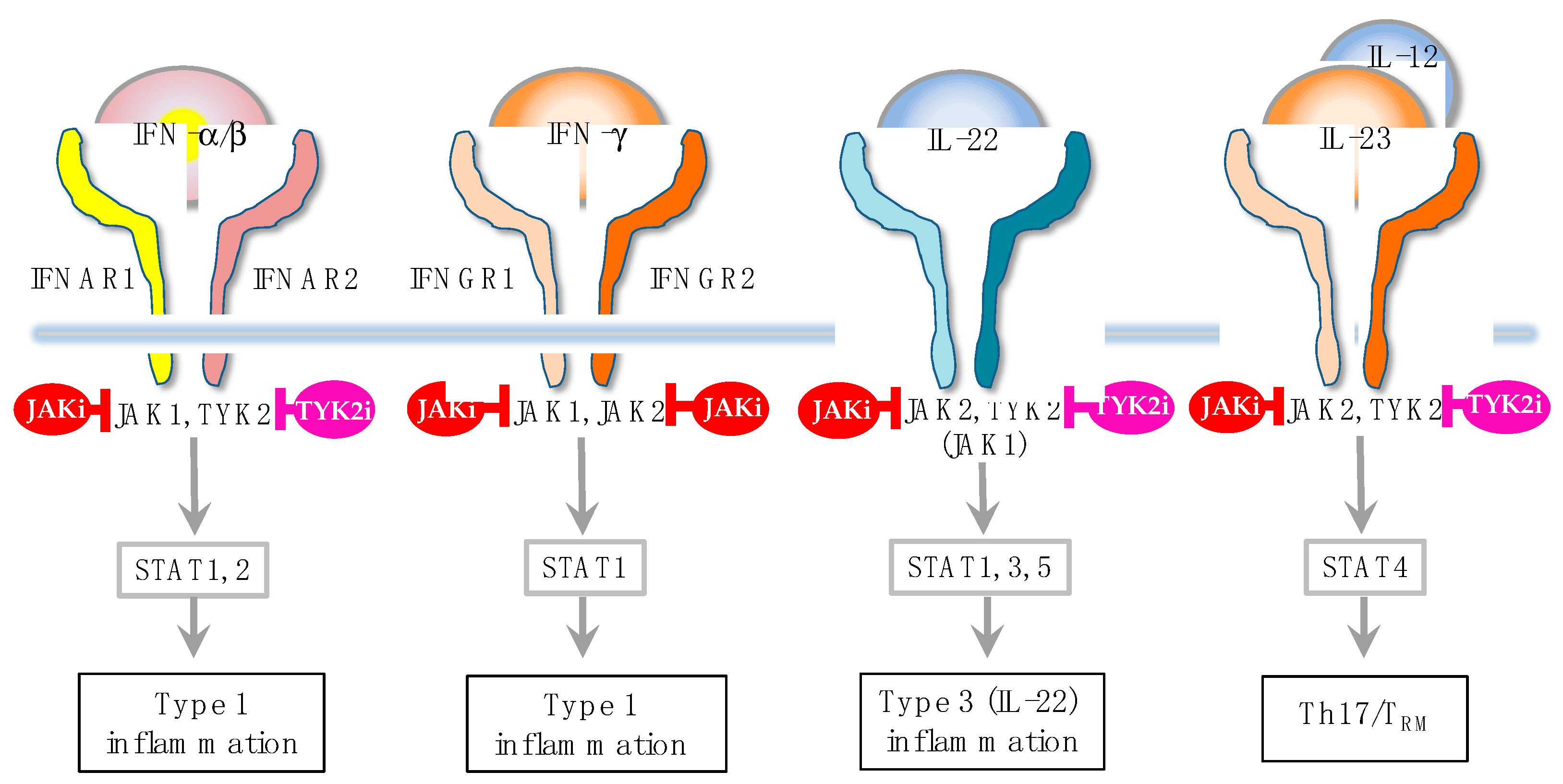
In addition to these biologics, Janus kinase (JAK) inhibitors, baricitinib [
47], upadacitinib [
48] and abrocitinib [
49], have recently been marketed for the treatment of AD. They mainly inhibit JAK1, which is crucial for the induction of AD [
6,
7,
50] (
Figure 2). In fact, JAK1 gain-of-function causes AD-like skin lesions, eosinophilia, and keratinization of the epidermis [
51,
52,
53]. Thus, it seems that JAK1 inhibitors have a therapeutic effect on type 2 inflammation, as represented by extrinsic AD, European American AD, and pediatric AD. However, JAK1 signaling transduces not only for type 2 cytokines, but also for IFN-a/b, IFN-g and IL-22 [
50,
54,
55] (
Figure 3). Given this broad ability to inhibit type 2, type 1 and type 3 cytokines, JAK inhibitors are potentially efficacious for Th1/Th2/Th17-dominant and Th2/eosinophil-inferior endotypes. In this sense, it is anticipated that JAL inhibitors exert therapeutic effects on the broad spectrum of AD.
7. Conclusions
While subtyping of AD was conducted based on the phenotype, recent findings have revealed importance of the endotype for the classification, leading to more accurate characterization of each patient. To choose the therapeutic option, such as cytokine- or receptor-targeted biologics and JAK inhibitors, information on AD endotyping may be helpful. The cytokine-based endotype is a representative and is categorized based on type 2, type 1, and/or type 3 cytokine-high properties [
1,
2,
3,
4]. In a comparison with the type 3-dominant condition of psoriasis [
56], the range of immune dysregulation of AD is uniquely wide, indicating that personalized therapeutic approach is a critical issue in AD patients.
On one hand, current endotype approach of AD has further clarified that AD is a heterogeneous and complicated disorder. On the other hand, investigators should reconsider the validity of criteria of AD, because the endotype analysis is performed in certain groups that are defined as AD with one of the classical criteria [
17,
57,
58]. Given that the AD criteria are based on the phenotype, disease history and comorbidities, the current endotype studies are conducted in patients classically diagnosed as AD with the phenotype and allergic history. This raises the possibility that some endotypically important AD groups have been excluded by the potentially biased criteria. In this context, it is interesting whether endotypes or clusters would be present in other eczematous dermatitis such as contact dermatitis [
59]. Combination of the phenotypic and endotypic signs seem to be currently an ideal way for subtyping of AD. Finally, the biomarker-based endotyping aims at individualized treatment options, but its daily clinical use is a future issue.
Author Contributions
Author Contributions: Conceptualization, Y.T. and T.S; writing – original draft preparation, Y.T.; writing and editing, T.S., S.K., Y.O. and M.O.; visualization, Y.T. and T.S.; supervision, Y.T. and M.O.; project administration, Y.T.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol Int. 2022, 71, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Honda, T.; Kabashima, K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol. 2018, 30, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Wu, J.; Kabashima, K.; Guttman-Yassky, E. ; Endophenotypic Variations of Atopic Dermatitis by Age, Race, and Ethnicity. J Allergy Clin Immunol Pract. 2018, 8, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J Allergy Clin Immunol Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Nomura, T.; Akiyama, M.; Sandilands, A.; Nemoto-Hasebe, I.; Sakai, K.; Nagasaki, A.; Ota, M.; Hata, H.; Evans, A.T.; Palmer, C.N.A.; et al. Specific filaggrin mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J Invest Dermatol. 2008, 128, 1436–1441. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J Am Acad Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Tokura, Y.; Phadungsaksawasdi, P.; Ito, T. Atopic dermatitis as Th2 disease revisited, J Cutan Immunol Allergy, 2018, 1, 158-164. [CrossRef]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef]
- Brenninkmeijer, E.E.; Spuls, P.I.; Legierse, C.M.; Lindeboom, R.; Smitt, J.H.; Bos, J.D. Clinical differences between atopic and atopiform dermatitis. J Am Acad Dermatol. 2008, 58, 407–414. [Google Scholar] [CrossRef]
- Kuriyama, S.; Kasuya, A.; Fujiyama, T.; Tatsuno, K.; Sakabe, J.; Yamaguchi, H.; Ito, T.; Tokura, Y. Leukoderma in patients with atopic dermatitis. J Dermatol. 2015, 42, 215–218. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakamura, M.; Bito, T.; Haruyama, S.; Kabashima, R.; Sugita, K,; Kobayashi, M.; Kabashima, K.; Tokura, Y.; et al. Chondrodermatitis of the auricle in patients with atopic dermatitis. Eur J Dermatol. 2010, 20, 813-814. [CrossRef]
- Sugita, K.; Kabashima, K.; Ota, T.; Tokura, Y. ; Angiohistiocytoid papules associated with atopic dermatitis. J Eur Acad Dermatol Venereol. 2008, 22, 403–404. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980, 92, 44–47. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Wen, H.C.; Czarnowicki, T.; Noda, S.; Malik, K.; Pavel, A.B.; Nakajima, S.; Honda, T.; Shin, J.U.; Lee, H.; Chou, M.; et al. Serum from Asian patients with atopic dermatitis is characterized by TH2/TH22 activation, which is highly correlated with nonlesional skin measures. J Allergy Clin Immunol. 2018, 142, 324-328, e11. [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. ; Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef]
- Mori, T.; Ishida, K.; Mukumoto, S.; Yamada, Y.; Imokawa, G.; Kabashima, K.; Kobayashi, M.; Bito, T.; Nakamura, M.; Ogasawara, K.; Tokura, Y.; et al. Comparison of skin barrier function and sensory nerve electric current perception threshold between ige-high extrinsic and ige-normal intrinsic types of atopic dermatitis. Br J Dermatol. 2009, 162, 83–90. [Google Scholar] [CrossRef]
- Kabashima-Kubo, R.; Nakamura, M.; Sakabe, J.I.; Sugita, K.; Hino, R.; Mori, T.; Kobayashi, M.; Bito, T.; Kabashima, K.; Ogasawara, K.; et al. A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: Possible immunological state of the intrinsic type. J Dermatol Sci. 2012, 67, 37–43. [Google Scholar] [CrossRef]
- Suarez-Farinas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kabashima-Kubo, R.; Bito, T.; Sakabe, J-I.; Shimauchi, T.; Ito, T.; Hirasawa, S.; Hirasawa, N.; Ogasawara, K.; Tokura, Y. High frequencies of positive nickel/cobalt patch tests and high sweat nickel concentration in patients with intrinsic atopic dermatitis. J Dermatol Sci. 2013, 72, 240-245. [CrossRef]
- Yamaguchi, H.; Hirasawa, N.; Asakawa, S.; Okita, K.; Tokura, Y. Intrinsic atopic dermatitis shows high serum nickel concentration. Allergol Int. 2015, 64, 282–284. [Google Scholar] [CrossRef]
- Sakabe, J-I.; Kamiya, K.; Yamaguchi, H.; Ikeya, S.; Suzuki, T.; Aoshima, M.; Tatsuno, K.; Fujiyama, T.; Suzuki, M.; Yatagai, T.; et al. Proteome analysis of stratum corneum from atopic dermatitis patients by hybrid quadrupole-orbitrap mass spectrometer. J Allergy Clin Immunol. 2014, 134, 957-960, e8. [CrossRef]
- Aoshima, M.; Phadungsaksawasdi, P.; Nakazawa, S.; Iwasaki, M.; Sakabe, J-I.; Umayahara, T.; Yatagai, T.; Ikeya, S.; Shimauchi, T.; Tokura, Y.; et al. Decreased expression of suprabasin induces aberrant differentiation and apoptosis of epidermal keratinocytes: Possible role for atopic dermatitis. J Dermatol Sci. 2019, 95, 107-112. [CrossRef]
- Nakazawa, S.; Shimauchi, T.; Funakoshi, A.; Aoshima, M.; Phadungsaksawasdi, P. ; Sakabe, J-I.; Asakawa, S.; Hirasawa, N.; Ito, T.; Tokura, Y. Suprabasin-null mice retain skin barrier function and show high contact hypersensitivity to nickel upon oral nickel loading. Sci Rep. 2020, 10, 14559. [CrossRef]
- Chan, T.C.; Sanyal, R.D.; Pavel, A.B.; Glickman, J.; Zheng, X.; Cho, Y.; Tsai, T.; Peng, X.; Krueger, J.G.; Guttman-Yassky, El. Atopic dermatitis in Chinese patients shows TH2/TH17 skewing with psoriasiform features. J Allergy Clin Immunol. 2018, 142, 1013–1017. [Google Scholar] [CrossRef]
- Margolis, D.; Mitra, N.; Wubbenhort, B.; D’Andrea, K.; Kraya, A.; Hoffstad, O.; Gupta, J.; kim, B.; Yan, A.; et al. Uncommon filaggrin variants are associated with persistent atopic dermatitis in African-Americans. J Invest Dermatol. 2018, 138, 1501–1506. [Google Scholar] [CrossRef]
- Sanyal, R.D.; Pavel, A.B.; Glickman, J.; Chan, T.C.; Zheng, X.; Zhang, N.; Cueto, Inna.; Peng, X.; Estrada, Y.; Fuentes-Duculan, J.; et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol. 2019, 122, 99-110.e6. [CrossRef]
- Czarnowicki, T.; He, H.; Canter, T.; Han, J.; Lefferdink, R.; Erickson, T.; Rangel, S.; Kameyama, N.; Kim, H.J.; Pavel, A.B.; et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol. 2020, 145, 215–228. [Google Scholar] [CrossRef]
- Thijs, J.L.; Strickland, I.; Bruijnzeel-Koomen, C.A.F.M.; Nierkens, S.; Giovannone, B.; Csomor, E.; Sellman, B.R.; Mustelin, T.; Sleeman, M.A.; de Bruin-Weller, M.S.; et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol. 2017, 140, 730–737. [Google Scholar] [CrossRef]
- Bakker, D.S.; Nierkens, S.; Knol, E.F.; Giovannone, B.; Delemarre, E.M.; van der Schaft, J.; Wijk, F.V.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Confirmation of multiple endotypes in atopic dermatitis based on serum biomarkers. J Allergy Clin Immunol. 2021, 147, 189–198. [Google Scholar] [CrossRef]
- Leonard, A.; Wang, J.; Yu, L.; Liu, H.; Estrada, Y.; Greenlees, L.; McPhee, R.; Ruzin, A.; Guttman-Yassky, E.; Howell, M.D. Atopic dermatitis endotypes based on allergen sensitization, reactivity to Staphylococcus aureus antigens, and underlying systemic Inflammation. J Allergy Clin Immunol Pract. 2020, 8, 236-247, e3. [CrossRef]
- Imai, Y. ILC2s in skin disorders, Allergol Int, 2023, 72, 201-206. [CrossRef]
- Guttman-Yassky, E.; Diaz, A.; Pavel, A.B.; Fernandes, M.; Lefferdink, R.; Erickson, T.; Canter, T.; Rangel, S.; Peng, X.; Li, R.; et al. Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children With Early-Onset Atopic Dermatitis. JAMA Dermatol. 2019, 155, 1358–1370. [Google Scholar] [CrossRef]
- Imai, Y.; Yasuda, K.; Sakaguchi, Y.; Haneda, T.; Mizutani, H.; Yoshimoto, T.; Nakanishi, K.; Yamanishi. K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013, 110, 13921-13926. [CrossRef]
- Imai, Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Koyasu, S. ; The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Tsang, M.S. X. Sun, Wong, C.K. The Role of New IL-1 Family Members (IL-36 and IL-38) in Atopic Dermatitis, Allergic Asthma, and Allergic Rhinitis. Curr Allergy Asthma Rep. 2020, 8, 40. [CrossRef]
- Halling, A.S.; Loft, N.; Silverberg, J.I.; Guttman-Yassky, E.; Thyssen, J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J Am Acad Dermatol. 2021, 84, 139–147. [Google Scholar] [CrossRef]
- Imai, Y.; Kusakabe, M.; Nagai, M.; Yasuda, K.; Yamanishi, K. Dupilumab Effects on Innate Lymphoid Cell and Helper T Cell Populations in Patients with Atopic Dermatitis. JID Innov. 2021, 1, 100003. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; Debusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018, 78, 863-871, e11. [CrossRef]
- Kabashima K.; Furue M.; Hanifin J.M.; Pulka G.; Wollenberg A.; Galus R.; Etoh, T.; Mihara, R.; Nakano, M.; Ruzicka, T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J Allergy Clin Immunol. 2018, 142, 1121-1130, e7. [CrossRef]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis, A randomized clinical trial. JAMA Dermatol. 2020, 156, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaci, D.; Chu, C.Y.; Hong, H.C.H.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021, 397, 2151–2168. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Thyssen, J.P.; Blauvelt, A.; Eyerich, K.; Soong, W.; Rice, Z.P.; Hong, H.C.H.; Katoh, N.; Valenzuela, F.; DiBonaventura, M.; et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022, 400, 273–282. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am J Clin Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.N.; Calis, J.J.A.; Buta, S.; Evrony, G.; Martin, J.C.; Uhl, S.A.; Caron, R.; Jarchin, L.; Dunkin, D.; Phelps, R.; et al. Complex autoinflammatory syndrome unveils fundamental principles of JAK1 kinase transcriptional and biochemical function, Immunity. 2020, 53, 672-684, e11. [CrossRef]
- Del Bel, K.L.; Ragotte, R.J.; Saferali, A.; Lee, S.; Vercauteren, S.M.; Mostafavi, S.A.; Mostafavi, S.A.; Schreiber, R.A.; Prendiville, J.S.; Phang, M.S.; Halparin, J.; et al. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol. 2017,139,2016-2020.e5. [CrossRef]
- Takeichi, T.; Lee, J.Y.W.; Okuno, Y.; Miyasaka, Y.; Murase, Y.; Yoshikawa, T.; Tanahashi, K.; Nishida, E.; Okamoto, T.; Ito, K.; et al. Autoinflammatory keratinization disease with hepatitis and autism reveals roles for JAK1 kinase hyperactivity in autoinflammation. Front Immunol. 2022, 12, 737747. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, D.; Dumoutier, L.; Constantinescu, S.; Kruijer, W.; Schuringa, J.J.; Renauld, J.C.; Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002, 277, 33676-33682. [CrossRef]
- Tokura, Y.; Mori, T.; Hino, R. Psoriasis and other Th17-mediated skin diseases. J UOEH. 2010, 32, 317–328. [Google Scholar] [CrossRef]
- Aoki, T.; Yoshida, H.; Furue, M.; Tagami, H.; Kaneko, F.; Ohtsuka, F.; Nishioka, K.; Toda, K.; Mizoguchi, M.; Ichihashi, M.; et al. English version of the concluding report published in 2001 by the Advisory Committee on Atopic Dermatitis Severity Classification Criteria of the Japanese Dermatological Association. J Dermatol. 2011, 38, 632–644. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Koppes, S.A.; Engebretsen, K.A.; Agner, T.; Angelova-Fischer, I.; Berents, T.; Brandner, J.; Brans, R.; Clausen, M.L.; Hummler, E.; et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis. 2017, 77, 1–16. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).