1. Introduction
Cathinone is the stimulant natural compound shown to be constituent of Catha edulis, Ephedra sinica, and Ephedra gerardiana sikkimensis, and recently have become the most common compound of new psychoactive substances [
1,
2,
3]. The approximate average concentration of cathinone in fresh khat leaves is 1 mg/gm [
4]. It is categorized as mind-manifesting substances in Schedules I of the Controlled Substances Act [
5]. Cathinone is closely resemble to pharmacological actions of amphetamine and cathine, and it has one third potency as those of amphetamine and up to ten times potency as those of cathine [
6,
7].
Administration of cathinone or Catha edulis was observed to produces mental alertness, excitement, euphoria, loquacity and social interaction, mydriasis, impair visual perception and discrimination, anorexia, insomnia, increased muscular activity, hyperthermia, hypertension, and tachycardia [
8]. These effects may produce via several neurotrasmitters releases and other biochemical changes. In this regard, it has been found cathinone has ability to increase the levels of dopamine, serotonin, norepinephrine, enkephalins, endorphins, glucose and free fatty acids [
6,
9,
10,
11].
Cathinone (IUPAC name: (2S)-2-amino-1-phenylpropan-1-one) is a monoamine alkaloid compound with molecular formula C9H11NO and molecular weight of 149.19. It has a pKa value of 7.55 and can undergo post-mortem redistribution effect [
12]. Cathinone is rapidly metabolized to norephedrine and norpseudoephedrine (cathine), however, cathine is also minor metabolite of pseudoephedrine [
13,
14,
15]. On the other hand, cathinone in fresh Catha edulis is unstable and can easily be reduced into cathine over time through exposure to air or heat [
6,
16]. As a result, determining and interpreting cathinone levels in forensic settings can be challenging.
Understanding the pharmacological, toxicological, and metabolic effects of cathinone necessitates an in-depth knowledge of their tissue distribution, pharmacokinetics, and metabolic profile. Few pharmacokinetic studies on cathinone have been conducted, and the tissue distribution pattern of the cathinone metabolic profile has yet to be explored. The present study will investigate the pharmacokinetic indices, tissue distribution, and metabolic profile in rats treated with single oral dose of Cathinone, which are important for identification, quantification, interpretation, and understanding the pharmacological and toxicological actions of cathinone and its metabolites.
This study provided evidence of the adverse effects associated with exposure to cathinone, which can be used to direct future experimental, clinical, and forensic research.
2. Results
The LC-IT/MS method was used to quantify the distribution of cathinone and cathine after a single oral dose of Cathinone. Kinetic indices for cathinone and cathine were determined using a non-compartment model. The UPLC-QTOF/MS analysis was used for initial screening. Only compounds with a 90% and above spectral similarity score in the NIST library were considered to be correctly identified.
2.1. Disposition Concentrations
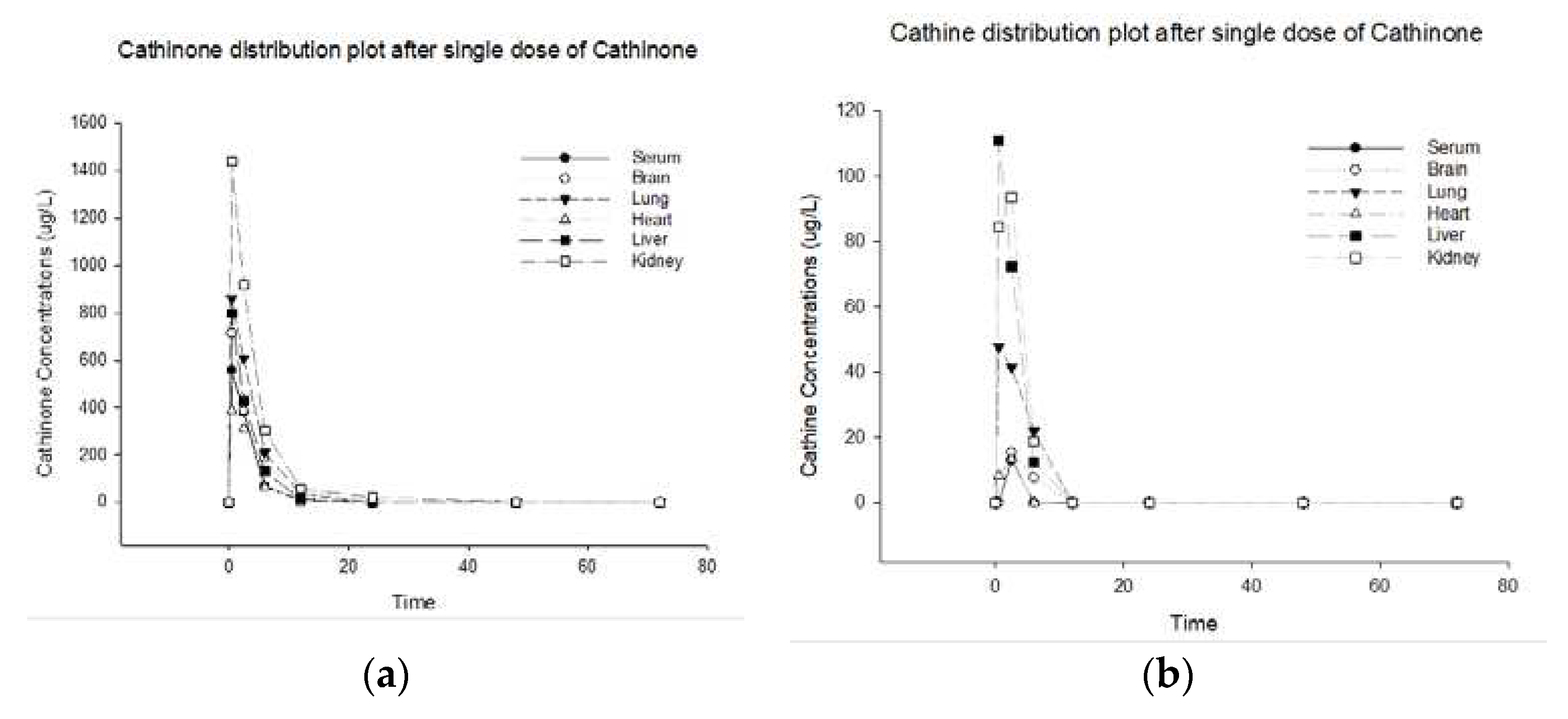
The cathinone and its metabolite cathine disposition curves in serum, brain, lung, heart, liver, and kidney after a single oral dose of cathinone are shown in
Figure 1 (a, b). This figure represents the distribution plot of cathinone and cathine concentrations in various rat organs over time, following a single dose of cathinone.
Two compounds cathinone and cathine were identified and quantified in all samples. The
Table 1 represent the disposition concentrations for cathinone and cathine after administering a single oral dose of 5 mg/kg cathinone.
The concentration of Cathinone in the serum is initially 558.3 ug/L at half an hour of cathinone administration, decreasing to 378.37 ug/L within two hours and half, and continuing to decreases until it becomes undetectable after 24-hours. During this time, the lungs, liver, and heart exhibited a high concentration of cathinone at 859, 798.9, and 385.8 ug/L, which reduced to 608.1, 429.3, and 309.1 ug/L within 2.5 hours, respectively. Subsequently, over the next 24 hours, these concentrations continued to diminish and eventually became undetectable. Importantly, the kidney showed the highest concentration of 1438.6 ug/L, which gradually decreased to 1.97 within 48 hours and disappeared by the 72 hours. On the other hand, the rat brain showcases a concentration of 712.7 ug/L for cathinone, which diminishes to 384.7 ug/L within 2.5 hours and subsequently decreases to 182.9 ug/L within 6 hours. After 12 hours, this concentration is undetectable.
After cathinone administration, cathine metabolite concentration peaks at 13.125 ug/L in 2.5 hours and becomes undetectable. The liver, following kidney and lung exhibited a high initial concentration of cathine at 110.8, 84.4, and 47.8 ug/L, respectively, which reduced and eventually became undetectable after 6 hours. The heart showed the initial concentration of 8.3 ug/L at half an hour, which peaked to 13.3 ug/L at 2.5 hours, and disappeared by the 6 hours. While, the brain peaked to 15.4 ug/L at 2.5 hours, and disappeared by the 12 hours.
2.2. Disposition Kinetics Indices
The disposition kinetics indices for two compounds cathinone and its metabolite cathine were determined in all tested samples. The
Table 2 and
Table 3 summarize the main kinetic indices for cathinone and cathine, respectively, after administering a single oral dose of 5 mg/kg Cathinone.
2.3. Metabolic Profiling
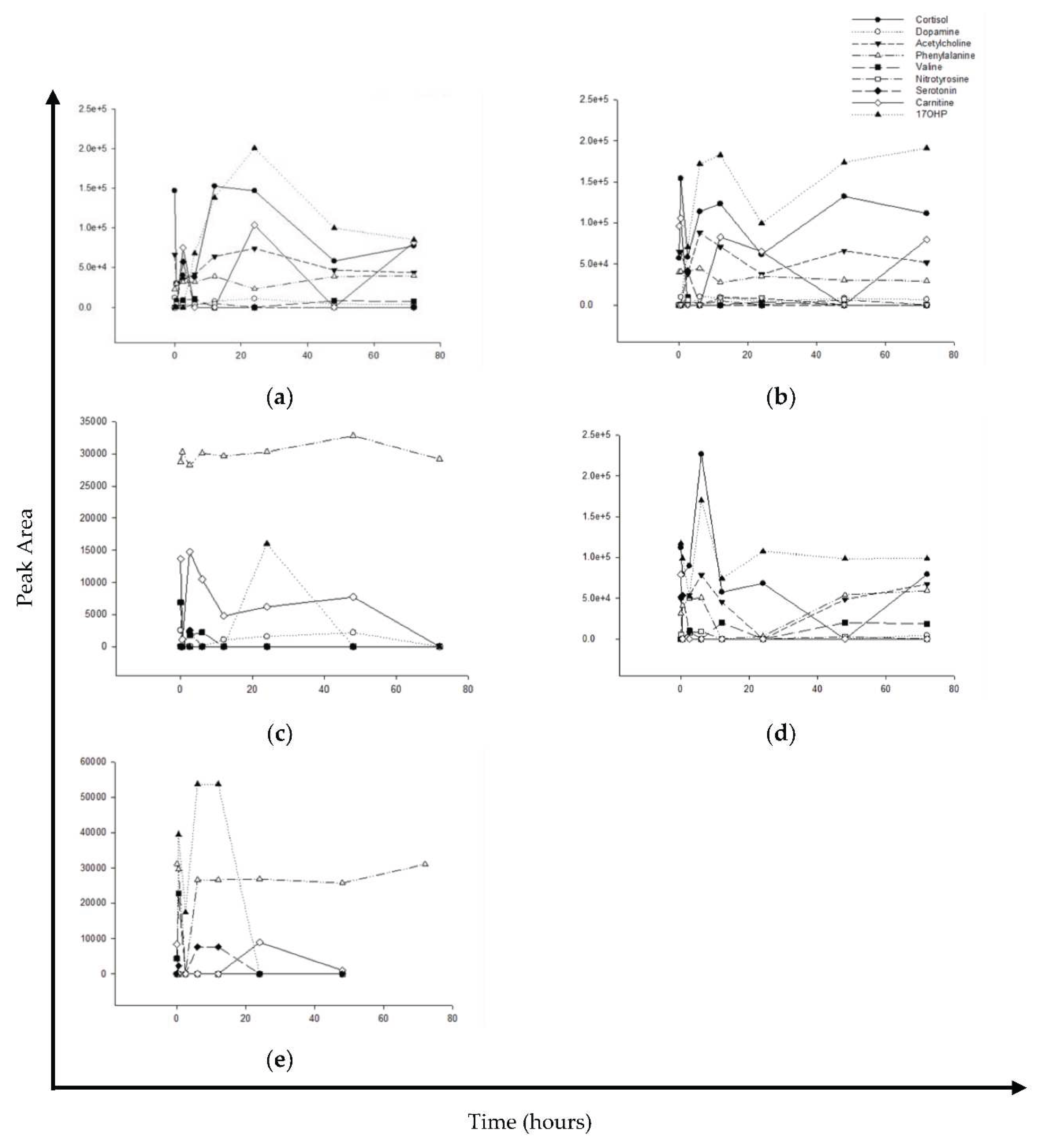
This study was also identified the metabolic profiles using UPLC-QTOF/MS analysis. The metabolic profile was detected in the serum, brain, heart, liver, and kidney of rats at different times after a single oral dose of cathinone. The main biomarkers, cortisol, dopamine, acetylcholine, phenylalanine, valine, nitrotyrosine, serotonin, carnitine, 17-hydroxyprogesterone (17OHP) were detected in the serum, brain, heart, liver, and kidney tissues after a single oral dose of cathinone in rats at different time points.
In response to a single dose of cathinone,
Figure 2 shows how various biomarkers are distributed over time. Upon administration of cathinone, different biomarkers respond differently. In serum and brain, an initial drop in cortisol and 17OHP, followed by an increase, was detected while in the liver, an initial increase followed by a decrease was detected. Similarly, in the kidney and heart, an initial drop in phenylalanine followed by an increase was detected. Carnitine was sharply dropped and then gradually decreased in the heart, kidney, brain, and serum. The distribution patterns of various biomarkers for different organs, include brain (
Figure 2b), heart (
Figure 2c), liver (
Figure 2d), and kidney (
Figure 2e) are detailed in their corresponding graphs.
3. Discussion
The toxicokinetic properties of synthetic cathinone remain an area of limited understanding. This research endeavored to shed light on the kinetic disposition and tissue biomarker profile of cathinone after administering a single dose to rats. The misuse of cathinone can have severe consequences, including rhabdomyolysis, cerebral and lung edema, and multiorgan failure [
17]. Cathinone transforms into phenylalkylamine derivatives, which are later metabolized into cathine, affecting neurotransmitter functions [
18].
HPLC-IT/MS was used to determining the concentrations of cathinone and cathine, and UPLC-QTOF/MS was used to analyze the metabolic profile post a single cathinone dose in rats. Cathinone and its metabolite cathine were found in significant concentrations across various tissues, with the kidney, liver, and lungs having the most pronounced levels. Different organs reached peak concentrations at distinct times, with some peaking faster than others.
Based on our investigation, both cathinone and cathine recorded C
max serum levels of 558 and 13.1 ug/L, after 0.5 and 2.5 hours, respectively. It is possible that the reason for the delayed C
max in cathine is due to the time required for the conversion of cathinone to cathine. This explanation is supported by previous findings that used both cathinone and cathine through khat administration [
19,
20]. In those studies, both cathinone and cathine reached their maximum serum concentrations at the same time. In addition, oral ingestion results in faster absorption and a higher C
max compared to chewing khat leaves [
4]. Similar to serum, a high concentrations of cathinone and cathine were distributed to the brain and heart. They reached their peak levels after 0.5 and 2.5 hours, respectively, indicating rapid distribution of cathinone to the brain and heart tissues. This rapid distribution of cathinone to these tissues could be the reason behind its harmful effects on the cardiovascular and neurovascular systems of khat users. In this regard, khat consumption can impair driving ability [
21]. Among fatalities involving khat, firearm injuries, hanging, and road traffic accidents were responsible for the majority of deaths [
22]. Therefore, it is necessary to conduct dose-response studies on cathinone and its metabolite cathine in these organs to better understand their effects. Based on current research, cathinone and cathine have a short MRT (mean residence time), ranging from 1.6 to 4.8 hours, consistent with earlier findings [
19]. This suggests that cathinone and cathine are quickly metabolized and eliminated from the body. As a result, it is advisable to perform autopsies as soon as possible for toxicological investigation in forensic settings.
In this study, UPLC-QTOF/MS analysis was used to detect several biomarkers. Most of these biomarkers are associated with the primary effects of cathinone. The distribution pattern and prolonged fluctuation of these biomarkers suggest that cathinone has post-effects, which is consistent with previous research linking cathinone and cathine with delayed effects [
19,
23]. It is noting that the biomarker 17OHP showed increased levels for an extended period, which hints at the possibility of an inflammatory reaction, such as in liver cirrhosis or insulin resistance [
24,
25]. Similarly, cortisol levels have been shown to be affected by cathinone in several studies, suggesting potential interactions with critical hormonal pathways [
26,
27].
The significance of elevated 17-OHP levels, particularly in the brain, highlights the need to better understand their complex physiological interactions. Our research findings indicate that there are fluctuations in dopamine levels that may require more frequent metabolic profiling. It is suggested that considering cathinone kinetics, increasing the number of time points for detection can provide a more accurate assessment of changes in dopamine levels. The prolonged effect of cathinone administration in our study suggests a post-cathinone effect. Carnitine, a precursor to acetylcholine [
28], also exhibited intriguing patterns in the serum, brain, and heart. Importantly, carnitine correlates with drug-induced lethal cardiomyopathy [
29]. It has a protective effect against various cardiovascular diseases, including arterial hypertension, cardiac inflammation, fibrosis, and myocardial infarction [
30,
31].
Our study found that there was a significant decrease in the levels of carnitine in heart tissue for an extended period of time, and after 48 hours, the levels became undetectable. This decrease in carnitine levels may leave the heart vulnerable to the toxic effects of cathinone, a stimulant that is known to have toxic effects on the cardiac cells [
23,
32,
33]. These finding suggests that the reason behind this delay may be related to the post cathinone effects on the heart, tend to delay seeking medical attention for cardiovascular symptoms [
23,
34]. This delay in seeking medical care can have serious consequences, including increased risk of heart toxicity. Further examination is necessary to confirm the metabolic profile findings and establish their correlation with cathinone concentration.
4. Materials and Methods
4.1. Study Design
Adult male Wistar albino rats (8 and 12 weeks old, 250-300 g weight) were obtained from the Experimental Animal Center of Medical Research Center, Jazan University, Jazan, Saudi Arabia. A total of 32 rats were randomly selected, group-caged by time points (4 Rats per group) and kept for 5 days prior to starting experiments. All rats will be fed with standard laboratory diet and had free access to water. Cathinone was prepared shortly prior to administration by dissolved in distill water at the final concentration of 5 mg/ml. The single target dose of 5 mg/kg body weight administered by oral gavage to the rats. All rats were food fasted prior to dosing and food will be withheld for 3 hours after dose administered.
Each group sacrificed at specific time schedules (Time zero (group1), 0.5 hour (group2), 2.5 hours (group3), 6 hours (group4), 12 hours (group5), 24 hours (group6), 48 hours (group7), and 72 hours (group8)). Blood, brain, lung, heart, liver, and kidney of each rat from each group collected at different time points for consequences analysis. Plasma separated from blood sample by centrifugation. Tissue samples were placed in a 0.9% sodium chloride solution and blot on a filter paper to remove the blood, thereafter weighted and collected in specimen collection tube. All separated serum and tissue sample stored at −20 °C for consequences experimental analysis.
4.2. Sample Preparations
One gram of each organ tissue was diluted with 1-mL deionised water. All organ tissue homogenized by the stomacher and centrifuged at 3,000
×g for 15 min. After that, 1-ml of centrifugated sample mixed with 1 ml phosphate buffer pH 6 and vortexed and made ready for extraction step. Cathine and cathinone are extracted from different samples matrices along with calibrators, controls and blanks by solid phase extraction method prescribed by SPE cartridges manufacturer (Clean Screen DAU Extraction Column 300mg 3mL, UCT, Philadelphia, USA) for amphetamine type stimulants (ATS) extraction from biological samples, as previously described [
35]. All samples were reconstituted with 100 µL of the aqueous part of the mobile phase (10mM ammonium formate with 0.11% formic acid) for liquid chromatography ion trap mass spectrometry (LC-IT/MS) method (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
4.3. LC-IT/MS Analysis
Cathinone and cathine were identified and quantified by LCQ Fleet Ion Trap LC/MS system, using LCQ fleet mass analyzer coupled with Surveyor Auto-Sampler and Surveyor Quaternary Pump and controlled by X-Caliber Software (Themo Scientific, USA). The method is validated and previously described [
35,
36]. Briefly, 10 µl of each sample injected by an autosampler. The chromatographic separation of cathine, cathinone and MDMA achieved by HPLC column (Hypersil GOLD, 5 μm, 150 x 4.6 mm, Thermo Scientific, USA). The mass analyzer runs in scan mode scanning for m/z 152 for cathine, m/z 150 for cathinone and m/z 194 for MDMA. Cathine, cathinone and MDMA are furtherly fragmented in the collision cell with helium gas by Pulsed Q collision induced dissociation (PQD) mode into m/z 134 and 117 for cathine, m/z 132 and 105 for cathinone and m/z 163 and 135 for MDMA. Quantitative and qualitative analysis are performed using X-Caliber software. This analytical method was optimized, validated, and verified by the staff of the Forensic Toxicology Services Administration in Jazan [
35,
36].
4.4. Analysis of Cathinone Pharmacokinetic Indices and Tissue Distribution
The distribution of cathinone and cathine in various tissues of rats were determined after a single oral dose. The tissues tested included serum, lung, heart, brain, liver, and kidney, and the experiment was conducted over different time intervals. Pharmacokinetic parameters of cathinone and cathinone in both serum and tissue samples were calculated using non-compartmental pharmacokinetic analysis with WinNonlin 2.1. The parameters calculated include the average maximal concentration (Cmax), time taken to reach Cmax (Tmax), area under the concentration-time curve (AUC), half-life (t 1/2), apparent volume of distribution (Vz/F), apparent clearance (CL/F), and mean residence time (MRTlast).
4.5. Metabolic Profile Analysis
Using the SCIEX X500R QTOF LC-MS/MS system, the metabolic profile after a single dose of Cathinone is identified as previously described [
35]. Briefly, Chromatographic separations will obtain on a SELECTRA C18 column (15 cm × 4.6 mm, 5µm) maintained at 45°C. Mass spectrometry was performed on an ExionLC™ System equipped with a Sciex X500R QTOF (Sciex, USA). Data acquired in SWATH mode using positive electrospray ionization.
4.6. Data Processing
The data obtained from the analysis was processed by SCIEX software. The data was then automatically processed using the streamlined workflow of the SCIEX OS (Sciex, USA) to identify the analytes and any proposed metabolites. The identification of compounds was based on retention time (±0.05 minutes), mass deviation (±10 mDa), and appropriate isotope profiles.
4.7. Statistical Analysis
The data were analyzed using SigmaPlot 11 for Windows. Mean, standard deviation, confidence intervals, and linear regression were calculated using student t-test or analysis of variation (ANOVA). Additionally, the pharmacokinetic model and PK indices were calculated using WinNonlin 2.1.
5. Conclusions
This study aimed to provide a comprehensive understanding of the toxicokinetic properties of cathinone in rats, including its metabolic effects and kinetic disposition after a single dose. Our findings indicate that cathinone and its metabolite cathine have different physiological impacts and are metabolized at varying rates. The concentration of cathinone and its metabolite cathine was found to be high in all tissues, with the highest concentration in the kidney tissue. Cathinone peaks quickly in the kidney, while cathine has a delayed peak. The liver and lungs were found to metabolize cathinone to cathine rapidly, more than other organs such as the serum, brain, heart, and kidney. Our study identified some biomarkers for specific organs that had prolonged effects, which suggests the need for further research to establish the link between cathinone and their influence on biomarker profiles.
Author Contributions
Conceptualization, F.S. and I.A.; methodology, I.A. and M.A.A. (Mohamed Ahmed Al-Kasim); software, I.A.; validation, M.O., A.A. and M.A.; formal analysis, I.A. and A.A.; investigation, E.S.; resources, F.S. and I.K.; data curation, I.A.; writing—original draft preparation, I.A. and F.S.; writing—review and editing, I.A., F.S., A.J., M.A.A. (Mohamed Ahmed Al-Kasim) and D.B.; visualization, I.A.; supervision, I.A. and M.A.A. (Mohamed Ahmed Al-Kasim); project administration, F.S.; funding acquisition, F.S., A.J., and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: W44-93.
Institutional Review Board Statement
The study protocol (reference number REC-43/12/282) was approved by the Standing Committee for Scientific Research Ethics at Jazan University (HAPO-10-Z-001).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant details are included in the article.
Acknowledgments
The authors express their appreciation to the Medical Research Center at Jazan University and Forensic Toxicology Services in Jazan, Saudi Arabia for their invaluable collaboration and significant contributions to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, N.B. Khat (Catha edulis Forsk)–and now there are three. Brain research bulletin 2019, 145, 92-96. [CrossRef]
- Correia, B.; Fernandes, J.; Botica, M.J.; Ferreira, C.; Quintas, A. Novel psychoactive substances: the razor’s edge between therapeutical potential and psychoactive recreational misuse. Medicines 2022, 9, 19. [CrossRef]
- Brenneisen, R.; Fisch, H.; Koelbing, U.; Geisshusler, S.; Kalix, P. Amphetamine-like effects in humans of the khat alkaloid cathinone. British journal of clinical pharmacology 1990, 30, 825-828. [CrossRef]
- Widler, P.; Mathys, K.; Brenneisen, R.; Kalix, P.; Fisch, H.U. Pharmacodynamics and pharmacokinetics of khat: a controlled study. Clinical Pharmacology & Therapeutics 1994, 55, 556-562. [CrossRef]
- Administration, D.E. Drugs of Abuse: A Dea Resource Guide; CreateSpace Independent Publishing Platform: 2020.
- Zelger, J.; Carlini, E. Influence of cathinone (α-aminopropiophenone) and cathine (phenylpropanolamine) on circling behavior and on the uptake and release of [3H] dopamine in striatal slices of rats. Neuropharmacology 1981, 20, 839-843. [CrossRef]
- Mereu, G.; Pacitti, C.; Argiolas, A. Effect of (-)-cathinone, a khat leaf constituent, on dopaminergic firing and dopamine metabolism in the rat brain. Life sciences 1983, 32, 1383-1389. [CrossRef]
- Brenneisen, R.; Fisch, H.; Koelbing, U.; Geisshusler, S.; Kalix, P. Amphetamine-like effects in humans of the khat alkaloid cathinone. British journal of clinical pharmacology 1990, 30, 825-828. [CrossRef]
- Coppola, M.; Mondola, R. Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicology letters 2012, 211, 144-149. [CrossRef]
- Nencini, P.; Ahmed, A.M. Naloxone-reversible antinociceptive activity of cathinone, the active principle of khat, in the mouse and rat. Pharmacological research communications 1982, 14, 759-770. [CrossRef]
- Nencini, P. Cathinone, active principle of the khat leaf: its effects on in vivo and in vitro lipolysis. Pharmacological research communications 1980, 12, 855-861. [CrossRef]
- Pélissier-Alicot, A.-L.; Gaulier, J.-M.; Champsaur, P.; Marquet, P. Mechanisms underlying postmortem redistribution of drugs: a review. Journal of analytical toxicology 2003, 27, 533-544. [CrossRef]
- Tseng, Y.L.; Shieh, M.-H.; Kuo, F.-H. Metabolites of ephedrines in human urine after administration of a single therapeutic dose. Forensic science international 2006, 157, 149-155. [CrossRef]
- Pokrajac, M.; Miljković, B.; Bisailović, B. Mass spectrometric investigation of 2-aminopropiophenones and some of their metabolites. Rapid communications in mass spectrometry 1991, 5, 59-61. [CrossRef]
- Scheline, R.R. Handbook of Mammalian Metabolism of Plant Compounds; CRC Press: 2017. [CrossRef]
- Nencini, P.; Ahmed, A.M. Khat consumption: a pharmacological review. Drug and alcohol dependence 1989, 23, 19-29. [CrossRef]
- Adebamiro, A.; Perazella, M.A. Recurrent acute kidney injury following bath salts intoxication. American Journal of Kidney Diseases 2012, 59, 273-275. [CrossRef]
- Silva, B.; Soares, J.; Rocha-Pereira, C.; Mladěnka, P.; Remião, F.; Researchers, O. Khat, a cultural chewing drug: a toxicokinetic and toxicodynamic summary. Toxins 2022, 14, 71. [CrossRef]
- Alamir, A.M.; Jeraiby, M.A.; Korashy, H.M.; Shaheen, E.S.; Attafi, M.A.; Oraiby, M.E.; Hakami, A.M.; Albeishy, M.Y.; Khardali, I.A.; Juraybi, I.A. Cathine and cathinone disposition kinetics and neurotransmitter profile in several organs of rats exposed to a single dose of Catha edulis (Vahl) Forssk. ex Endl. extract. Drug Metabolism and Personalized Therapy 2023, 38, 199-207. [CrossRef]
- Toennes, S.W.; Harder, S.; Schramm, M.; Niess, C.; Kauert, G.F. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. British journal of clinical pharmacology 2003, 56, 125-130. [CrossRef]
- Toennes, S.W.; Kauert, G.F. Driving under the influence of khat—alkaloid concentrations and observations in forensic cases. Forensic science international 2004, 140, 85-90. [CrossRef]
- Shaikhain, G.; Gaballah, M.; Alhazmi, A.; Khardali, I.; Hakami, A.; Oraiby, M.; Alharbi, S.; Tobaigi, M.; Ghalibi, M.; Fageeh, M. Fatalities Involving Khat in Jazan, Saudi Arabia, 2018 to 2021. Toxics 2023, 11, 506. [CrossRef]
- Mohan, S.; Abdelwahab, S.I.; Hobani, Y.H.; Syam, S.; Al-Zubairi, A.S.; Al-Sanousi, R.; Oraiby, M.E. Catha edulis extract induces H9c2 cell apoptosis by increasing reactive oxygen species generation and activation of mitochondrial proteins. Pharmacognosy Magazine 2016, 12, S321. [CrossRef]
- Gambella, G.; Bellotti, S.; Cutolo, M.; Modesti, A.; Scarpa, S.; Novelli, A.; Ravera, G. Changes in fibronectin production in rat liver during cirrhotic evolution due to treatment with CCl4 and steroid hormones: correlation with plasmatic fibronectin. Pathologica 1992, 84, 343-361.
- Waters, T.P.; Schultz, B.A.; Mercer, B.M.; Catalano, P.M. Effect of 17α-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstetrics & Gynecology 2009, 114, 45-49. [CrossRef]
- Nyongesa, A.W.; Oduma, J.A.; Nakajima, M.; Odongo, H.O.; Adoyo, P.A.; al’Absi, M. Dose-response inhibitory effects of purified cathinone from khat (Catha edulis) on cortisol and prolactin release in vervet monkeys (C hlorocebus aethiops). Metabolic Brain Disease 2014, 29, 451-458. [CrossRef]
- Patel, N. Mechanism of action of cathinone: the active ingredient of Khat (Catha Edulis. East African Medical Journal 2000, 77. [CrossRef]
- White, H.L.; Scates, P.W. Acetyl-L-carnitine as a precursor of acetylcholine. Neurochemical research 1990, 15, 597-601. [CrossRef]
- Taibjee, S.; Ramani, P.; Brown, R.; Moss, C. Lethal cardiomyopathy in epidermolysis bullosa associated with amitriptyline. Archives of disease in childhood 2005, 90, 871-872. [CrossRef]
- Blanca, A.J.; Ruiz-Armenta, M.V.; Zambrano, S.; Miguel-Carrasco, J.L.; Arias, J.L.; Arévalo, M.; Mate, A.; Aramburu, O.; Vázquez, C.M. Inflammatory and fibrotic processes are involved in the cardiotoxic effect of sunitinib: protective role of L-carnitine. Toxicology Letters 2016, 241, 9-18. [CrossRef]
- Mathew, S.; Menon, P.; Kurup, P. Effect of administration of carnitine on the severity of myocardial infarction induced by isoproterenol in rats. Australian journal of experimental biology and medical science 1986, 64, 79-87. [CrossRef]
- Taibjee, S.M.; Ramani, P.; Brown, R.; Moss, C. Lethal cardiomyopathy in epidermolysis bullosa associated with amitriptyline. Archives of disease in childhood 2005, 90, 871-872. [CrossRef]
- Malone, J.I.; Cuthbertson, D.D.; Malone, M.A.; Schocken, D.D. Cardio-protective effects of carnitine in streptozotocin-induced diabetic rats. Cardiovascular diabetology 2006, 5, 1-6. [CrossRef]
- Ali, W.M.; Zubaid, M.; Al-Motarreb, A.; Singh, R.; Al-Shereiqi, S.Z.; Shehab, A.; Rashed, W.; Al-Sagheer, N.Q.; Saleh, A.H.; Al Suwaidi, J. Association of khat chewing with increased risk of stroke and death in patients presenting with acute coronary syndrome. In Proceedings of the Mayo Clinic Proceedings, 2010; pp. 974-980. [CrossRef]
- Alamir, A.M.; Jeraiby, M.A.; Korashy, H.M.; Shaheen, E.S.; Attafi, M.A.; Oraiby, M.E.; Hakami, A.M.; Albeishy, M.Y.; Khardali, I.A.; Juraybi, I.A.; et al. Cathine and cathinone disposition kinetics and neurotransmitter profile in several organs of rats exposed to a single dose of Catha edulis (Vahl) Forssk. ex Endl. extract. Drug Metabolism and Personalized Therapy 2023, 38, 199-207. [CrossRef]
- Alamir, A.; Watterson, J.; Attafi, I.Development and Validation of a Uplc-Qtof-Ms Method for Blood Analysis of Isomeric Amphetamine-Related Drugs. Separations 2022, 9, 285. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).