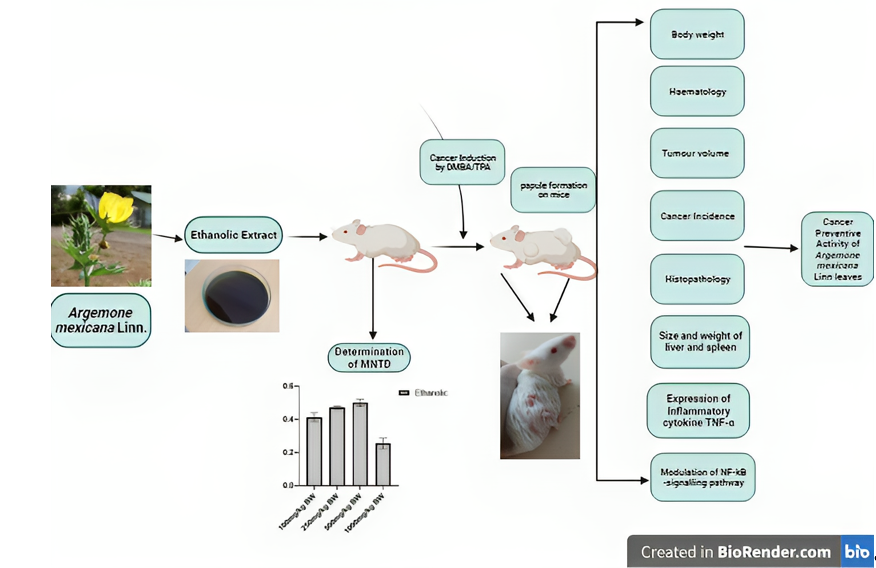
1. Introduction
Skin cancer is one the prominent cancers in the world which accounts to 2-3 million of cases diagnosed in world in past 5 years. This makes it as the 5th most common cancers in the world which brings it as a burden on economy of any country [
1]. The cases of skin cancer are increasing at an exponential rate because of prolonged exposure to UV light, chemicals all over the skin care products and immune suppressing lifestyle of population [
2]. The majority of cancers are caused by a genetic predisposition, environmental pollutants like excessive alcohol or tobacco use, as well as exposure to hazardous chemicals and radiation. These factors can either be internal body processes like spontaneous mutations, hormones, and nutrient metabolism, or they can be external stimuli [
3].
Indian sub-continent is a well-known biological hot spot with a large variety of flora and this could be taken in use for treating many diseases and ailments including cancer, the literature of Ayurved has treasured many plants that look of no use but contain a treasure of phytochemicals including steroids, flavonoids, saponins etc, that could be used to treat the cancer [
4].
Argemone mexicana Linn (A.M), from family Paperveraceae. It is an exotic wild plant toxic in nature, with wide-spread distribution in many tropical and sub-tropical countries including west Africa, India, South America, etc [
5]. It is a prickly annual herb of and has yellow scentless flowers on it. It is an important medicinal plant and extensively used in Ayurveda, Siddha, Unani and Homeopathic medicines.
Argemone mexicana is reported to have antimicrobial, antimalarial, larvicidal, nematocidal, antifungal, anti-cancerous activity etc. In India it is taken into use for treatment of various disease using different parts of the plants for treating jaundice, scabies, fungal infections, ulcers, asthma, intestinal infections skin, cough, and other disease etc [
6,
7,
8]. A study in Mali, S. Africa also stated about the human trail in use of plant extract/sap against malaria. The study was conducted in clinical trials in Mali and Switzerland [
9].
Animal study plays a very important role in validation of such results and hence, drawing reference from studies been reported by Sharmila and Manoharan, 2011, where they have used naturally derived Rosmaniric acid from the leaves of Rosemary plant and investigated on its anti-cancer potential against DMBA and TPA induced skin cancer in Swiss albino mice [
10]. The mouse skin model of multi-stage chemical carcinogenesis represents one of the best-established
in vivo models for the study of the sequential and stepwise development of tumors and the former chemicals used are well-known carcinogens. Similar studies have been reported by Manoharan and Selvan, 2012 where they have used geraniol, a synthetic product for treatment of skin cancer in Swiss albino mice [
11].
For the molecular pathway regulation studies, NF-kB and TNF-a play a critical role in the link between inflammation and cancer through NF-kB ability to up regulate tumour promoting cytokine and survival genes [
12]. Wu et al, 2010 defined the combination of TNF-α and NF-kB as an emerging treatment for cancers. The canonical and alternative main NF-κB pathways have been thoroughly described. In the conventional pathway, the inhibitor of κB (IκB) sequesters NF-κB (often a heterodimer of p65 and p50) in the cytoplasm when it is not triggered [
13]. A number of upstream signalling pathways target IκB, activating an IκB kinase (IKK) complex made up of one regulatory subunit, NF-κB essential modulator (NEMO, also known as IKKγ), and at least two kinases, IKKα and IKKβ. IKKα and IKKβ have the ability to phosphorylate IκB directly, which causes ubiquitination and 26S proteasome destruction of the protein [
14,
15].
Several studies have been made in ethnopharmacological evaluation of anti-cancerous and cytotoxic activity of Argemone mexicana leaves and isolated compounds in in vitro cell lines. No animal study has been reported linked to its therapeutic potential against skin cancer. Here in this study, an attempt has been made to study the cancer-preventive and cancer treatment in DMBA/TPA induced skin cancer in mice. The study aims to evaluate the effect of ethanolic extract of Argemone mexicana Linn leaves on cancer-prevention and expression pattern of TNF-α along with modulation p65 subunit of NF-kB signalling pathway in the cancer induced mice model.
2. Materials and Methods
2.1. Chemicals
Chemicals, DMBA (7,12-dimethylbenz[a]-anthracene), TPA (12-O-Tetradecanoylphorbol-13-acetate) were purchased from Sigma-Aldrich, USA. The standard chemotherapeutic drugs Doxorubicin was purchased from Pfizer, USA. TNF-α (Kit Catalogue No: ELK9154) and NF-kB (Kit Catalogue No: ELK1387) kits were purchased from ELK Biotechnology.
2.2. Sample Collection, Authentication and Preparation of Extracts
The leaves of
Argemone mexicana Linn were collected from local areas and villages surrounding GLA University, Mathura, during February and March, (Coordinates: 27.49ºN and 77.69ºE). The leaves were authenticated from CIMAP (Central Institute of Medicinal and Aromatic Plants), Lucknow and the specimen sample was deposited with Voucher no. CIMAP/Bot-pharm/2021/09. Soxhlet apparatus was used to prepare the ethanolic extract for testing concentrated using rotary vacuum evaporator (Yamato Scientific Co., Japan) [
16].
2.3. Animal Care and Handling
Swiss Albino mice of 4-6 weeks ~25 gm was purchased from the animal house of NIB (National Institute of Biologicals), Noida, U.P under sanction No. GLAIPR/IAEC/21/PHD/04 and housed in Animal House Facility, Institute of Pharmaceutical research, GLA University, Mathura (No. 1260/PO/Re/09/CPCSEA). The animals were housed in standard husbandry conditions and subjected to acclimatization period of 21 days at 22±2 ºC temperature and 12 hrs light/dark cycle. Food was provided in the form of pellet and water was provided ad libitum.
2.4. Determination of Non-Toxic Dose of Argemone Mexicana Leaves (AML) Extract
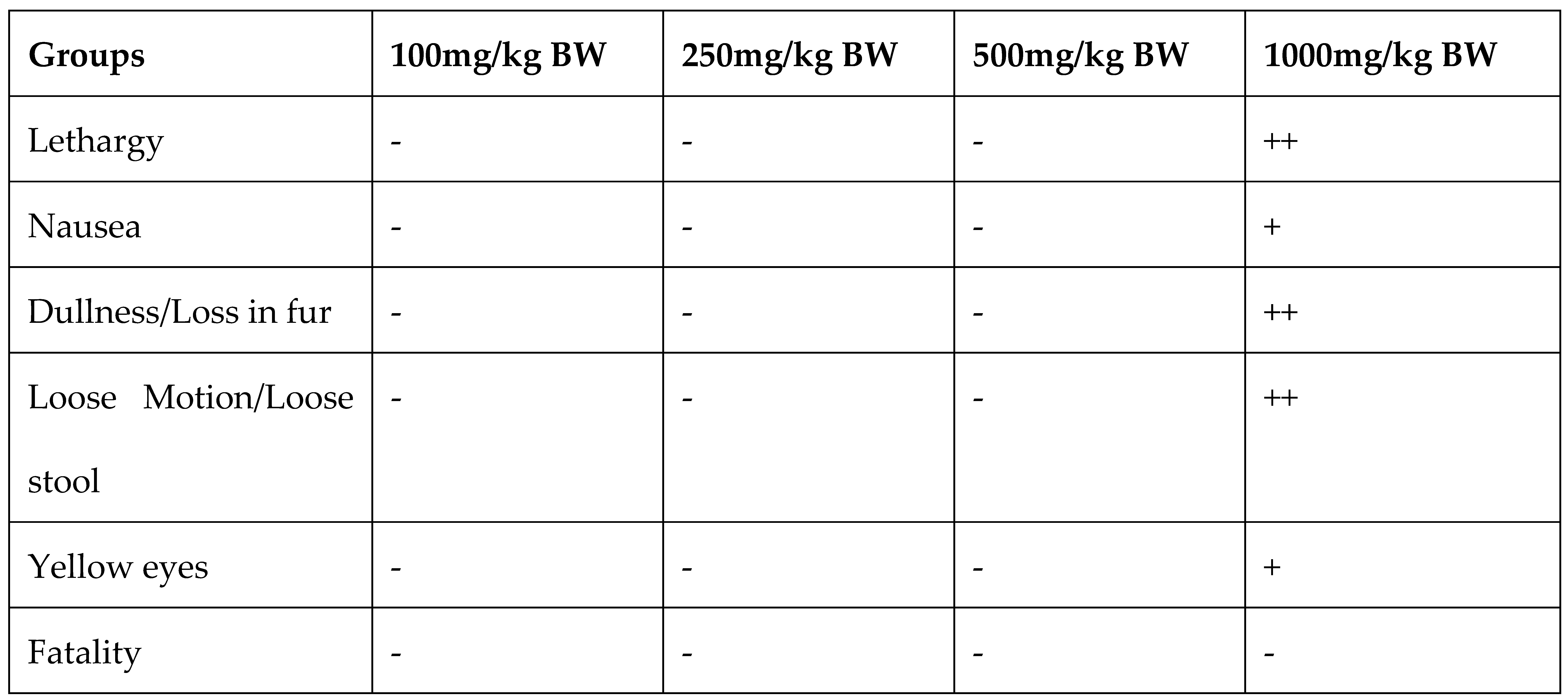
For the determination of Maximum nontoxic concentration of Argemone mexicana Linn leaves (AML) extract, the animals were divided into five groups of 8 animals each. The first group consist of the animals, which are fed with vehicle 0.01% of DMSO daily for 21 days. From 2nd to 5th group animals were fed orally with 100mg/kg, 250mg/kg, 500mg/kg and 1000mg/kg BW of AML extract for 3 weeks. Different physical and hematological parameters were measured in the blood of the animals after 21 days of treatment.
2.5. Determination of Skin Cancer Preventive activity of Argemone Mexicana Leaves (AML) Extract
2.5.1. Induction of Skin Cancer in Experimental Animals
The two-stage carcinogenesis method using DMBA and TPA was used for cancer induction in mice as per quoted in Cold Spring Harbour protocol by Kemp et al., 2015 [
17] and repeatedly used by researchers including Surien et al.,2022 [
18]. The protocol is well-known for its localized effect and comparatively less time-consuming formation of cancer papilloma on skin. The method includes shaving a particular area on the back of mice with epilator or depilating cream and application of DMBA and TPA. 250 µg/ml stock solution of DMBA was prepared in acetone and 0.1ml of this stock was applied twice a week [
19] whereas, 2.5µg/ml stock solution of TPA was prepared in acetone and0.2ml was applied once a week on the shaved area [
20]. The DMBA is the initiator for cancer and TPA is a promoter. DMBA & TPA treatment was stopped in the animals where cancer lesions started appearing while continued in the other animals up to the appearance of cancer lesion on the skin to determine the cancer preventive activity of the AML extract.
The animals were divided into six groups of 8 animals each:
1st Group: Normal vehicle group, which are fed with 0.01% DMSO during the experiment and 0.1% acetone was applied on the shaved skin.
2nd Group: Negative Control group, which are fed with 0.01% DMSO and cancer was induced by DMBA/TPA method.
3rd Group: Animals were pretreated with 100mg/ kg BW of AML extract for 3 weeks prior to start the application of carcinogens on the skin.
4th Group: Animals were pretreated with 250mg/ kg BW of AML extract for 3 weeks prior to start the application of carcinogens on the skin.
5th Group: Animals were pretreated with 500mg/ kg BW of AML extract for 3 weeks prior to start the application of carcinogens on the skin.
6th Group: The animals were treated with 500mg/ kg BW of AML extract along with the application of carcinogens on the skin. The group was said to be the Post treated (P.T) group.
In all the experimental groups AML extract feeding was continued till the end of the experiment.
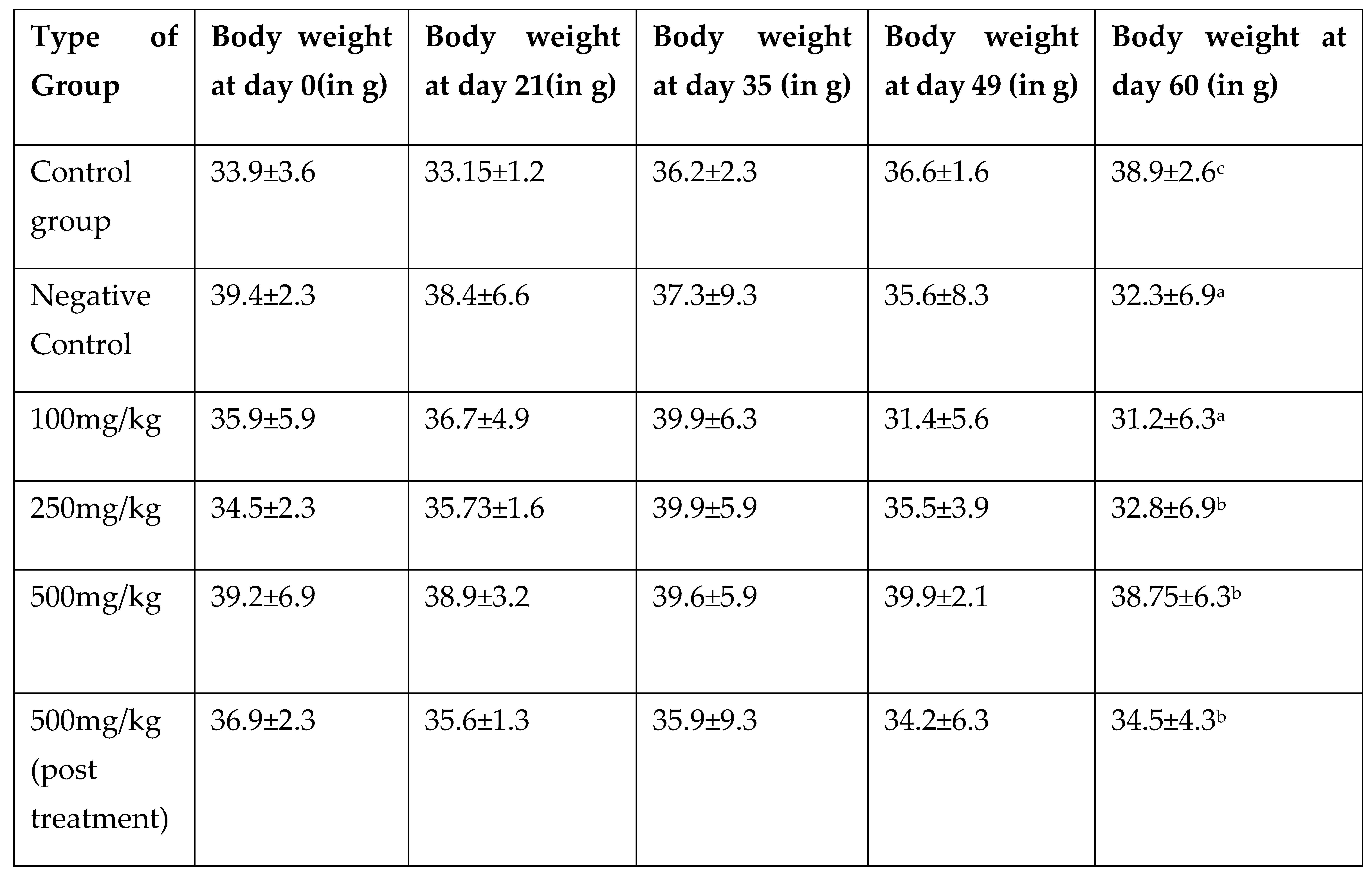
2.5.2. Effect on Body Weight
The weight of mice was noted every week in all the groups using the weighing scale and average % change in weight of mice along the groups was noted upto 60 days.
2.5.3. Effect of AML on Tumour Volume
The animals were subjected to measurement of tumour volume across the groups using Vernier calliper. The formula v=
where D1, D2, D3 are diameters of the tumours (Sharmila and Manoharan, 2012) [
10]. The burden was calculated with the formula at 0, 30th and 60th day by multiplying tumour volume and number of tumours on animal.
2.5.4. Effect on Cancer Induction
After starting the DMBA/TPA treatment, the percentage of animals having cancer lesions was noted at every two weeks interval. The readings were taken on the basis of pre-cancerous lesion occurrence after which the treatment was stopped. The study was conducted till 60 days for observation of tumour development and treatment was continued in the groups which didn’t have tumour incident till the time all animals developed tumour. This gave the evidence of the study of cancer preventive activity of the dose and the time it helped in cancer resistance. After sometime, the lesions transformed into gross papules. Two animals from each group were sacrificed after 60 days of starting the experiment. The blood was collected from these animals and used for haematological analysis and determination of TNF-α and NF-kB signalling. Histopathological analysis of skin tumour tissue was performed. The individual animal was noted and studied along with the statistical analysis for the same which helped in conclusion of the result.
2.5.5. Effect on Histopathological Parameters of Skin Cancer Tissue
The tissue samples of skin tumours were cleaned by removing the extra skin. The tumour tissues were fixed in Neutral Buffer Formalin (NBF), washed in running water overnight to get rid of formalin. The tissues were then embedded in paraffin for section cutting using microtome and then loaded on the slides. The slides were stained in the standard series of Haematoxylin and Eosin and fixed with Dibutylphthalate Polystyrene Xylene (DPX). The slides were then observed under microscope.
2.5.6. Effect on Haematological Parameters
The blood samples from the sacrificed mice from each group were analysed for RBC, Hb, MCV, HCT, MCH, MCHC, WBC, DLC, and Platelets count.
2.5.7. Effect on Weight and Size of Liver and Spleen
The animals were scarified and the weight of the organs including Spleen, liver was measured with the help of weighing balance.
2.5.8. Effect on Inflammatory Cytokine TNF-α Concentration in Serum
The concentrations of TNF-α in serum of each sacrificed animals was measured using ELISA kit, (Catalogue No: ELK9154) as per the manufacturers protocol. The plates were pre coated with the capture antibody. Plates were incubated with 100µl of serum from each animal/ different concentrations of TNF- α standard according to the format and incubated for 90mins. wells were washed with washing buffer further incubated with detection antibody. TMB is used as substrate for the development of colour. The reaction was stopped with stop reagent and reading were taken at 450nm.
2.5.9. Effect on p65 Subunit Concentration of NF-kB Signalling Pathway in Serum
The concentrations of p65 subunit of NF-kB signalling pathway were determined in serum of each using ELISA kit, (Kit Catalogue No: ELK1387) as per the manufacturers protocol. The given protocol was similar to the TNF-α as given above. The reading of developed colour was taken at 450nm.
2.6. Determination of Skin Cancer Treatment Activity of Argemone Mexicana Leaves (AML) Extract
Skin cancer was induced by DMBA/TPA method in 18 normal animals. After the appearance of cancer lesions in the skin of all the animals (approx. 60 days), they were grouped into three groups. The first group act as Negative control, where no treatment was given to cure the cancer. While in the second group 500mg/kg body weight of AML extract was given orally while in third group Doxorubicin chemotherapeutic drug treatment (10mg/kg BW) was given to cure the tumour. The increase/ decrease in size of the tumour was measured in each group up to 120 days.
4. Discussion
One out of every six recorded fatalities worldwide are caused by some sort of malignancy, which is a worrisome statistic as tumours, cancers, and other forms of malignancy have been proven to grow over time. One of them, skin cancer, is a fatal cancer that is common in the United States. Since the skin is the largest organ in the body and cancer often begins in the epidermis, DNA damage brought on by oxidative stress may be to contributing factors [
1,
21]. Chemotherapy, radiotherapy, surgery, and oral medications make up the foundation of a patient's treatment, but the situation never seems to stabilise [
22]. Exploration of benefits of the plant derived phytoconstituents for the chemo preventive activity are been researched on for skin cancer, similar evidences could be derived from researchers like Klos and Chlubek, 2022, gave the comprehensive study on plant terpenoids for anti-melanoma and their Synergistic action of combining terpenoids with other substances [
23].
Argemone mexicana Linn (AM) is one such plant which posses anti-microbial [
24], Cytotoxic [
25], anti-fungal [
26] and anti-inflammatory activity [
27]. Its pharmacological activity was recently explored by Jaiswal
et al., 2023 [
28]. It has been experimented for its anti-cancerous property by different researchers including Chang
et al., 2003, Singh
et al., 2016 and Prabhakaran
et al, 2017 for its of different plant parts and found a significant result [
29,
30,
31]. Exploring the anti-cancerous activity of AM is still an area for potential research but it holds a suspicion as it is well known for its toxic nature and notorious for the case of epidemic dropsy [
32]. Because of presence of Sanguinarine and its analogues seeds and flowers are toxic, but leaves of
Argemone mexicana do not contain these components and are non-toxic and utilized in the present study. A study conducted in clinical trials in Mali and Switzerland also gave evidence supporting the use of plant extract/sap against malaria in humans [
9]. The TNF-α, Nuclear factor-kB (
NF-kB),
STAT3, AKT and
COX-2 and linked with different stages of cancer progression and reported to regulate cancer proliferation, apoptosis, invasion, metastasis and angiogenesis. This highlights the important role of such factors in cancer prevention. In 2003, Ueda and Richmond reported about expression of inflammatory cytokines, NF-kB pathway stimulation and other immunological events that leads to inflammation and eventually to cancer [
33].
In the present study, the ethanolic extract of
Argemone mexicana Linn leaves which was found to possess the best anti-cancer activity in the
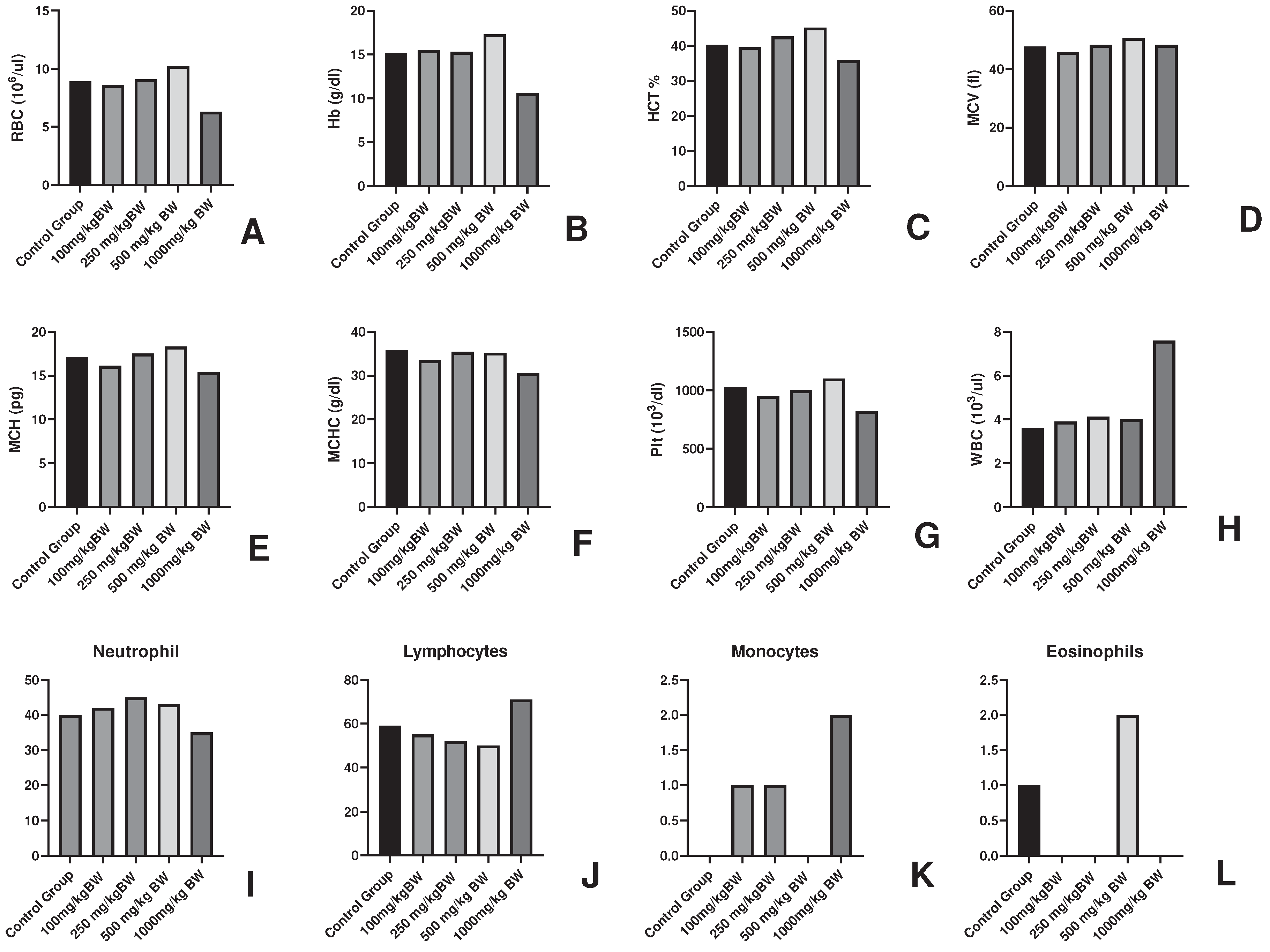
In vitro analysis (unpublished data) was employed for the in vivo study in mice model. The non-toxic dose determination was done with the different doses and 1000mg/kg body weight of the dose was found to be non-toxic as it induced lethargy, weight loss and alterations in haematological findings of the experimental animals. The average weight loss by 1000mg/kg body weight of the dose was -43.3% which denoted the toxicity. On the other hand, a slight increase in body weight could be seen in 250mg/kg and 500mg/kg dose of 22.8% and 21.56 % respectively. This gain in body weight could be accountable to the immunomodulatory property of
Argemone mexicana Linn [
27,
34] which is supported by the 4-5% increase in RBC, Hb, MCV, MCH, Plt at 250mg/kg BW and 500mg/kg BW. The 1000mg/kg body weight dose caused sub-acute toxicity and no causality has been observed in the animals. The study of chemo preventive potential of the AML was carried in DMBA/TPA induced skin cancer in mice model along with the doses 100mg/kg, 250mg/kg and 500mg/kg body weight. The mice model by DMBA/TPA method has been employed for the skin cancer studies by researches including Manoharan and Sharmila where they used Rosmanaric acid for its anti-cancer activity [
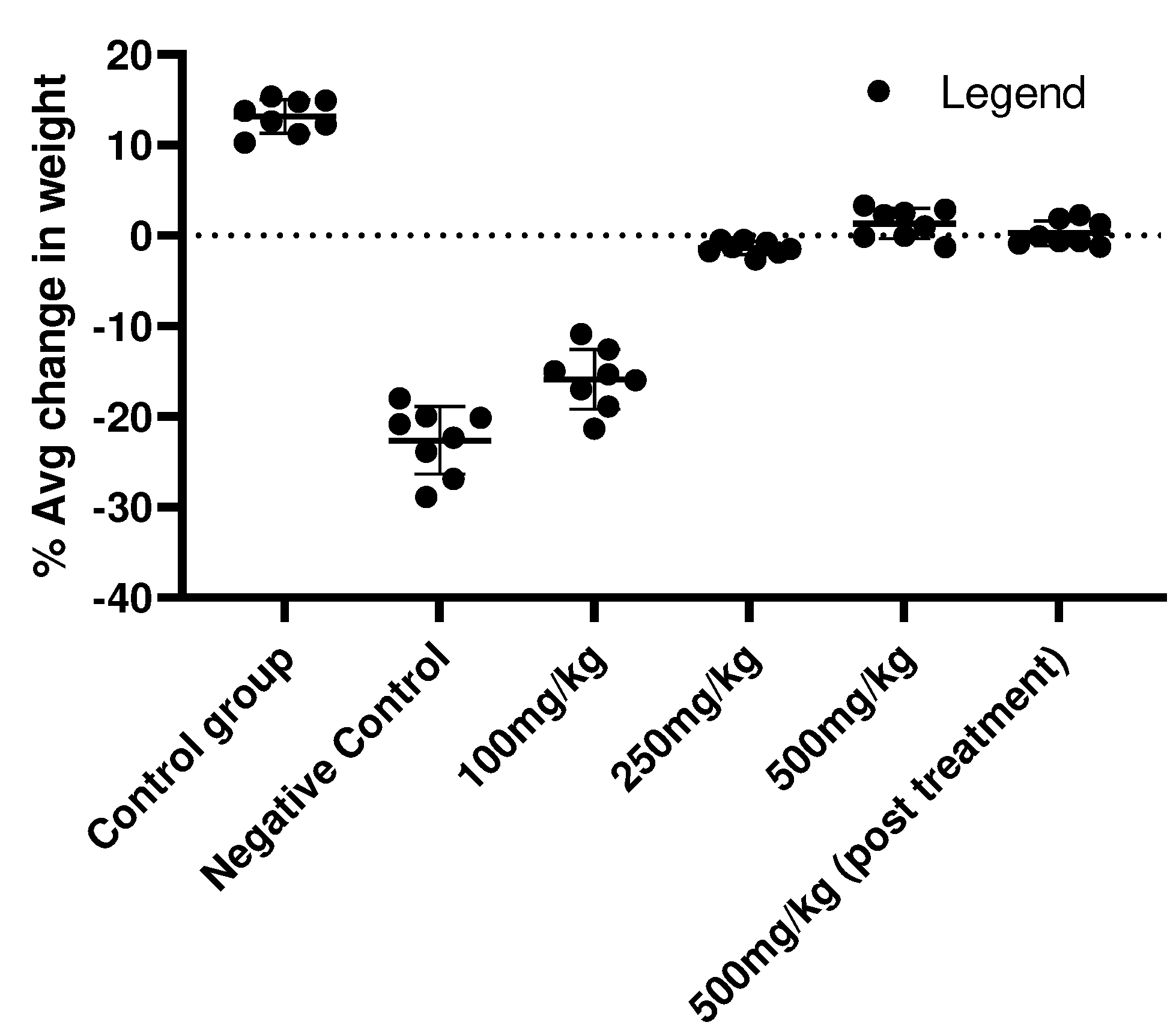
11]. The animals were examined through various parameter including change in weight, incident of cancer induction, blood parameters, histopathology, etc. Analysis of body weight gave a clear picture of cancer induction in the animals where average weight loss in Negative Control (-18%), 100mg/kg BW (-15%), 250mg/kg BW (-5%), 500mg/kg BW (0.015%), 500 mg/kg BW (-1.2%) in comparison to the Control vehicle group where the average body weight gain was 13.8%.
The DMBA/TPA induction resulted in 100% tumour in Negative Control group which was fed with 0.1% DMSO (1089.69 mm
3). The tumour volume and burden could also be correlated with the loss in weight, the average tumour burden could be found significant (P<0.001) in the 100mg/kg body weight in comparison to the Negative Control group where the total burden of tumour was the highest (895.89mm
3). Tumour burden could also be observed in 250mg/kg (423.96mm
3) and 500mg/kg (296.89mm
3) (P.T) groups on 60
th day of experiment, and only a few animals 2 in number showed a pre-cancerous lesion of 500mg/kg group. The animals which already showed the pre-cancerous lesions were stopped with the DMBA/TPA treatment, but the animals not showing the cancer induction were continued with the treatment. The experiment gave the time interval till which the 500mg/kg ethanolic extract of AML resisted the growth of cancer. it could be concluded that with this experiment, the extract resisted the cancer growth till ±32 days in comparison to Negative Control group where all the animals have developed the cancer. The pre-cancerous lesions in 500mg/kg BW group could be observed after ±56 days in comparison to the Negative Control group i.e after the 500mg/kg BW group showed precancerous lesion after ±32 days than that of Negative Control group. A difference ±15 days of cancer induction could be observed in 500mg/kg pre and post treated group which showed the cancer-preventive activity of the AML extracts. Similar experiment was quoted by Sharmila and Mahoharan, 2012 [
10].
In the other parameters including blood parameters, a surge in the parameters could be seen in the Negative Control group and the best results could be found in 500mg/kg group which supported the anti-cancerous activity of the group at blood level. The decrease in number of RBC and increase in WBC was also quoted in [
35].
Histopathology revealed the occurrence of distorted cells with enlarged nucleus, formation of keratin pearls and hyperplastic lesions in the Negative Control group which gave the evidence of squamous cell carcinoma in the animals. The 100mg/kg BW group showed similar feature as the Negative Control which indicated that the AML extract had non-significant effect on the cancer prevention in the animals at this dose. On the other hands, the presence of a greater number of normal cells than the distorted hyperplastic cell and few of them on a verge of turning cancerous highlighted effect of 250mg/kg dose of AML. In 500mg/kg BW group, the histopathology was found to be similar as of the normal skin, but with a bit thickening and abnormality in the epidermal layer which could be corelated to the occurrence of pre-cancerous lesion the animal. The 500 mg/kg (P.T) BW of the group showed the presence of hyperplasia at certain sites and the other was found to be normal. The pretreatment at 500mg/kg BW dose was better than post treatment 500mg/kg group in resisting the cancer induction. This could be supported by the fact that the Ayurved always focuses on prevention rather than cure. The results were compared and correlated with the studies given by Manhoran and Selvan, 2012 [
11], Chen
et al.,2009 [
36], Paolino
et al., 2017 [
37].
The effect of dose of AML leaves could also be seen on the weight of organs extracted from the sacrificed animals where a splenomegaly could be observed in the spleen of Negative Control animals due to increased no. in WBC under influence of cancer induction. The findings were also mentioned by Goel, 2020 [
27] where the study highlighted the immunomodulatory response of AML aquoues extract. The variation in size of spleen could be noticed in a dose-dependent manner where the weight of 500mg/kg BW group was of almost similar to the weight of Control vehicle animal spleen. The liver could only be seen enlarged in the Negative Control group and was similar normal in other groups. The findings were in synchronization with the severity of cancer induction amongst the groups .
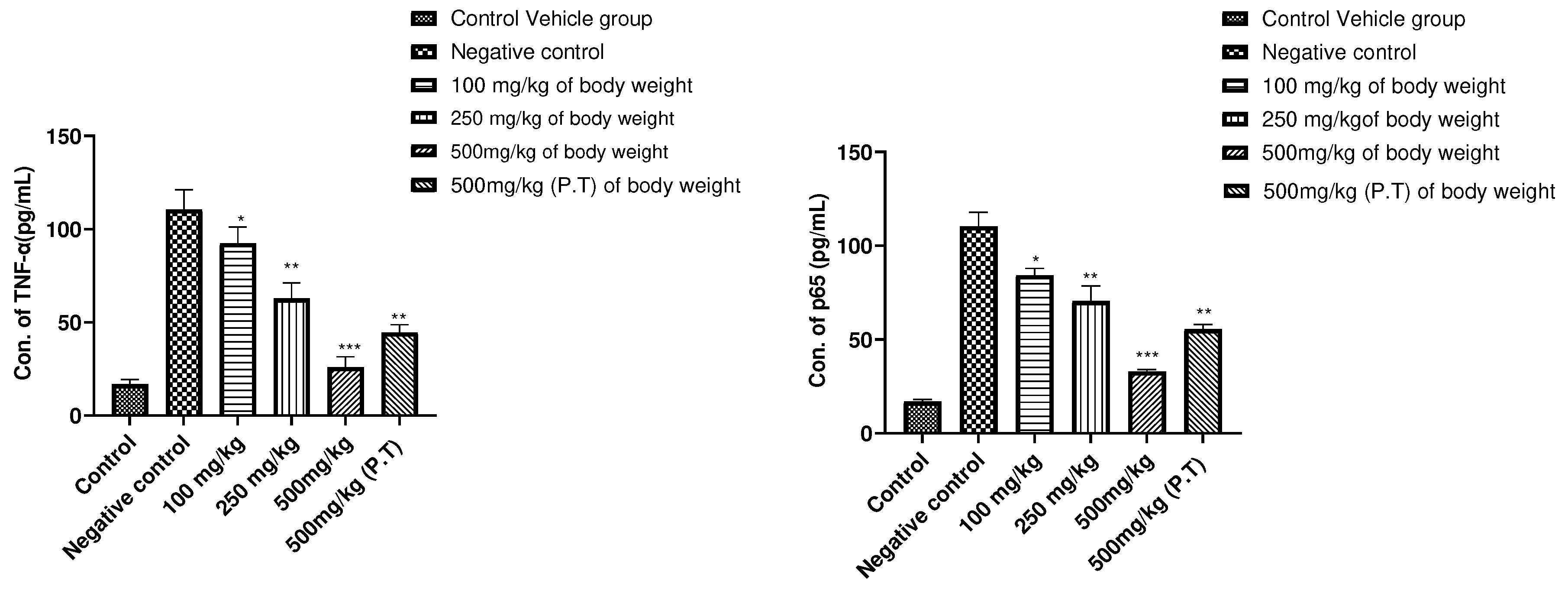
The TNF-α is a pro-inflammatory cytokine involved in tumorigenic role [
38]. As the DMBA/TPA carcinogenesis process involves TNF-α mediated increase in nuclear translocation of NF-kB (A transcription factor) which facilitates the survival and proliferation of neoplastic cells [
39]. On the cytokine level, the TNF-α concentration was found to be decrease significantly in 500mg/kg BW vs Negative Control group (P<0.001), also 100mg/kg, 250mg/kg, 500mg/kg BW (P.T) (P>0.05) statistically. And in case of NF-kB signalling pathway, the concentration of p65 subunit was found to be regulated in 500mg/kg BW group significantly (P<0.001) as compared to the Control group. P<0.05 in 100mg/kg, 250mg/kg and 500 mg/kg PT group. This study confirms the blocking of NF-kB inflammatory pathway for the production of different inflammatory cytokines as well as supports the above findings where cancer was prevented maximally by AML extract at 500mg/kg BW of the dose in experimental animals
As the treatment of AML extract as well as standard Doxorubicin drug did not show any curative effect, thus found insignificant. This again emphasize on the better results of the pretreatment with AYUSH drugs.
Author Contributions
Conceptualization, S.K, A.G.; validation, A.G., N.J.S, S.F, B.S; formal analysis, S.K, A.G; Investigation, S.K, A.G.; Resources, S.K, A.G, N.J.S, S.F, B.S; Data curation, S.K, A.G; writing—original draft preparation, S.K. A.G; writing—review and editing, A.G. N.J.S, S.F, B.S; Supervision, A.G Financial Support, N.J.S, S.F. All authors have read and agreed to the published version of the manuscript
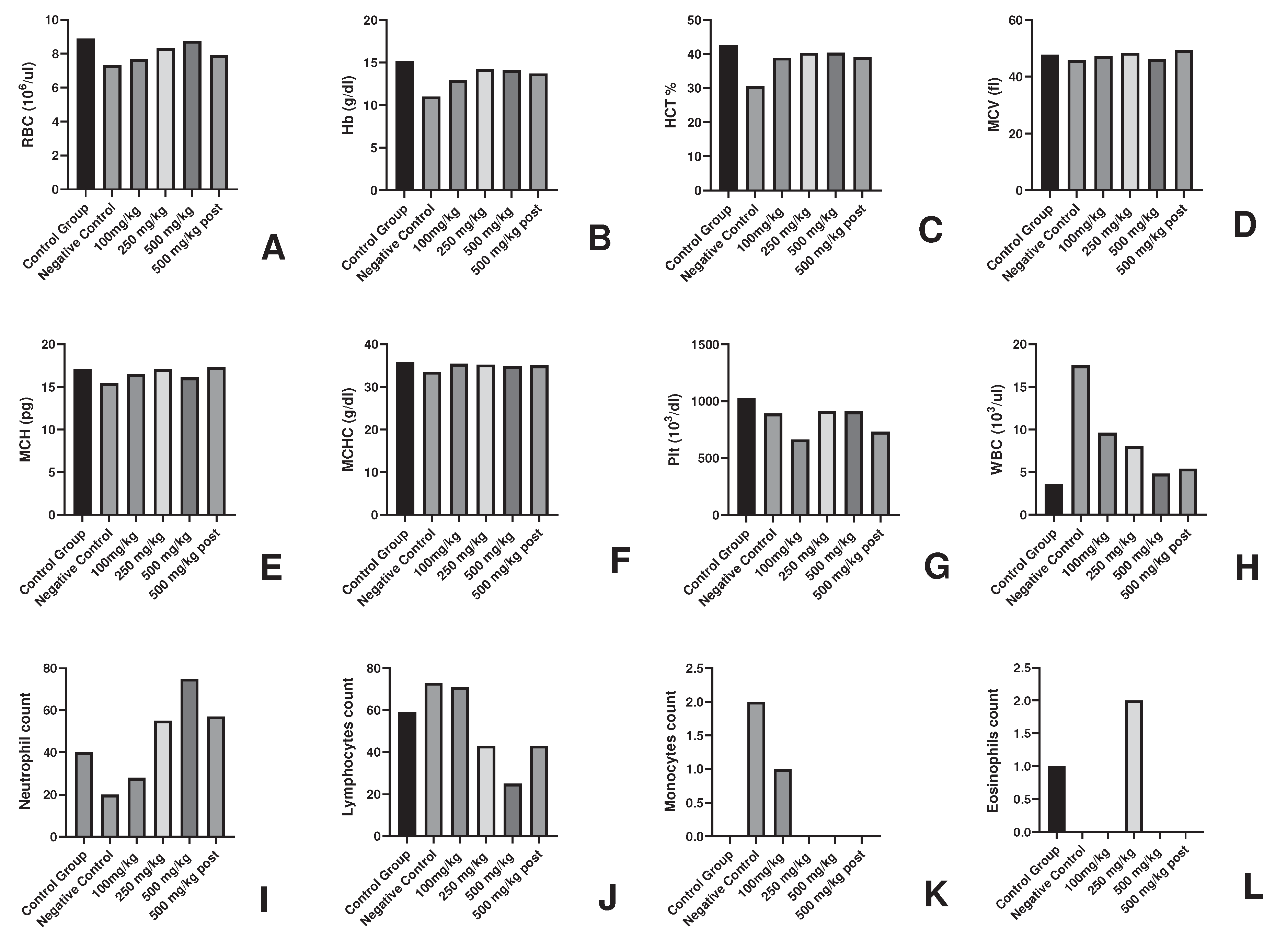
Figure 1.
Haematological parameters in AML fed groups of experimental animals for determination of Non-toxic dose (A-L).
Figure 1.
Haematological parameters in AML fed groups of experimental animals for determination of Non-toxic dose (A-L).
Figure 2.
Percentage of weight change in cancer induced experimental animals at 60th day.
Figure 2.
Percentage of weight change in cancer induced experimental animals at 60th day.
Figure 3.
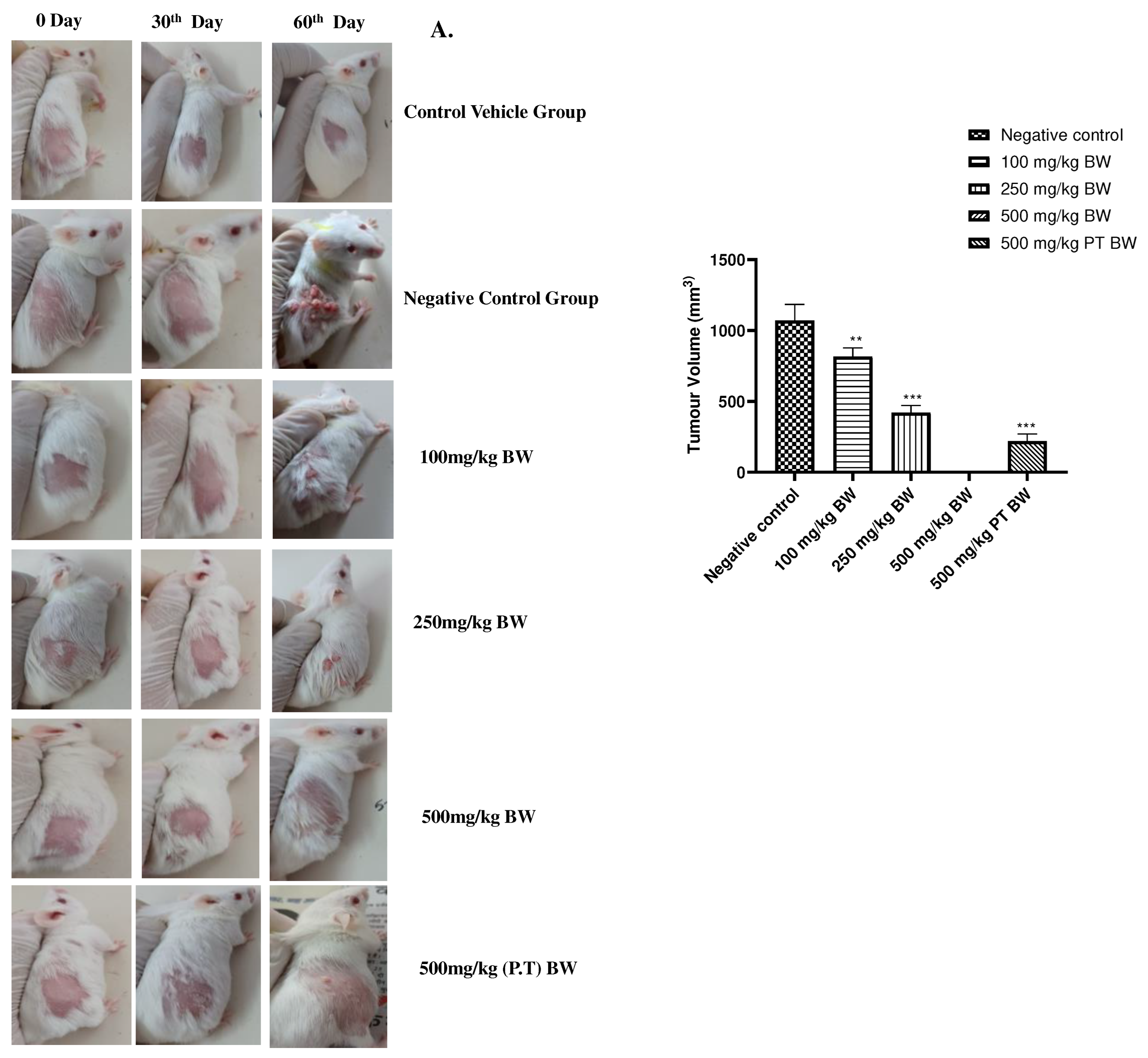
Prevention of cancer induction by AML. A.) The gross tumour appearance in mice and variation in sizes amongst the group at 30th and 60th day of post-cancer induction. Highest tumour load could be observed in Negative Control group and no tumour growth or lesion in 500mg/kg BW group. B.) The tumour volume was found to significantly reduced (P<0.05) in 100mg/kg BW 250mg/kg BW, 500 mg/kg BW (P.T). 100mg/kg BW was found to be significant statistically P<0.001 in the most in Negative Control group and significantly no tumour growth in 500mg/kb body weight group.
Figure 3.
Prevention of cancer induction by AML. A.) The gross tumour appearance in mice and variation in sizes amongst the group at 30th and 60th day of post-cancer induction. Highest tumour load could be observed in Negative Control group and no tumour growth or lesion in 500mg/kg BW group. B.) The tumour volume was found to significantly reduced (P<0.05) in 100mg/kg BW 250mg/kg BW, 500 mg/kg BW (P.T). 100mg/kg BW was found to be significant statistically P<0.001 in the most in Negative Control group and significantly no tumour growth in 500mg/kb body weight group.
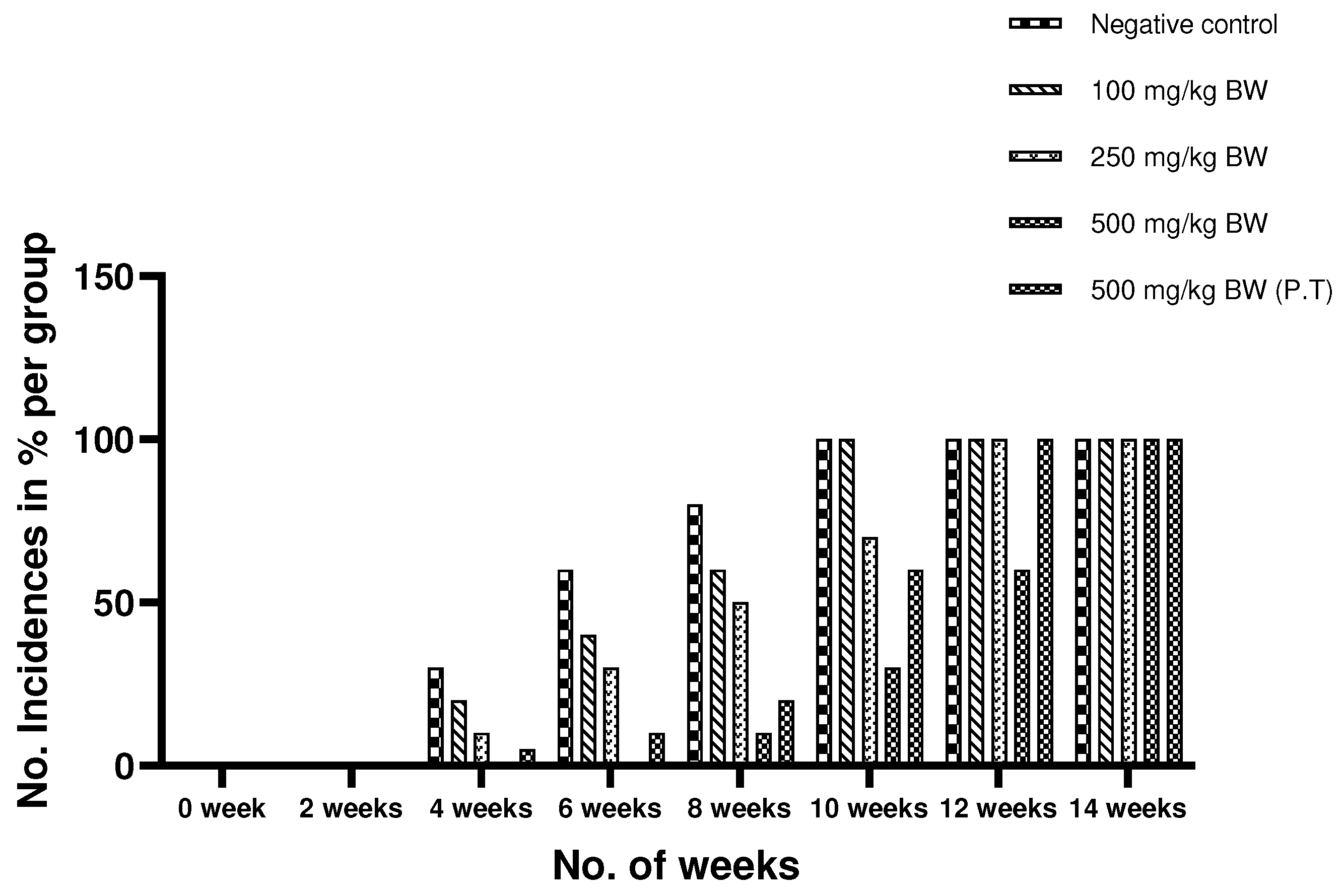
Figure 4.
Incidence of cancer induction in percentage experimental animals in a group using DMBA/TPA method. The observations have been made on the basis of occurrence of pre-cancerous lesion in the groups.
Figure 4.
Incidence of cancer induction in percentage experimental animals in a group using DMBA/TPA method. The observations have been made on the basis of occurrence of pre-cancerous lesion in the groups.
Figure 5.
Histopathological examination of skin tissue from the sacrificed mice of all experimental groups. (A.) shows the normal skin from the Control vehicle group where all the layers of skin including epidermis, dermis and hypodermis in their normal condition along with the hair follicles, sebaceous gland. The Negative Control group (B.) shows highly distorted cells, hyperplastic lesions along with the formation of keratin pearl, large, dark coloured and uneven shaped nucleus indicated the squamous cell carcinoma. The 100mg/kg BW group (C.) shows similar structures as of B, the formation of keratin pearl, distorted cell arrangements indicating the cancer formation. The 250mg/kg BW group (D.) showed mixture of disarranged cells and normal cells. Hyperplastic lesions could be observed along with benign types of cells. E.) depicting the 500mg/kg BW group shows similar structure like the Control vehicle group, with a slight thickening in the epidermis layer. Whereas in the F.) The disorderly arranged cells could only be seen at some sights. Hyperplastic thickening could be observed.
Figure 5.
Histopathological examination of skin tissue from the sacrificed mice of all experimental groups. (A.) shows the normal skin from the Control vehicle group where all the layers of skin including epidermis, dermis and hypodermis in their normal condition along with the hair follicles, sebaceous gland. The Negative Control group (B.) shows highly distorted cells, hyperplastic lesions along with the formation of keratin pearl, large, dark coloured and uneven shaped nucleus indicated the squamous cell carcinoma. The 100mg/kg BW group (C.) shows similar structures as of B, the formation of keratin pearl, distorted cell arrangements indicating the cancer formation. The 250mg/kg BW group (D.) showed mixture of disarranged cells and normal cells. Hyperplastic lesions could be observed along with benign types of cells. E.) depicting the 500mg/kg BW group shows similar structure like the Control vehicle group, with a slight thickening in the epidermis layer. Whereas in the F.) The disorderly arranged cells could only be seen at some sights. Hyperplastic thickening could be observed.
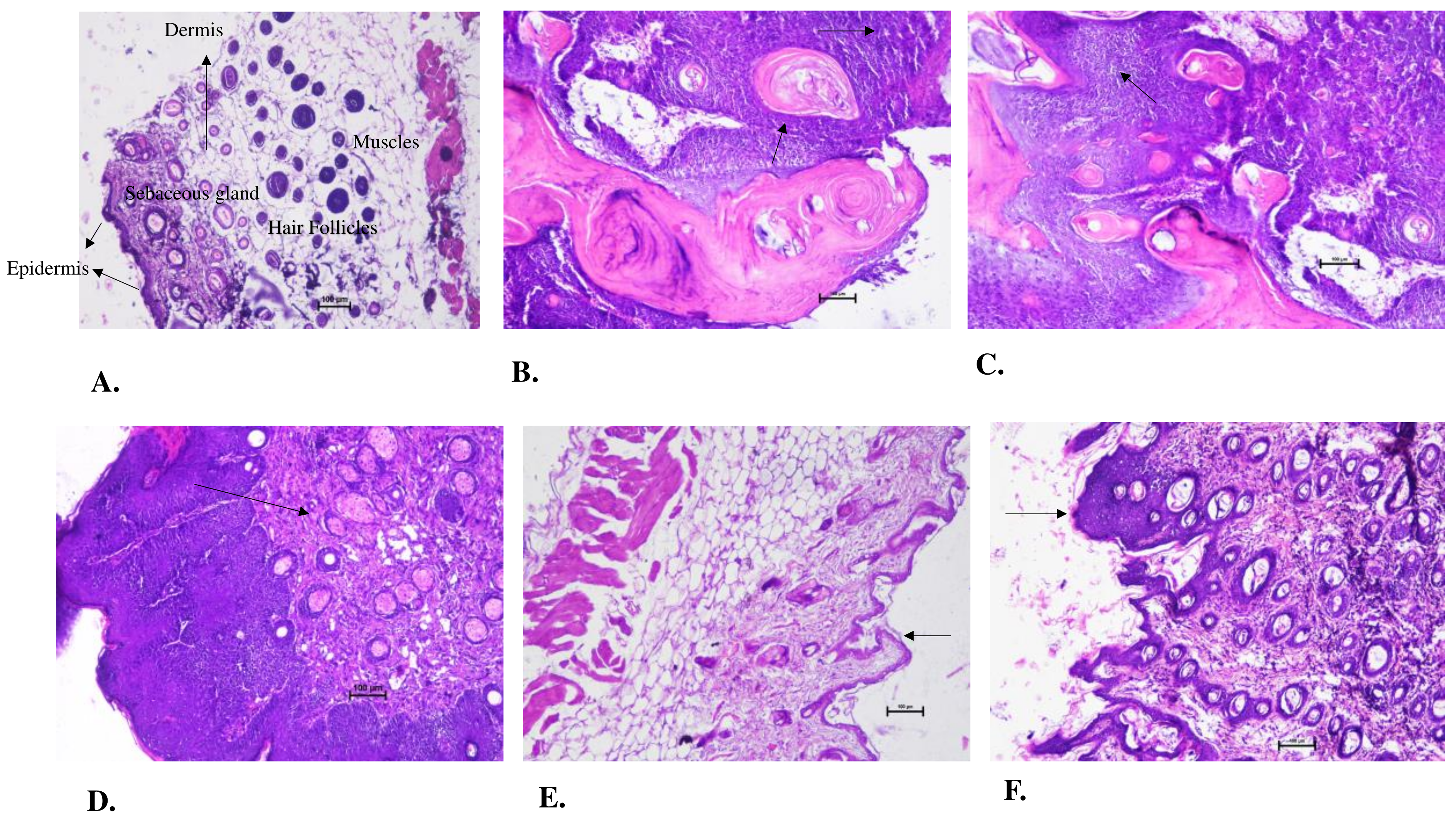
Figure 6.
Effect of AML extract of Haematology of cancer induced experimental animals.
Figure 6.
Effect of AML extract of Haematology of cancer induced experimental animals.
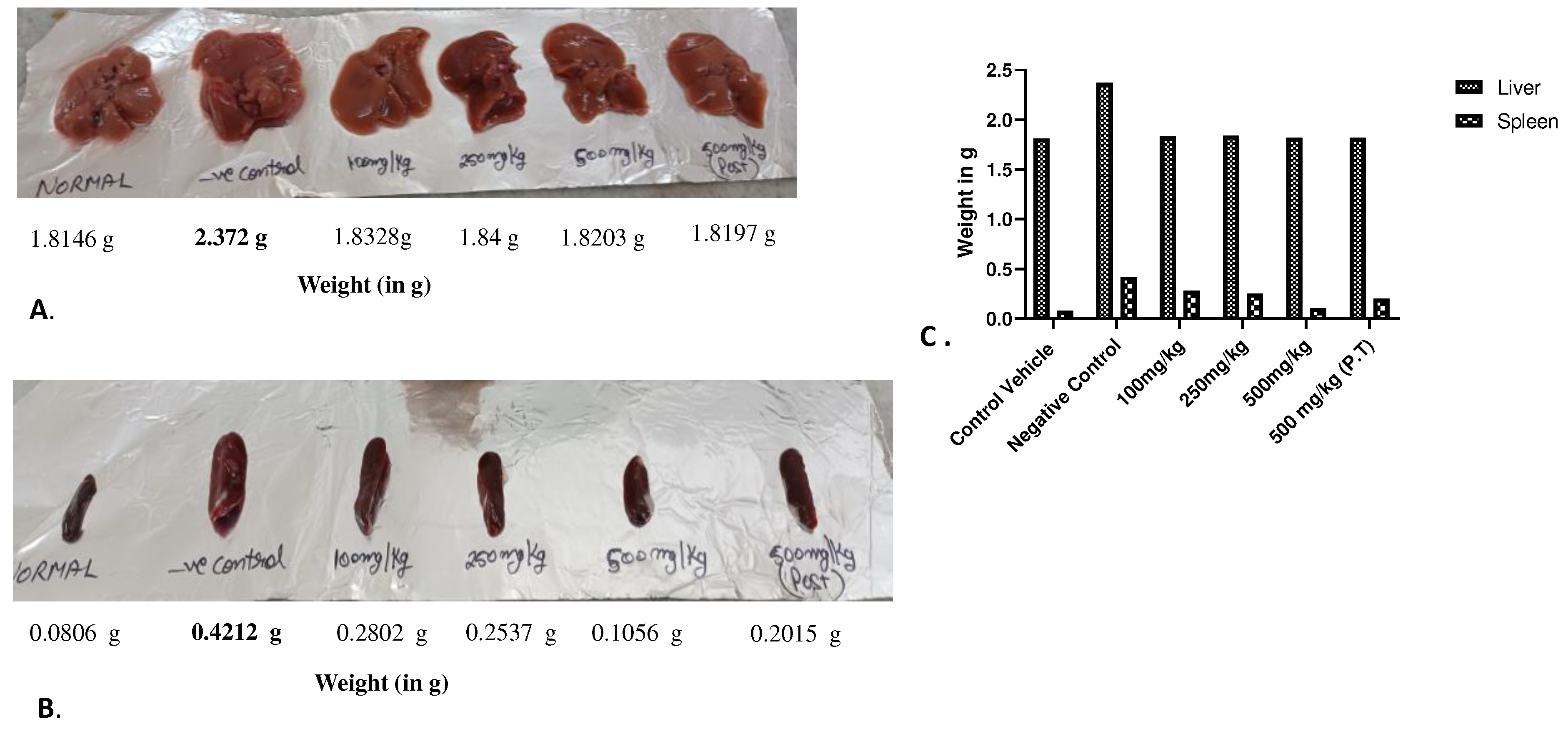
Figure 7.
Size and weight of A.) liver B.) The Spleen C.) Average weight of organ from the sacrificed experimental animals on 60th day of experiment. It could be seen that the liver in A.) in the Negative Control group is enlarged and normal in other groups whereas in case of spleen B.) splenomegaly could be observed in Negative Control group. Other groups also exhibit increase size in spleen which is visibly resisted by the 500mg/kg BW of AML extract.
Figure 7.
Size and weight of A.) liver B.) The Spleen C.) Average weight of organ from the sacrificed experimental animals on 60th day of experiment. It could be seen that the liver in A.) in the Negative Control group is enlarged and normal in other groups whereas in case of spleen B.) splenomegaly could be observed in Negative Control group. Other groups also exhibit increase size in spleen which is visibly resisted by the 500mg/kg BW of AML extract.
Figure 8.
The alteration in pattern of (A.) TNF-α expression and (B.) p65 subunit of NF-kB signalling pathway from the mice serum where it could be seen that the concentration of TNF-α was found to be downregulated in 500mg/kg BW groups significantly (P<0.001). ***P<0.001, **P<0.01, *P<0.05 defines significance decrease in concentration level in comparison to the Negative Control group. The results were calcualted using One way ANOVA followed by Dunnett test.
Figure 8.
The alteration in pattern of (A.) TNF-α expression and (B.) p65 subunit of NF-kB signalling pathway from the mice serum where it could be seen that the concentration of TNF-α was found to be downregulated in 500mg/kg BW groups significantly (P<0.001). ***P<0.001, **P<0.01, *P<0.05 defines significance decrease in concentration level in comparison to the Negative Control group. The results were calcualted using One way ANOVA followed by Dunnett test.
Table 1.
Physical features in experimental animals for determination of Non-toxic dose of Argemone mexicana Linn leaves extract.
Table 1.
Physical features in experimental animals for determination of Non-toxic dose of Argemone mexicana Linn leaves extract.
Table 2.
Average change in weight of experimental animals for determination of Maximum Non-toxic dose where ***P<0.001 is significant decrease in weight in comparison to Control Group. The values are expressed as mean ±SD for 8 animals. The results were calcualted using One way ANOVA followed by Dunnett test.
Table 2.
Average change in weight of experimental animals for determination of Maximum Non-toxic dose where ***P<0.001 is significant decrease in weight in comparison to Control Group. The values are expressed as mean ±SD for 8 animals. The results were calcualted using One way ANOVA followed by Dunnett test.
Table 3.
Average change in body weight in cancer induced animals along with the given treatment of AML where significant decrease (***P<0.001) in Negative Control group was observed. The values are expressed as means ±SD for 8 animals. Values indicating a***P<0.001, b**P<0.05, c*P<0.05 were found to be statistically significant. The results were calcualted using One way ANOVA followed by Dunnett test.
Table 3.
Average change in body weight in cancer induced animals along with the given treatment of AML where significant decrease (***P<0.001) in Negative Control group was observed. The values are expressed as means ±SD for 8 animals. Values indicating a***P<0.001, b**P<0.05, c*P<0.05 were found to be statistically significant. The results were calcualted using One way ANOVA followed by Dunnett test.