Submitted:
21 November 2023
Posted:
22 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Search methods
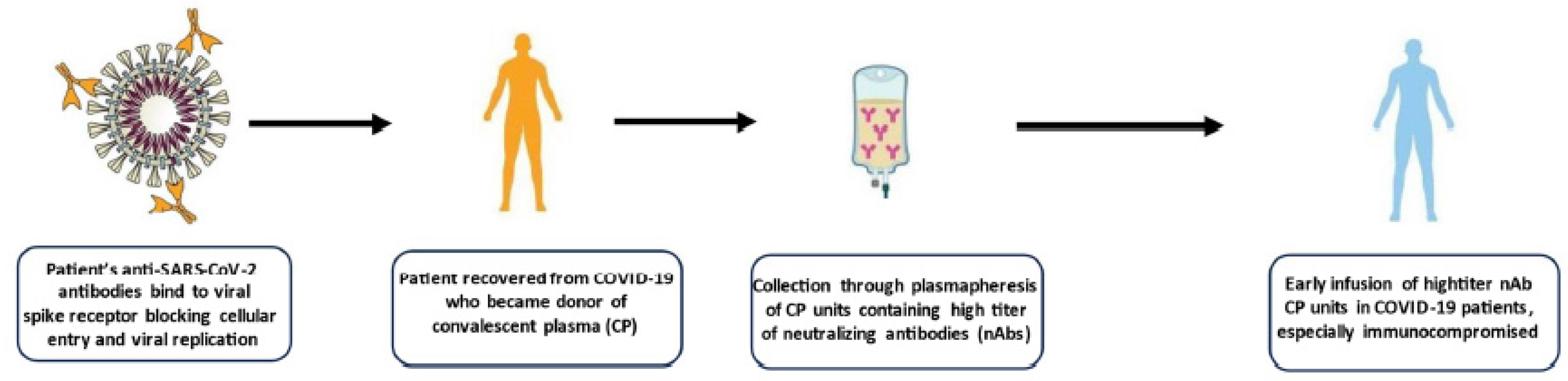
3. Convalescent plasma
3.1. Characteristics of CCP
3.2. Safety of CCP
3.3. Clinical indications of CCP
a) Hospitalized patients
b) Outpatients
c) Immunocompromised patients
4. Hyperimmune immunoglobulins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus disease (COVID-19) pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey={adgroupsurvey}&gclid=EAIaIQobChMI09ix0OX0gAMV1pNoCR0H-w_aEAAYASAAEgJQpfD_BwE (accessed on 14 November 2023).
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Nguyen-Van-Tam, J.S.; Beck, C.R.; et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Vaglio, S.; Pupella, S.; Facco, G.; Catalano, L.; Liumbruno, G.M.; Grazzini, G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016, 14, 152–7. [Google Scholar] [CrossRef] [PubMed]
- Luke, T.C.; Casadevall, A.; Watowich, S.J.; Hoffman, S.L.; Beigel, J.H.; Burgess, T.H. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010, 38, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.G.; van Helmond, N.; Senefeld, J.W.; Wiggins, C.C.; Klassen, S.A.; Baker, S.E.; Larson, K.F.; Murphy, B.M.; Andersen, K.J.; Ford, S.K.; et al. Convalescent Plasma for Infectious Diseases: Historical Framework and Use in COVID-19. Clin Microbiol Newsl. 2021, 43, 23–32. [Google Scholar] [CrossRef]
- Bloch, E.M.; Shoham, S.; Casadevall, A.; Sachais, B.S.; Shaz, B.; Winters, J.L.; van Buskirk, C.; Grossman, B.J.; Joyner, M.; Henderson, J.P.; et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020, 130, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Grossman, B.J.; Henderson, J.P.; Joyner, M.J.; Shoham, S.; Pirofski, L.A.; Paneth, N. The Assessment of Convalescent Plasma Efficacy against COVID-19. Med. 2020, 1, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Wendel, S.; Land, K.; Devine, D.V.; Daly, J.; Bazin, R.; Tiberghien, P.; Lee, C.K.; Arora, S.; Patidar, G.K.; Khillan, K.; et al. Lessons learned in the collection of convalescent plasma during the COVID-19 pandemic. Vox Sang. 2021, 116, 872–879. [Google Scholar] [CrossRef]
- Franchini, M.; Marano, G.; Velati, C.; Pati, I.; Pupella, S.; Maria Liumbruno, G. Operational protocol for donation of anti-COVID-19 convalescent plasma in Italy. Vox Sang. 2021, 116, 136–137. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M. Impact of pathogen-reduction technologies on COVID-19 convalescent plasma potency. Transfus Clin Biol. 2021, 28, 132–134. [Google Scholar] [CrossRef]
- Del Fante, C.; Franchini, M.; Baldanti, F.; Percivalle, E.; Glingani, C.; Marano, G.; Mengoli, C.; Mortellaro, C.; Viarengo, G.; Perotti, C.; et al. A retrospective study assessing the characteristics of COVID-19 convalescent plasma donors and donations. Transfusion. 2021, 61, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Casadevall, A. Pathogen reduction technologies need to evaluate Fc-mediated antibody functions. Transfusion. 2022, 62, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.; Carlton, L.; Paterson, A.; et al. Neutralization capacity of convalescent plasma against SARS-CoV-2 omicron sublineages: Implications for donor selection. Vox sanguinis 2023. [CrossRef] [PubMed]
- Focosi, D.; Joyner, M.J.; Casadevall, A. Recent Hybrid Plasma Better Neutralizes Omicron Sublineages Than Old Hyperimmune Serum. Clin Infect Dis. 2023, 76, 554. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M.; Pirofski, L.A.; Burnouf, T.; Fairweather, D.; Joyner, M.J.; Casadevall, A. COVID-19 Convalescent Plasma Is More than Neutralizing Antibodies: A Narrative Review of Potential Beneficial and Detrimental Co-Factors. Viruses. 2021, 13, 1594. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Maggi, F.; Focosi, D. ABO blood group-related mechanism of infection of SARS-CoV-2: an overview of systematic reviews. Clin Chem Lab Med. 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Arthur, C.M.; Wang, J.; Verkerke, H.; Josephson, C.D.; Kalman, D.; et al. The SARS-CoV-2 receptor-binding domain preferentially recognizes blood group A. Blood Adv 2021, 5, 1305–9. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, T.; Ma, L.; Zhang, H.; Wang, H.; Wei, W.; et al. The impact of ABO blood group on COVID-19 infection risk and mortality: a systematic review and meta-analysis. Blood Rev 2021, 48, 100785. [Google Scholar] [CrossRef] [PubMed]
- Huaman, M.A.; Raval, J.S.; Paxton, J.H.; Mosnaim, G.S.; Patel, B.; Anjan, S.; Meisenberg, B.R.; Levine, A.C.; Marshall, C.E.; Yarava, A.; et al. Transfusion reactions associated with COVID-19 convalescent plasma in outpatient clinical trials. Transfusion. 2023, 63, 1639–1648. [Google Scholar] [CrossRef]
- Franchini, M.; Cruciani, M.; Casadevall, A.; et al. Safety of COVID-19 convalescent plasma. A definitive systematic review and meta-analysis of randomized controlled trials. Transfusion 2023.
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Perotti, C.; Baldanti, F.; Bruno, R.; Del Fante, C.; Seminari, E.; Casari, S.; Percivalle, E.; Glingani, C.; Musella, V.; Belliato, M.; et al. Covid-Plasma Task Force. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020, 105, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Joyner, M.J.; Pirofski, L.A.; Senefeld, J.W.; Shoham, S.; Sullivan, D.; Paneth, N.; Focosi, D. Convalescent plasma therapy in COVID-19: Unravelling the data using the principles of antibody therapy. Expert Rev Respir Med. 2023, 17, 381–95. [Google Scholar] [CrossRef] [PubMed]
- Senefeld, J.W.; Gorman, E.K.; Johnson, P.W.; Moir, M.E.; Klassen, S.A.; Carter, R.E.; Paneth, N.S.; Sullivan, D.J.; Morkeberg, O.H.; Wright, R.S.; et al. Rates Among Hospitalized Patients With COVID-19 Treated With Convalescent Plasma: A Systematic Review and Meta-Analysis. Mayo Clin Proc Innov Qual Outcomes. 2023, 7, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Iannizzi, C.; Chai, K.L.; Piechotta, V.; Valk, S.J.; Kimber, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; McQuilten, Z.; et al. Convalescent plasma for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2023, 5, CD013600. [Google Scholar] [CrossRef]
- Casadevall, A.; Dragotakes, Q.; Johnson, P.W.; Senefeld, J.W.; Klassen, S.A.; Wright, R.S.; Joyner, M.J.; Paneth, N.; Carter, R.E. Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality. Elife. 2021, 10, e69866. [Google Scholar] [CrossRef]
- Lang, K. Is convalescent plasma still useful as a covid treatment? BMJ. 2023, 383, 2185. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M.; Pirofski, L.A.; et al. COVID-19 Convalescent Plasma and Clinical Trials: Understanding Conflicting Outcomes. Clin Microbiol Rev. 2022, 35, e0020021. [Google Scholar] [CrossRef]
- Menichetti, F.; Popoli, P.; Puopolo, M.; et al. TSUNAMI Study group. Effect of High-Titer Convalescent Plasma on Progression to Severe Respiratory Failure or Death in Hospitalized Patients with COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Netw Open. 2021, 4, e2136246. [Google Scholar] [CrossRef]
- Körper, S.; Weiss, M.; Zickler, D.; Wiesmann, T.; Zacharowski, K.; Corman, V.M.; Grüner, B.; Ernst, L.; Spieth, P.; Lepper, P.M.; et al. Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19. J Clin Invest. 2021, 131, e152264. [Google Scholar] [CrossRef]
- Ortigoza, M.B.; Yoon, H.; Goldfeld, K.S.; Troxel, A.B.; Daily, J.P.; Wu, Y.; Li, Y.; Wu, D.; Cobb, G.F.; Baptiste, G.; et al. Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients: A Randomized Clinical Trial. JAMA Intern Med. 2022, 182, 115–126. [Google Scholar] [CrossRef]
- De Santis, G.C.; Oliveira, L.C.; Garibaldi, P.M.M.; Almado, C.E.L.; Croda, J.; Arcanjo, G.G.A.; Oliveira, É.A.F.; Tonacio, A.C.; Langhi DMJr Bordin, J.O.; et al. High-Dose Convalescent Plasma for Treatment of Severe COVID-19. Emerg Infect Dis. 2022, 28, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bennett-Guerrero, E.; Romeiser, J.L.; Talbot, L.R.; Ahmed, T.; Mamone, L.J.; Singh, S.M.; Hearing, J.C.; Salman, H.; Holiprosad, D.D.; Freedenberg, A.T.C.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York: A Double-Blind Randomized Trial. Crit Care Med. 2021, 49, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H.; et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020, 324, 460–470, Erratum in: JAMA. 2020, 324, 519. [Google Scholar] [CrossRef]
- Gharbharan, A.; Jordans, C.; Zwaginga, L.; Papageorgiou, G.; van Geloven, N.; van Wijngaarden, P.; den Hollander, J.; Karim, F.; van Leeuwen-Segarceanu, E.; Soetekouw, R.; et al. CoV-Early study group. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial. Clin Microbiol Infect. 2023, 29, 208–214. [Google Scholar] [CrossRef]
- Lacombe, K.; Hueso, T.; Porcher, R.; Mekinian, A.; Chiarabini, T.; Georgin-Lavialle, S.; Ader, F.; Saison, J.; Martin-Blondel, G.; De Castro, N.; et al. Use of covid-19 convalescent plasma to treat patients admitted to hospital for covid-19 with or without underlying immunodeficiency: open label, randomised clinical trial. BMJ Med. 2023, 2, e000427. [Google Scholar] [CrossRef] [PubMed]
- Misset, B.; Piagnerelli, M.; Hoste, E.; Dardenne, N.; Grimaldi, D.; Michaux, I.; De Waele, E.; Dumoulin, A.; Jorens, P.G.; van der Hauwaert, E.; et al. Convalescent Plasma for Covid-19-Induced ARDS in Mechanically Ventilated Patients. N Engl J Med. 2023, 389, 1590–600. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.A.; Henderson, J.P.; Shah, P.K.; et al. COVID-19 and Cancer Consortium. Association of Convalescent Plasma Therapy with Survival in Patients with Hematologic Cancers and COVID-19. JAMA Oncol, 2021; 7, 1167–1175. [Google Scholar]
- Donato, M.L.; Park, S.; Baker, M.; et al. Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma. JCI Insight 2021, 6, e143196. [Google Scholar] [CrossRef]
- Briggs, N.; Gormally, M.V.; Li, F.; et al. Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. PLoS One 2021, 16, e0254453. [Google Scholar] [CrossRef]
- Zhou, C.K.; Bennett, M.M.; Villa, C.H.; et al. Multi-center matched cohort study of convalescent plasma for hospitalized patients with COVID-19. PLoS One 2022, 17, e0273223. [Google Scholar] [CrossRef]
- Franchini, M.; Corsini, F.; Focosi, D.; Cruciani, M. Safety and Efficacy of Convalescent Plasma in COVID-19: An Overview of Systematic Reviews. Diagnostics 2021, 11, 1663. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M. Home and Out-of-Hospital Therapy with COVID-19 Convalescent Plasma in Europe. Life 2022, 12, 1704. [Google Scholar] [CrossRef]
- Focosi, D.; Casadevall, A. Convalescent plasma in outpatients with COVID-19. Lancet Respir Med. 2022, 10, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Pérez Marc, G.; Wappner, D.; et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Gebo, K.A.; Shoham, S.; et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N Engl J Med. 2022, 386, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Baksh, S.N.; Heath, S.L.; Fukuta, Y.; Shade, D.; Meisenberg, B.; Bloch, E.M.; Tobian, A.A.R.; Spivak, E.S.; Patel, B.; Gerber, J.; et al. Symptom Duration and Resolution with Early Outpatient Treatment of Convalescent Plasma for Coronavirus Disease 2019: A Randomized Trial. J Infect Dis. 2023, 227, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Gebo, K.A.; Heath, S.L.; Fukuta, Y.; Zhu, X.; Baksh, S.; Abraham, A.G.; Habtehyimer, F.; Shade, D.; Ruff, J.; Ram, M.; et al. CSSC-004 Consortium. Early antibody treatment, inflammation, and risk of post-COVID conditions. mBio. 2023, 19, e0061823. [Google Scholar] [CrossRef] [PubMed]
- Gharbharan, A.; Jordans, C.; Zwaginga, L.; Papageorgiou, G.; van Geloven, N.; van Wijngaarden, P.; den Hollander, J.; Karim, F.; van Leeuwen-Segarceanu, E.; Soetekouw, R.; et al. CoV-Early study group. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial. Clin Microbiol Infect. 2023, 29, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Alemany, A.; Millat-Martinez, P.; Corbacho-Monné, M.; Malchair, P.; Ouchi, D.; Ruiz-Comellas, A.; Ramírez-Morros, A.; Rodríguez Codina, J.; Amado Simon, R.; Videla, S.; et al. CONV-ERT Group. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med. 2022, 10, 278–288. [Google Scholar] [CrossRef]
- Levine, A.C.; Fukuta, Y.; Huaman, M.A.; Ou, J.; Meisenberg, B.R.; Patel, B.; Paxton, J.H.; Hanley, D.F.; Rijnders, B.J.A.; Gharbharan, A.; et al. Coronavirus Disease 2019 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-Analysis of Individual Participant Data From 5 Randomized Trials. Clin Infect Dis. 2023, 76, 2077–2086. [Google Scholar] [CrossRef]
- Stadler, E.; Chai, K.L.; Schlub, T.E.; Cromer, D.; Khan, S.R.; Polizzotto, M.N.; Kent, S.J.; Beecher, C.; White, H.; Turner, T.; et al. Determinants of passive antibody efficacy in SARS-CoV-2 infection: a systematic review and meta-analysis. Lancet Microbe. 2023, 4, e883–e892. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Focosi, D.; Hanley, D.F.; et al. Outpatient regimens to reduce COVID-19 hospitalisations: a systematic review and meta-analysis of randomized controlled trials. medRxiv, 2227. [Google Scholar] [CrossRef]
- Hoertel, N.; Boulware, D.R.; Sánchez-Rico, M.; et al. Prevalence of Contraindications to Nirmatrelvir-Ritonavir among Hospitalized Patients with COVID-19 at Risk for Progression to Severe Disease. JAMA Netw Open 2022, 5, e2242140. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M. Potential use of convalescent plasma for SARS-CoV-2 prophylaxis and treatment in immunocompromised and vulnerable populations. Expert Rev Vaccines. 2022, 21, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.M.; Focosi, D.; Shoham, S.; Senefeld, J.; Tobian, A.A.R.; Baden, L.R.; Tiberghien, P.; Sullivan, D.J.; Cohn, C.; Dioverti, V.; et al. Guidance on the Use of Convalescent Plasma to Treat Immunocompromised Patients with Coronavirus Disease 2019. Clin Infect Dis. 2023, 76, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M.; Joyner, M.J.; Henderson, J.P.; Casadevall, A. Convalescent plasma in oncohematological patients. Hematol Oncol. 2023, 41, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Senefeld, J.W.; Joyner, M.J.; Sullivan, D.; Casadevall, A.; Bloch, E.M.; Franchini, M. Lower anti-spike levels in B-cell-depleted patients after convalescent plasma transfusion suggest the need for repeated doses. Br J Haematol. 2023, 200, e22–e24. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M. COVID-19 neutralizing antibody-based therapies in humoral immune deficiencies: A narrative review. Transfus Apher Sci. 2021, 60, 103071. [Google Scholar] [CrossRef] [PubMed]
- Senefeld, J.W.; Franchini, M.; Mengoli, C.; Cruciani, M.; Zani, M.; Gorman, E.K.; Focosi, D.; Casadevall, A.; Joyner, M.J. COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023, 6, e2250647. [Google Scholar] [CrossRef] [PubMed]
- Denkinger, C.M.; Janssen, M.; Schäkel, U.; Gall, J.; Leo, A.; Stelmach, P.; Weber, S.F.; Krisam, J.; Baumann, L.; Stermann, J.; et al. Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial. Nat Cancer. 2023, 4, 96–107. [Google Scholar] [CrossRef]
- Hueso, T.; Godron, A.S.; Lanoy, E.; Pacanowski, J.; Levi, L.I.; Gras, E.; Surgers, L.; Guemriche, A.; Meynard, J.L.; Pirenne, F.; et al. Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis. Leukemia. 2022, 36, 1025–1034. [Google Scholar] [CrossRef]
- Franchini, M.; Casadevall, A.; Senefeld, J.W.; Joyner, M.J.; Sullivan, D.J.; Focosi, D. Recommendations on the use of COVID-19 convalescent plasma to treat immunocompromised patients. Semin Thromb Hemost 2023. [CrossRef]
- Guo, W.; Zheng, Y.; Feng, S. Omicron related COVID-19 prevention and treatment measures for patients with hematological malignancy and strategies for modifying hematologic treatment regimes. Front Cell Infect Microbiol. 2023, 13, 1207225. [Google Scholar] [CrossRef]
- Franchini, M.; Casadevall, A.; Joyner, M.J.; et al. WHO Is Recommending against the Use of COVID-19 Convalescent Plasma in Immunocompromised Patients? Life 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Tuccori, M.; Franchini, M. The Road towards Polyclonal Anti-SARS-CoV-2 Immunoglobulins (Hyperimmune Serum) for Passive Immunization in COVID-19. Life 2021, 11, 144. [Google Scholar] [CrossRef]
- Ali, S.; Uddin, S.M.; Shalim, E.; Sayeed, M.A.; Anjum, F.; Saleem, F.; et al. Hyperimmune anti-COVID-19 IVIG (CIVIG) treatment in severe and critical COVID-19 patients: A phase I/II randomized control trial. EClinicalMedicine. 2021, 36, 100926. [Google Scholar] [CrossRef]
- Parikh, D.; Chaturvedi, A.; Shah, N.; Patel, P.; Patel, R.; Ray, S. Safety and efficacy of COVID-19 hyperimmune globulin (HIG) solution in the treatment of active COVID-19 infection - Findings from a Prospective, Randomized, Controlled, Multi-Centric Trial. 2021. Preprint at https://wwwmedrxivorg/content/101101/2021072621261119v1.
- Huygens, S.; Hofsink, Q.; Nijhof, I.S.; Goorhuis, A.; Kater, A.P.; Te Boekhorst, P.A.W.; et al. Hyperimmune Globulin for Severely Immunocompromised Patients Hospitalized With Coronavirus Disease 2019: A Randomized, Controlled Trial. J Infect Dis. 2023, 227, 206–10. [Google Scholar] [CrossRef]
- ITAC (INSIGHT 013) Study Group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet. 2022, 399, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Kimber, C.; Valk, S.J.; Chai, K.L.; Piechotta, V.; Iannizzi, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; McQuilten, Z.; et al. Hyperimmune immunoglobulin for people with COVID-19. Cochrane Database Syst Rev. 2023, 1, CD015167. [Google Scholar] [CrossRef] [PubMed]
- Maor, Y.; Shinar, E.; Izak, M.; Rahav, G.; Brosh-Nissimov, T.; Kessler, A.; Rahimi-Levene, N.; Benin-Goren, O.; Cohen, D.; Zohar, I.; et al. A Randomized Controlled Study Assessing Convalescent Immunoglobulins vs Convalescent Plasma for Hospitalized Patients with Coronavirus 2019. Clin Infect Dis. 2023, 77, 964–971. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M.; Nicastri, E.; Sullivan, D.J.; Casadevall, A. Convalescent Plasma Versus Hyperimmune Immunoglobulins. Clin Infect Dis. 2023, ciad406. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M. Passive immunotherapies for COVID-19: The subtle line between standard and hyperimmune immunoglobulins is getting invisible. Rev Med Virol. 2022, 32, e2341. [Google Scholar] [CrossRef]
| Study, year [reference] | Cases/controls | Results | Signs of efficacy |
|---|---|---|---|
| TSUNAMI, 2021 [30] | 487 patients (241 CCP + ST/246 ST) |
The primary end point occurred in 59 of 231 patients (25.5%) treated with CCP + ST and in 67 of 239 patients (28.0%) who received ST (OR, 0.88; 95% CI, 0.59-1.33; P = 0.54) | In patients with COVID-19 at an early stage at baseline, the primary end point occurred less frequently in the group treated with CP plus ST (8 of 69 [11.6%]) vs those who received ST (16 of 73 [21.9%]) (OR 0.47; 95% CI, 0.19-1.18; P = 0.059) |
| CAPSID, 2021 [31] | 105 patients (53 CCP + ST/52 ST) |
The primary end point occurred in 43.4% of patients in the CCP + ST group and in 32.7% of patients in the ST group (P = 0.32) | In the subgroup that received a higher cumulative amount of nAbs, significantly shorter intervals to clinical improvement (20 vs. 66 days, P < 0.05) and to hospital discharge (21 vs. 51 days, P = 0.03) and better survival (day-60 probability of survival 91.6% vs. 68.1%, P = 0.02) were observed in comparison with the control group. |
| CONTAIN, 2022 [32] | 941 patients (468 CCP/473 placebo) |
The cumulative adjusted OR (caOR) for the primary outcome was 0.94 (95% CI, 0.75-1.18) | A possible benefit of CCP was observed in the subgroup of patients treated during the first pandemic wave (April-June 2020) when steroids and remdesivir where not still in use (caOR 0.72; 95% CI 0.46-1.13). |
| RBR-7f4mt9f, 2022 [33] | 107 patients (36 CCP + ST/71 ST) |
No statistically significant reduction in mortality, requirement for invasive ventilation, and duration of hospital stay was observed between cases and controls | At day 30, death rates were 22% for CCP group and 25% for control group; at day 60, rates were 31% for CCP and 35% for control. |
| Bennet Guerrero, 2021 [34] | 74 patients (59 CCP/15 SP) |
No difference in ventilator-free days or mortality (27% vs 33%) was observed at day 28 in CCP group versus SP group | All-cause mortality through 90 days was numerically lower in the CCP group than standard plasma group (27% vs 33%; P = 0.63). |
| Li, 2020 [35] | 103 patients (52 CCP + ST/51 ST) |
No significant difference was observed in time to clinical improvement within 28 days between CCP and control groups | A 8.3% (15.7% versus 24.0%) absolute difference in mortality rate at day + 28 was observed in favor of CCP treated patients. |
| ConCOVID, 2023 [36] | 86 patients (43 CCP/43 ST) |
CCP had no effect on the disease course and did not improve survival. | Mortality in CCP group was 14% (6 out of 43) vs 26% in control group (11 out of 43) (OR, 0.47; 95% CI 0.15-1.38) |
| Lacombe, 2023 [37] | 120 patients (60 CCP/60 ST) |
No difference in early outcomes between CCP and standard care group was observed. | The survival rate at day +14 and day +28 was higher in the CCP group than in standard care group (mortality rate: 5% versus 13% at day +14 and 12% versus 20% at day +28). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





