Submitted:
20 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analysis of viscosity
2.2.2. Analysis of flexural strength
2.2.3. Analysis of hardness
2.2.4. Analysis of tensile strength
3. Results and Discussion
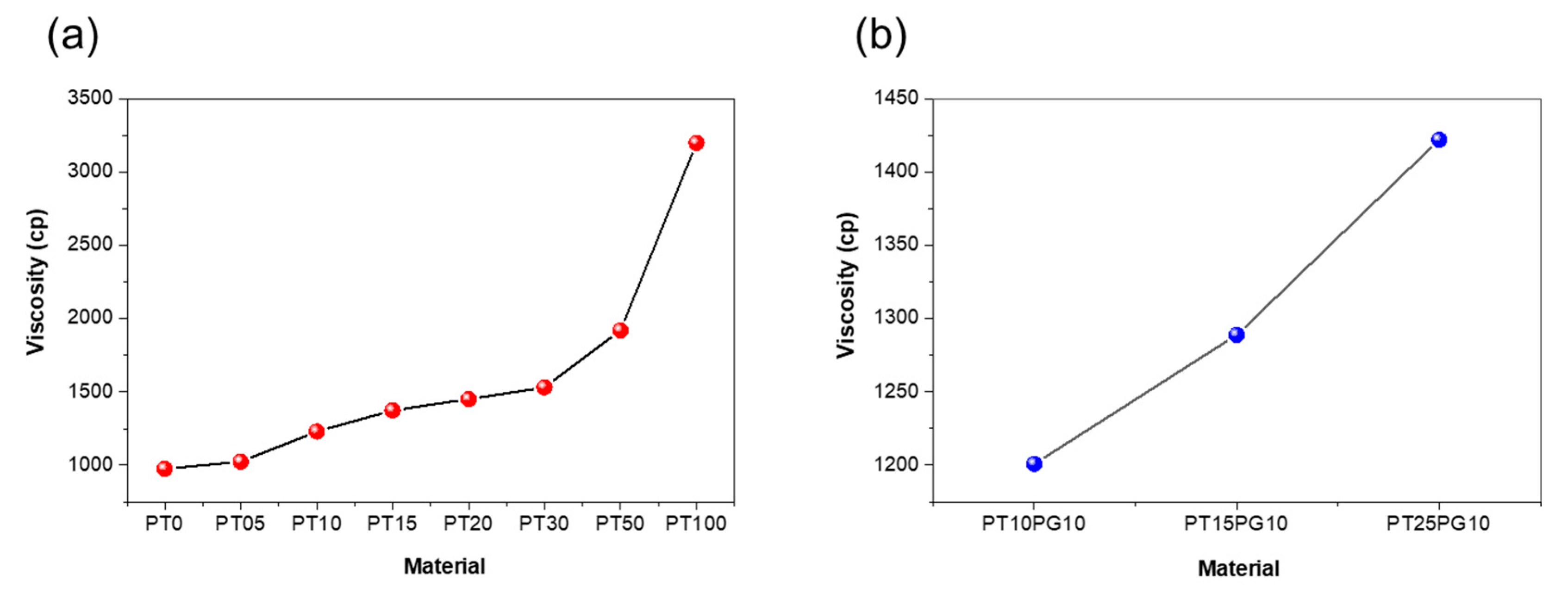
3.1. Viscosity of potassium titanate/acrylate resin using ceramic-based additive
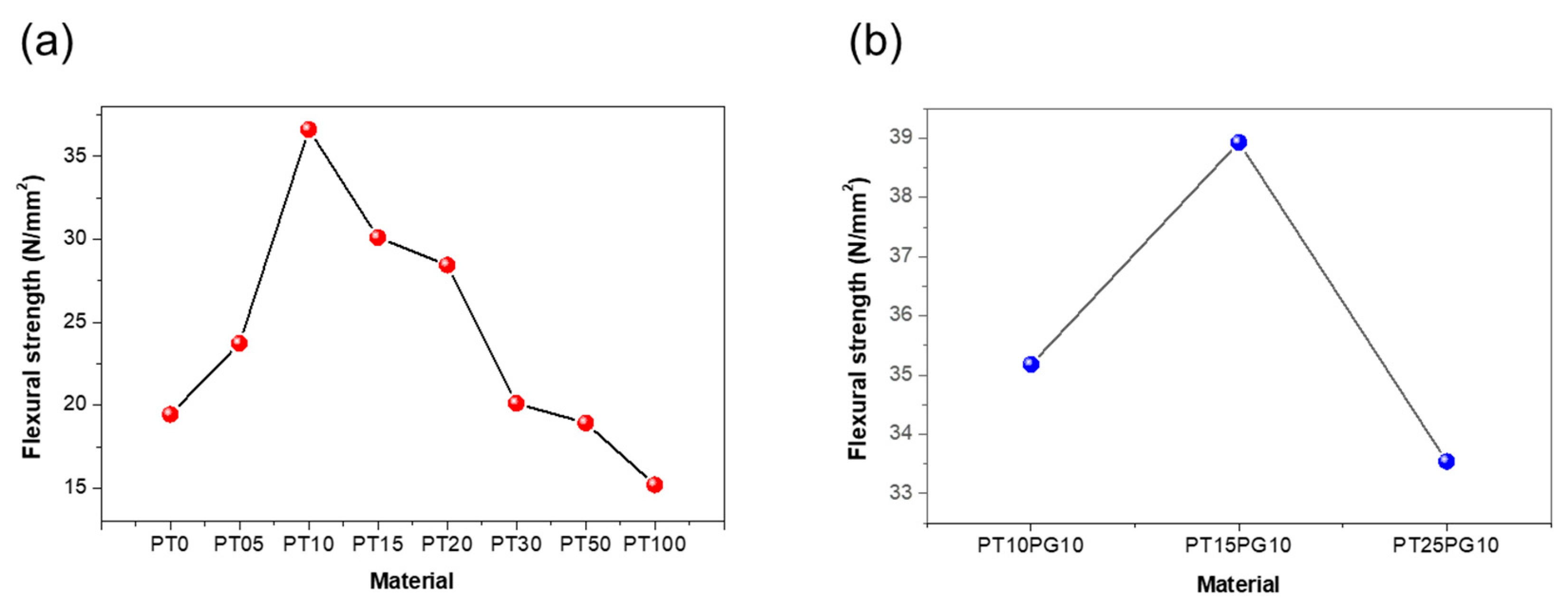
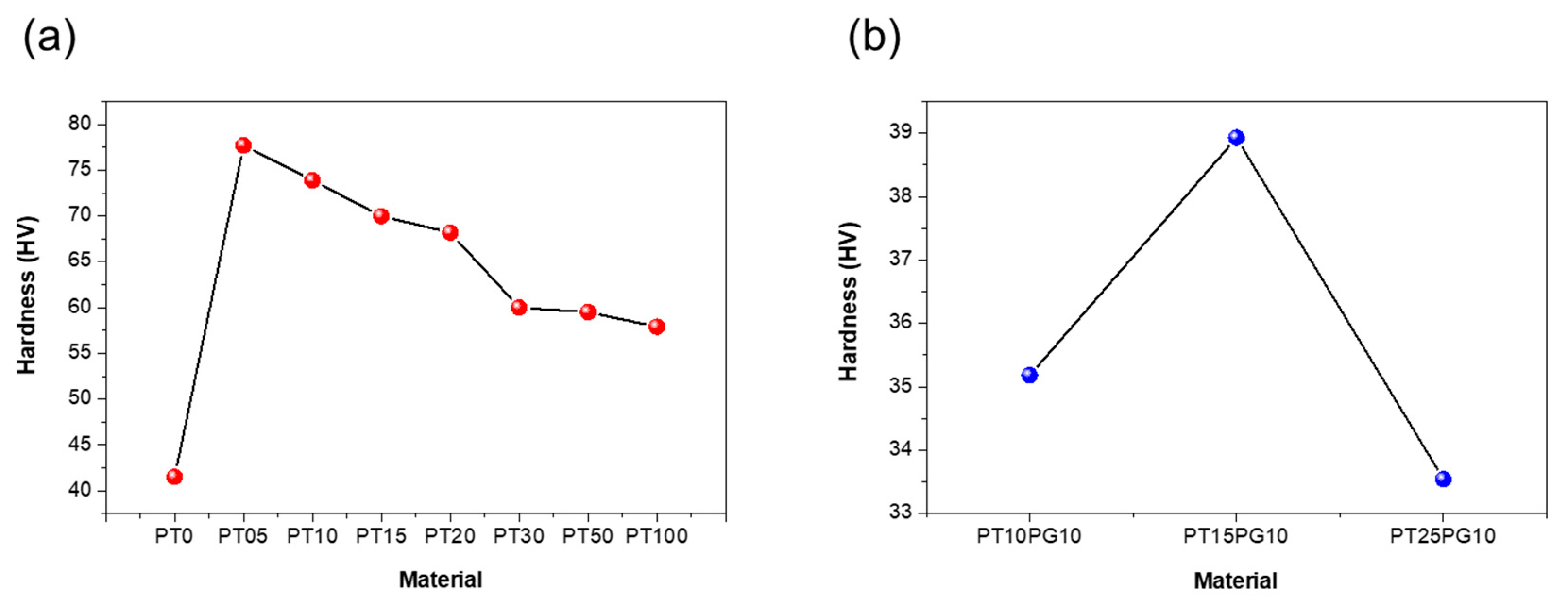
3.2. Mechanical properties of potassium titanate/acrylate resin using ceramic-based additive
3.3. 3D Printing of potassium titanate/acrylate resin using ceramic-based additive
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chemical Reviews 2017, 117, 10212-10290. [CrossRef]
- Jain, A.; Bansal, K.K.; Tiwari, A.; Rosling, A.; Rosenholm, J.M. Role of Polymers in 3D Printing Technology for Drug Delivery - An Overview. Curr Pharm Des 2018, 24, 4979-4990. [CrossRef]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chemical Reviews 2016, 116, 1496-1539. [CrossRef]
- Wendel, B.; Rietzel, D.; Kühnlein, F.; Feulner, R.; Hülder, G.; Schmachtenberg, E. Additive Processing of Polymers. Macromolecular Materials and Engineering 2008, 293, 799-809. [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Business Horizons 2012, 55, 155-162. [CrossRef]
- Kwon, I.K.; Matsuda, T. Photo-polymerized microarchitectural constructs prepared by microstereolithography (muSL) using liquid acrylate-end-capped trimethylene carbonate-based prepolymers. Biomaterials 2005, 26, 1675-1684. [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121-6130. [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Advanced Materials 2018, 30, 1706344. [CrossRef]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems.
- Harynska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing-Characterization and Fabrication. Materials (Basel) 2019, 12, 887. [CrossRef]
- Ivanova, O.; Williams, C.; Campbell, T. Additive manufacturing (AM) and nanotechnology: promises and challenges. Rapid Prototyping Journal 2013, 19, 353-364. [CrossRef]
- Ullett, J.S.; Schultz, J.W.; Chartoff, R.P. Novel liquid crystal resins for stereolithography - processing parameters and mechanical analysis. Rapid Prototyping Journal 2000, 6, 8-17. [CrossRef]
- Vaezi, M.R.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. The International Journal of Advanced Manufacturing Technology 2013, 67, 1721-1754. [CrossRef]
- Revilla-León, M.; Özcan, M. Additive Manufacturing Technologies Used for Processing Polymers: Current Status and Potential Application in Prosthetic Dentistry: Polymer Additive Manufacturing for Prosthodontics. Journal of Prosthodontics 2018, 28, 146-158. [CrossRef]
- Hector Sandoval, J.; Wicker, R.B. Functionalizing stereolithography resins: effects of dispersed multi-walled carbon nanotubes on physical properties. Rapid Prototyping Journal 2006, 12, 292-303. [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Additive Manufacturing 2018, 20, 44-67. [CrossRef]
- Shukla, V.; Bajpai, M.; Singh, D.K.; Singh, M.; Shukla, R. Review of basic chemistry of UV-curing technology. Pigment & Resin Technology 2004, 33, 272-279. [CrossRef]
- Cornelio, R.B.; Wikant, A.; Mjosund, H.; Kopperud, H.M.; Haasum, J.; Gedde, U.W.; Ortengren, U.T. The influence of bis-EMA vs bis GMA on the degree of conversion and water susceptibility of experimental composite materials. Acta Odontol Scand 2014, 72, 440-447. [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Advanced Engineering Materials 2014, 16, 729-754. [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds.
- Kim, G.-T.; Go, H.-B.; Yu, J.-H.; Yang, S.-Y.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Cytotoxicity, Colour Stability and Dimensional Accuracy of 3D Printing Resin with Three Different Photoinitiators. Polymers 2022, 14, 979. [CrossRef]
- Zhang, J.; Launay, K.; Hill, N.S.; Zhu, D.; Cox, N.; Langley, J.; Lalevée, J.; Stenzel, M.H.; Coote, M.L.; Xiao, P. Disubstituted Aminoanthraquinone-Based Photoinitiators for Free Radical Polymerization and Fast 3D Printing under Visible Light. Macromolecules 2018, 51, 10104-10112. [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: advances in photopolymerizations initiated by thioxanthones. Polymer Chemistry 2015, 6, 6595-6615. [CrossRef]
- Meereis, C.T.; Leal, F.B.; Lima, G.S.; de Carvalho, R.V.; Piva, E.; Ogliari, F.A. BAPO as an alternative photoinitiator for the radical polymerization of dental resins.
- Zhang, K.; Xie, C.; Wang, G.; He, R.; Ding, G.; Wang, M.; Dai, D.; Fang, D. High solid loading, low viscosity photosensitive Al2O3 slurry for stereolithography based additive manufacturing. Ceramics International 2019, 45, 203-208. [CrossRef]
- Ding, G.; He, R.; Zhang, K.; Xia, M.; Feng, C.; Fang, D. Dispersion and stability of SiC ceramic slurry for stereolithography. Ceramics International 2020, 46, 4720-4729. [CrossRef]
- Lemon, M.T.; Jones, M.S.; Stansbury, J.W. Hydrogen bonding interactions in methacrylate monomers and polymers. J Biomed Mater Res A 2007, 83, 734-746. [CrossRef]
- Crivello, J.V.; Reichmanis, E. Photopolymer Materials and Processes for Advanced Technologies. Chemistry of Materials 2014, 26, 533-548. [CrossRef]
- Ferracane, J.L. Resin composite--state of the art. Dent Mater 2011, 27, 29-38. [CrossRef]
- Song, Y.; Zheng, Y.L.; Tang, Y.F.; Yang, H.B. Fabrication and Stability of CoAl2O4 Ceramic Pigment for 3D Printing. Materials Science Forum 2017, 898, 1935-1939. [CrossRef]
- Chung, K.; Greener, E.H. Degree of conversion of seven visible light-cured posterior composites. J Oral Rehabil 1988, 15, 555-560. [CrossRef]
- dos Santos, G.B.; Alto, R.V.; Filho, H.R.; da Silva, E.M.; Fellows, C.E. Light transmission on dental resin composites. Dent Mater 2008, 24, 571-576. [CrossRef]
- Zhang, X.; Wu, X.; Shi, J. Additive manufacturing of zirconia ceramics: a state-of-the-art review. Journal of Materials Research and Technology 2020, 9, 9029-9048. [CrossRef]
- Qin, W.; Majidi, H.; Yun, J.; van Benthem, K. Electrode Effects on Microstructure Formation During FLASH Sintering of Yttrium-Stabilized Zirconia. Journal of the American Ceramic Society 2016, 99, 2253-2259. [CrossRef]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. Journal of Dentistry 2007, 35, 819-826. [CrossRef]
- Suominen, J.M.; Frankberg, E.J.; Vallittu, P.K.; Levänen, E.; Vihinen, J.; Vastamäki, T.; Kari, R.; Lassila, L.V.J. Three-dimensional printing of zirconia: characterization of early stage material properties. Biomaterial Investigations in Dentistry 2019, 6, 23-31. [CrossRef]
- Wesemann, C.; Spies, B.C.; Sterzenbach, G.; Beuer, F.; Kohal, R.; Wemken, G.; Krügel, M.; Pieralli, S. Polymers for conventional, subtractive, and additive manufacturing of occlusal devices differ in hardness and flexural properties but not in wear resistance. Dent Mater 2021, 37, 432-442. [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1-25. [CrossRef]
- Alarifi, I.M. A performance evaluation study of 3d printed nylon/glass fiber and nylon/carbon fiber composite materials. Journal of Materials Research and Technology 2022, 21, 884-892. [CrossRef]
- Marchand, R.; Brohan, L.; Tournoux, M. TiO2(B) a new form of titanium dioxide and the potassium octatitanate K2Ti8O17. Materials Research Bulletin 1980, 15, 1129-1133. [CrossRef]
- Suganuma, K.; Fujita, T.; Niihara, K.; Suzuki, N. AA6061 composite reinforced with potassium titanate whisker. Journal of Materials Science Letters 1989, 8, 808-810. [CrossRef]
- Minjie Qu, X.J.W.H.E.G.L. Performance of Potassium Titanate Whisker Reinforced PPESK Composites. Journal of Materials Sciences and Technology 2009, 20, 445-447.
- Jiang, W.; Tjong, S.C. Thermal stability of polycarbonate composites reinforced with potassium titanate whiskers: effect of coupling agent addition. Polymer Degradation and Stability 1999, 66, 241-246. [CrossRef]
- Song, S.Y.; Park, M.S.; Lee, D.; Lee, J.W.; Yun, J.S. Optimization and characterization of high-viscosity ZrO2 ceramic nanocomposite resins for supportless stereolithography. Materials & Design 2019, 180, 107960. [CrossRef]
- Faes, M.; Vleugels, J.; Vogeler, F.; Ferraris, E. Extrusion-based additive manufacturing of ZrO2 using photoinitiated polymerization. CIRP Journal of Manufacturing Science and Technology 2016, 14, 28-34. [CrossRef]
- Lian, Q.; Sui, W.; Wu, X.; Yang, F.; Yang, S. Additive manufacturing of ZrO ceramic dental bridges by stereolithography. Rapid Prototyping Journal 2018, 24, 114-119. [CrossRef]
- Jl, F.; EH, G. Fourier transform infrared analysis of degree of polymerization in unfilled resins--methods comparison. J Dent Res 1984, 63, 1093-1095. [CrossRef]
- Pelka, M.; Danzl, C.; Distler, W.; Petschelt, A. A new screening test for toxicity testing of dental materials. Journal of Dentistry 2000, 28, 341-345. [CrossRef]
- Goncalves, F.; Campos, L.M.P.; Rodrigues-Junior, E.C.; Costa, F.V.; Marques, P.A.; Francci, C.E.; Braga, R.R.; Boaro, L.C.C. A comparative study of bulk-fill composites: degree of conversion, post-gel shrinkage and cytotoxicity. Braz Oral Res 2018, 32, e17. [CrossRef]
- Warr, C.; Valdoz, J.C.; Bickham, B.P.; Knight, C.J.; Franks, N.A.; Chartrand, N.; Van Ry, P.M.; Christensen, K.A.; Nordin, G.P.; Cook, A.D. Biocompatible PEGDA Resin for 3D Printing. ACS Applied Bio Materials 2020, 3, 2239-2244. [CrossRef]
- Lin, C.-H.; Lin, Y.-M.; Lai, Y.-L.; Lee, S.-Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. The Journal of Prosthetic Dentistry 2020, 123, 349-354. [CrossRef]
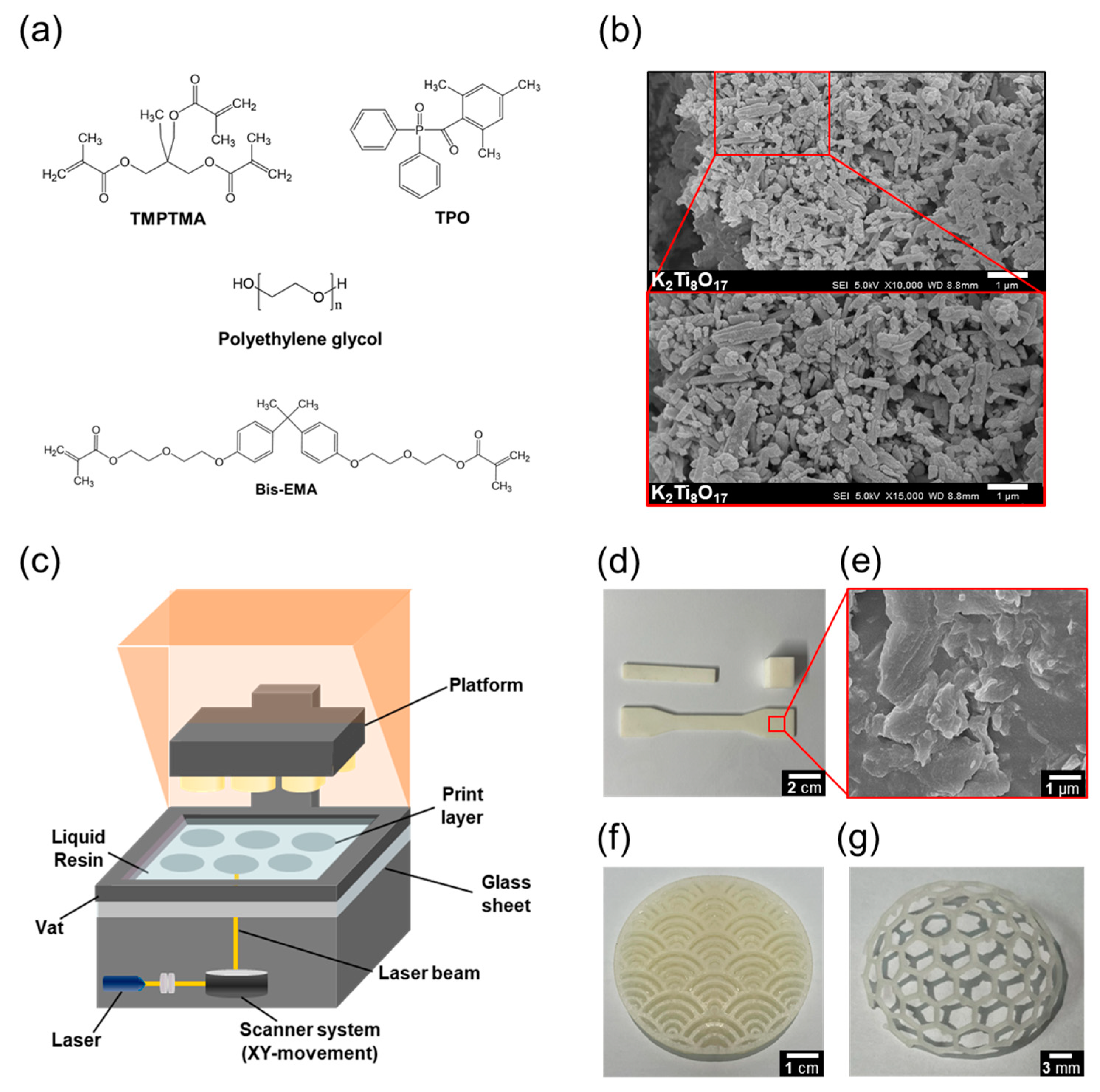
| Name | Composition (wt%) | Viscosity (cp) | ||
|---|---|---|---|---|
| Resin | PEG | Potassium titanate | ||
| PT0 | 100 | 0 | 0 | 975 |
| PT05 | 99.5 | 0 | 0.5 | 1025 |
| PT10 | 99 | 0 | 1.0 | 1231 |
| PT15 | 98.5 | 0 | 1.5 | 1375 |
| PT20 | 98 | 0 | 2.0 | 1452 |
| PT30 | 97 | 0 | 3.0 | 1532 |
| PT50 | 95 | 0 | 5.0 | 1922 |
| PT100 | 90 | 0 | 10.0 | 3202 |
| PT10PG10 | 89 | 10 | 1.0 | 1201 |
| PT15PG10 | 88.5 | 10 | 1.5 | 1289 |
| PT25PG10 | 87.5 | 10 | 2.5 | 1422 |
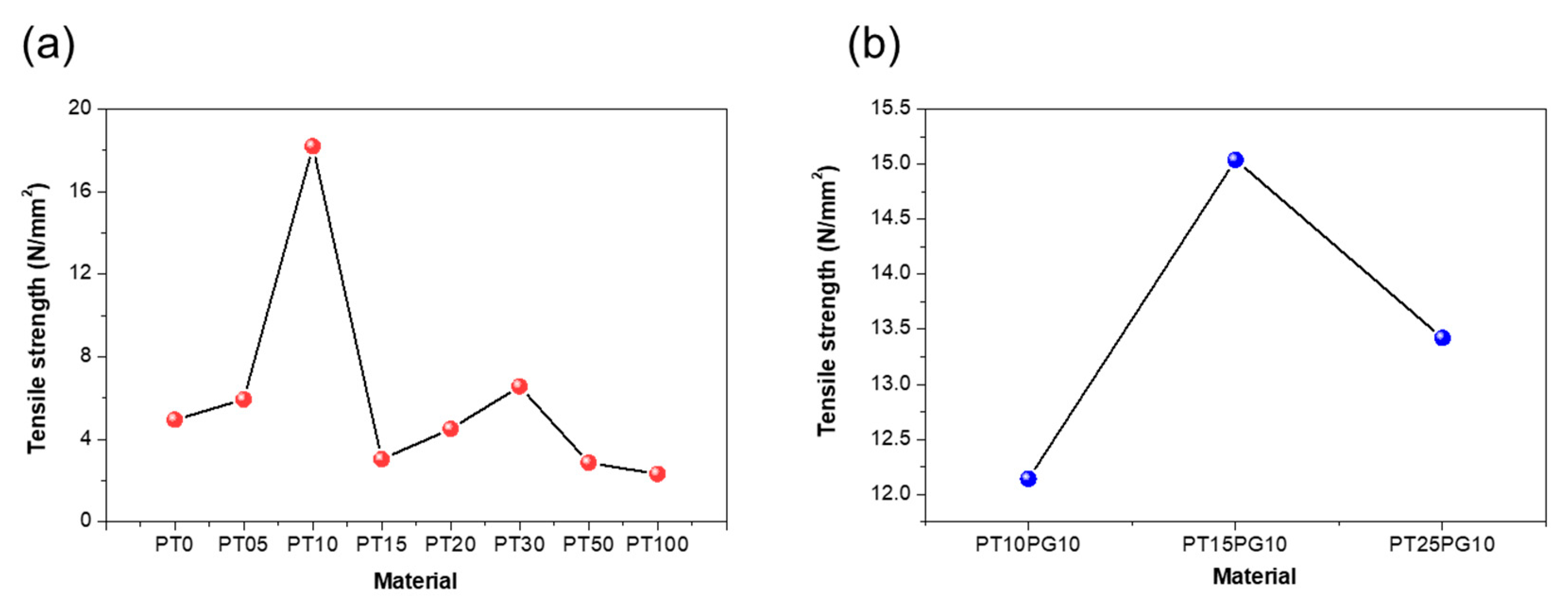
| Name | Flexural strength (N/mm2) |
Hardness (HV) |
Tensile strength (N/mm2) |
|---|---|---|---|
| PT0 | 19.45 | 41.5 | 4.95 |
| PT05 | 23.74 | 77.7 | 5.93 |
| PT10 | 36.63 | 73.9 | 18.2 |
| PT15 | 30.12 | 70.0 | 3.03 |
| PT20 | 28.45 | 68.2 | 4.51 |
| PT30 | 20.11 | 60.0 | 6.56 |
| PT50 | 18.94 | 59.5 | 2.86 |
| PT100 | 15.21 | 57.9 | 2.32 |
| PT10PG10 | 35.18 | 73.0 | 12.14 |
| PT15PG10 | 38.93 | 79.0 | 15.04 |
| PT25PG10 | 33.54 | 69.0 | 13.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





