Submitted:
15 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of P. emblica Loaded Wound Dressing Materials
2.2. Swelling Tests
2.3. BET Analysis
2.4. Morphology Analysis
2.5. Cell Culture
2.6. Cultivation of HaCaT Cells on Cryogels
2.7. MTT Assay
2.8. Trypan Blue Exclusion Assay
2.9. Live-Dead Staining Assay
2.10. Phase Contrast Microscopy
2.11. Giemsa Staining
2.12. Immunofluorescence Staining
2.13. SEM Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Cryogels
3.2. Swelling Studies
3.3. BET Analysis
3.4. Morphology Analysis
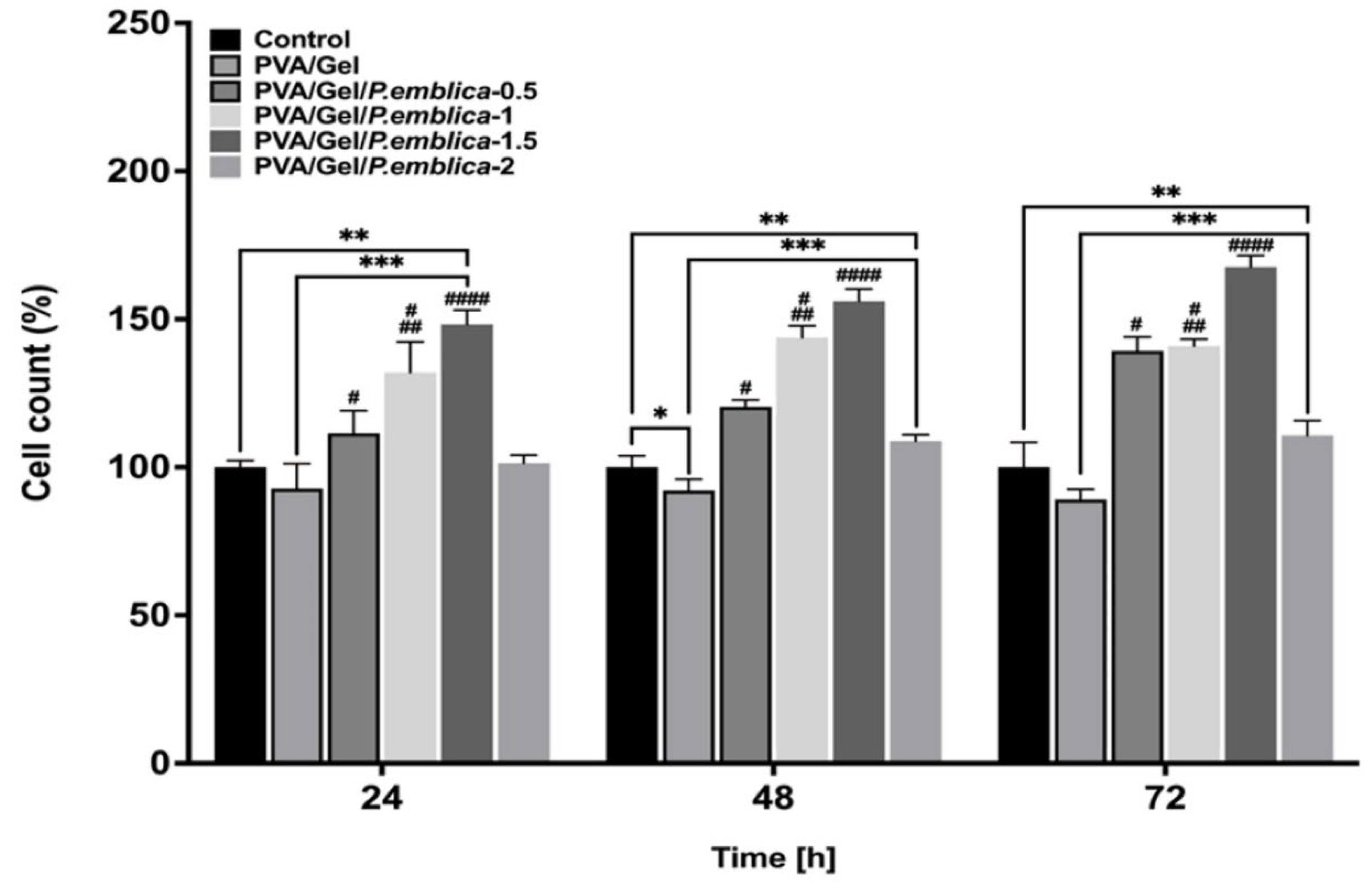
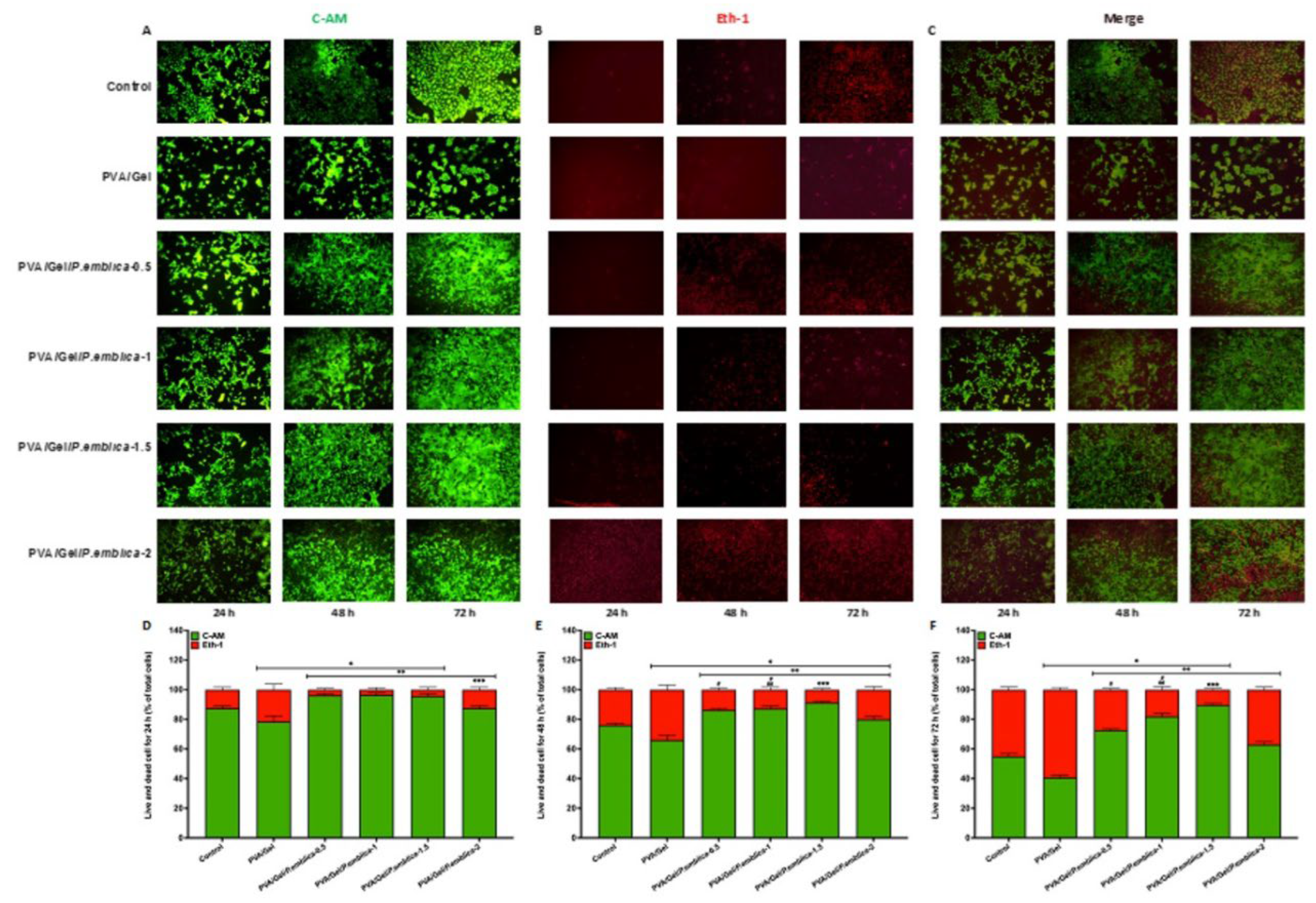
3.5. Assessment of Cell Viability of Cryogels by MTT Assay, Trypan Blue Exclusion Assay and Live/Dead Staining
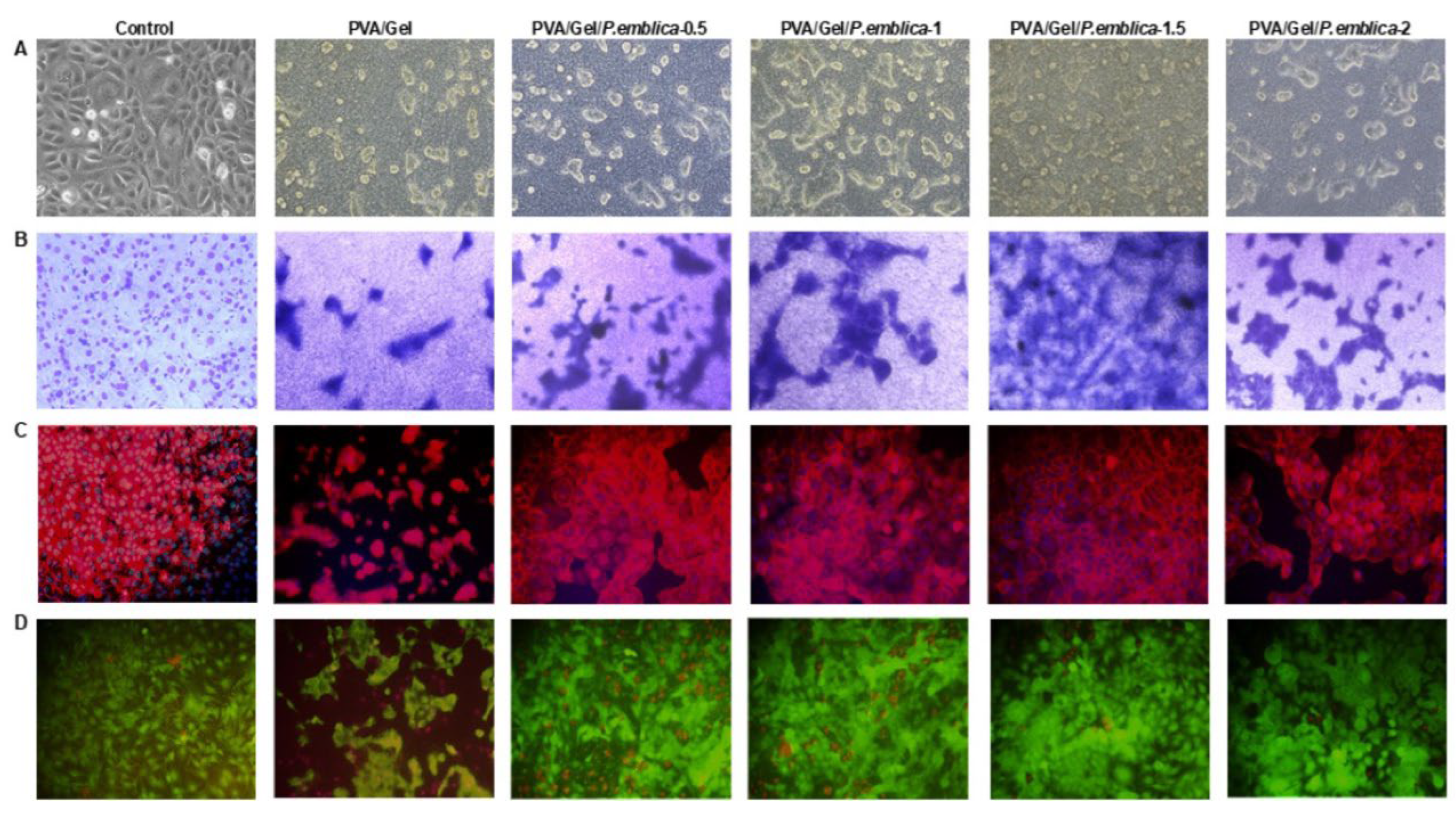
3.6. Investigation of Cell Morphology, Adhesion, Viability, Cell-Cell and Cell-Matrix Interactions on Cryogels via Phase Contrast Microscopy, Giemsa Staining, Immunofluorescence Staining and SEM
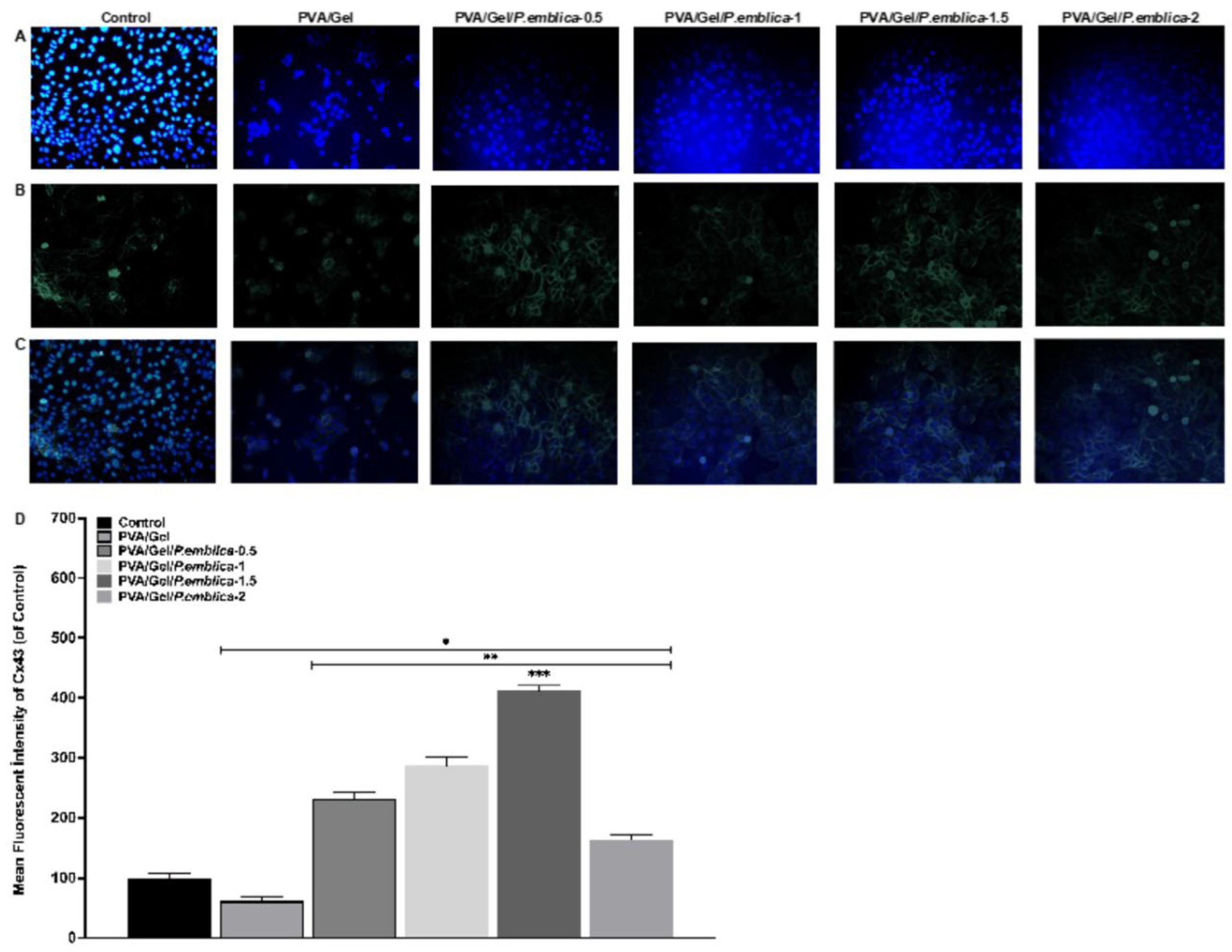
3.7. Cell adhesion and Infiltration on PVA/Gel and PVA/Gel/P.emblica Cryogels by DAPI and F-Actin Staining
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Farley A, McLafferty E, Hendry C. Cells, tissues, organs and systems. Nurs Sand. 2012;26(52):40. [CrossRef]
- Dai T, Kharkwal GB, Tanaka, M, et al. Animal models of external traumatic wound infections. Virulence. 2011;2(4):296-315. [CrossRef]
- Gobi R, Ravichandiran P, Babu R, et al. Biopolymer and synthetic polymer-based nanocomposites in wound dressing applications: a review. Polymers. 2021;13(12):1962. [CrossRef]
- Koyutürk A, Soyaslan D. Yara ve yanık tedavisinde kullanılan örtüler. MAKÜ FEBED. 2016;7(Special Issue 1): p. 58-65.
- Dabiri G, Damstetter E, Phillips T. Choosing a wound dressing based on common wound characteristics. Adv Wound Care. 2016;5(1): 32-41. [CrossRef]
- Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599-610. [CrossRef]
- Schoukens G. Advanced Textiles for Wound Care. 2nd ed. Cambridge: Woodhead Publishing; 2019. Bioactive dressings to promote wound healing; p. 135–167. [CrossRef]
- Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687-12722. [CrossRef]
- Kamoun E. A, Kenawy E. R. S, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8(3):217-233. [CrossRef]
- Akin B, Ozmen M. M. Antimicrobial cryogel dressings towards effective wound healing. Prog Biomater. 2022:1-16. [CrossRef]
- Tripathi A, Vishnoi T, Singh D, et al. Modulated crosslinking of macroporous polymeric cryogel affects in vitro cell adhesion and growth. Macromol Biosci. 2013;13(7): 838-850. [CrossRef]
- Shirbin S. J, Lam S. J, Chan N. J. A, Ozmen M. M, et al. Polypeptide-based macroporous cryogels with inherent antimicrobial properties: the importance of a macroporous structure. ACS Macro Lett. 2016:5(5); 552-557. [CrossRef]
- Memic A, Colombani T, Eggermont L. J, et al. Latest advances in cryogel technology for biomedical applications. Adv Ther. 2019;2(4):1800114. [CrossRef]
- Eigel D, Werner C, Newland B. Cryogel biomaterials for neuroscience applications. Neurochem Int. 2021;(147):105012. [CrossRef]
- 15. Moura L. I, Dias A. M, Carvalho E, et al. (2013). Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013;9(7):7093-7114. [CrossRef]
- Vishnoi, T. In 3D Printing in Medicine and Surgery. Cambridge: Woodhead Publishing; 2021. 3D bioprinting of tissue systems; p. 171-197.
- Singh S, Gaikwad K. K, Lee Y. S. Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. Int J Biol Macromol. 2018;107:1879-1887. [CrossRef]
- Liu X, Jia G. Modern wound dressing using polymers/biopolymers. J Material Sci Eng. 2018;7(3). [CrossRef]
- Maver T, Maver U, Kleinschek K. S, et al. A review of herbal medicines in wound healing. Int J Dermatol. 2015;54(7):740-751. [CrossRef]
- Gaspar-Pintiliescu A, Stanciuc A. M, Craciunescu O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int J Biol Macromol .2019;(138): 854-865. [CrossRef]
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, (2019). Wound dressings: Current advances and future directions. J Appl Polym Sci. 2019;136(27):47738. [CrossRef]
- Joseph N, Rao MPB, Geevarughese NM, et al. Bioactive Dietary Factors and Plant Extracts in Dermatology. Humana Press: Totowa; 2013. Amla (Emblica officinalis Gaertn.) the Indian indigenous berry in skin care. p. 113-123. [CrossRef]
- Khan K. H. Roles of Emblica officinalis in medicine-A review. Bot Res Int. 2009; 2(4): 218-228. ISSN 1995-8951.
- Jayaweera DMA. Medicinal plants (Indigenous and exotic) used in Ceylon. Colombo: National Science Council of Sri Lanka; 1980.
- Bhandari P. R, Kamdod M. A. Emblica officinalis (Amla): A review of potential therapeutic applications. Int J Green Pharm. 2012:6(4);257. [CrossRef]
- Kulkarni K. V, Ghurghure S. M. Indian gooseberry (Emblica officinalis): Complete pharmacognosy review. Int J Chem Stud. 2018; 2(2):5-11. ISSN 2581-348X.
- Khan M. S, Qais F. A, Ahmad I. New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads. Amsterdam (Netharlands): Elsevier Academic Press; 2019. Indian berries and their active compounds: Therapeutic potential in cancer prevention; p. 179-201. [CrossRef]
- Talekar Y. P, Apte K. G, Paygude S. V, et al. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J Ayurveda Integr Med. 2017;8(2):73-81. [CrossRef]
- Ahmad B, Hafeez N, Rauf A, et al. Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S Afr J Bot. 2021;138: 278-310. [CrossRef]
- Chularojmontri L, Suwatronnakorn M, Wattanapitayakul S. K. Phyllanthus emblica L. enhances human umbilical vein endothelial wound healing and sprouting. Evid Based Complement Alternat Med. 2013;2013:720728. [CrossRef]
- Klimis-Zacas D, Rodriguez-Mateos A. Berries and Berry Bioactive Compounds in Promoting Health. United Kingdom: Royal Society of Chemistry; 2022.
- Perçin I, Idil N, Denizli A. Molecularly imprinted poly (N-isopropylacrylamide) thermosensitive based cryogel for immunoglobulin G purification. Process Biochemistry. 2019;80, 181-189. [CrossRef]
- Fan M, Gong L, Huang Y, Wang D, Gong Z. Facile preparation of silver nanoparticle decorated chitosan cryogels for point-of-use water disinfection. Science of the Total Environment. 2018;613, 1317-1323. [CrossRef]
- Gun’ko V. M, Savina I. N, Mikhalovsky S. V. Cryogels: Morphological, structural and adsorption characterisation. Adv Colloid Interface Sci.2013;187:1-46. [CrossRef]
- Górska A, Krupa A, Majda D, et al. Poly (Vinyl alcohol) cryogel membranes loaded with resveratrol as potential active wound dressings. AAPS PharmSciTech. 2021; 22:1-14. [CrossRef]
- Xu N, Yuan Y, Ding L, et al. Multifunctional chitosan/gelatin@ tannic acid cryogels decorated with in situ reduced silver nanoparticles for wound healing. Burns Trauma. 2022;10. [CrossRef]
- Nikhil A, Kumar A. Evaluating potential of tissue-engineered cryogels and chondrocyte derived exosomes in articular cartilage repair. Biotechnol Bioeng. 2022;119(2):605-625. [CrossRef]
- Hobro A. J, Smith N. I. An evaluation of fixation methods: Spatial and compositional cellular changes observed by Raman imaging. Vib Spectrosc. 2017;91:31-45. [CrossRef]
- Zhang T, Wang Y, Xia Q, et al. Propofol mediated protection of the brain from ischemia/reperfusion injury through the regulation of microglial connexin 43. Front Cell Dev Biol. 2021;9:637233. [CrossRef]
- Gülnar B, Canatar İ, Özdaş S. Antiadipogenic and antiobesogenic effects of pterostilbene in 3T3-L1 preadipocyte models. Turk J Biol. 2023;47(2): 130-140. [CrossRef]
- Gugliuzza A. Encyclopedia of Membranes. Berlin, Heidelberg: Springer; 2016. Solvent Swollen Polymer; p. 1801–1802. [CrossRef]
- Shetty G. R, Rao B. L. Preparation and characterization of silk fibroin-polyvinyl alcohol (PVA) blend films for food packaging materials. Materials Today: Proceedings. 2022;55:194-200. [CrossRef]
- Choi S. M, Singh D, Kumar A, et al. Porous three-dimensional PVA/gelatin sponge for skin tissue engineering. Int J Polym Mater Polym Biomater.2013;62(7):384-389. [CrossRef]
- Jones LO, Williams L, Boam T, et al. Cryogels: recent applications in 3D-bioprinting, injectable cryogels, drug delivery, and wound healing. Beilstein J Org Chem. 2021;17(1): 2553-2569. [CrossRef]
- Derazshamshir A, Baydemir G, Andac M, et al. Molecularly imprinted PHEMA-based cryogel for depletion of hemoglobin from human blood. Macromol Chem Phy. 2010;211(6):657-668. [CrossRef]
- Huang M. R, Li X. G, Yang Y. Oxidative polymerization of o-phenylenediamine and pyrimidylamine. Polym Degrad Stab. 2000;71(1): 31-38. [CrossRef]
- Du Toit J. P, Pott R. W. Transparent polyvinyl-alcohol cryogel as immobilisation matrix for continuous biohydrogen production by phototrophic bacteria. Biotechnol Biofuels. 2020;13(1): 1-16. [CrossRef]
- Liu Y, Vrana N.E, Cahill P.A, et al. Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility. J Biomed Mater Res B Appl Biomater. 2009;90: 492-502. [CrossRef]
- Razavi M, Qiao Y, Thakor A. S. Three-dimensional cryogels for biomedical applications. J Biomed Mater Res A. 2019;107(12): 2736-2755. [CrossRef]
- Teixeira, V. S., Kalckhoff, J. P., Krautschneider, W., Schroeder, D. Bioimpedance analysis of L929 and HaCaT cells in low frequency range. Current directions in biomedical engineering. 2018; 4(1): 115-118. [CrossRef]
- Shakya A. K, Holmdahl R, Nandakumar K. S, et al. Polymeric cryogels are biocompatible, and their biodegradation is independent of oxidative radicals. J Biomed Mater Res A. 2014;102(10):3409-3418. [CrossRef]
- Kathuria N, Tripathi A, Kar K. K, et al. Synthesis and characterization of elastic and macroporous chitosan–gelatin cryogels for tissue engineering. Acta Biomater. 2009;5(1):406-418. [CrossRef]
- Kao H. H, Kuo C. Y, Chen K. S, et al. . Preparation of gelatin and gelatin/hyaluronic acid cryogel scaffolds for the 3D culture of mesothelial cells and mesothelium tissue regeneration. Int J Mol Sci. 2019;20(18): 4527. [CrossRef]
- Muppalaneni S, Omidian H. Polyvinyl alcohol in medicine and pharmacy: a perspective. J. Dev. Drugs. 2013;2(3):1-5. [CrossRef]
- Farris S, Song J, Huang Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J Agric Food Chem. 2010;58(2):998-1003. [CrossRef]
- He Y, Wang C, Wang C, et al. An overview on collagen and gelatin-based cryogels: fabrication, classification, properties and biomedical applications. Polymers, 2021;13(14): 2299. [CrossRef]
- Charron P. N, Jacobs J. I, Yao S. X, et al. Effects of cryo-processing on the mechanical and biological properties of poly (vinyl alcohol)-gelatin theta-gels. Biointerphases. 2020;15(5). [CrossRef]
- Cheaburu-Yilmaz C. N, Yilmaz O, Aydin Kose F, et al. Chitosan-graft-poly (N-isopropylacrylamide)/PVA cryogels as carriers for mucosal delivery of voriconazole. Polymers. 2019;11(9):1432. [CrossRef]
- Vrana N. E, Cahill P. A, McGuinness G. B. Endothelialization of PVA/gelatin cryogels for vascular tissue engineering: Effect of disturbed shear stress conditions. J Biomed Mater Res A. 2010;94(4):1080-1090. [CrossRef]
- Liao T. T, Sukpat S, Chansriniyom C, et al. Topical combined Phyllanthus emblica Linn. and simvastatin improves wound healing in diabetic mice by enhancing angiogenesis and reducing neutrophil infiltration. Biomed Rep. 2023;18(4): 1-11. [CrossRef]
- Suvandee W, Teeranachaideekul V, Jeenduang N, et al. One-Pot and Green Preparation of Phyllanthus emblica Extract/Silver Nanoparticles/Polyvinylpyrrolidone Spray-On Dressing. Polymers. 2022;14(11):2205. [CrossRef]
- Lin Y. Y, Lu S. H, Gao R, et al. A novel biocompatible herbal extract-loaded hydrogel for acne treatment and repair. Oxid Med and Cell Longev. 2021;2021. [CrossRef]
- Abd El-Aziz A. M, El-Maghraby A, Ewald A, et al. In-vitro cytotoxicity study: cell viability and cell morphology of carbon nanofibrous scaffold/hydroxyapatite nanocomposites. Molecules. 2021;26(6):1552. [CrossRef]
- Pavez Lorie E, Stricker N, Plitta-Michalak B, et al. Characterisation of the novel spontaneously immortalized and invasively growing human skin keratinocyte line HaSKpw. Sci Rep. 2020;10(1):15196. [CrossRef]
- Pessina A, Raimondi A, Cerri A, et al. High sensitivity of human epidermal keratinocytes (HaCaT) to topoisomerase inhibitors. Cell Prolif. 2021;34(4):243-252. [CrossRef]
- Stoker M. G. P, Rubin H. Density dependent inhibition of cell growth in culture. Nature.1967;215(5097):171-172. [CrossRef]
- Guo B, Dong R, Liang Y, et al. Haemostatic materials for wound healing applications. Nat Rev Chem. 2021;5(11):773-791. [CrossRef]
- Lemos N. E, Brondani L. D. A, Dieter C, et al. Use of additives, scaffolds and extracellular matrix components for improvement of human pancreatic islet outcomes in vitro: A systematic review. Islets. 2017;9(5):73-86. [CrossRef]
- Liu M, Dai Y, Li Y, et al. Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Med. 2008;74(08):809-815. [CrossRef]
- Boudreau N. J, Jones P. L. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339(3):481-488. [PubMed]
- Entcheva E, Bien H, Yin L, et al. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials.2004;25(26):5753-5762. [CrossRef]
- Ma Y, Wang X, Su T, et al. Recent advances in macroporous hydrogels for cell behavior and tissue engineering. Gels. 2022;8(10):606. [CrossRef]
- Eyckmans J, Chen C. S. 3D culture models of tissues under tension. J Cell Sci. 2017;130(1): 63-70. [CrossRef]
- Boukamp P, Petrussevska R. T, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761-771. [CrossRef]
- Borowiec A. S, Delcourt P, Dewailly E, et al. Optimal differentiation of in vitro keratinocytes requires multifactorial external control. PloS one. 2013;8(10):e77507. [CrossRef]
- Breitkreutz D, Stark H. J, Plein P, et al. Differential modulation of epidermal keratinization in immortalized (HaCaT) and tumorigenic human skin keratinocytes (HaCaT-ras) by retinoic acid and extracellular Ca2+. Differentiation. 1993;54(3):201-217. [CrossRef]
- Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of β-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167(1):59-69. [CrossRef]
- Langlois S, Maher A. C, Manias J. L, et al. Connexin levels regulate keratinocyte differentiation in the epidermis. J Biol Chem. 2007;282(41):30171-30180. [CrossRef]
- Faniku C, O’Shaughnessy E, Lorraine C, et al. The connexin mimetic peptide Gap27 and Cx43-knockdown reveal differential roles for Connexin43 in wound closure events in skin model systems. Int J Mol Sci. 2018;19(2):604. [CrossRef]
- Langer R, Tirrell D. A. Designing materials for biology and medicine. Nature. 2004;428(6982):487-492. [CrossRef]
- Iandolo D, Pennacchio F. A, Mollo V, et al. Electron microscopy for 3D scaffolds–cell biointerface characterization. Adv Biosyst. 2019;3(2):1800103. [CrossRef]
- Deb P, Deoghare A. B, Borah A, et al. Scaffold development using biomaterials: a review. Mater Today Proc. 2018;5(5):12909-12919. [CrossRef]
- Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998-1008. [CrossRef]
- Afanasiev SA, Muslimova EF, Nashchekina YA et al. Peculiarities of Cell Seeding on Electroformed Polycaprolactone Scaffolds Modified with Surface-Active Agents Triton X-100 and Polyvinylpyrrolidone. Bull Exp Biol Med. 2020;169:600-604. [CrossRef]
- Datta H. S, Mitra S. K, Patwardhan B. Wound healing activity of topical application forms based on ayurveda. Evid Based Complement Alternat Med. 2011;2011:134378. [CrossRef]
- Hixon K. R, Lu T, Sell S. A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017;62:29-41. [CrossRef]


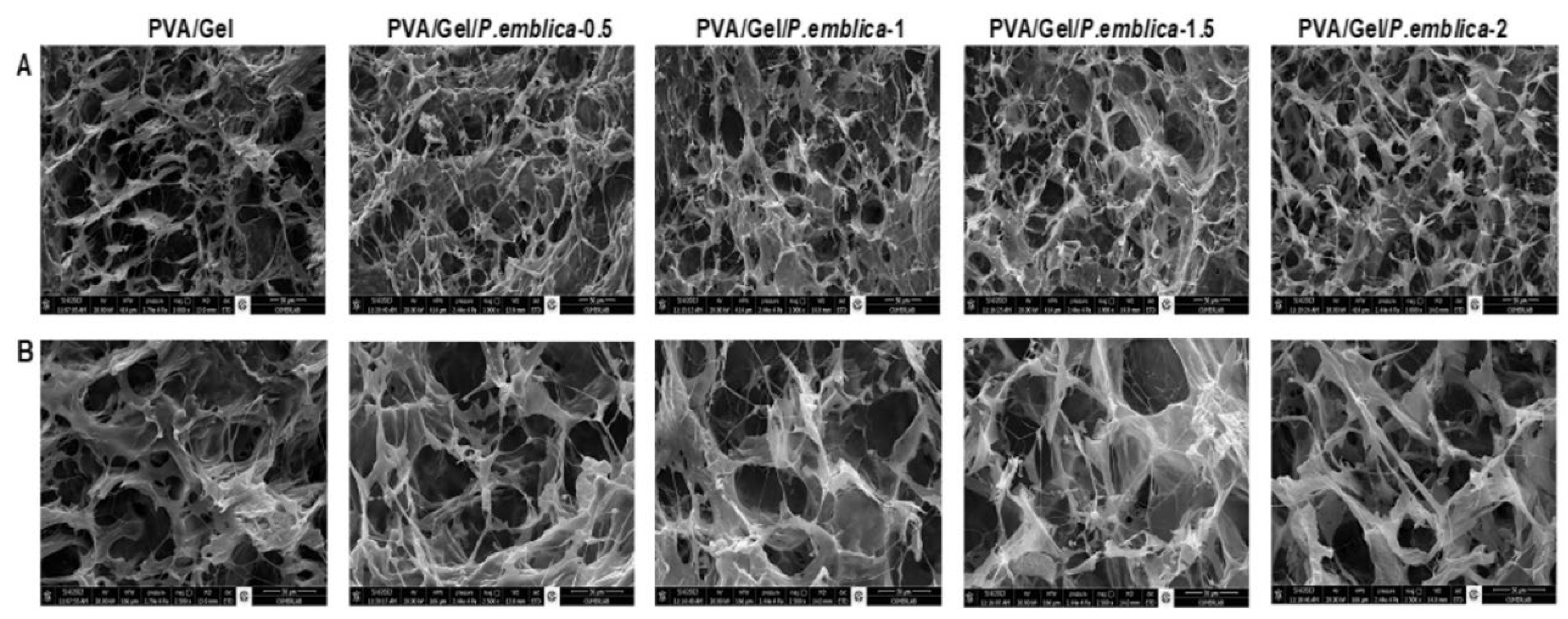
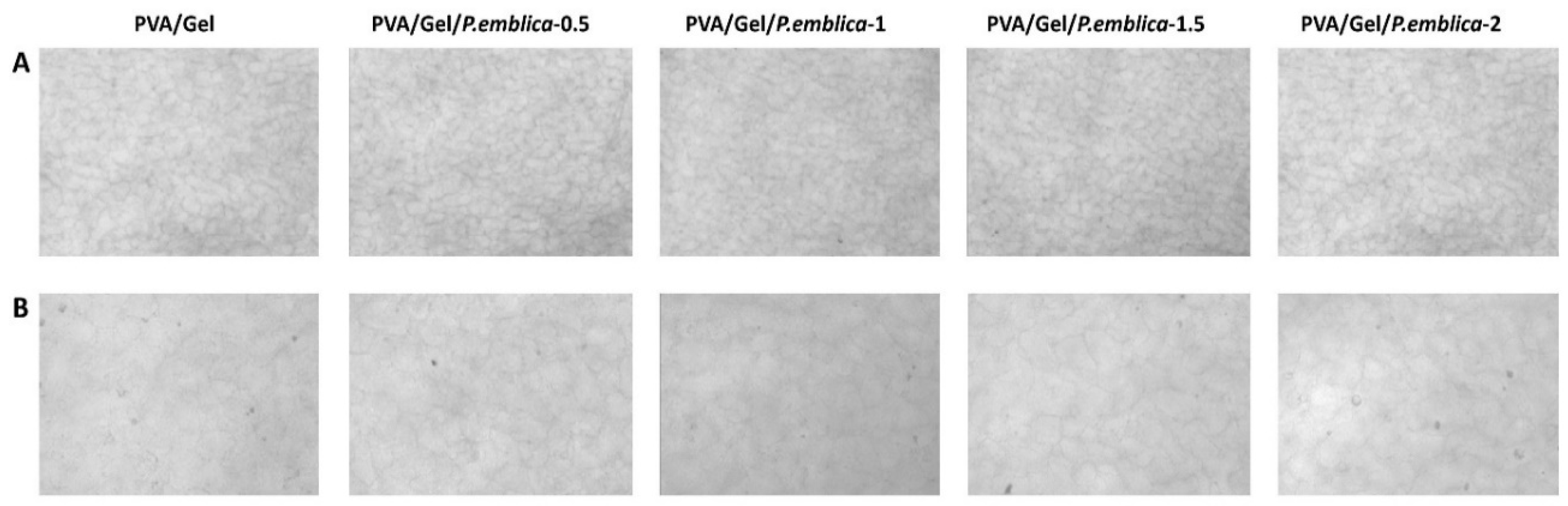
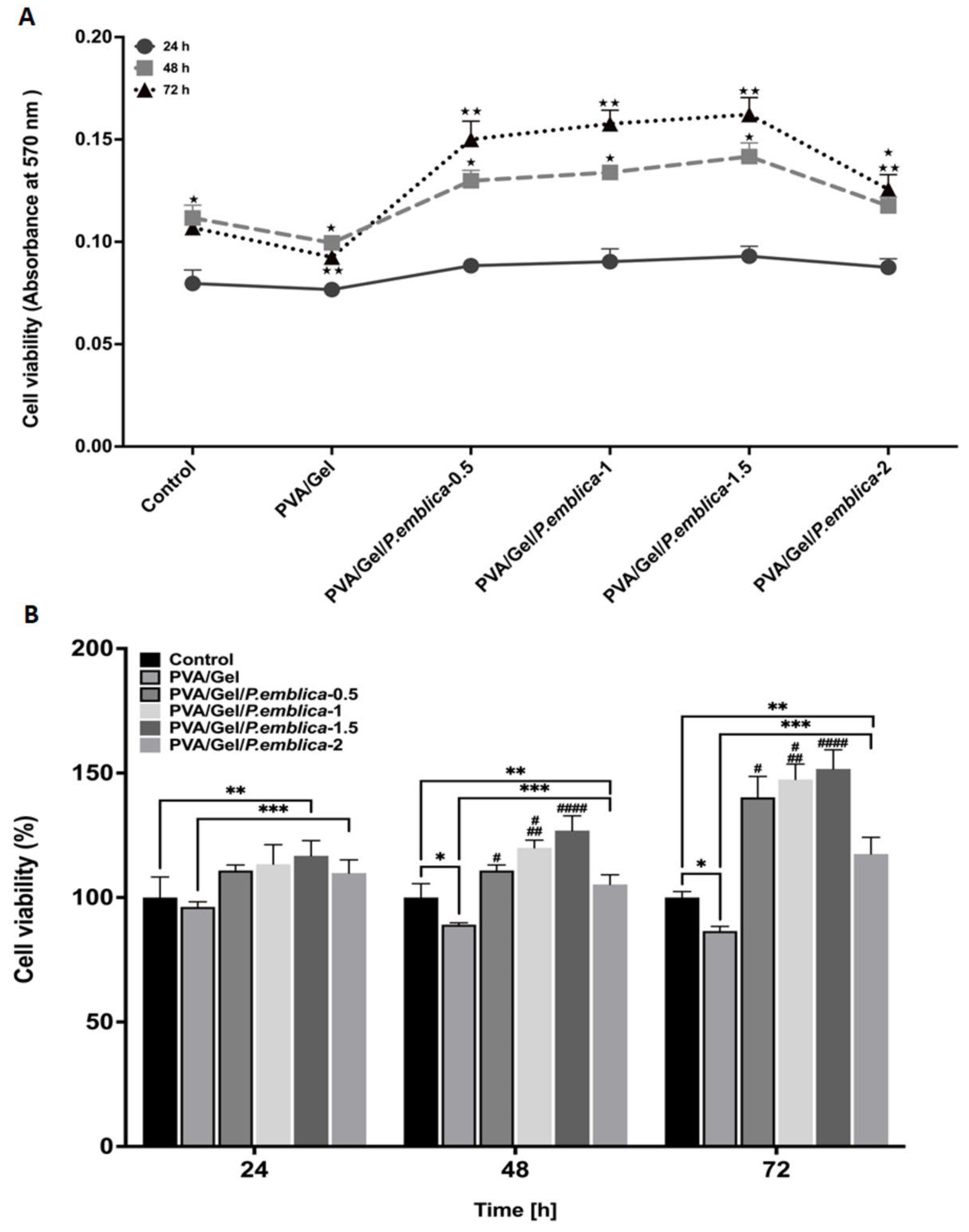


| Swelling Rate (%) | Polymerization Yield (%) | Macroporosity (%) | |
|---|---|---|---|
| PVA/Gel | 450 ± 4.7 | 92 ± 2.1 | 82 ± 2.1 |
| PVA/Gel/P.emblica-0.5 | 452 ± 1.2 | 93 ± 0.6 | 82 ± 1 |
| PVA/Gel/P.emblica-1 | 457 ± 2.5 | 92 ± 1.2 | 82 ± 2 |
| PVA/Gel/P.emblica-1.5 | 458 ± 2.6 | 93 ± 1 | 83 ± 1.2 |
| PVA/Gel/P.emblica-2 | 446 ± 5.5 | 91 ± 2.5 | 81 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





