1. Introduction
The genus
Eucalyptus encompasses approximately 900 species and subspecies distributed worldwide [
1]. Members of this genus are multipurpose trees cultivated for their ornamental characteristics, timber production, and cut foliage [
2].
Eucalyptus Leaves, a by-product of tree cutting, are particularly rich in essential oil that has been attributed antioxidant, antimicrobial, repellent, insecticidal, herbicidal and nematicidal activities, among others [
1,
3,
4]. Due to their numerous biological activities,
Eucalyptus oils are widely used in various industrial sectors, including cosmeceuticals, fragrances, foods, pharmaceuticals, agrochemicals, and household products [
5]. They are also used in different traditional medicine systems to treat colds, cough, influenza, sore throat, and sinus congestion [
6]. Recent applications of
Eucalyptus essential oils include the treatment of gastrointestinal disorders (diarrhea, colic, and dysentery) and respiratory diseases (asthma, laryngitis, trachealgia, and pharyngitis) in addition to its anti-inflammatory, wound healing, analgesic, anti-nociceptive, cytotoxic, and anti-diabetic properties [
7].
Because of these intriguing activities and relevant applications, the main representing species of the genus
Eucalyptus are extensively studied for the essential oil composition of their foliage. Previous phytochemical investigations pointed to the presence of common compounds including oxygenated monoterpenes (1,8-cineole, citronellol, piperitone, isopulegol, citronellal, α-terpineol, linalool, terpinyl acetate, citronellyl acetate, etc.), monoterpene hydrocarbons (α-, β-pinene, p-cymene, limonene, camphene, γ-terpinene, etc.), oxygenated sesquiterpenes (spathulenol, caryophyllene oxide, etc.) and sesquiterpene hydrocarbons (β-caryophyllene, aromadendrene, α-copaene, bicyclogermacrene, etc.) [
1,
7,
8,
9].
However, Eucalyptus essential oils' quality and subsequent biological activities are somewhat variable, depending on plant species/subspecies, origin, season, organ, extraction and analytical conditions. Consequently, different chemotypes within different populations of the same species have been described [
1]. Given their wide medicinal, agronomic and industrial applications, analysis of Eucalyptus essential oils and their chemodiversity is paramount to defining potential applications and drawing the best strategy for their conservation and naturalization.
In Tunisia, the genus Eucalyptus is represented by 117 species naturalized into 30 arboretums [
10]. Most of them are cultivated as ornamental and honey trees, and for timber and firewood production. In folk medicine, the Eucalyptus leaves are used to treat colds, coughs and respiratory disorders, namely pharyngitis, bronchitis and sinusitis [
9]. Earlier compositional studies reported interspecific variations in essential oil composition and its biological activities [
9,
10,
11,
12,
13,
14]. However, most of these studies are focused on particular species such as
E. camaldulensis and
E. globulus [
10,
12,
13,
14] and little is known about the remaining species. The main objective of this present study was to determine the essential oil composition of different Eucalyptus species and their chemodiversity. Antimicrobial activity was also assessed.
2. Results and discussion
2.1. Yields and chemical composition of essential oils
From the leaves, pale yellowish essential oils with average yields of 0.96, 0.35, 0.55, 0.32, and 0.14 % (w/w) were obtained for
E. astringens,
E. camaldulensis,
E. lehmannii,
E. leucoxylon and
E. sideroxylon, respectively (
Table 1). These values match those reported for
E. oleosa [16],
E. camaldulensis,
E. saligna [
1],
E. gomphocornuta, E. paniculata [
8],
E. bosistoana, E. mellidiora, E. odorata, and
E. paniculata [17], but they are considerably lower than those observed in
E. globulus, E. cinerea, E. citriodora [
1],
E. accedens, E. cladoalyx, E. lesouefi, E. melliodora, E. punctate, E. robusta, E. wandoo [
9],
E. melliodora and E. maidenii, among others [
8]. These discrepancies could be attributed to genetic factors, pedo-climatic conditions, season, plant age, processing and extraction methodology. In our case, differences in the essential oil yields were unequivocally attributed to genetic differences (species) as they were cultivated and processed under the same conditions.
The chromatographic analysis identified 48 components covering more than 90% of the total peak area. Irrespective of
Eucalyptus species, all oil samples are terpenoid-rich essential oils. Terpenoids (oxygenated mono-, and sesquiterpenes) are particularly abundant in
E. lehmanii (71.67%),
E. leucoxylon (70.57%) and
E. sideroxylon (76.74%). The oxygenated monoterpene 1,8-cineole (eucalyptol) was by far the major component (22-51%) in all investigated essential oils. Therefore, the studied
Eucalyptus species could be categorized as 1,8-cineole chemotype. Other significant compounds, including aromadendrene, globulol, pinoarvone and α-pinene were identified in
E. astringens. Aromadendrene and globulol were also detected in appreciable amounts in
E. sideroxylon. In the E. camaldulensis essential oil, spathulenol and p-cymene were abundant. The essential oil of
E. lehmannii had the highest percentages of α-pinene, α-terpineol and terpinyl acetate. The monoterpene hydrocarbons α-pinene, terpinolene, p-cymene, and the oxygenated sesquiterpene globulol were the most plentiful components in the essential oil of E. leucoxylon. Compared with earlier compositional studies, the pattern of abundance of the main compounds was reported in
Eucalyptus sp. For example, the profile 1,8-cineole> α-pinene has been previously described in the Tunisian specimens of
E. leucoxylon [
11],
E. lehmanii and
E. astringens [
8]. In contrast, the latter authors reported that spathulenol and o-cymene were dominants in the essential oil of E. camaldulensis. At this point, it can be inferred that this species represents different chemotypes. For instance, The p-cymene/1,8-cineole chemotype has been described in Turkish specimens of
E. camaldulensis [
6].
Other chemotypes, including 1,8-cineole/p-cymene [18]; 1,8-cineole/limonene [19]; 1,8-cineole/α-pinene [20,21]; α-phellandrene/β-pinene [22]; Linalool/1,8-cineole [23]; spathulenol/p-cymene [24] and α-pinene/ p-cymene [25] have been reported in the
E. camaldulensis specimens from Argentina, Brazil, Egypt, Tunisia, India, Pakistan, Spain and Taiwan. The α-pinene/1,8-cineole chemotype has been recorded for Tunisian
E. astringens [
13] and
E. leucoxylon [
10,21] specimens. Regarding
E. sideroxylon from the same origin, the presence of at least two chemotypes, 1,8-cineole/globulol (this study) and 1,8-cineole/α-pinene [
11] may be confirmed. In contrast, it seems that the 1,8-cineole/α-pinene chemotype dominated the leaf essential oil of
E. lehmannii species [
8,
11,
12,
13]. In general, it appeared that the chemical composition of the essential oil of
Eucalyptus sp. is particularly prone to qualitative and quantitative changes depending on genetics (species, subspecies, and cultivars), season, climate, soil type and agricultural practices. Given their industrial importance as a source of essential oil, a better categorization of
Eucalyptus species based on the definition of some specific distinctive volatile markers will be of great importance for authentication purposes.
2.2. Specific markers in Eucalyptus essential oils
A compound was designed as a marker if its presence or absence was confirmed in all samples from the same geographic origin [26,27]. Based on this concept, a list of chemically defined volatile markers has been established (
Table 2). As shown, the presence of γ-maaliene and γ-gurjunene is the apanage of the essential oil of
E. astringens. Dehydrosabinene, 4-carene, p-cymenene, 2-methyl-3-phenyl-propanal, phellandral, thymol, p-cymen-7-ol, and copaborneol distinguished the essential oil of
E. camaldulensis.
The presence of camphene, α-campholenal, carvacrol, α-terpinyl acetate versus the absence of isocarveol, rosifoliol and humulene epoxide II ruled out the essential oil of E. Lehmanii from the remaining species. The essential oil of E. leucoxylon was typified by the presence of β-pinene, β-myrcene, terpinolene and piperitone, while it was exempted from viridiflorene. The absence of borneol seems be characteristic of the essential oil of E. sideroxylon. From a practical standpoint, the aforementioned chemical markers could provide baseline information for the quality assessment of the commercialized leaf essential oils of Eucalyptus species growing in the region of Korbous.
2.3. Principal component analysis (PCA)
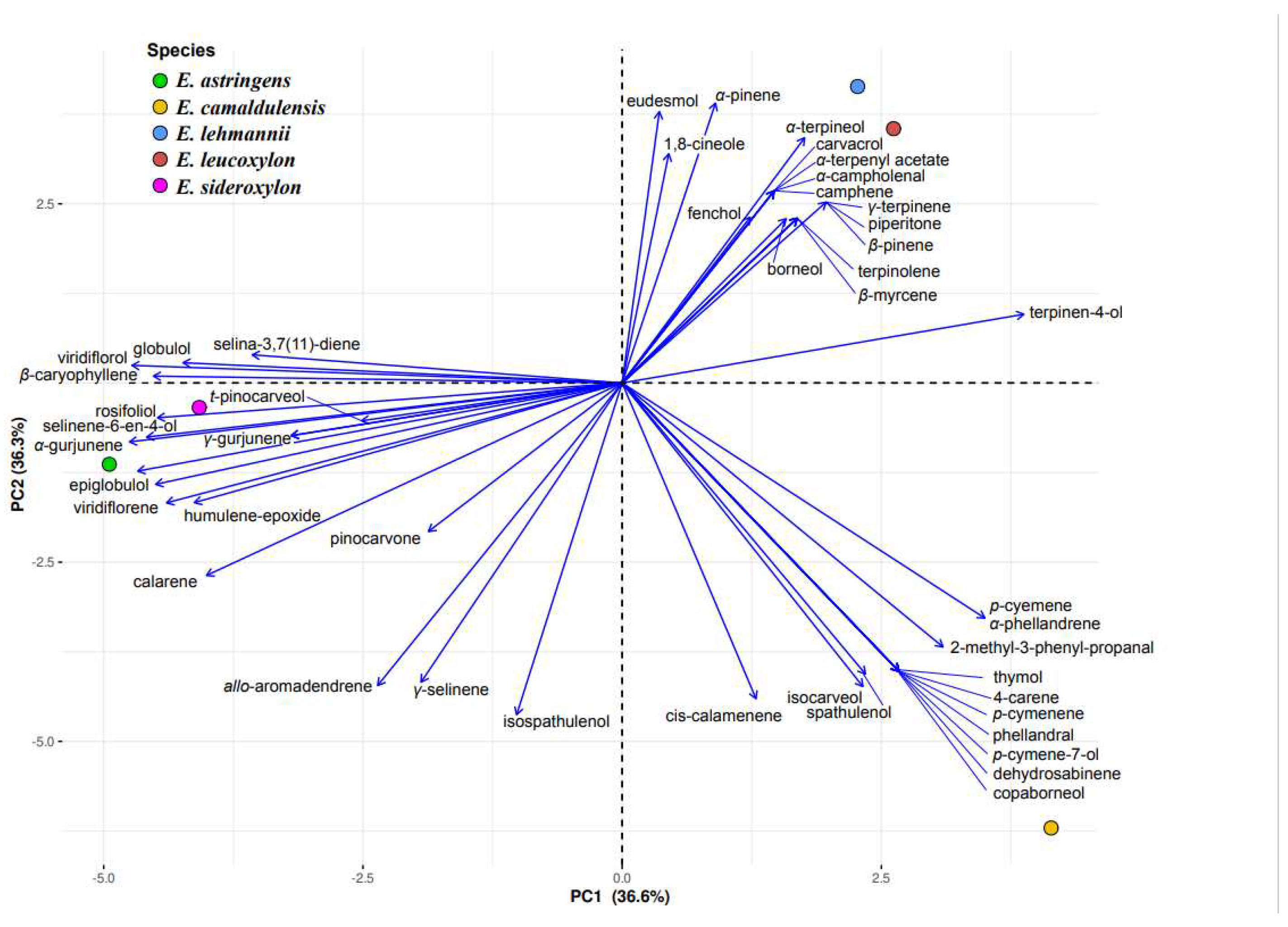
In order to validate our chemical marker selection and confirm the interspecific chemical polymorphism, a PCA analysis (
Figure 1) based on the entire volatile profile was performed. From the PCA biplot, which explains 73.3% of the total variance (36.6 and 36.3% for Dim 1 and Dim 2, respectively), three distinctive groups can be easily distinguished. The first group pertaining to essential oils of
E. astringens and
E. sideroxylon exhibiting similar profiles that consist of aromadendrene, globulol, viridiflorene, viridiflorol, rosifoliol, selina-6-en-4-ol, humulene epoxide II, α-gurjunene, and β-caryophyllene. The second group brings together
E. lehmannii and
E. leucoxylon with high amounts of α-pinene, γ-terpinene and α-terpineol. The
E. camaldulensis species was clearly separated from the other species owing to its high content of spathulenol, cis-calamenene, isocarveol, and p-cymene, in addition to its marker components listed above. Taking into account that all
Eucalyptus species of the same age and cultivated and processed under the same conditions (i.e,. collection of leaves, drying, extraction of essential oils and their analysis), the genetic dissimilarity between
Eucalyptus spp. was confirmed again based on their essential oil composition.
Considering that the bioactivity of an essential oil is primarily determined by its chemical composition, it will be of great significance to evaluate the antimicrobial activity of Eucalyptus spp.
2.3. Antimicrobial activity of Eucalyptus spp. Essential oils
Results of the antimicrobial activity of the five
Eucalyptus essential oils were summarized in
Table 3. All essential oils strongly inhibited the growth of all the tested strains with the gram-positive S. aureus (diameter of inhibition zone 25-35 mm) and E. faecium (diameter of inhibition zone 29.5-34.5 mm) strains being the most sensitive. They were particularly inhibited by essential oils of
E. camaldulensis,
E. lehmannii and
E. sideroxylon. The essential oils from
E. camldulensis and
E. leucoxylon were, in turn, most effective against the gram-negative bacteria
E. coli and
S. typhimurium. The former essential oil (
E. camaldulensis) was also distinguished by its high efficiency against the yeast
C. albicans with a halo of inhibition similar to that of the standard antibiotic nystatin. These results agreed with previous reports showing the potent antimicrobial activity of
Eucalyptus essential oils against the gram-positive bacterial strains, particularly
E. aureus and the yeast
C. albicans [
1]. The sensitivity of the gram-positive bacteria was attributed to the presence of a thick peptidoglycan wall associated with the lipophilic ends of lipoteichoic acid, facilitating the entry of hydrophobic components into the cell membrane [28]. Studies linking the antimicrobial activity of
Eucalyptus essential oils and their main components are abundant. In this context, it has been reported that the essential oil of
E. camaldulensis strongly inhibited the growth of the gram-positive
S. aureus and Bacillus cereus [
6].
Eucalyptus camaldulensis essential oil which was described as the most active antibiotic among
Eucalyptus species was also found to be effective against the yeast
C. albicans [29] supporting our findings. Similar results have also been reported for the essential oil derived from
E. sideroxylon [30],
E. astringens,
E. lehmanii [
8], and
E. leucoxylon [31], among others.
A direct evidence of the antimicrobial activity of the main components of Eucalyptus essential oils has also been provided. Especially actives are 1,8-cineole [32], α-pinene [33], terpinyl acetate [34,35], α-terpineol [36], globulol [37], aromdendrene [38], p-cymene [39], Spathulenol [40], trans-pinocarveol [41], and terpinen-4-ol [42], among others. These compounds’ synergistic and additive effects have also been described for the essential oil of E. globulus [38]. In their checkerboard assay, the study authors successfully shown that the combination of 1,8-cineole and aromadendrene greatly enhanced the antimicrobial effect against the methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus faecalis via an additive or synergistic interactions. Four years later, Yang et al. [43] showed that combinations involving p-cymene, terpinen-4-ol, α-terpineol, and linalool exhibited an additive antibacterial effect against some food-borne pathogens, including E. coli O157:H7, S. aureus, S. mutans, S. sanguinis, S. enterica, Lysteria monocytogenes and Vibrio parahaemolyticus. More recently, it has been shown that the combination of terpinen-4-ol and α-terpineol synergistically inhibited the growth of S. aureus, MRSA, E. coli, and Pseudomonas aeruginosa [44].
From a mechanistic standpoint, the identified components (solely or in combination) could exert their antimicrobial activity by interfering with the lipophilic core of the membrane, leading to increased fluidity and, ultimately, the leakage of vital macromolecules (e.g. nucleic acids and proteins), potassium ions and protons [45]. Other mechanisms of action include alteration of the fatty acid composition, impairment of metabolic pathways, inhibition of cellular respiratory chain with a concomitant interruption of oxidative phosphorylation, decrease in ATP pool, interference with the glucose and oxygen uptake, denaturation of cell proteins, disruption of nucleic acid synthesis, installation of oxidative stress, and inhibition of enzyme activity have been proposed [28,36,46,47,48]. Although the exact mechanism of the antimicrobial effect of Eucalyptus essential oils was not fully understood, the implication of one or more of the mechanisms mentioned above could explain the strong antimicrobial activity of the studied Eucalyptus species. Whatever the case, these data provide evidence for the current use of their essential oils as a natural antiseptic and food preservative.
3. Materials and Methods
3.1. Plant materials
Leaf samples were taken from five specimens of 52-year-old trees of E. astringens, E. camaldulensis, E. lehmannii, E. leucoxylon and E. sideroxylon growing in the arboretum of Korbous (Northeastern Tunisia, latitude: 36°50’N, longitude: 10°23’E, Altitude: 180 m above sea level; climate: sub-humid). For each species, ten trees were selected within each plot (based on health status and size), and a branch (ca. 3–4 m high and 1 m long) was cut from each tree, handpicking 100 g of fresh matter sample of mature foliage. Leaves were dried at room temperature (20 ± 2°C), pulverized into fine powders, and examined for their essential oils composition.
3.2. Isolation of essential oils
The dried leaf samples were hydrodistilled for three hours using a Clevenger-type apparatus. The essential oil samples were dried over anhydrous sodium sulfate Na2SO4 and stored in sealed amber vials at -20°C until analyzed.
3.3. Analysis of essential oils
Samples of essential oils were diluted 20-fold in hexane and analyzed using an HP 6890 (II) gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) fitted with a HP-5 (30 m × 0.32 mm ID, 0.25µm film thickness; Supelco, Bellefonte, PA, USA) capillary column. Operating conditions were as follows: The oven temperature was programmed at 5°C/min from an initial temperature 40°C (kept isothermal for 10 min) to 280°C, which was kept for 10 min. Injector and FID detector temperature were maintained at 230°C; the injection volume was 0.5 µL; split ratio of 1:20 and the flow rate of the carrier nitrogen gas was 1.2 mL/min.
For the gas chromatography-mass spectrometry (GC-MS) analysis, an HP 6890 gas chromatograph coupled to an HP 5973 mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) was used. The analytical conditions and the column used for individual component separation were the same as used for GC-FID analysis, except helium was used as carrier gas. The mass spectrometer was operated in electron-impact (EI) mode; ionization energy, 72 eV; ion source temperature, 270°C; scan time, 1 s, and mass range, 50-550 amu.
The identification of constituents was based on the comparison of their retention indices (RI) relative to (C7-C40) n-alkanes with those of literature [
15] and/or with those of authentic standards when available, and by matching the database NIST05a MS library. The relative content of the identified components was obtained by electronic integration of the FID-peak areas without correcting for response factors.
3.4. Antimicrobial activity
From the leaves, pale yellowish essential oils with average yields of 0.96, 0.35, 0.55, 0.32, and 0.14 % (w/w) were obtained for
E. astringens,
E. camaldulensis,
E. lehmannii,
E. leucoxylon and
E. sideroxylon, respectively (
Table 1). These values match those reported for E. oleosa [
16], E. camaldulensis, E. saligna [
1],
E. gomphocornuta, E. paniculata [
8],
E. bosistoana, E. mellidiora, E. odorata, and
E. paniculata [
17], but they are considerably lower than those observed in
E. globulus, E. cinerea, E. citriodora [
1],
E. accedens, E. cladoalyx, E. lesouefi, E. melliodora, E. punctate, E. robusta, E. wandoo [
9],
E. melliodora and E. maidenii, among others [
8]. These discrepancies could be attributed to genetic factors, pedo-climatic conditions, season, plant age, processing and extraction methodology. In our case, differences in the essential oil yields were unequivocally attributed to genetic differences (species) as they were cultivated and processed under the same conditions.
3.4. Antimicrobial activity
The antimicrobial activity of Eucalyptus spp. essential oils was evaluated qualitatively using the disc-diffusion assay described by the National Committee for Clinical Laboratory Standards (NCCLS, 1997). The Gram-positive bacteria Staphylococcus aureus (ATCC 6538) and Enterococcus feacium (ATCC 19434); the Gram-negative bacteria Escherichia coli (ATCC 8739) and Salmonella Typhimurium (ATCC 14028), and the yeast Candida albicans (ATCC 10231) were used as the test microorganisms. All microorganisms were obtained from the culture collection center of the Institut National de Recherche et d’Analyse Physico-Chimique (INRAP, Sidi thabet, Tunisia). Bacterial strains were cultured in sterile Mueller Hinton agar (MHA) medium and incubated at 37°C for 24 h, while fungal strains were cultured in Sabouraud dextrose agar (SDA) at 30°C for 48 h.
Briefly, 100 µL of microbial suspension comprising 1-2 × 108 CFU/mL of bacterial cells or 1-5 × 106 CFU/mL for yeast were spread onto petri plates containing MHA or SDA culture mediums, respectively. Sterile filter paper disks (6 mm in diameter) were impregnated with 10µL of essential oil (10 mg/mL in DMSO) and placed on the inoculated plates and left to stand for 2 h at 4°C before being incubated at 37°C for 24 h for bacteria and 30°C for 48 h for yeast. The diameter of the inhibition zone was accurately measured. Ampicillin and gentamycin were used as positive controls for bacteria and yeast, respectively.
3.5. Statistical analysis
Principal component analysis (PCA) based on the whole composition of essential oils was performed to elucidate the inter-relationships between all species. Data on the antimicrobial activity was presented as mean ± SD of triplicate. All analyses were carried out using the statistical R 2.14.1 packages (Wirtschaftsuniversität Wien, Vienna, Austria).
5. Conclusions
This The compositional investigation of leaf essential oils of Eucalyptus spp. revealed a significant chemical polymorphism, which was determined genetically (species effect). The 1,8-cineole-rich essential oils were categorized by using a set of distinctive chemical markers. Differentiation between the different Eucalyptus spp. and their resulting essential oils extracted under the same conditions was achieved. This chemical identification can serve as a method for determining the oils’ specific Eucalyptus species of origin. The studied oils exhibited a strong antimicrobial activity, presumably owing to their high 1,8-cineole contents and/or other putative compounds acting synergistically or additively.
Based on these results, it can be suggested that the studied Eucalyptus spp. oils could represent candidates as natural flavors and conservators for food/feed, cosmetic, pharmaceutic, agrochemicals and used on household applications, with particular relevance for highly perishables and products susceptible to microbial contamination. Further studies discovering other new activities for Eucalyptus essential oils and details on the mechanisms of their actions should be reported.
Author Contributions
Conceptualization, H.A and K.H.; methodology, H.A., Y.M. and K.H.; software, K.H; validation, H.A., S.L and K.H.; formal analysis, H.A., Y.M. and K.H.; investigation, H.A., Y.M., S.H., W.S., S.A., and K.H.; resources, H.A., S.H. and K.H.; data curation, H.A. and K.H.; writing—original draft preparation, H.A. and K.H.; writing—review and editing, H.A., S.H., M.C., M. de H.M., W.S., S.A., S.L and K.H.; visualization, H.A., S.H.,M.C., M. de H.M., S.A., S.L. and K.H; supervision, H.A., M.C., M. de H.M. and K.H., project administration, H.A., W.S. and K.H; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable..
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors are thankful to the International Center for Agricultural Research in the Dry Areas (ICARDA) and the Livestock and Climate CGIAR Initiatives of the OneCGIAR. The opinions expressed in this work do not necessarily reflect the views of ICAR, ICARDA, or the One CGIAR. Authors are also thankful to the Researchers Supporting Project number (RSP2021/390), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical Variability and Biological Activities of Eucalyptus spp. Essential Oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef]
- Caputo, L.; Smeriglio, A.; Trombetta, D.; Cornara, L.; Trevena, G.; Valussi, M.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemical Composition and Biological Activities of the Essential Oils of Leptospermum petersonii and Eucalyptus gunnii. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Mossi, A.J.; Astolfi, V.; Kubiak, G.; Lerin, L.; Zanella, C.; Toniazzo, G.; Oliveira, D.; Devilla, I.; Cansian, R.; Restello, R. Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae). J. Sci. Food Agric. 2011, 91, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, J.C.; do Nascimento, J.E.; Jacob, R.G.; Fiorentini, Â.M.; da Silva, W.P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Dogan, G.; Kara, N.; Bagci, E.; Gur, S. Chemical Composition and Biological Activities of Leaf and Fruit Essential Oils from Eucalyptus camaldulensis. Z. Naturforsch. C 2017, 72, 483–489. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crop. Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Limam, H.; Ben Jemaa, M.; Tammar, S.; Ksibi, N.; Khammassi, S.; Jallouli, S.; Del Re, G.; Msaada, K. Variation in chemical profile of leaves essential oils from thirteen Tunisian Eucalyptus species and evaluation of their antioxidant and antibacterial properties. Ind. Crops Prod. 2020, 158, 112964. [Google Scholar] [CrossRef]
- Ameur, E.; Sarra, M.; Yosra, D.; Mariem, K.; Nabil, A.; Lynen, F.; Larbi, K.M. Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complement. Med. Ther. 2021, 21, 209. [Google Scholar]
- Jemaa, J.M.B.; Haouel, S.; Bouaziz, M.; Khouja, M.L. Seasonal variations in chemical composition and fumigant activity of five Eucalyptus EOs against three moth pests of stored dates in Tunisia. J. Stored Prod. Res. 2012, 48, 61–67. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Chemical composition of 8 Eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12, 1. [Google Scholar] [CrossRef]
- Slimane, B.B.; Ezzine, O.; Dhahri, S.; Jamaa, M.L.B. Essential oils from two Eucalyptus from Tunisia and their insecticidal action on Orgyia trigotephras (Lepidotera, Lymantriidae). Biol. Res. 2014, 47, 29. [Google Scholar] [CrossRef]
- Hamdi, S.H.; Hedjal-Chebheb, M.; Kellouche, A.; Khouja, M.L.; Boudabous, A.; Jemaa, J.M.B. Management of three pests’ population strains from Tunisia and Algeria using Eucalyptus essential oils. Ind. Crops Prod. 2015, 74, 551–556. [Google Scholar] [CrossRef]
- Yangui, I.; Zouaoui Boutiti, M.; Boussaid, M.; Messaoud, C. Essential Oils of Myrtaceae Species Growing Wild in Tunisia: Chemical Variability and Antifungal Activity Against Biscogniauxia mediterranea, the Causative Agent of Charcoal Canker. Chem. Biodivers. 2017, 14, e1700058. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography-mass spectroscopy. J. Am. Soc. Mass Spectrom. 2001, 6, 25–86. [Google Scholar]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Khouja, M.L.; Bouajila, J. Eucalyptus oleosa Essential Oils: Chemical Composition and Antimicrobial and Antioxidant Activities of the Oils from Different Plant Parts (Stems, Leaves, Flowers and Fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Kouki, H.; Polito, F.; De Martino, L.; Mabrouk, Y.; Hamrouni, L.; Amri, I.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemistry and bioactivities of six Tunisian Eucalyptus species. Pharmaceuticals 2022, 15, 1265. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Licastro, S.; Zerba, E.; Gonzalez, A.P.; Masuh, H. Sensitivity of Aedes aegypti adults (Diptera: Culicidae) to the vapors of Eucalyptus EOs. Bioresour. Technol. 2009, 100, 6083–6087. [Google Scholar] [CrossRef]
- Batista-Pereira, L.G.; Fernandes, J.B.; Silva, M.F.G.F.; Vieira, P.C.; Bueno, O.C.; Correêa, A.G. Electrophysiological responses of Atta sexdens rubropilosa workers to EOs of Eucalyptus and its chemical composition. Z. Naturforsch. 2006, 61, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Abo Elgat, W.A.A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeterior. Biodegrad. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Jemaa, J.M.B.; Haouel, S.; Khouja, M.L. Efficacy of Eucalyptus EOs fumigant control against Ectomyelois ceratoniae (Lepidoptera: Pyralidae) under various space occupation conditions. J. Stored Prod. Res. 2013, 53, 67–71. [Google Scholar] [CrossRef]
- Debbarma, J.; Kishore, P.; Nayak, B.B.; Kannuchamy, N.; Gudipati, V. Antibacterial activity of ginger, Eucalyptus and sweet orange peel EOs on fish-borne bacteria. J. Food Process. Preserv. 2013, 37, 1022–1030. [Google Scholar] [CrossRef]
- Ghaffar, A.; Yameen, M.; Kiran, S.; Kamal, S.; Jalal, F.; Munir, B.; Saleem, S.; Rafiq, N.; Ahmad, A.; Saba, I.; et al. Chemical composition and in-vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules 2015, 20, 20487–20498. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Blazquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus EOs in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Cheng, S.S.; Huang, C.G.; Chen, Y.J.; Yu, J.J.; Chen, W.J.; Chang, S.T. Chemical compositions and larvicidal activities of leaf EOs from two Eucalyptus species. Bioresour. Technol. 2009, 100, 452–456. [Google Scholar] [CrossRef]
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Ashour, H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2008, 7, 399–403. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Chemical composition of 8 Eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Song, X.; Fang, C.; Xing, R.; Liu, L.; Zhao, X.; Zou, Y.; Li, L.; Jia, R.; Ye, G.; Shi, F.; Zhou, X.; Zhang, Y.; Wan, H.; Wei, Q.; Yin, Z. 1,8-Cineole Inhibits Biofilm Formation and Bacterial Pathogenicity by Suppressing luxS Gene Expression in Escherichia coli. Front. Pharmacol. 2022, 13, 988245. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, antimicrobial evaluation, and structure-activity relationship of alpha-pinene derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552. [Google Scholar] [CrossRef]
- Fidan, H., stefanova, G., Kostova, I., Stankov, S., Damyanova, S., Stoyanova, A., Zhzljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [CrossRef]
- Badr, M.M.; Badawy, M.E.I.; Taktak, N.E.M. Characterization, antimicrobial activity, and antioxidant activity of the nanoemulsions of Lavandula spica essential oil and its main monoterpenes. J. Drug Deliv. Sci. Technol. 2021, 65, 102732. [Google Scholar] [CrossRef]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014, 45, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus labill. Nat. Prod. Res. 2008, 22, 569–575. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.M.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Dzul-Beh, A.d.J.; García-Sosa, K.; Uc-Cachón, A.H.; Bórquez, J.; Loyola, L.A.; Barrios-García, H.B.; Peña-Rodríguez, L.M.; Molina-Salinas, G.M. In vitro growth inhibition and bactericidal activity of spathulenol against drug-resistant clinical isolates of Mycobacterium tuberculosis. Rev. Bras. Farmacogn. 2019, 29, 798–800. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Klepser, M.E.; Ernst, E.J.; Keele, D.; Roling, E.; Van Vuuren, S.; Demirci, B.; Baser, K.F.C.; van Wyk, B.-E.; Jäger, A.K. The composition and antimicrobial activity of the essential oil of the resurrection plant Myrothamnus flabellifolius. S. Afr. J. Bot. 2002, 68, 100–105. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-Ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Johansen, B.; Duval, R.E.; Sergere, J.C. First evidence of a combination of terpinen-4-ol and α-terpineol as a promising tool against ESKAPE pathogens. Molecules 2022, 27, 7472. [Google Scholar] [CrossRef] [PubMed]
- Zomorodian, K.; Moein, M.; Pakshir, K.; Karami, F.; Sabahi, Z. Chemical composition and antimicrobial activities of the essential oil from Salvia mirzayanii leaves. J. Evid. Based Complementary Altern. Med. 2017, 22, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Melkina, O.E.; Plyuta, V.A.; Khmel, I.A.; Zavilgelsky, G.B. The Mode of Action of Cyclic Monoterpenees (−)-Limoneneand (+)-α-Pinene on Bacterial Cells. BioMolecules 2021, 11, 806. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crop Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





 Absence
Absence




