Submitted:
18 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial characterization
2.1.1. Bacterial strain
2.1.2. Culture conditions of bacterial strain.
2.1.3. Genotypic characterization of the organism
2.1.4. Antimicrobial susceptibility testing
2.1.5. Antimicrobial resistance genes
2.1.6. Search Against the Virulence Factor Database
2.1.7. Hemolytic activity
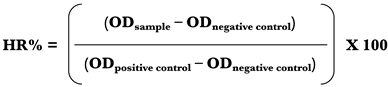
2.1.8. Cytotoxicity in Vero cells
2.2. Human safety assessment
2.2.1. Study design
2.2.2. Power and sample size consideration
2.2.3. Subjects
2.2.4. Trial Intervention
2.2.5. Sample Collection, Processing, and Data Management
2.3. Safety assessment
2.3.1. Clinical Determinations
2.3.2. Occurrence of adverse events determination
2.3.3. Statistical Analyses
3. Results
3.1. Species and strain identification
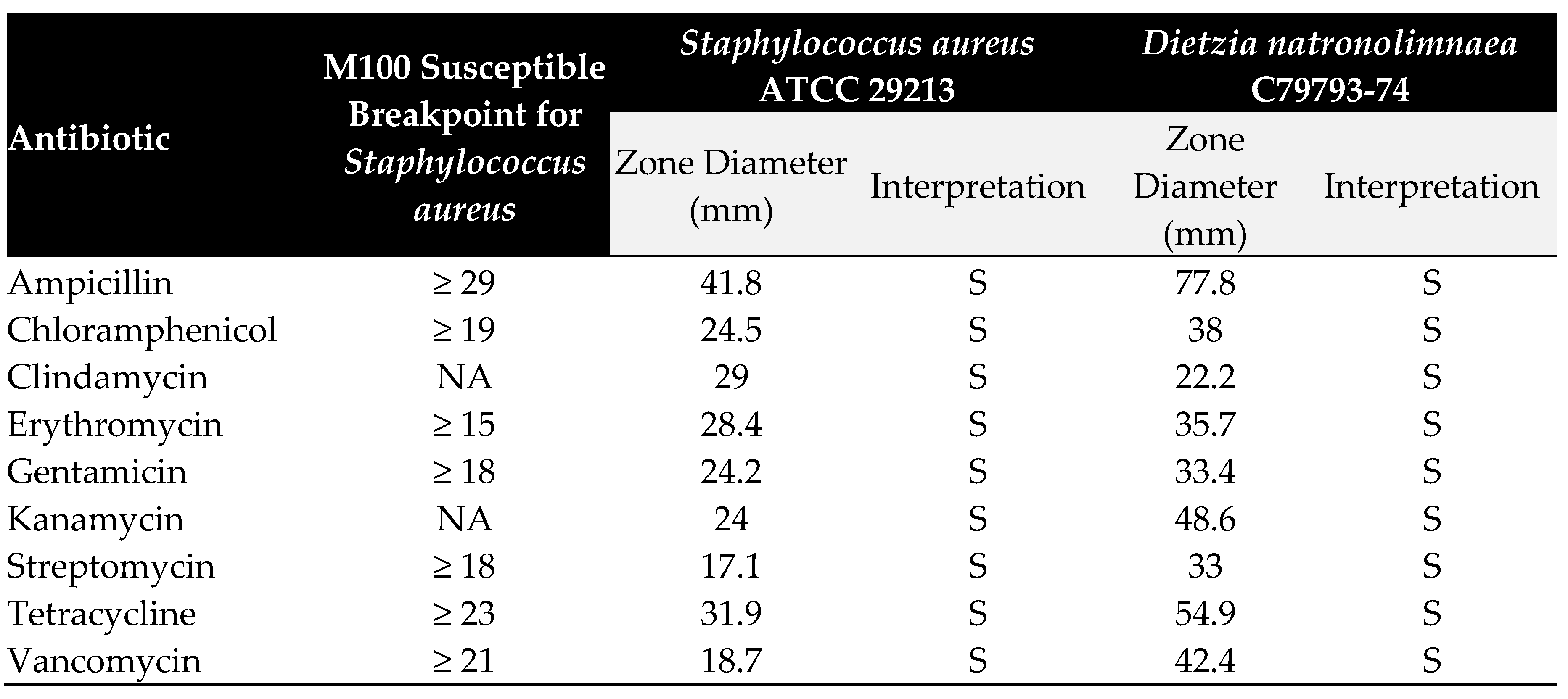
3.2. Antimicrobial resistance and Virulence factors
3.3. Hemolytic activity and cytotoxicity
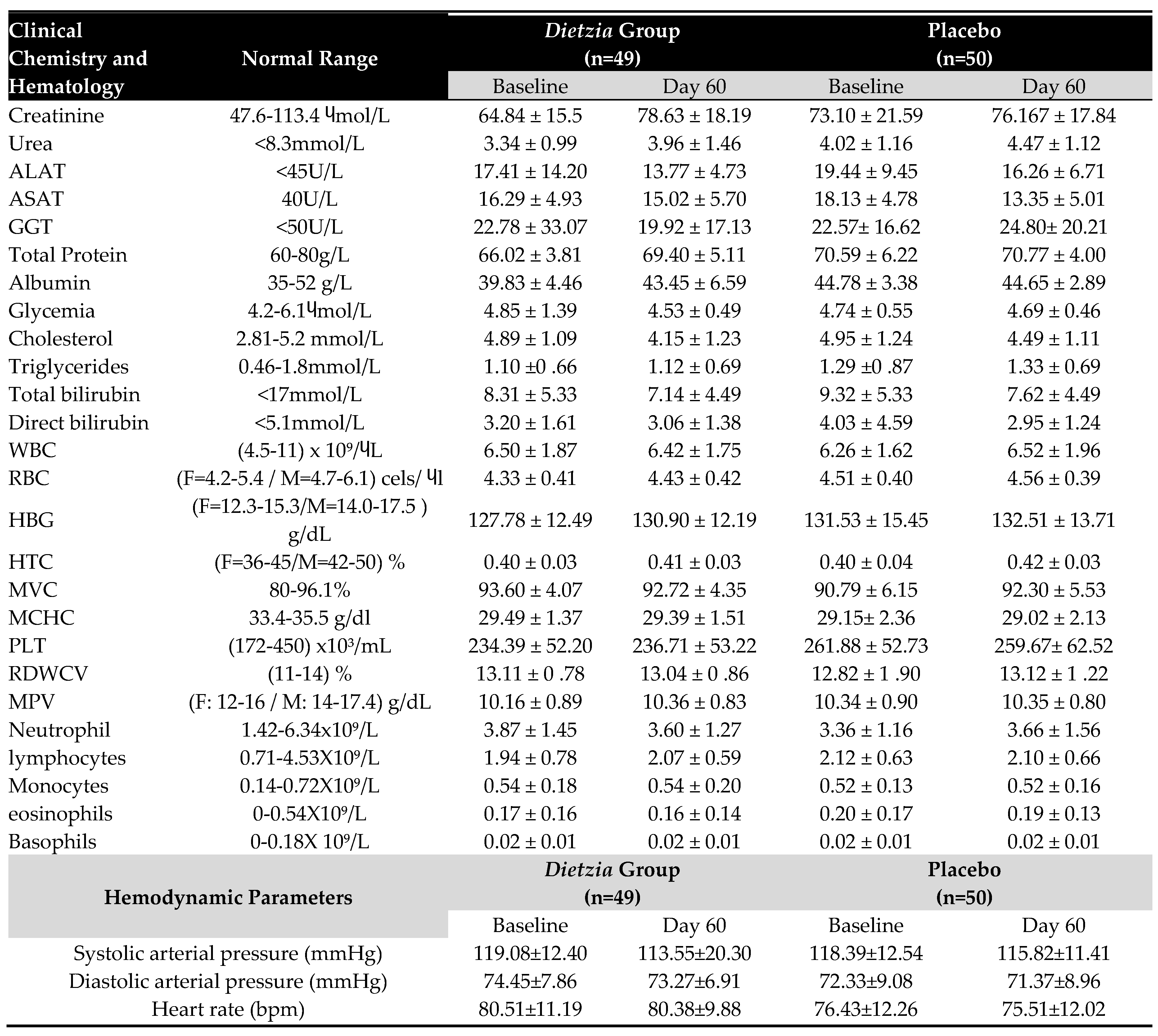
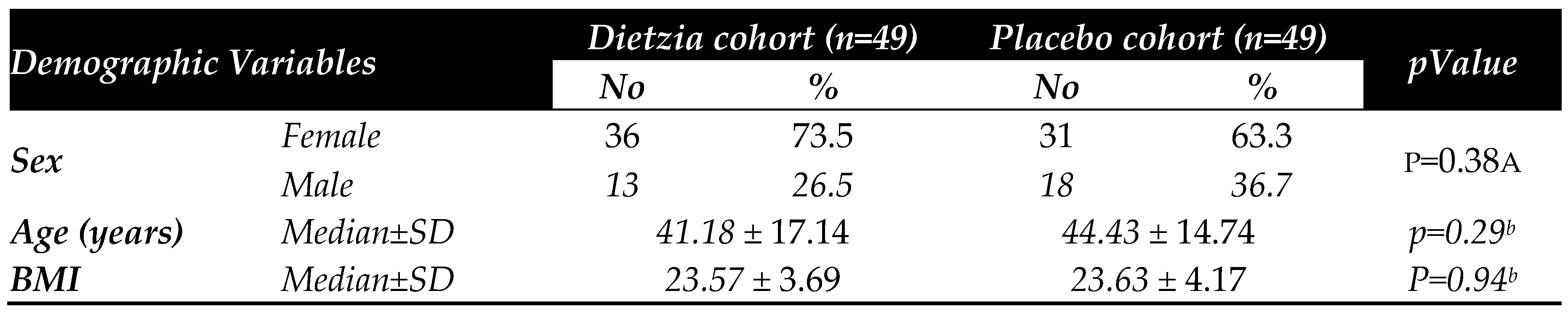
3.4. Clinical determinations
 |
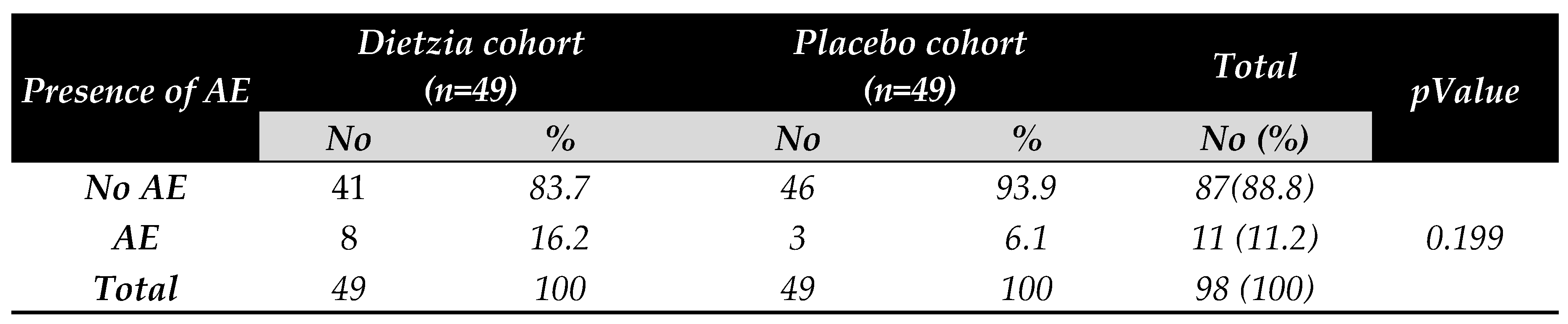
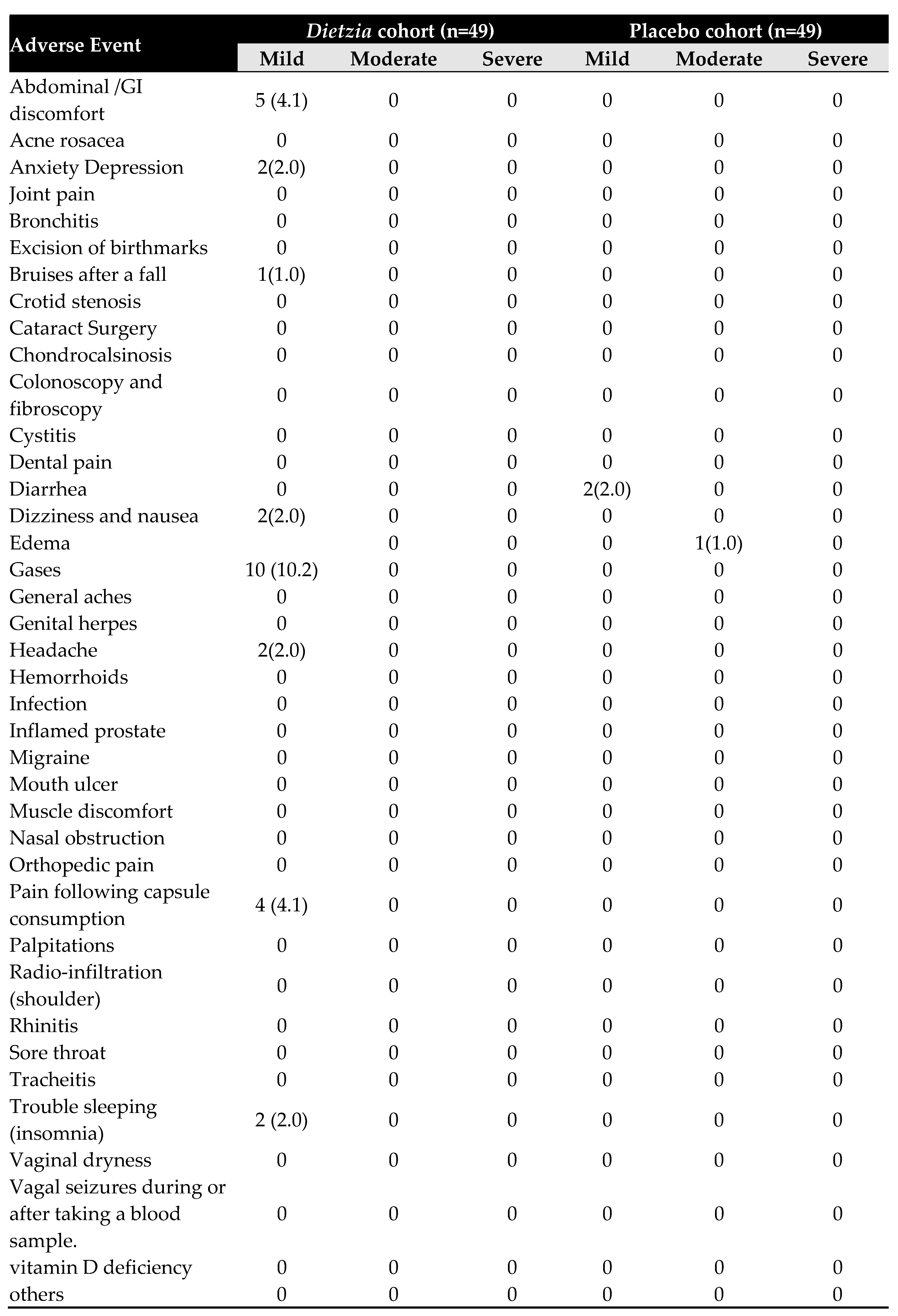
3.5. Occurrence of Adverse Effect determination
3.6. Health questionnaire analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salminen, S., et al., Functional food science and gastrointestinal physiology and function. Br J Nutr, 1998. 80 Suppl 1: p. S147-71.
- Bodke, H. and S. Jogdand, Role of Probiotics in Human Health. Cureus, 2022. 14(11): p. e31313.
- Yumoto, I., et al., Dietzia psychralcaliphila sp. nov., a novel, facultatively psychrophilic alkaliphile that grows on hydrocarbons. Int J Syst Evol Microbiol, 2002. 52(Pt 1): p. 85-90.
- Richards, W. In vitro and in vivo inhibition of Mycobacterium paratuberculosis by iron deprivation-a hypothesis.[Conference paper]. in International Conference on Johne's disease, Parkville, Vic.(Australia), 1988. 1989. CSIRO. [CrossRef]
- Click, R.E., A Potential 'Curative' Modality for Crohn's Disease---Modeled after Prophylaxis of Bovine Johne's Disease. Mycobact Dis, 2012. 2: p. 117.
- Dow, C.T., Mycobacterium avium subspecies paratuberculosis—An environmental trigger of type 1 diabetes mellitus. 2012.
- Kuenstner, J.T., et al., The Consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) Conference 2017. Front Public Health, 2017. 5: p. 208. [CrossRef]
- Zarei-Kordshouli, F., B. Geramizadeh, and A. Khodakaram-Tafti, Prevalence of Mycobacterium avium subspecies paratuberculosis IS 900 DNA in biopsy tissues from patients with Crohn's disease: histopathological and molecular comparison with Johne's disease in Fars province of Iran. BMC Infect Dis, 2019. 19(1): p. 23. [CrossRef]
- Ekundayo, T.C. and A.I. Okoh, Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911-2019: A Growth Analysis of Association with Human Autoimmune Diseases. Microorganisms, 2020. 8(8). [CrossRef]
- Agrawal, G., T.J. Borody, and W. Chamberlin, 'Global warming' to Mycobacterium avium subspecies paratuberculosis. Future Microbiol, 2014. 9(7): p. 829-32. [CrossRef]
- Yumoto I Fau - Nakamura, A., et al., Dietzia psychralcaliphila sp. nov., a novel, facultatively psychrophilic alkaliphile that grows on hydrocarbons. (1466-5026 (Print)).
- Nesterenko, O.A., et al., Rhodococcus luteus nom. nov. and Rhodococcus maris nom. nov. International Journal of Systematic and Evolutionary Microbiology, 1982. 32(1): p. 1-14.
- Rainey, F.A., et al., Dietzia, a new genus including Dietzia maris comb. nov., formerly Rhodococcus maris. (0020-7713 (Print)).
- Rodriguez, R.L., et al., The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res, 2018. 46(W1): p. W282-w288.
- Humphries, R., et al., Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J Clin Microbiol, 2021. 59(12): p. e0021321.
- McArthur, A.G., et al., The comprehensive antibiotic resistance database. Antimicrobial agents and chemotherapy, 2013. 57(7): p. 3348-3357.
- Chen, L., et al., VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res, 2005. 33(Database issue): p. D325-8. [CrossRef]
- Lefevre, M., et al., Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regulatory Toxicology and Pharmacology, 2017. 83: p. 54-65. [CrossRef]
- Sæbø, I.P., et al., Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int J Mol Sci, 2023. 24(3). [CrossRef]
- Soto, C., et al., Sticholysin II-mediated cytotoxicity involves the activation of regulated intracellular responses that anticipates cell death. Biochimie, 2018. 148: p. 18-35. [CrossRef]
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. 2191. [CrossRef]
- Moher, D., et al., CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International journal of surgery, 2012. 10(1): p. 28-55.
- Vilagut, G., et al., El Cuestionario de Salud SF-36 español: una década de experiencia y nuevos desarrollos. Gaceta sanitaria, 2005. 19: p. 135-150. [CrossRef]
- Altschul, S.F., et al., Basic local alignment search tool. (0022-2836 (Print)).
- Yumoto, I., et al., Dietzia psychralcaliphila sp. nov., a novel, facultatively psychrophilic alkaliphile that grows on hydrocarbons. International Journal of Systematic and Evolutionary Microbiology, 2002. 52(1): p. 85-90.
- Rodriguez-R, L.M., et al., The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic acids research, 2018. 46(W1): p. W282-W288. [CrossRef]
- Oppong, Y.E.A., et al., Genome-wide analysis of Mycobacterium tuberculosis polymorphisms reveals lineage-specific associations with drug resistance. BMC Genomics, 2019. 20(1): p. 252.
- Wang, W., et al., Determination of critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against para-aminosalicylic acid with clinical isolates with thyA, folC and dfrA mutations. Annals of Clinical Microbiology and Antimicrobials, 2022. 21(1): p. 1-9.
- Kalayanarooj, S., Clinical Manifestations and Management of Dengue/DHF/DSS. Trop Med Health, 2011. 39(4 Suppl): p. 83-7. [CrossRef]
- Gharibzahedi, S.M.T., S.H. Razavi, and M.J.A.o.m. Mousavi, Potential applications and emerging trends of species of the genus Dietzia: a review. 2014. 64(2): p. 421-429. [CrossRef]
- Roe, A.L., et al., Considerations for determining safety of probiotics: A USP perspective. Regul Toxicol Pharmacol, 2022. 136: p. 105266. [CrossRef]
- Gharibzahedi, S.M.T., S.H. Razavi, and S.M. Mousavi, Characterization of bacteria of the genus Dietzia: an updated review. Annals of Microbiology, 2014. 64(1): p. 1-11. [CrossRef]
- Gharibzahedi, S.M.T., S.H. Razavi, and S.M.J.A.o.m. Mousavi, Characterization of bacteria of the genus Dietzia: an updated review. 2014. 64(1): p. 1-11. [CrossRef]
- Meier-Kolthoff, J.P., H.P. Klenk, and M. Göker, Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. Int J Syst Evol Microbiol, 2014. 64(Pt 2): p. 352-356. [CrossRef]
- Luo, C., R.L. Rodriguez, and K.T. Konstantinidis, MyTaxa: an advanced taxonomic classifier for genomic and metagenomic sequences. Nucleic Acids Res, 2014. 42(8): p. e73. [CrossRef]
- Alcock, B.P., et al., CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic acids research, 2020. 48(D1): p. D517-D525. [CrossRef]
- Oliveira, P.H., et al., The chromosomal organization of horizontal gene transfer in bacteria. Nat Commun, 2017. 8(1): p. 841. [CrossRef]
- Ouwehand, A.C., et al., Probiotic approach to prevent antibiotic resistance. Ann Med, 2016. 48(4): p. 246-55.
- Alderwick, L.J., et al., The C-terminal domain of the Arabinosyltransferase Mycobacterium tuberculosis EmbC is a lectin-like carbohydrate binding module. PLoS Pathog, 2011. 7(2): p. e1001299. [CrossRef]
- Rahlwes, K.C., I.L. Sparks, and Y.S. Morita, Cell Walls and Membranes of Actinobacteria. Subcell Biochem, 2019. 92: p. 417-469. [CrossRef]
- Bottai, D., M.I. Gröschel, and R. Brosch, Type VII Secretion Systems in Gram-Positive Bacteria. Curr Top Microbiol Immunol, 2017. 404: p. 235-265.
- Nath, Y., S.K. Ray, and A.K. Buragohain, Essential role of the ESX-3 associated eccD3 locus in maintaining the cell wall integrity of Mycobacterium smegmatis. Int J Med Microbiol, 2018. 308(7): p. 784-795.
- Wang, M., Y. Nie, and X.-L. Wu, Membrane vesicles from a Dietzia bacterium containing multiple cargoes and their roles in iron delivery. Environmental Microbiology, 2021. 23(2): p. 1009-1019. [CrossRef]
- Sanders, M.E., et al., Safety assessment of probiotics for human use. Gut Microbes, 2010. 1(3): p. 164-85. [CrossRef]
- Bourebaba, Y., et al., Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed Pharmacother, 2022. 153: p. 113138. [CrossRef]
- Yasmin, I., et al., In vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms, 2020. 8(3). [CrossRef]
- Dewanjee, S., et al., Probiotics: evolving as a potential therapeutic option against acetaminophen-induced hepatotoxicity. Biomedicines, 2022. 10(7): p. 1498.
- Ayala, D.I., et al., A Systematic Approach to Identify and Characterize the Effectiveness and Safety of Novel Probiotic Strains to Control Foodborne Pathogens. 2019. 10. [CrossRef]
- Hong, H.A., et al., The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J Appl Microbiol, 2008. 105(2): p. 510-20.
- Elham, N., et al., Selective Cytotoxic effect of Probiotic, Paraprobiotic and Postbiotics of <i>L.casei</i> strains against Colorectal Cancer Cells: <i>Invitro</i> studies. Brazilian Journal of Pharmaceutical Sciences, 2022. 58.
- Click, R.E., A 60-day probiotic protocol with Dietzia subsp. C79793-74 prevents development of Johne's disease parameters after in utero and/or neonatal MAP infection. J Virulence, 2011. 2(4): p. 337-347.
- Sudhindra, P., G. Wang, and R.B. Nadelman, Identification of Dietzia spp. from Cardiac Tissue by 16S rRNA PCR in a Patient with Culture-Negative Device-Associated Endocarditis: A Case Report and Review of the Literature. Case Rep Infect Dis, 2016. 2016: p. 8935052.
- Sweeney, R., et al., Paratuberculosis (Johne's disease) in cattle and other susceptible species. 2012. 26(6): p. 1239-1250.
- Ewald, P.W. and H.A.S.J.C.O.i.G. Ewald, An evolutionary perspective on the causes and treatment of inflammatory bowel disease. 2013. 29(4): p. 350-356.
- Rondanelli, M., et al., Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes, 2017. 8(6): p. 521-543.
- Nikolova, V.L., et al., Acceptability, Tolerability, and Estimates of Putative Treatment Effects of Probiotics as Adjunctive Treatment in Patients With Depression: A Randomized Clinical Trial. JAMA Psychiatry, 2023. 80(8): p. 842-847. [CrossRef]
- Sotoudegan, F., et al., Reappraisal of probiotics’ safety in human. Food and Chemical Toxicology, 2019. 129: p. 22-29.
- Click, R.E., Successful treatment of asymptomatic or clinically terminal bovine Mycobacterium avium subspecies paratuberculosis infection (Johne's disease) with the bacterium Dietzia used as a probiotic alone or in combination with dexamethasone: Adaption to chronic human diarrheal diseases. J Virulence, 2011. 2(2): p. 131-143.
- Click, R.E., Crohn's disease therapy with Dietzia: the end of anti-inflammatory drugs. Future Microbiology, 2015. 10(2): p. 147-150.
- Click, R.E., A Potential'Curative'Modality for Crohn's Disease---Modeled after Prophylaxis of Bovine Johne's Disease. Mycobacterial diseases: tuberculosis & leprosy, 2012. 2: p. 117-117.
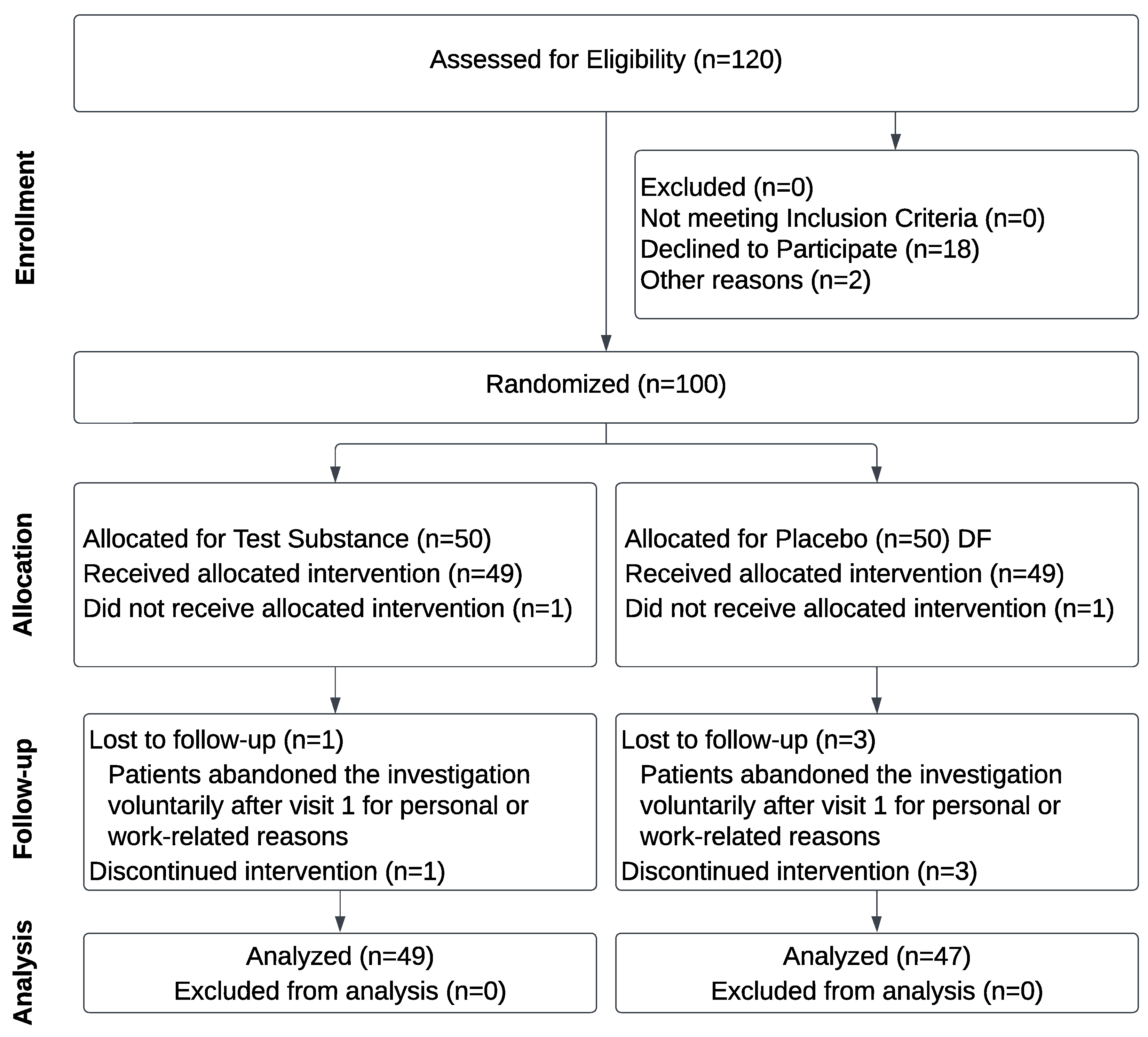
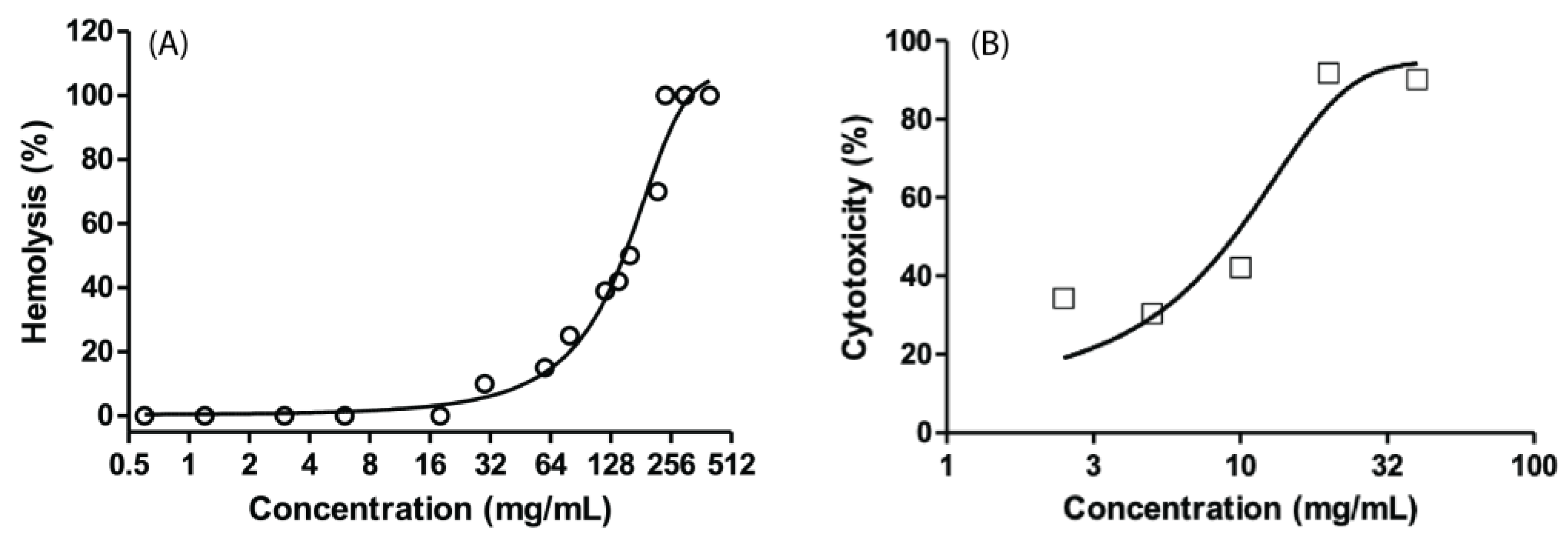
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





