Submitted:
19 November 2023
Posted:
21 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
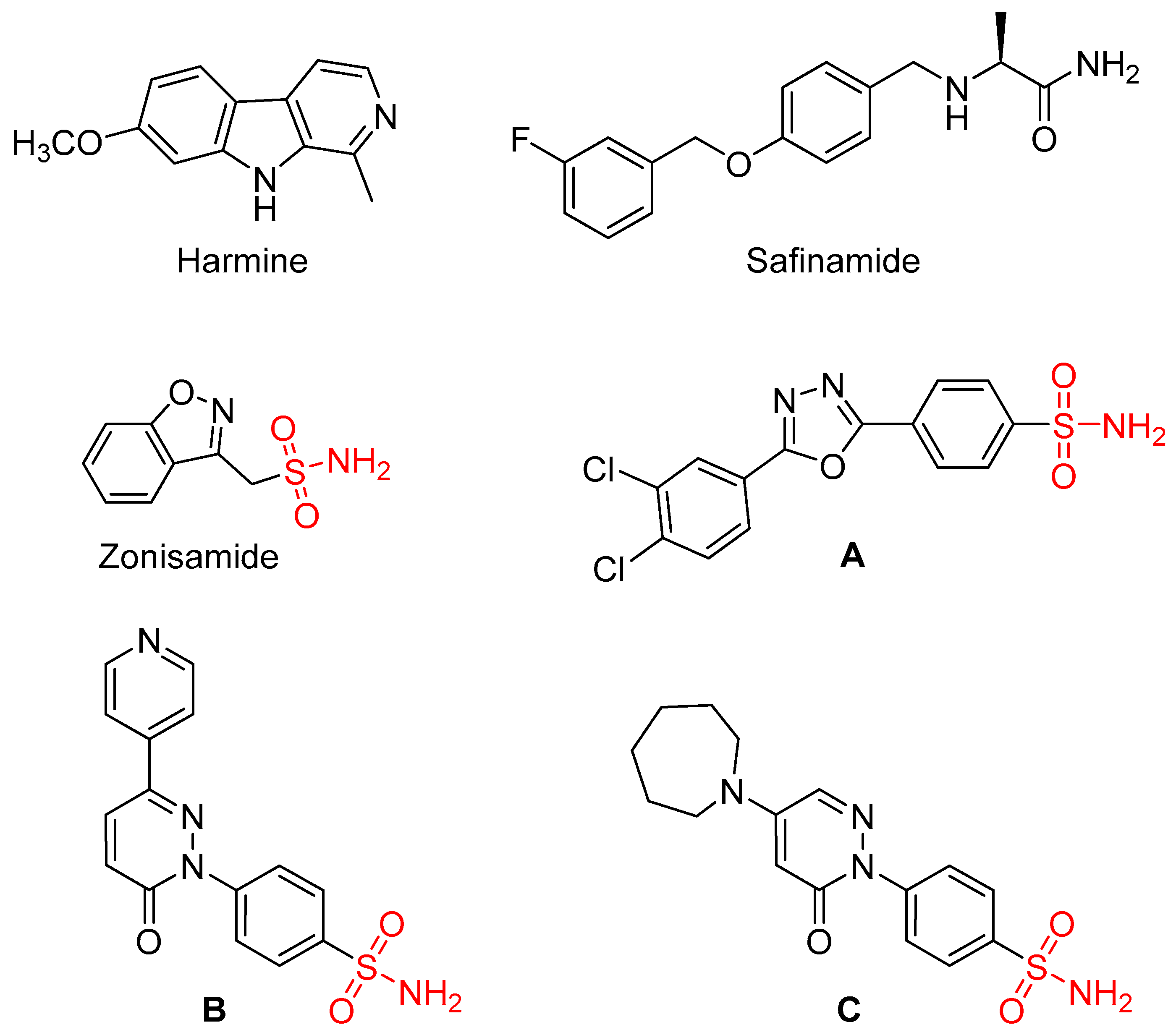
2. Results and Discussion
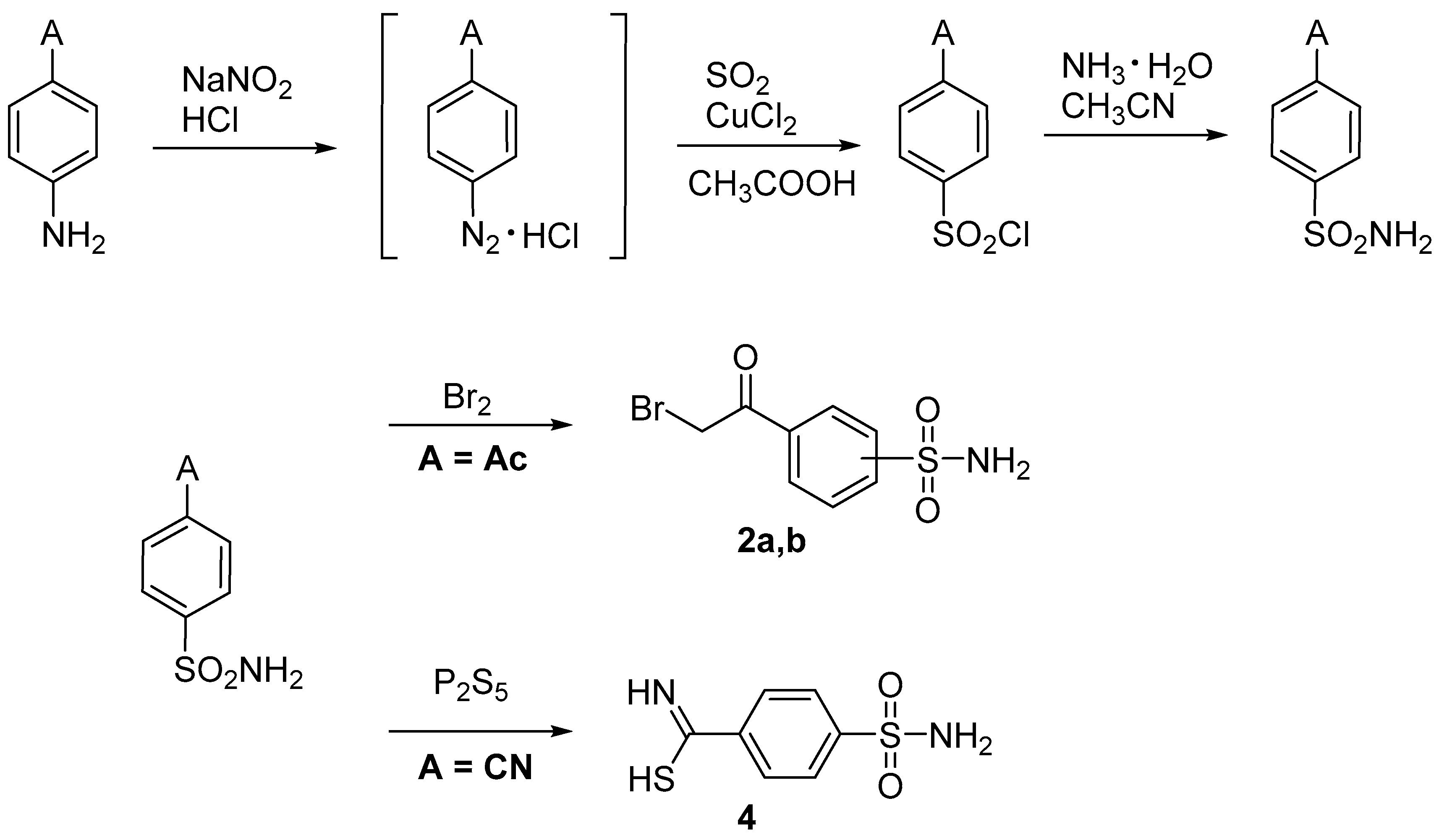
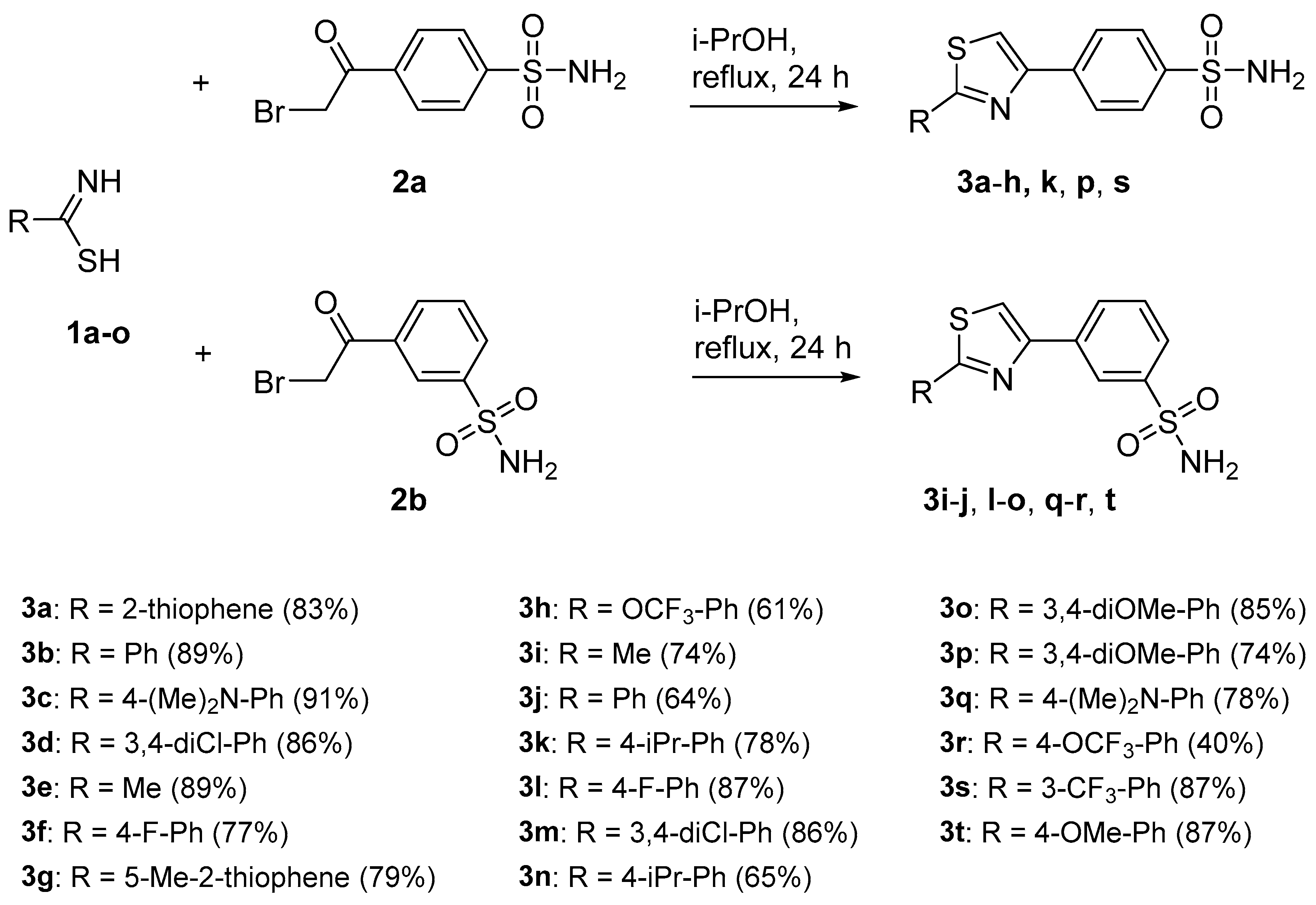
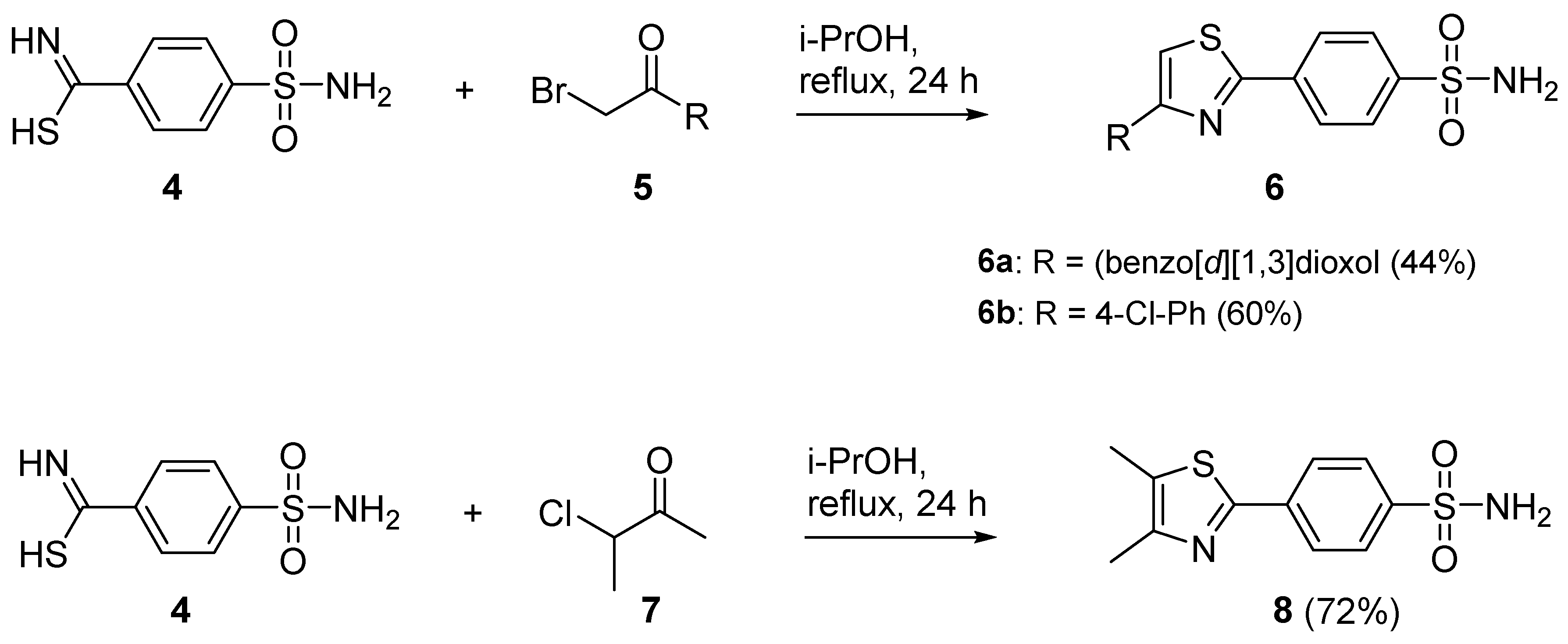
2.1. Chemistry
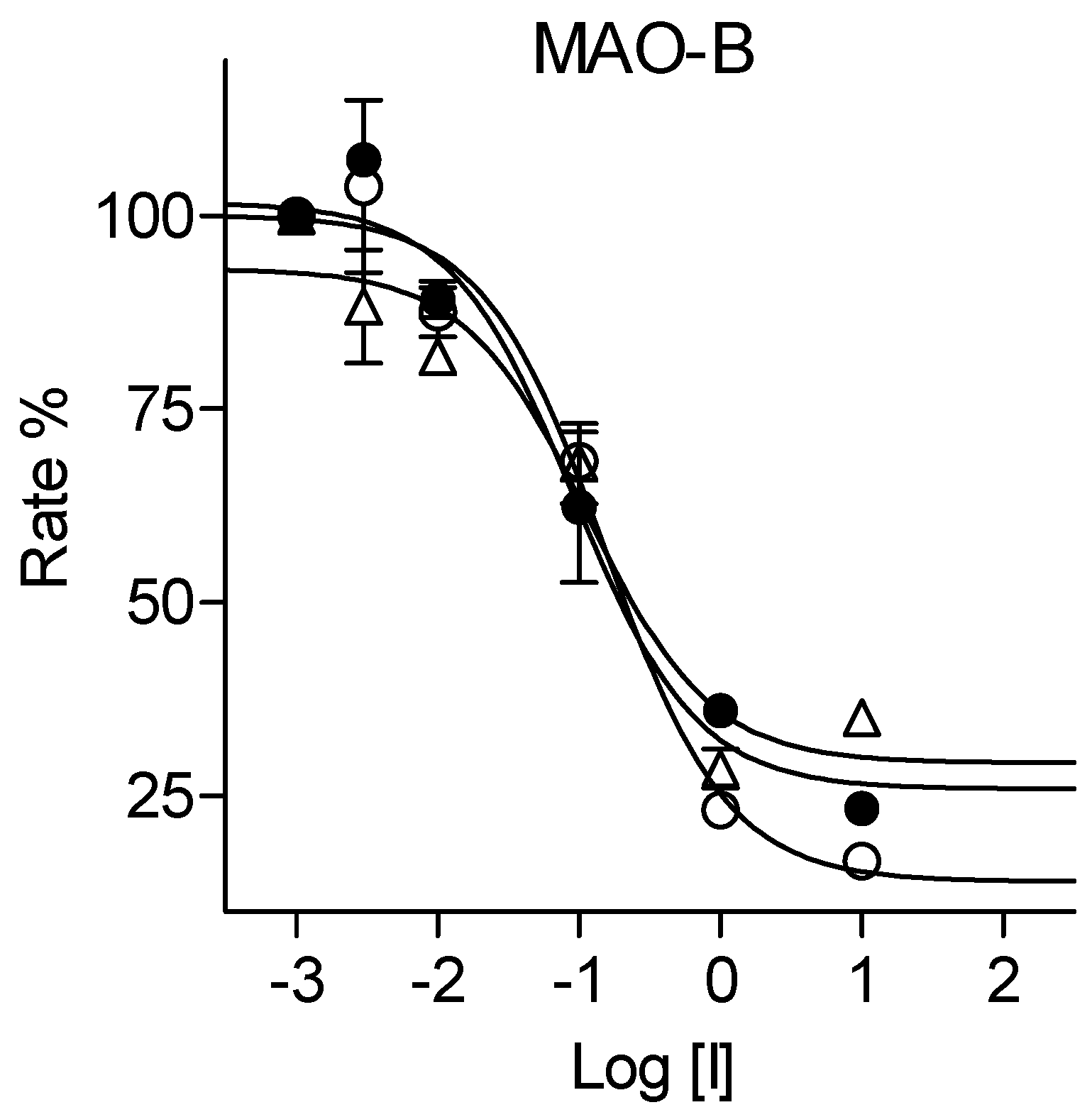
2.2. In vitro biological evaluation – MAO inhibition
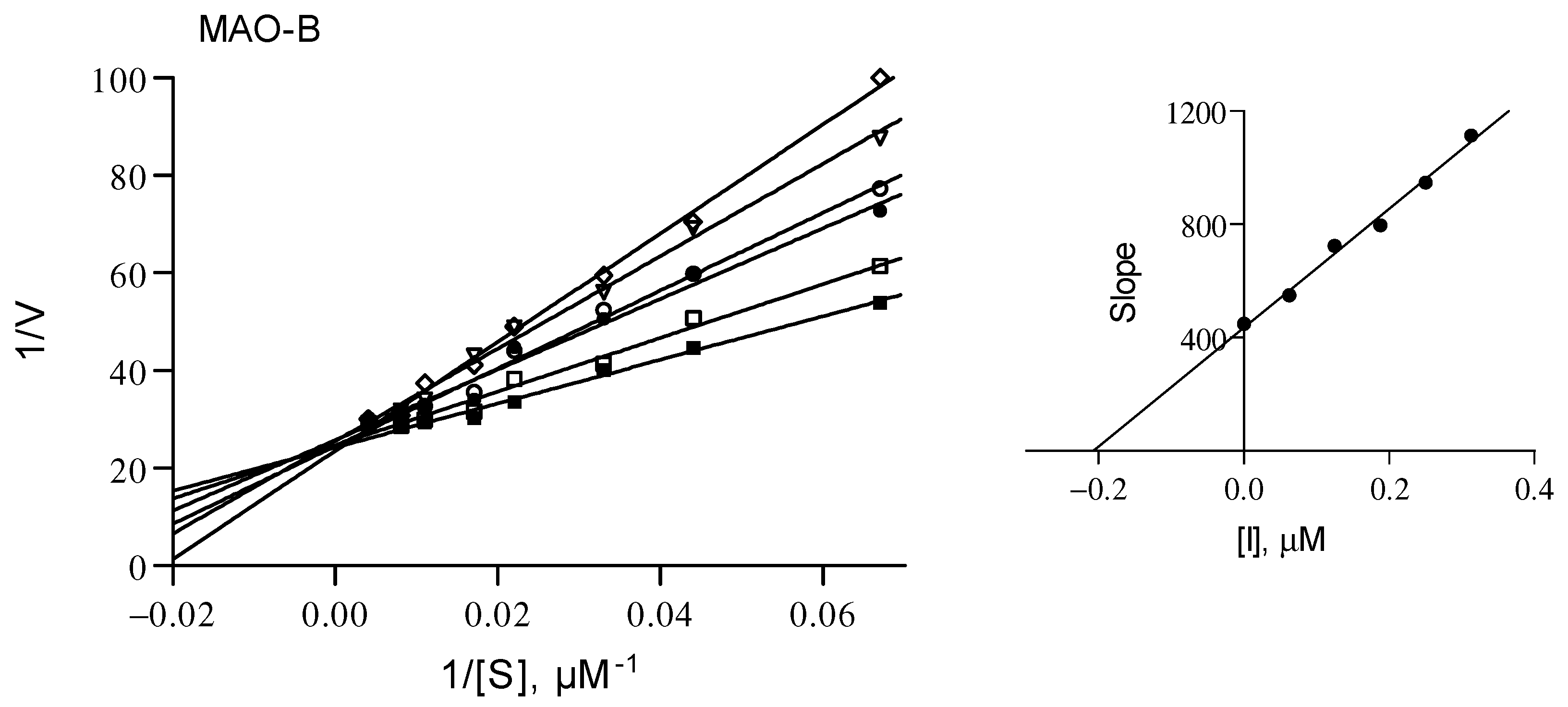
2.3. Lineweaver-Burk graphs for the inhibition of MAO-B by 3j
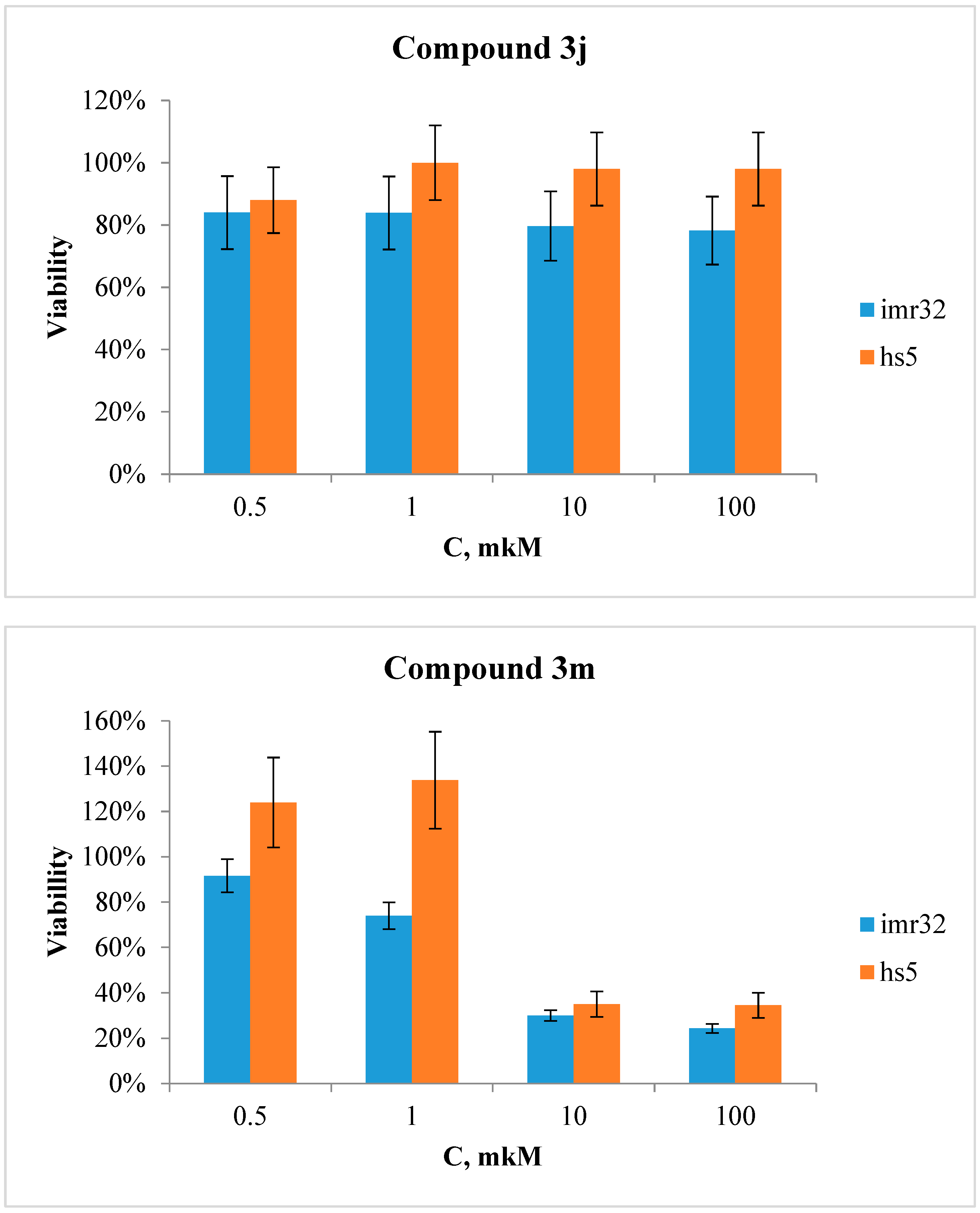
2.4. Cytotoxicity in vitro
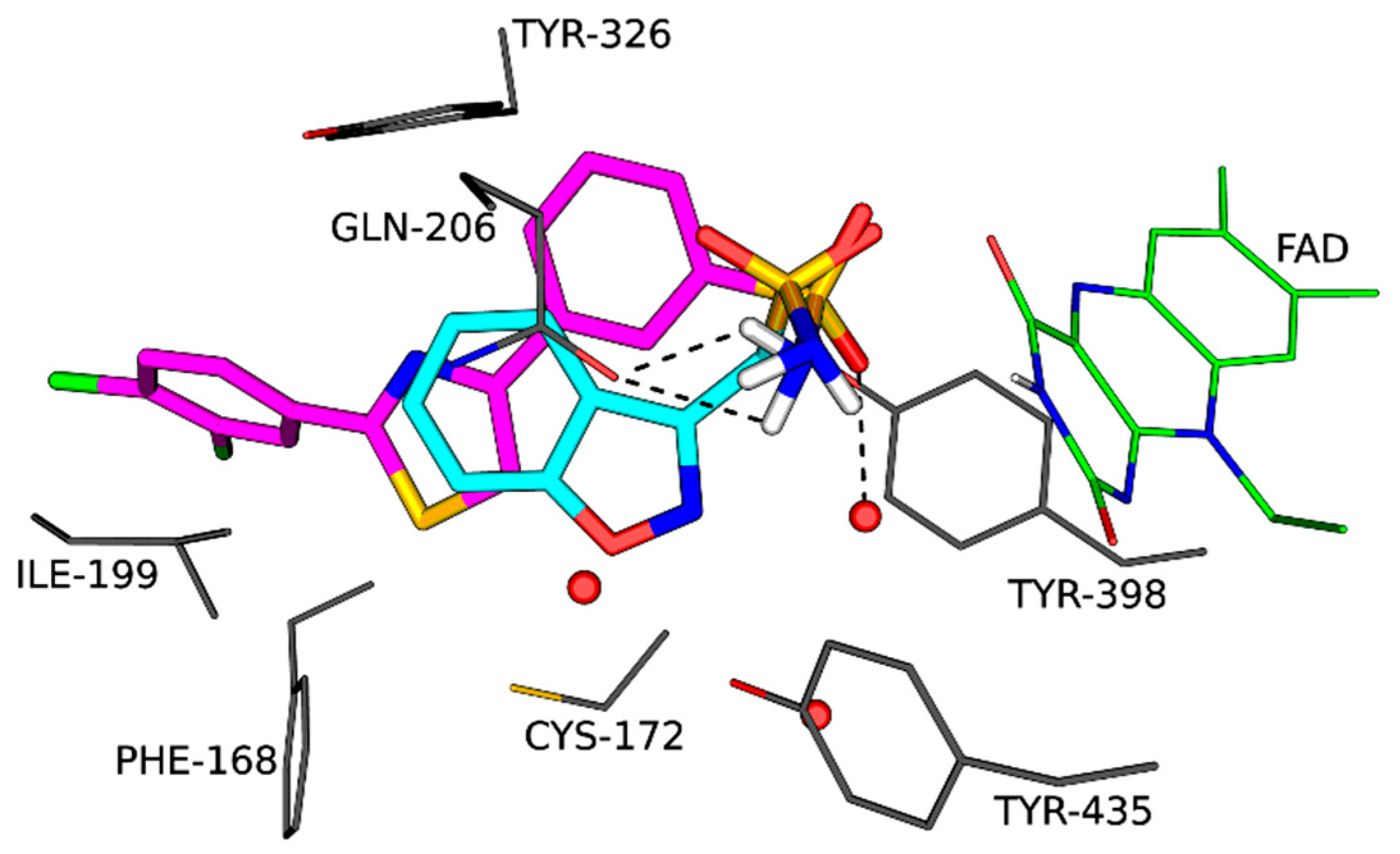
2.5. Molecular docking
3. Conclusion
4. Materials and Methods
4.1. Chemicals and instrumentation
4.2. General procedure for the preparation of thiazoles (3) (GP1).
4.3. Preparation and characterization of isomeric thiazoles (6)
4.4. Preparation and characterization of the thiazole 8
4.7. Procedure for measuring MAO inhibition potency
4.8. Procedure for measuring cytotoxicity
4.9. Procedure for performing molecular docking
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alborghetti, M.; Bianchini, E.; De Carolis, L.; Galli, S.; Pontieri, F.E.; Rinaldi, D. Type-B monoamine oxidase inhibitors in neurological diseases: Clinical applications based on preclinical findings. Neural Regen Res 2024, 19, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.W.; Lan, N.C.; Johnson, D.L.; Abell, C.W.; Bembenek, M.E.; Kwan, S.W.; Seeburg, P.H.; Shih, J.C. Cdna cloning of human liver monoamine oxidase a and b: Molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A 1988, 85, 4934–4938. [Google Scholar] [CrossRef] [PubMed]
- Bala, A. A need for clinical trial: Re-purposing the monoamine oxidase inhibitors (MAO-I) for rheumatoid arthritis (RA). Inflammopharmacology 2023. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Aldeco, M.; Mattevi, A.; Edmondson, D.E. Interactions of monoamine oxidases with the antiepileptic drug zonisamide: Specificity of inhibition and structure of the human monoamine oxidase b complex. J Med Chem 2011, 54, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Newton-Vinson, P.; Hubalek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase b, a drug target for the treatment of neurological disorders. Nat Struct Biol 2002, 9, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of human monoamine oxidase b complexes with selective noncovalent inhibitors: Safinamide and coumarin analogs. J Med Chem 2007, 50, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Andersen, J.K. Dopaminergic neurons. Int J Biochem Cell Biol 2005, 37, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.E. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: Biological implications. Curr Pharm Des 2014, 20, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Wiberg, A.; Oreland, L.; Marcusson, J.; Winblad, B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J Neural Transm 1980, 49, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.S.; Volkow, N.D.; Wang, G.J.; Logan, J.; Pappas, N.; Shea, C.; MacGregor, R. Age-related increases in brain monoamine oxidase b in living healthy human subjects. Neurobiol Aging 1997, 18, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Gradiz, R.; Silva, H.C.; Carvalho, L.; Botelho, M.F.; Mota-Pinto, A. MIA PaCa-2 and PANC-1 – pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci. Rep. 2016, 6, 21648. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Carradori, S.; D'Agostino, I.; Campestre, C.; Petzer, J.P. An updated patent review on monoamine oxidase (mao) inhibitors. Expert Opin Ther Pat 2022, 32, 849–883. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, H.; Ma, Y.; Zhao, Z.; An, Q.; Zhao, J.; Shi, C. Monoamine oxidase a (maoa): A promising target for prostate cancer therapy. Cancer Lett 2023, 563, 216188. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, F.; Binda, C.; Khalil, A.; Li, M.; Mattevi, A.; Castagnoli, N.; Edmondson, D.E. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase b by specific reversible inhibitors. J Biol Chem 2005, 280, 15761–15766. [Google Scholar] [CrossRef] [PubMed]
- Kaboudin, B.; Elhamifar, D. Phosphorus pentasulfide: A mild and versatile reagent for the preparation of thioamides from nitriles. Synthesis-Stuttgart 2006, 224–226. [Google Scholar] [CrossRef]
- Kaludercic, N.; Mialet-Perez, J.; Paolocci, N.; Parini, A.; Di Lisa, F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol 2014, 73, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, V.P.; Kumar, V. A perspective on monoamine oxidase enzyme as drug target: Challenges and opportunities. Curr Drug Targets 2017, 18, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Lum, C.T.; Stahl, S.M. Opportunities for reversible inhibitors of monoamine oxidase-a (rimas) in the treatment of depression. CNS Spectr 2012, 17, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.E.; Rigacci, S.; Borchi, E.; Bargelli, V.; Miceli, C.; Giordano, C.; Raimondi, L.; Nediani, C. Monoamine oxidase is overactivated in left and right ventricles from ischemic hearts: An intriguing therapeutic target. Oxid Med Cell Longev 2016, 2016, 4375418. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Ginovart, N.; Boovariwala, A.; Sagrati, S.; Hussey, D.; Garcia, A.; Young, T.; Praschak-Rieder, N.; Wilson, A.A.; Houle, S. Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 2006, 63, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mostert, S.; Petzer, A.; Petzer, J.P. Indanones as high-potency reversible inhibitors of monoamine oxidase. ChemMedChem 2015, 10, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Mulinari, S. Monoamine theories of depression: Historical impact on biomedical research. J Hist Neurosci 2012, 21, 366–392. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Okumura, M.; Ozawa, M.; Mimori, M.; Maku, T.; Shiraishi, T.; Kitagawa, T.; Takatsu, H.; Sato, T.; Komatsu, T. , et al. Effects of monotherapy with a monoamine oxidase b inhibitor on motor symptoms in parkinson's disease are dependent on frontal function. Neurol Sci 2023, 44, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Novaroli, L.; Reist, M.; Favre, E.; Carotti, A.; Catto, M.; Carrupt, P.A. Human recombinant monoamine oxidase b as reliable and efficient enzyme source for inhibitor screening. Bioorg Med Chem 2005, 13, 6212–6217. [Google Scholar] [CrossRef] [PubMed]
- Petzer, A.; Shetnev, A.; Efimova, J.; Korsakov, M.; Filimonov, S.; Petzer, P.J. Pyridazinone-substituted benzenesulfonamides demonstrate inhibition of monoamine oxidase. Letters in Drug Design & Discovery 2023, 20, 1–8. [Google Scholar]
- Pizzinat, N.; Copin, N.; Vindis, C.; Parini, A.; Cambon, C. Reactive oxygen species production by monoamine oxidases in intact cells. Naunyn Schmiedebergs Arch Pharmacol 1999, 359, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S. The two-century journey of parkinson disease research. Nat Rev Neurosci 2017, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Scobie, M. Mth1 Inhibitors for Treatment of Inflammatory and Autoimmune Conditions. U.S. Patent US16/120,170, 31 August 2018. [Google Scholar]
- Shetnev, A.; Shlenev, R.; Efimova, J.; Ivanovskii, S.; Tarasov, A.; Petzer, A.; Petzer, J.P. 1,3,4-oxadiazol-2-ylbenzenesulfonamides as privileged structures for the inhibition of monoamine oxidase b. Bioorg Med Chem Lett 2019, 29, 126677. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C. Monoamine oxidase isoenzymes: Genes, functions and targets for behavior and cancer therapy. J Neural Transm (Vienna) 2018, 125, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu Rev Neurosci 1999, 22, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of human monoamine oxidase a at 2.2-a resolution: The control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A 2008, 105, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Sonsalla, P.K.; Wong, L.Y.; Winnik, B.; Buckley, B. The antiepileptic drug zonisamide inhibits mao-b and attenuates mptp toxicity in mice: Clinical relevance. Exp Neurol 2010, 221, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Suchting, R.; Tirumalajaru, V.; Gareeb, R.; Bockmann, T.; de Dios, C.; Aickareth, J.; Pinjari, O.; Soares, J.C.; Cowen, P.J.; Selvaraj, S. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: A systematic review and network meta-analysis. J Affect Disord 2021, 282, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Umbarkar, P.; Singh, S.; Arkat, S.; Bodhankar, S.L.; Lohidasan, S.; Sitasawad, S.L. Monoamine oxidase-a is an important source of oxidative stress and promotes cardiac dysfunction, apoptosis, and fibrosis in diabetic cardiomyopathy. Free Radic Biol Med 2015, 87, 263–273. [Google Scholar] [CrossRef]
- Weissbach, H.; Smith, T.E.; Daly, J.W.; Witkop, B.; Udenfriend, S. A rapid spectrophotometric assay of mono-amine oxidase based on the rate of disappearance of kynuramine. J Biol Chem 1960, 235, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Mitsudera, H.; Shundoh, T. Highly regioselective direct halogenation: A simple and efficient method for preparing 4-halomethyl-5-methyl-2-aryl-1,3-thiazoles. Tetrahedron Letters 2004, 45, 69–73. [Google Scholar] [CrossRef]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in parkinson's disease and depressive illness. Br J Pharmacol 2006, 147 Suppl 1, S287–296. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Zisook, S. A clinical overview of monoamine oxidase inhibitors. Psychosomatics 1985, 26, 240–246, 251. [Google Scholar] [CrossRef] [PubMed]
| ID | Structure | IC50 (µM ± SD)a | ||
|---|---|---|---|---|
| MAO-A | MAO-B | SIb | ||
| 3a | 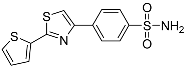 |
11.9 ± 1.49 | 13.8 ± 1.04 | 0.9 |
| 3b | 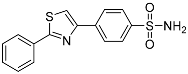 |
23.3 ± 1.88 | 6.62 ± 0.162 | 3.5 |
| 3c | 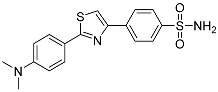 |
32.6 ± 3.80 | NIc | – |
| 3d | 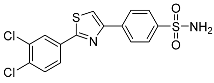 |
88.4 ± 25.3 | 0.407 ± 0.061 | 217 |
| 3e | 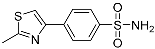 |
NIc | 79.1 ± 15.2 | – |
| 3f | 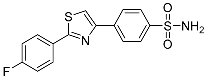 |
18.7 ± 0.226 | 0.484 ± 0.140 | 39 |
| 3g | 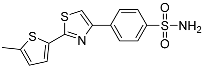 |
42.7 ± 3.66 | 9.85 ± 4.95 | 4.3 |
| 3h | 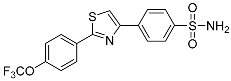 |
NIc | 30.5 ± 0.552 | – |
| 3i | 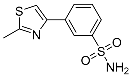 |
57.7 ± 4.32 | 79.6 ± 12.4 | 0.7 |
| 3j | 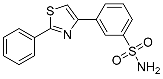 |
28.7 ± 1.46 | 0.103 ± 0.0027 | 279 |
| 3k | 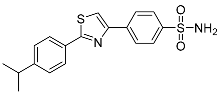 |
NIc | 12.6 ± 0.021 | – |
| 3l | 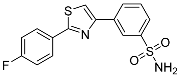 |
21.9 ± 1.10 | 0.193 ± 0.028 | 113 |
| 3m | 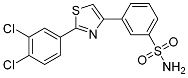 |
17.2 ± 0.212 | 0.172 ± 0.049 | 100 |
| 3n | 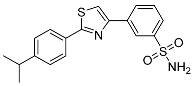 |
45.9 ± 8.64 | 3.60 ± 3.28 | 13 |
| 3o | 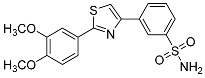 |
NIc | NIc | – |
| 3p | 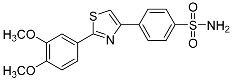 |
NIc | 77.1 ± 47.6 | – |
| 3q | 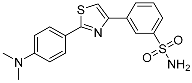 |
47.4 ± 8.03 | 0.404 ± 0.106 | 117 |
| 3r | 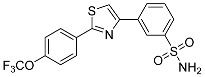 |
56.2 ± 8.25 | 29.0 ± 2.96 | 1.9 |
| 3s | 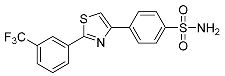 |
NIc | 40.5 ± 4.40 | – |
| 3t | 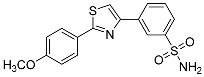 |
29.4 ± 3.06 | 2.80 ± 0.324 | 11 |
| 6a | 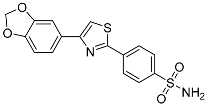 |
50.0 ± 3.50 | 58.3 ± 10.7 | 0.9 |
| 6b | 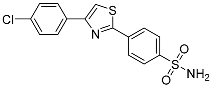 |
NIc | 0.412 ± 0.021 | – |
| 8 | 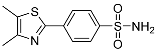 |
NIc | NIc | – |
| Toloxatoned | 1.67 ± 0.418 | – | – | |
| Safinamided | – | 0.240 ± 0.080 | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





