Submitted:
19 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Symptom Assessment Tools
2.2.2. Assessment of Autonomic Nervous System Function
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Participants
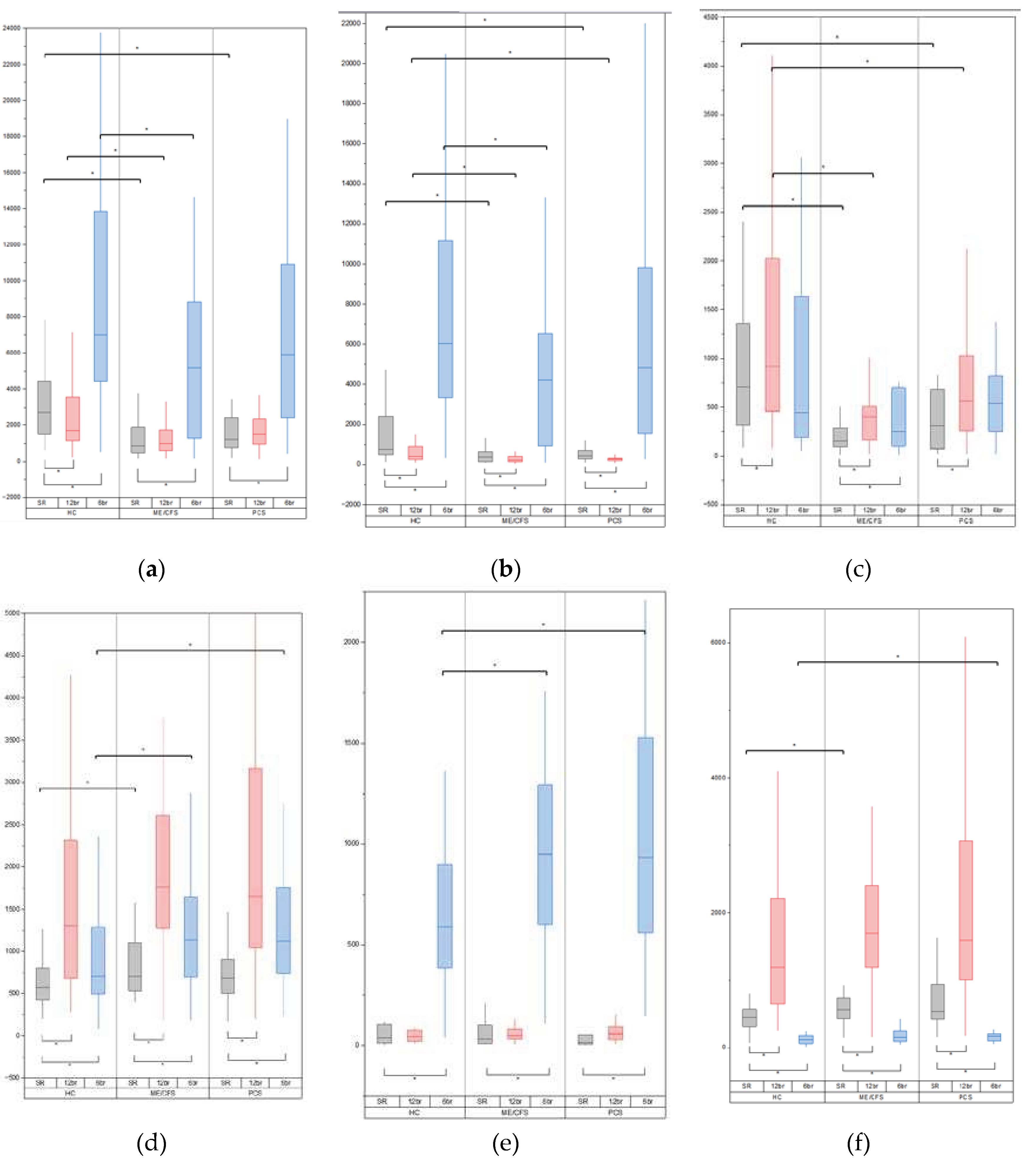
3.2. Baseline Variability Indices
3.3. Variability Indices during Breathing at 12 Breaths Per Minute
3.4. Variability Indices during Breathing at 6 Breaths per Minute
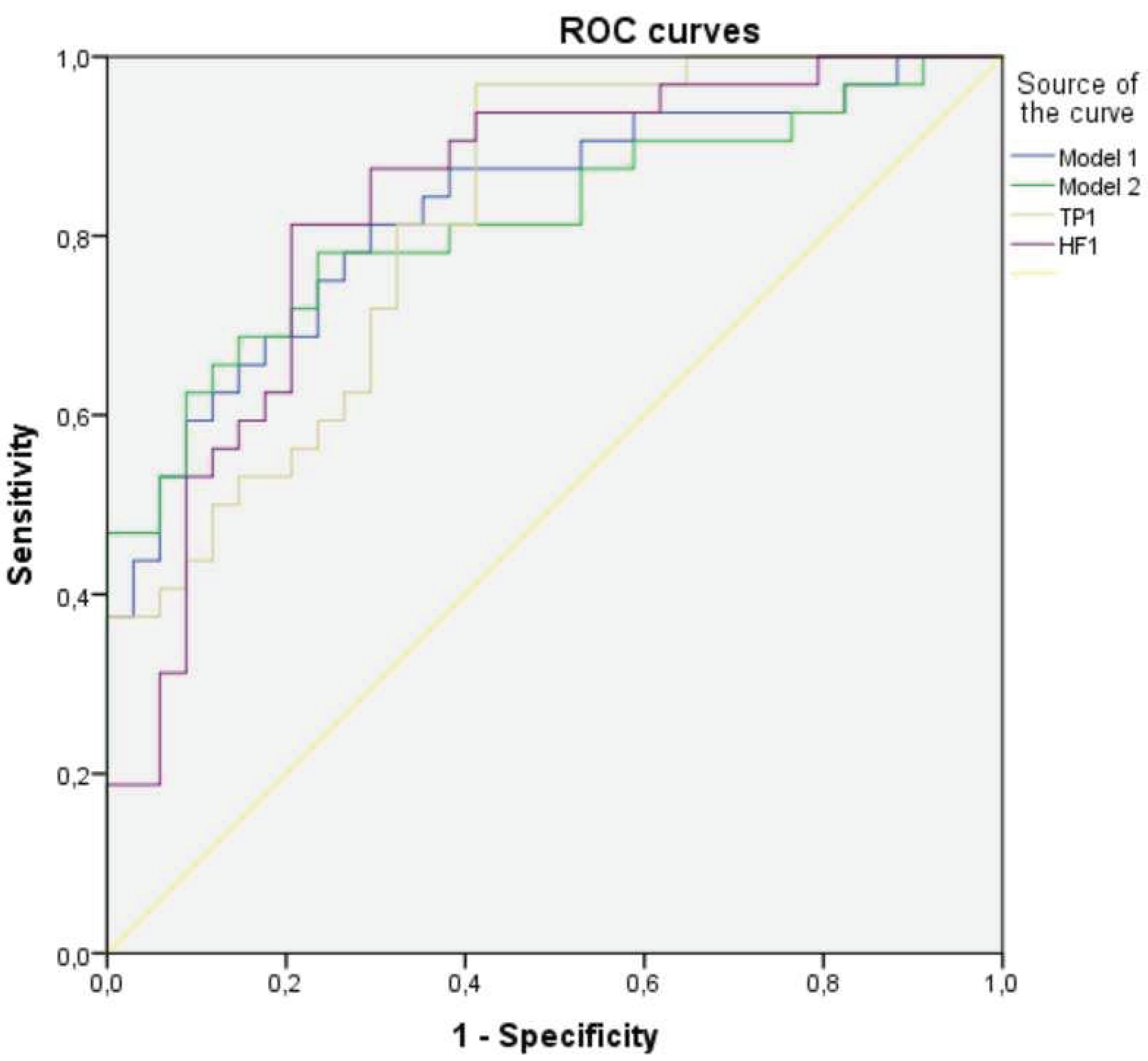
3.5. Multifactorial Logistic Regression Analysis in ME/CFS Group
3.6. Correlations of Heart Rate, Arterial Blood Pressure and Respiration Variability Parameters with Clinical Characteristics in Patient Groups
3.7. Baroreflex Sensitivity and Baroreflex Effectiveness Index
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Chronic Fatigue Syndr 2003, 11, 7–115. [CrossRef]
- Nacul L, Jérôme Authier F, Scheibenbogen C, Lorusso L, Bergliot Helland I, Alegre Martin J, et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina (B Aires) 2021, 57, 510. [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023, 21, 133–46. [Google Scholar] [CrossRef]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr 2019, 7, 427132. [Google Scholar] [CrossRef]
- Jason LA, Dorri JA. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol Int 2023 2022, 15, 1–11. [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022, 22, e102–7. [Google Scholar] [CrossRef] [PubMed]
- Komaroff AL, Lipkin WI. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med 2023, 10, 1187163. [CrossRef] [PubMed]
- Macarova JA, Malakhova SA, Novitskaya TA, Shapkina VA, Churilov LP. COVID-19 and Vasa vasorum: New Atherogenic Factor? A Case Report and Autopsy Findings. Diagnostics (Basel). 2023, 13, 1097. [CrossRef] [PubMed]
- Nunes JM, Kell DB, Pretorius E. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses. Blood Rev. 2023, 60, 101075. [CrossRef]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann Intern Med 1994, 121, 953. [CrossRef]
- IOM (Institute of Medicine). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC, USA: National Academies Press; 2015. [CrossRef]
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995, 39, 315–25. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosom Res 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Romanchuk, A.; Guzii, O. Variability and Pattern of Spontaneous Respiration in Different Types of Cardiac Rhythm Regulation of Highly Trained Athletes. Int J Hum Mov Sport Sci 2020, 8, 483–93. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Parati, G.; Castiglioni, P.; Tordi, R.; Mancia, G.; Pedotti, A. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol 2001, 280. [Google Scholar] [CrossRef] [PubMed]
- Skytioti, M.; Elstad, M. Respiratory Sinus Arrhythmia is Mainly Driven by Central Feedforward Mechanisms in Healthy Humans. Front Physiol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Saboul, D.; Pialoux, V.; Hautier, C. The breathing effect of the LF/HF ratio in the heart rate variability measurements of athletes. Eur J Sport Sci 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Santarelli, D.M.; O’Rourke, D. The physiological effects of slow breathing in the healthy human. Breathe (Sheffield, England) 2017, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Zaccaro A, Piarulli A, Laurino M, Garbella E, Menicucci D, Neri B, et al. How Breath-Control Can Change Your Life: A Systematic Review on Psycho-Physiological Correlates of Slow Breathing. Front Hum Neurosci 2018, 12. [CrossRef] [PubMed]
- Frith J, Zalewski P, Klawe JJ, Pairman J, Bitner A, Tafil-Klawe M, et al. Impaired blood pressure variability in chronic fatigue syndrome—a potential biomarker. QJM An Int J Med 2012, 105, 831–8. [CrossRef]
- Escorihuela RM, Capdevila L, Castro JR, Zaragozà MC, Maurel S, Alegre J, et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med 2020, 18, 1–12. [CrossRef]
- Boissoneault, J.; Letzen, J.; Robinson, M.; Staud, R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav 2019, 13, 789–97. [Google Scholar] [CrossRef] [PubMed]
- Reyes del Paso, G.A.; Contreras-Merino, A.M.; de la Coba, P.; Duschek, S. The cardiac, vasomotor, and myocardial branches of the baroreflex in fibromyalgia: Associations with pain, affective impairments, sleep problems, and fatigue. Psychophysiology 2021, 58, e13800. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.R.; Ray, C.; Benitez, A.; Robinson-Papp, J. Reduced cardiovagal baroreflex sensitivity is associated with postural orthostatic tachycardia syndrome (POTS) and pain chronification in patients with headache. Front Hum Neurosci 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Skow RJ, Garza NA, Nandadeva D, Stephens BY, Wright AN, Grotle AK, et al. Impact of COVID-19 on cardiac autonomic function in healthy young adults: potential role of symptomatology and time since diagnosis. Am J Physiol Hear Circ Physiol 2022, 323, H1206–H1211. [CrossRef] [PubMed]
- Brognara, F.; Castania, J.A.; Kanashiro, A.; Dias, D.P.M.; Salgado, H.C. Physiological Sympathetic Activation Reduces Systemic Inflammation: Role of Baroreflex and Chemoreflex. Front Immunol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Amorim MR, de Deus JL, Pereira CA, da Silva LEV, Borges GS, Ferreira NS, et al. Baroreceptor denervation reduces inflammatory status but worsens cardiovascular collapse during systemic inflammation. Sci Reports 2020 101 2020, 10, 1–13. [CrossRef] [PubMed]
- Okin, D.; Medzhitov, R. Review Evolution of Inflammatory Diseases. Curr Biol 2012, 22, R733–R740. [Google Scholar] [CrossRef]
- Gorji, A. Neuroinflammation: The Pathogenic Mechanism of Neurological Disorders. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Tate, W.P.; Walker, M.O.M.; Peppercorn, K.; Blair, A.L.H.; Edgar, C.D. Towards a Better Understanding of the Complexities of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Long COVID. Int J Mol Sci 2023, 24, 5124. [Google Scholar] [CrossRef]
- Braga J, Lepra M, Kish SJ, Rusjan PM, Nasser Z, Verhoeff N, et al. Neuroinflammation After COVID-19 With Persistent Depressive and Cognitive Symptoms. JAMA Psychiatry 2023, 80, 787–95. [CrossRef]
- Wang, M.; Pan, W.; Xu, Y.; Zhang, J.; Wan, J.; Jiang, H. Microglia-Mediated Neuroinflammation: A Potential Target for the Treatment of Cardiovascular Diseases. J Inflamm Res 2022, 15, 3083–94. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.G. Atherosclerosis and Blood Pressure Variability. Hypertension. 2018, 71, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Zambach C, Fedorowski A, Gerward S, Johansson M, Engström G, Hamrefors V. Subclinical atherosclerosis and risk factors in relation to autonomic indices in the general population. J Hypertens. 2023, 41, 759–767. [CrossRef] [PubMed]
- akahashi M, Miyai N, Nagano S, Utsumi M, Oka M, Yamamoto M, Shiba M, Uematsu Y, Nishimura Y, Takeshita T, Arita M. Orthostatic Blood Pressure Changes and Subclinical Markers of Atherosclerosis. Am J Hypertens. 2015, 28, 1134–40. [CrossRef] [PubMed]
- Sheng, Y.; Zhu, L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol 2018, 10, 17. [Google Scholar] [PubMed]
- Chistiakov, D.A.; Ashwell, K.W.; Orekhov, A.N.; Bobryshev, Y.V. Innervation of the arterial wall and its modification in atherosclerosis. Auton Neurosci 2015, 193, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Xia, S.; Pelliccioni, G.; Olivieri, F. Autonomic nervous system imbalance during aging contributes to impair endogenous anti-inflammaging strategies. GeroScience 2023. [CrossRef] [PubMed]
- Rivera, M.C.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Simula S, Vanninen E, Lehto S, Hedman A, Pajunen P, Syvänne M, et al. Heart rate variability associates with asymptomatic coronary atherosclerosis. Clin Auton Res 2014, 24, 31–7. [CrossRef]
- Huikuri H, V. , Jokinen V, Syvänne M, Nieminen MS, Airaksinen KEJ, Ikäheimo MJ, et al. Heart Rate Variability and Progression of Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol 1999, 19, 1979–85. [Google Scholar] [CrossRef]
| Variable | ME/CFS | PCС | HC | p-Value ME/CFS vs HC |
p-Value PCС vs HC |
|---|---|---|---|---|---|
| Age (years) | 35.00 (30.0-41.25) | 35.00 (29.50-41.50) | 34.50 (21.25-43.75) | 0.190 | 0.174 |
| BMI (kg/m2) | 23.41 (19.45-28.70) | 23.51 (20.54-27.86) | 22.07 (20.14-25.41) | 0.530 | 0.323 |
| Gender | |||||
| Male | 8 (23.53%) | 6 (20.69%) | 10 (31.25%) | 0.482 | 0.395 |
| Female | 26 (76.47%) | 23 (79.31%) | 22 (68.75%) | ||
| HADS | |||||
| Anxiety | 10.00 (5.75-12.00) | 10.00 (6.00-12.00) | 6.00 (2.00-8.00) | 0.001 | 0.001 |
| Depression | 10.00 (7.75-11.25) | 9.00 (6.00-10.00) | 3.00 (1.25-4.00) | 0.000 | 0.000 |
| MFI-20 | |||||
| General fatigue | 19.00 (17.75-20.00) | 18.00 (14.50-20.00) | 8.00 (5.25-9.75) | 0.000 | 0.000 |
| Physical fatigue | 17.50 (15.00-19.00) | 16.00 (14.00-18.00) | 7.00 (5.00-9.75) | 0.000 | 0.000 |
| Reduced activity | 18.00 (16.00-19.00) | 16.00 (12.5-19.00) | 7.50 (4.25-11.00) | 0.000 | 0.000 |
| Reduced motivation | 13.50 (11.00-16.00) | 12.00 (9.50-15.00) | 8.00 (6.00-9.00) | 0.000 | 0.000 |
| Mental fatigue | 16.50 (15.00-18.00) | 13.00 (11.00-16.00) | 6.00 (4.00-9.00) | 0.000 | 0.000 |
| IPAQ | |||||
| Total score IPAQ (MET-min/Week) | 1857.00 (590.25-2841.00) | 1506.00 (1039.50-2814.00) | 3027.00 (1606.50-5399.00) | 0.004 | 0.011 |
| Variable | ME/CFS | PCC | HC | p-Value ME/CFS vs HC |
p-Value PCC vs HC |
|
|---|---|---|---|---|---|---|
| Heart rate variability | ||||||
| TP (ms2) | 852.45 (469.03-1912.88) | 1358.10 (834.15-2687.15) | 2709.05 (1483.38-4454.36) | 0.000 | 0.008 | |
| LF (ms2) | 367.10 (137.45-635.13) | 429.20 (279.65-867.70) | 759.30 (477.33-2480.68) | 0.000 | 0.038 | |
| HF (ms2) | 152.90 (91.93-284.78) | 335.00 (102.45-717.10) | 703.70 (311.00-1394.80) | 0.000 | 0.012 | |
| VLF (ms2) | 256.15 (192.03-619.38) | 469.20 (237.10-814.60) | 727.50 (431.85-1014.80) | 0.000 | 0.017 | |
| LF/HF | 2.14 (1.20-4.13) | 1.46 (1.02-4.01) | 1.25 (0.71-2.31) | 0.029 | 0.155 | |
| Beat-to-beat systolic arterial blood pressure variability | ||||||
| TP (ms2) | 50.15 (23.38-66.78) | 42.20 (26.85-65.80) | 41.35 (22.15-63.15) | 0.434 | 0.598 | |
| LF (ms2) | 13.95 (6.45-21.85) | 13.50 (8.80-23.30) | 9.50 (6.30-19.68) | 0.438 | 0.157 | |
| HF (ms2) | 7.80 (4.38-16.73) | 8.40 (3.60-11.45) | 8.50 (4.48-14.28) | 0.944 | 0.718 | |
| VLF (ms2) | 20.30 (10.08-31.83) | 17.50 (7.80-29.60) | 12.90 (5.63-33.60) | 0.178 | 0.470 | |
| LF/HF | 1.63 (0.77-2.43) | 1.79 (1.20-2.92) | 1.11 (0.65-2.85) | 0.369 | 0.053 | |
| Beat-to-beat diastolic arterial blood pressure variability | ||||||
| TP (ms2) | 12.95 (7.38-25.28) | 12.80 (8.05-25.35) | 11.75 (7.60-20.53) | 0.559 | 0.488 | |
| LF (ms2) | 5.15 (2.93-7.98) | 5.30 (3.15-8.70) | 4.45 (2.25-7.90) | 0.585 | 0.593 | |
| HF (ms2) | 1.50 (0.90-2.15) | 1.10 (0.70-2.50) | 1.45 (0.70-2.68) | 0.832 | 0.772 | |
| VLF (ms2) | 5.75 (3.03-12.15) | 6.20 (2.80-13.15) | 4.45 (2.83-13.48) | 0.847 | 0.868 | |
| LF/HF | 3.39 (1.84-5.53) | 3.91 (2.35-6.83) | 3.02 (2.17-5.74) | 0.807 | 0.573 | |
| Respiration variability | ||||||
| TP (ms2) | 703.00 (523.63-1122.15) | 722.90 (480.45-993.45) | 576.10 (418.93-809.75) | 0.022 | 0.153 | |
| LF (ms2) | 32.65 (11.20-101.05) | 20.60 (7.20-182.80) | 39.45 (13.33-105.73) | 0.748 | 0.263 | |
| HF (ms2) | 560.85 (421.90-769.85) | 481.80 (350.90-951.95) | 449.85 (309.00-569.55) | 0.024 | 0.137 | |
| VLF (ms2) | 3.05 (2.05-4.88) | 3.40 (2.15-5.45) | 3.15 (2.25-6.20) | 0.572 | 0.960 | |
| LF/HF | 0.06 (0.02-0.13) | 0.03 (0.02-0.26) | 0.08 (0.03-0.29) | 0.403 | 0.132 | |
| Variable | ME/CFS | PCC | HC | p-Value ME/CFS vs HC |
p-Value PCC vs HC |
|
|---|---|---|---|---|---|---|
| Heart rate variability at 12 breaths/minute | ||||||
| TP (ms2) | 998.90 (573.93-1729.15) | 1506.80 (948.90-2410.35) | 1682.35 (1120.95-3607.90) | 0.001 | 0.088 | |
| LF (ms2) | 226.55 (140.28-399.68) | 272.60 (198.90-329.50) | 402.7 (237.35-908.75) | 0.002 | 0.018 | |
| HF (ms2) | 399.15 (162.38-529.75) | 564.50 (253.85-1095.35) | 920.45 (439.75-2023.70) | 0.000 | 0.038 | |
| Beat-to-beat systolic arterial blood pressure variability at 12 breaths/minute | ||||||
| TP (ms2) | 49.30 (24.85-65.45) | 42.40 (26.00-97.60) | 33.00 (18.85-49.18) | 0.101 | 0.231 | |
| LF (ms2) | 11.90 (5.93-21.33) | 9.60 (4.75-23.10) | 5.75 (3.70-10.85) | 0.007 | 0.029 | |
| HF (ms2) | 14.74 (6.08-32.05) | 12.80 (6.40-35.80) | 13.80 (8.38-22.50) | 0.773 | 0.960 | |
| Beat-to-beat dyastolic arterial blood pressure variability at 12 breaths/minute | ||||||
| TP (ms2) | 12.35 (7.48-22.78) | 13.90 (7.15-20.60) | 9.35 (4.63-14.55) | 0.034 | 0.067 | |
| LF (ms2) | 4.10 (2.13-6.90) | 4.10 (1.85-8.15) | 2.70 (1.73-4.70) | 0.061 | 0.161 | |
| HF (ms2) | 3.35 (1.45-5.83) | 2.60 (1.00-6.40) | 1.45 (0.93-3.15) | 0.010 | 0.126 | |
| Respiration variability at 12 breaths/minute | ||||||
| TP (ms2) | 1763.45 (1247.75-2613.40) | 1647.10 (1020.70-3323.25) | 1303.65 (683.95-2337.68) | 0.124 | 0.126 | |
| LF (ms2) | 51.10 (34.28-81.90) | 57.50 (27.90-112.10) | 45.20 (22.50-75.28) | 0.216 | 0.166 | |
| HF (ms2) | 1692.00 (1154.40-2413.70) | 1591.30 (988.10-3197.25) | 1189.25 (645.40-2238.68) | 0.118 | 0.126 | |
| Heart rate variability at 6 breaths/minute | ||||||
| TP (ms2) | 5179.75 (1260.90-8857.20) | 5891.10 (2363.10-11520.45) | 7007.30 (4443.65-14608.75) | 0.022 | 0.220 | |
| LF (ms2) | 4220.15 (921.70-6590.25) | 4824.30 (1528.40-9957.70) | 6022.40 (3333.65-11796.05) | 0.013 | 0.112 | |
| HF (ms2) | 249.90 (98.28-698.98) | 536.10 (231.55-1099.25) | 442.90 (183.50-1643.08) | 0.057 | 0.931 | |
| Beat-to-beat systolic arterial blood pressure variability at 6 breaths/minute | ||||||
| TP (ms2) | 77.20 (43.58-136.63) | 84.40 (48.90-157.35) | 62.90 (39.38-96.05) | 0.184 | 0.137 | |
| LF (ms2) | 53.60 (22.05-106.18) | 57.70 (27.30-122.60) | 39.25 (22.23-69.70) | 0.225 | 0.054 | |
| HF (ms2) | 5.35 (2.80-10.85) | 5.20 (2.65-8.95) | 3.15 (1.93-6.23) | 0.026 | 0.086 | |
| Beat-to-beat dyastolic arterial blood pressure variability at 6 breaths/minute | ||||||
| TP (ms2) | 21.15 (11.45-39.48) | 17.90 (12.70-35.20) | 15.30 (10.45-27.23) | 0.359 | 0.295 | |
| LF (ms2) | 14.25 (5.23-26.93) | 12.60 (6.35-27.00) | 9.40 (5.05-20.80) | 0.333 | 0.245 | |
| HF (ms2) | 1.70 (1.10-2.53) | 2.10 (1.40-3.80) | 1.10 (0.70-3.38) | 0.386 | 0.079 | |
| Respiration variability at 6 breaths/minute | ||||||
| TP (ms2) | 1133.80 (688.13-1664.75) | 1122.20 (724.45-1865.40) | 701.45 (492.33-1332.78) | 0.030 | 0.022 | |
| LF (ms2) | 947.55 (591.03-1304.13) | 933.80 (537.65-1612.40) | 588.45 (370.00-937.15) | 0.013 | 0.015 | |
| HF (ms2) | 157.40 (91.43-249.58) | 165.50 (97.20-214.10) | 116.55 (61.58-183.60) | 0.072 | 0.033 | |
| Variable | Adj. B | Adj. OR (95% CI) | P value |
|---|---|---|---|
| Model 1 | |||
| TP of HRV at spontaneous breathing | -0.001 | 0.999 (0.999-1.002) | 0.001 |
| TP of RV at spontaneous breathing | 0.002 | 1.002 (1.000-1.004) | 0.044 |
| Hosmer Lemeshow test, p-value=0.952; constant = 0.504 | |||
| Model 2 | |||
| LF of HRV at spontaneous breathing | -0.001 | 0.999 (0.998-1.000) | 0.012 |
| HF of HRV at 12 breaths/minute | -0.001 | 0.999 (0.998-1.000) | 0.047 |
| TP of RV at spontaneous breathing | 0.002 | 1.002 (1.000-1.003) | 0.088 |
| Hosmer Lemeshow test, p-value=0.730; constant = 0.562 | |||
| Variable | TP of HRV at SR | HF of HRV at SR | Model 1 | Model 2 |
|---|---|---|---|---|
| AUC (95% CI) | 0.819 (0.720-0.918) | 0.834 (0.735-0.933) | 0.830 (0.730-0.929) | 0.817 (0.712-0.922) |
| Сut-off (Maximum Youden’s index) | 1047.95 | 286.95 | 0.580 | 0.496 |
| Sensitivity %, (95% CI) | 96.9% | 81.3% | 73.5% | 85.3% |
| Specificity %, (95% CI) | 58.8% | 79.4% | 75% | 68.8% |
| Cut-off (Se=Sp) | 1587.55 | 296.95 | 0.603 | 0.612 |
| Sensitivity %, (95% CI) | 71.9% | 78.1% | 70.6% | 76.5% |
| Specificity %, (95% CI) | 70.6% | 79.4% | 81.3% | 75.% |
| Variable | ME/CFS | PCC | HC | |||
|---|---|---|---|---|---|---|
| (+) | (-) | (+) | (-) | (+) | (-) | |
| HADS_D | BMI DBPV_HF_6br/min |
RM HADS_A DBPV_TP_12br/min |
GF, PF, RA, RM, MF | |||
| HADS_A | RV_TP_6br/min RV_LF_6br/min RV_HF_6br/min |
HRV_TP_12br/min |
SBPV_TP_SR SBPV_VLF_SR RV_TP_6br/min RV_LF_6br/min |
GF, PF, RM RV_HF_6br/min |
HRV_HF_SR | |
| General fatigue | PF, RA SBPV_HF_SR DBPV_LF_12br/min SBPV_LF_6br/min DBPV_LF_6br/min |
HRV_TP_SR HRV_HF_SR HRV_VLF_SR HRV_TP_12br/min HRV_LF_12br/min HRV_HF_12br/min |
PF, RA | PF, RA, RM, MF HADS_D |
||
| Physical fatigue | GF, RA SBPV_TP_6br/min SBPV_LF_6br/min DBPV_TP_6br/min DBPV_LF_6br/min |
GF, RA SBPV_TP_12br/min SBPV_LF_12br/min DBPV_LF_12br/min SBPV_TP_6br/min SBPV_LF_6br/min DBPV_TP_6br/min DBPV_LF_6br/min |
GF, RA, RM, MF HADS_D, HADS_A DBPV_VLF_SR |
Age | ||
| Reduced activity | GF, PF |
HRV_HF_SR |
GF, PF, RM, MF DBPV_TP_12br/min |
GF, PF, RM, MF HADS_D SBPV_VLF_SR |
||
| Reduced motivation | SBPV_TP_SR SBPV_LF_SR |
RA, MF HADS_D |
GF, PF, RA, MF HADS_D, HADS_A |
|||
| Mental fatigue | RA, RM DBPV_HF_6br/min |
GF, PF, RA, RM HADS_D SBPV_TP_SR DBPV_TP_SR DBPV_HF_SR |
||||
| Age | SBPV_TP_6br/min SBPV_LF_6br/min SBPV_HF_6br/min |
RV_TP_SR RV_HF_SR RV_TP_6br/min RV_LF_6br/min |
HRV_LF_SR SBPV_LF_SR DBPV_LF_SR DBPV_HF_SR SBPV_TP_12br/min SBPV_HF_12br/min DBPV_HF_12br/min HRV_TP_6br/min HRV_LF_6br/min HRV_HF_6br/min |
SBPV_TP_6br/min SBPV_LF_6br/min |
PF DBPV_HF_SR HRV_TP_12br/min HRV_LF_12br/min HRV_TP_6br/min HRV_LF_6br/min HRV_HF_6br/min |
|
| BMI | RV_TP_12br/min RV_LF_12br/min RV_HF_12br/min RV_TP_6br/min RV_LF_6br/min RV_HF_6br/min |
HADS_D HRV_TP_SR HRV_HF_SR HRV_LF_SR DBPV_HF_6br/min |
RV_TP_12br/min RV_HF_12br/min SBPV_HF_6br/min RV_HF_6br/min |
HRV_HF_12br/min SBPV_HF_12br/min HRV_TP_6br/min HRV_LF_6br/min HRV_HF_6br/min |
SBPV_HF_12br/min |
|
| IPAQ | GF, PF; DBPV_TP_12br/min RV_LF_6br/min |
DBPV_TP_12br/min | ||||
| Variable | ME/CFS | PCC | HC | p-Value ME/CFS vs HC |
p-Value PCC vs HC |
|---|---|---|---|---|---|
| BRSup | 4.42 (2.88-6.28) | 5.91 (3.54-7.92) | 7.40 (4.90-14.03) | 0.000 | 0.041 |
| BRSdown | 4.85 (2.93-7.42) | 5.24 (3.97-8.48) | 9.15 (6.42-12.01) | 0.000 | 0.002 |
| BRSmean | 4.60 (3.12-6.40) | 5.99 (3.88-8.48) | 8.45 (5.25-13.40) | 0.000 | 0.016 |
| BEI_up | 0.57 (0.43-0.80) | 0.64 (0.44-0.78) | 0.70 (0.56-0.88) | 0.024 | 0.049 |
| BEI_down | 0.45 (0.37-0.62) | 0.44 (0.29-0.77) | 0.49 (0.36-0.82) | 0.434 | 0.470 |
| BR_up | 16.00 (7.50-25.00) | 18.00 (10.00-27.50) | 18.50 (9.25-29.00) | 0.362 | 0.942 |
| BR_down | 19.00 (9.00-25.75) | 12.00 (6.00-24.00) | 19.5 (7.00 - 32.75) | 0.529 | 0.191 |
| BRX_up | 10.5 (4.75-18.00) | 9.00 (5.50-14.00) | 5.50 (2.00-9.00) | 0.009 | 0.018 |
| BRX_down | 18.5 (9.00-25.00) | 14.00 (5.00-23.00) | 16.00 (5.00-21.50) | 0.218 | 0.931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





