Submitted:
21 November 2023
Posted:
21 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Characteristics of materials
Methods
Materials
3. Results
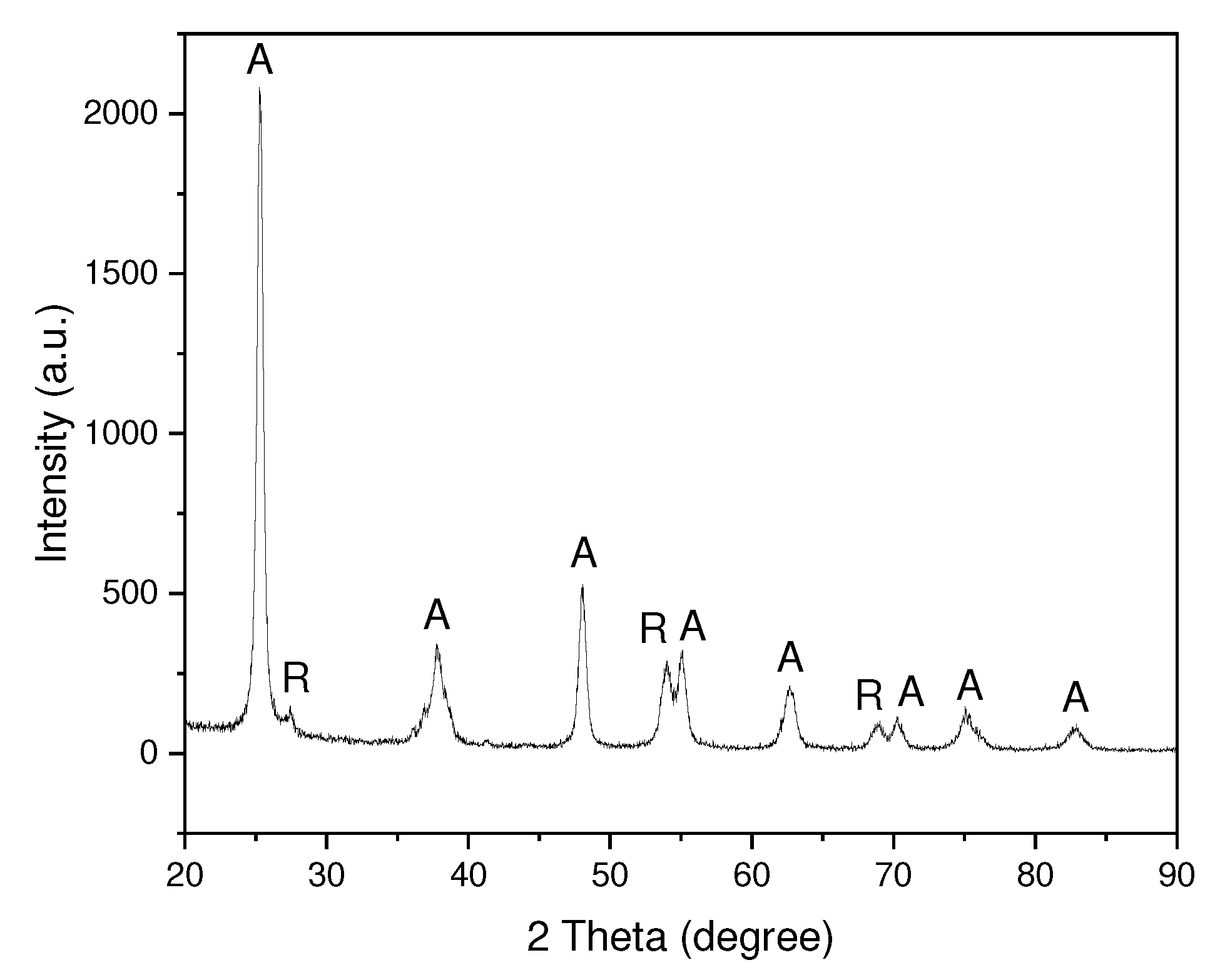
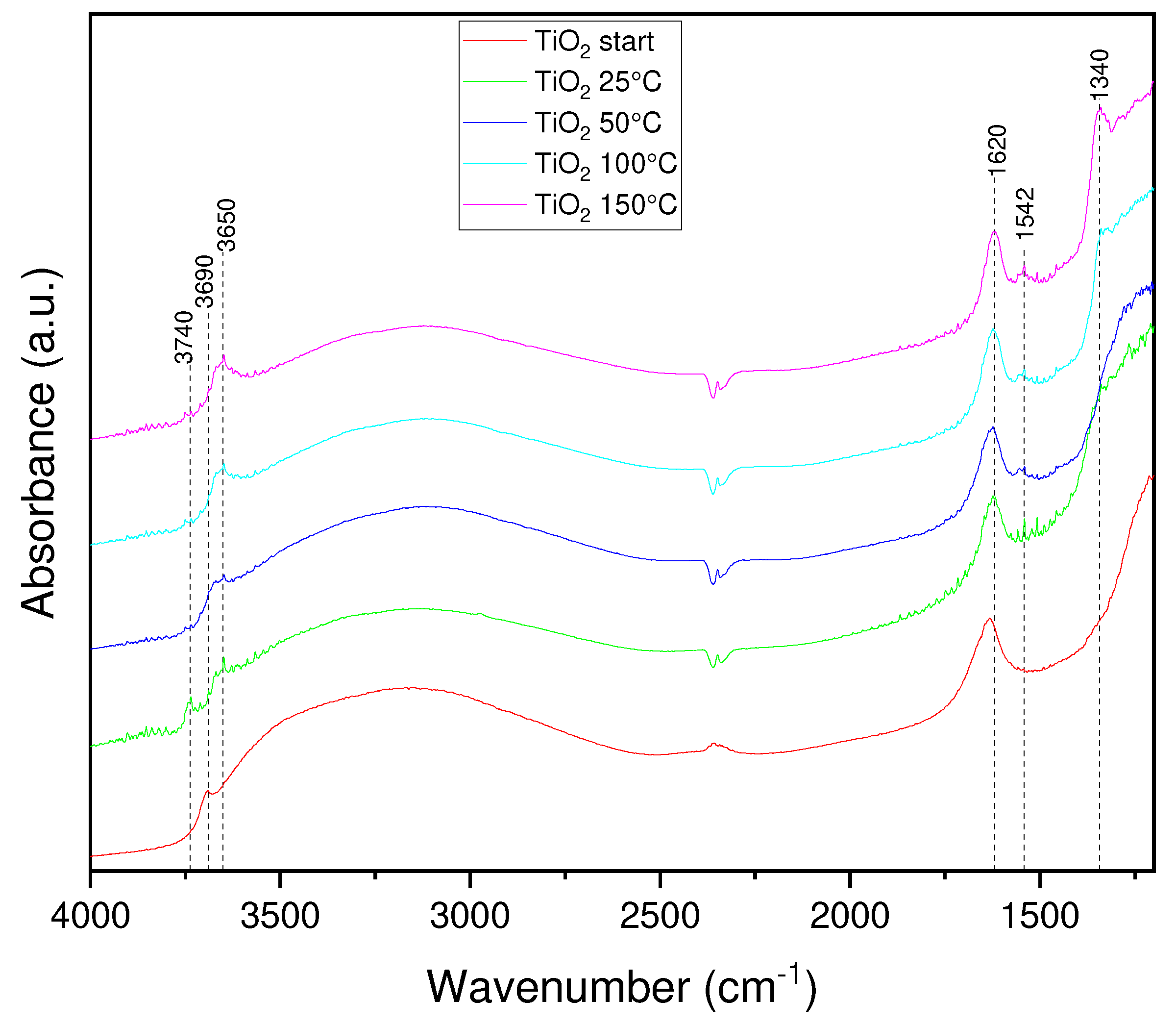
3.1. Physicochemical properties of TiO2
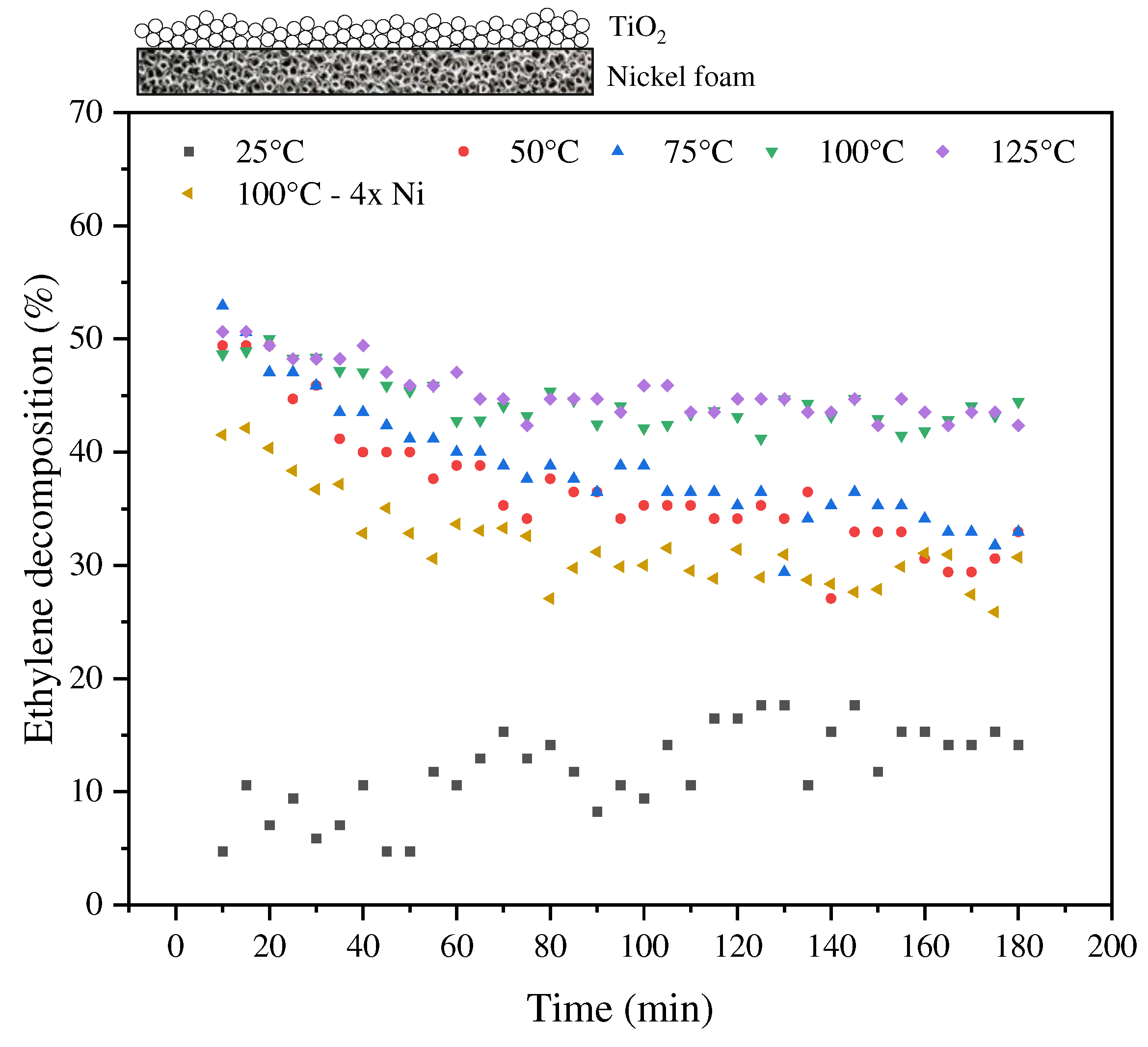
3.2. Thermo-photocatalytic decomposition of ethylene at the presence of TiO2 and TiO2/nickel foam under UV light
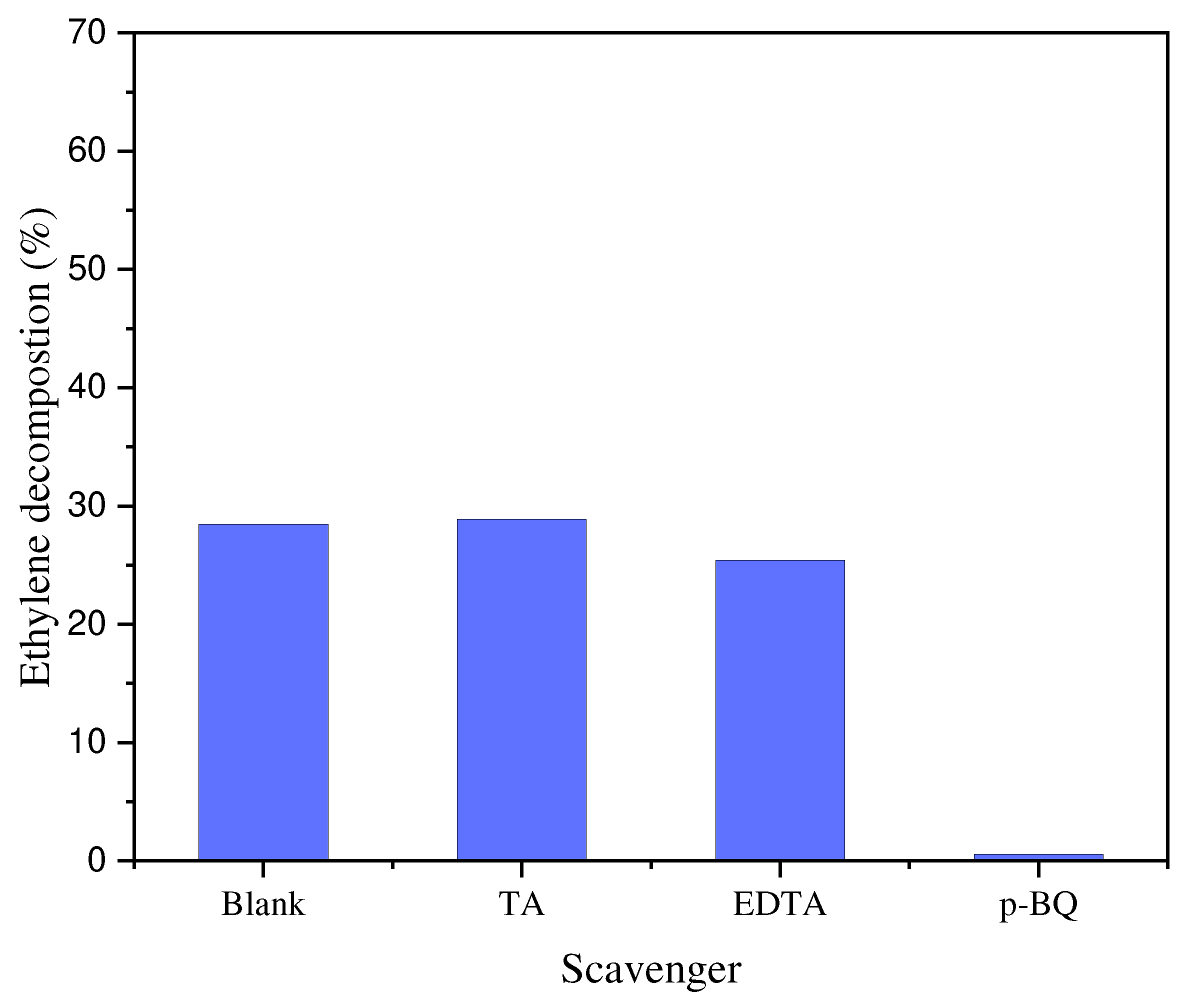
3.3. Thermo-photocatalytic decomposition of ethylene at the presence of radicals scavengers
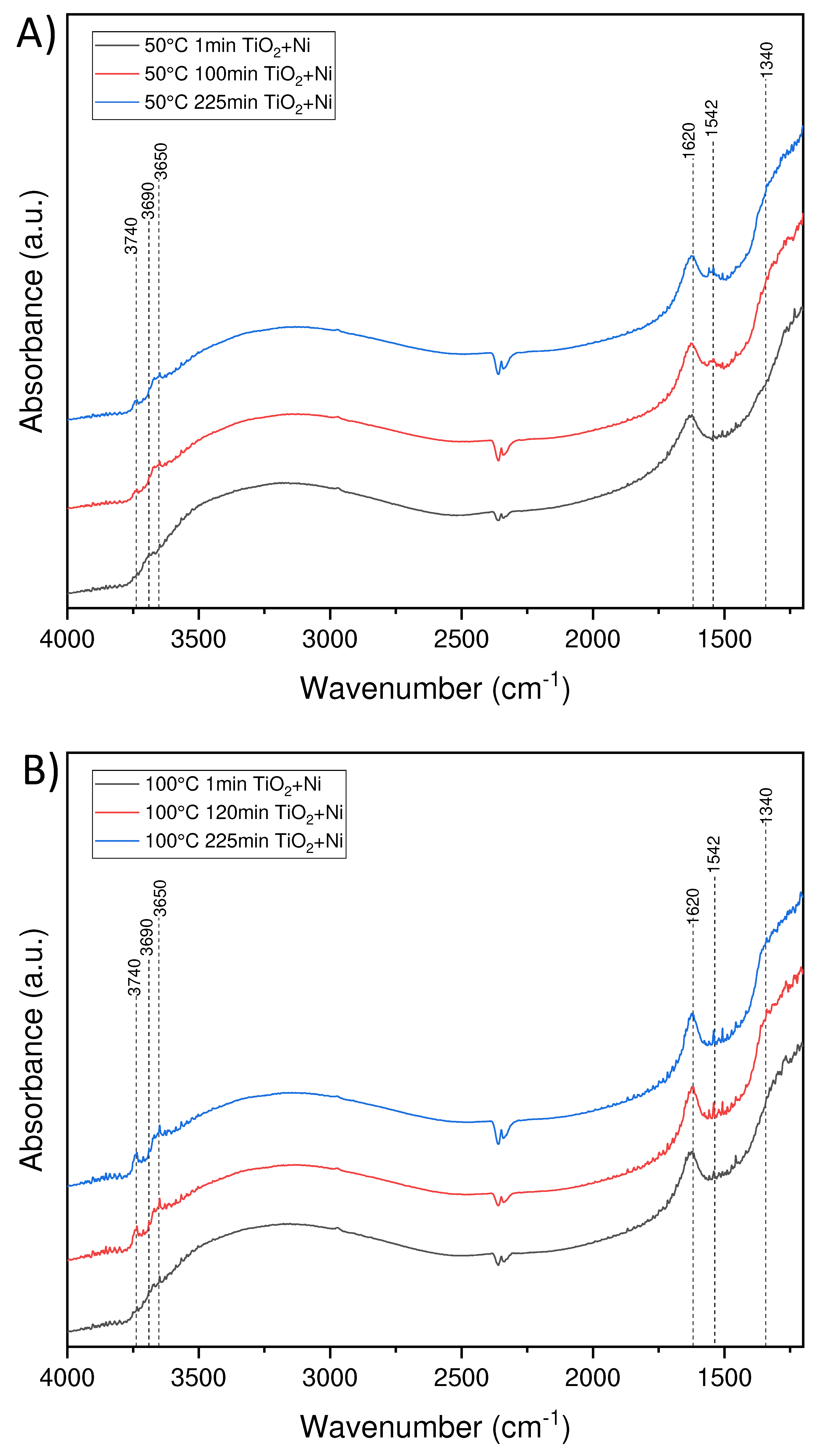
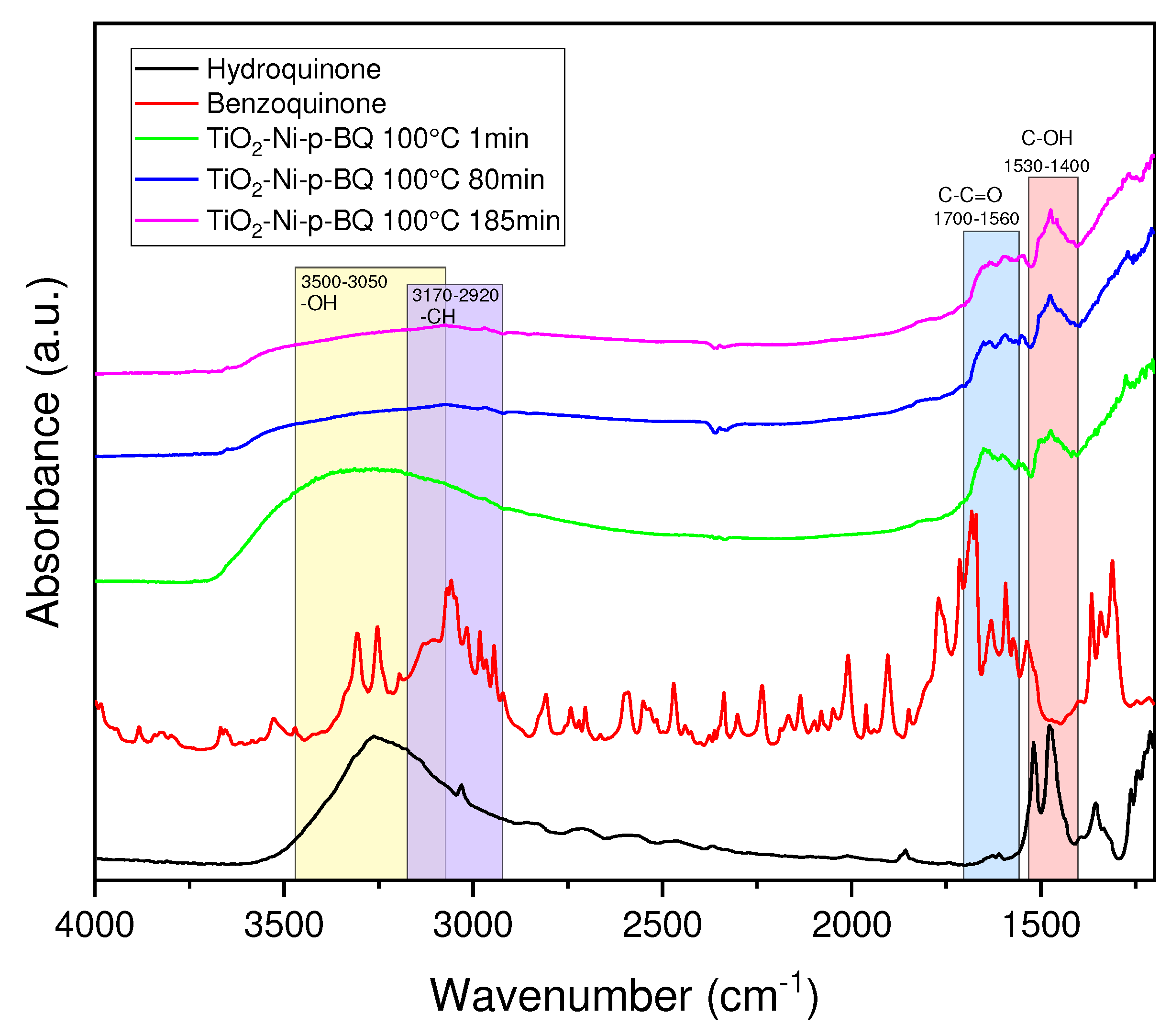
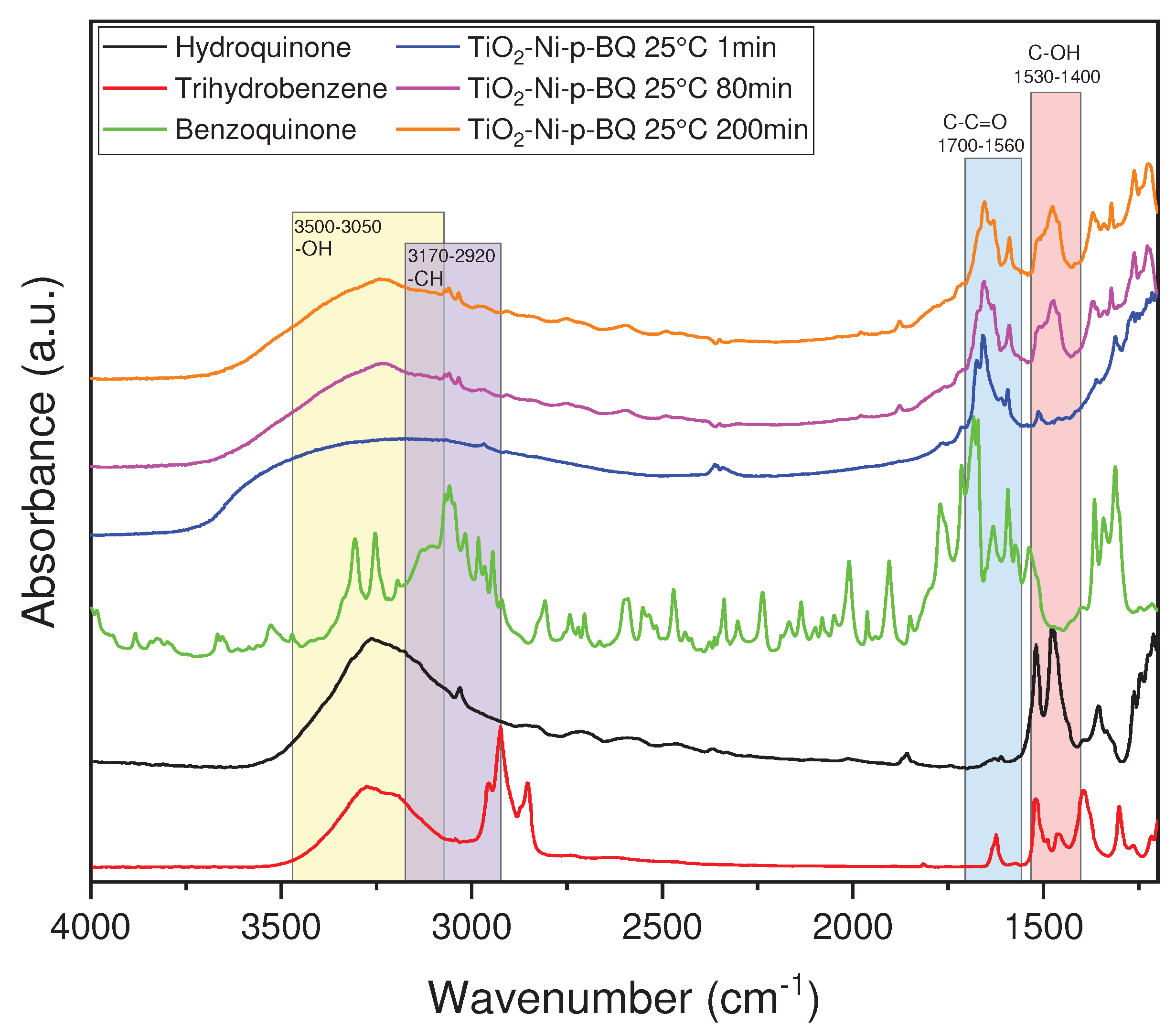
3.4. FTIR spectra of the photocatalyst surface measured at the condition of the photocatalytic process of ethylene decomposition
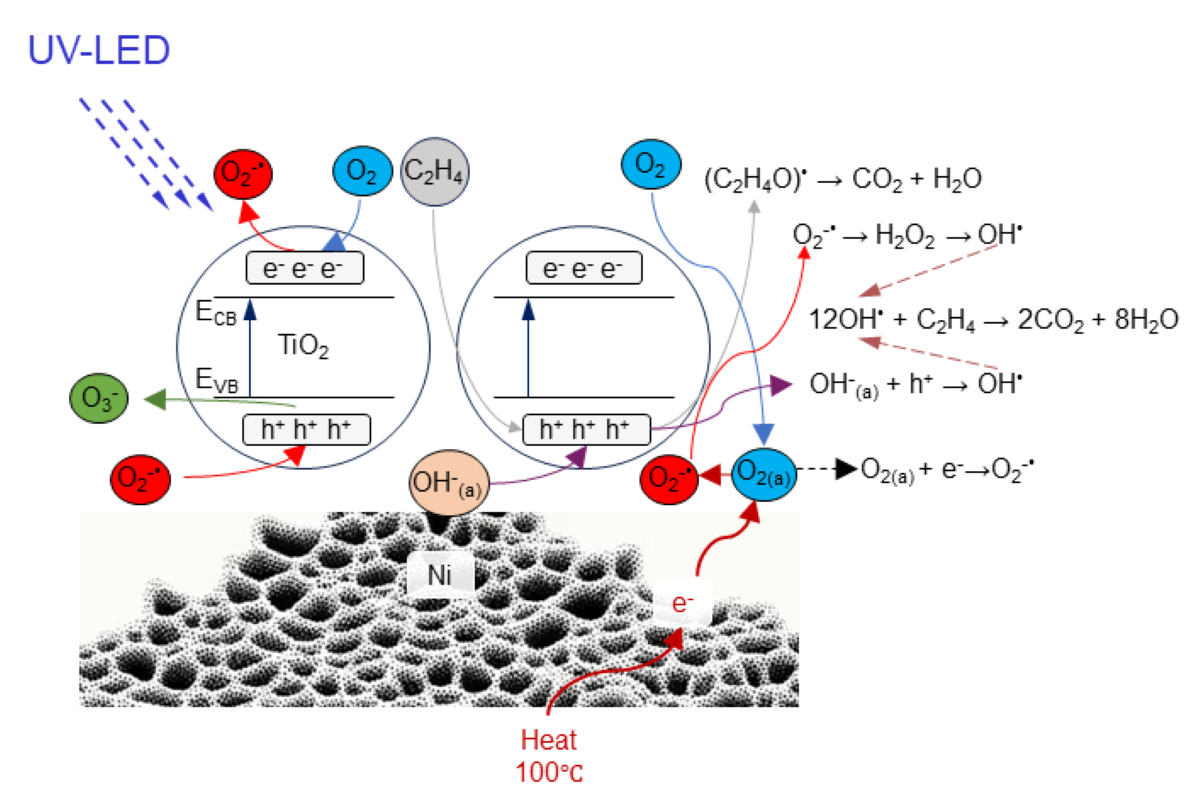
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P.V. Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Elsgaard, L. Ethylene Removal by a Biofilter with Immobilized Bacteria. Appl. Environ. Microbiol. 1998, 64, 4168–4173. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, H.Z.; Kheirkhah, B.; Kariminik, A. Ethylene Removal by Bio-filters in order to Increase Storage Life of Bananas. Int. J. Life Sci. 2015, 9, 62–65. [Google Scholar] [CrossRef]
- Smith, A.W.J.; Poulston, S.; Rowsell, L.; Terry, L.A.; Anderson, J.A. A New Palladium-Based Ethylene Scavenger to Control Ethylene-Induced Ripening of Climacteric Fruit. Platin. Met. Rev. 2009, 53, 112–122. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to Maintain Postharvest Fruit and Vegetable Quality through the Inhibition of Ethylene Action: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Caleb, O.J.; Rauh, C.; Mahajan, P.V. Efficacy of photocatalysis and photolysis systems for the removal of ethylene under different storage conditions. Postharvest Biol. Technol. 2019, 147, 68–77. [Google Scholar] [CrossRef]
- Hussain, M.; Russo, N.; Saracco, G. Photocatalytic abatement of VOCs by novel optimized TiO2 nanoparticles. Chem. Eng. J. 2011, 166, 138–149. [Google Scholar] [CrossRef]
- Kumar, S.; Fedorov, A.G.; Gole, J.L. Photodegradation of ethylene using visible light responsive surfaces prepared from titania nanoparticle slurries. Appl. Catal. B Environ. 2005, 57, 93–107. [Google Scholar] [CrossRef]
- Shi, G.; Mahmood, A.; Lu, G.; Wang, X.; Tong, S.; Ge, M.; Xie, X.; Sun, J. Adsorption and Photodegradation of Acetaldehyde and Ethylene on TiO2 (001) Surface: Experimental and First Principle Studies. Catal. Lett. 2019, 149, 2728–2738. [Google Scholar] [CrossRef]
- Rychtowski, P.; Tryba, B.; Skrzypska, A.; Felczak, P.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Nishiguchi, H.; Toyoda, M. Role of the Hydroxyl Groups Coordinated toTiO2 Surface on the Photocatalytic Decomposition of Ethylene at Different Ambient Conditions. Catalysts 2022, 12, 386. [Google Scholar] [CrossRef]
- Xia, J.; Dong, L.; Song, H.; Yang, J.; Zhu, X. Preparation of doped TiO2 nanomaterials and their applications in photocatalysis. Bull. Mater. Sci. 2023, 46, 13. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Varma, S.; Tripathi, A.K.; Bharadwaj, S.R.; Tyagi, A.K. Mechanistic Insight by in Situ FTIR for the Gas Phase Photo-oxidation of Ethylene by V-Doped Titania and Nano Titania. J. Phys. Chem. B 2009, 113, 5917–5928. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Xie, X.; Wang, X.; Mahmood, A.; Tong, S.; Ge, M.; Sun, J. Defect Chemistry of Er3+-Doped TiO2 and Its Photocatalytic Activity for the Degradation of Flowing Gas-Phase VOCs. J. Phys. Chem. C 2019, 123, 12321–12334. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, S.; Chen, X.; Song, X.; Li, L.; Huang, X. Photocatalytic degradation of ethylene using titanium dioxide nanotube arrays with Ag and reduced graphene oxide irradiated by γ-ray radiolysis. Appl. Catal. B Environ. 2017, 203, 673–683. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Weng, C.-H.; Srivastav, A.L.; Lin, Y.-T.; Tzeng, J.-H. Facile Synthesis and Characterization of N-Doped TiO2Photocatalyst and Its Visible-Light Activity for Photo-Oxidation of Ethylene. J. Nanomater. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Chen, F.-Y. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kang, H.-J. Aluminum sheet-based S-doped TiO2 for photocatalytic decomposition of toxic organic vapors. Chin. J. Catal. 2014, 35, 1189–1195. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Mei, X.; Yuan, H.; Li, C. Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance. Sustainability 2023, 15, 1403. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Song, X.; Luo, S.; Ye, S.; Situ, W. Photocatalytic degradation of ethylene in cold storage using the nanocomposite photocatalyst MIL101(Fe)-TiO2-rGO. Chem. Eng. J. 2021, 424, 130407. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, W.; Duan, J.; Fu, L.; Ma, L.; Yang, Z. Immobilized Ag3PO4/GO on 3D nickel foam and its photocatalytic degradation of norfloxacin antibiotic under visible light. RSC Adv. 2020, 10, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, F.; Chang, X.; He, D. Comparison of Nickel Foam/Ag-Supported ZnO, TiO2, and WO3for Toluene Photodegradation. Mater. Manuf. Process. 2014, 29, 789–794. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Shi, J.; Hu, H.; Shangguan, W. Design consideration of photocatalytic oxidation reactors using TiO2-coated foam nickels for degrading indoor gaseous formaldehyde. Catal. Today 2007, 126, 359–368. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, W.; Yuan, J.; Shi, J.; Shangguan, W. TiO2/SiO2Composite Films Immobilized on Foam Nickel Substrate for the Photocatalytic Degradation of Gaseous Acetaldehyde. Int. J. Photoenergy 2008, 2008, 679421. [Google Scholar] [CrossRef]
- Tryba, B.; Miądlicki, P.; Rychtowski, P.; Trzeciak, M.; Wróbel, R.J. The Superiority of TiO2 Supported on Nickel Foam over Ni-Doped TiO2 in the Photothermal Decomposition of Acetaldehyde. Materials 2023, 16, 5241. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, W.; Yuan, J.; Shi, J.; He, D.; Shangguan, W. High photocatalytic activity and stability for decomposition of gaseous acetaldehyde on TiO2/Al2O3 composite films coated on foam nickel substrates by sol-gel processes. J. Sol-Gel Sci. Technol. 2008, 45, 1–8. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, X.; Wang, X.; Wang, Y.; Lu, G.; Pui, D.Y.; Sun, J. Enhanced photocatalytic performance of Ag@TiO2 for the gaseous acetaldehyde photodegradation under fluorescent lamp. Chem. Eng. J. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Parka, D.R.; Zhang, J.; Ikeueb, K.; Yamashitab, H.; Anpo, M. Photocatalytic Oxidation of Ethylene to CO2 and H2O on Ultrafine Powdered TiO2 Photocatalysts in the Presence of O2 and H2O. J. Catal. 1999, 185, 114–119. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Zeltner, W.A.; Anderson, M.A. Effects of reaction temperature and water vapor content on the heterogeneous photocatalytic oxidation of ethylene. J. Photochem. Photobiol. A Chem. 1996, 97, 181–186. [Google Scholar] [CrossRef]
- Mphuthi, L.E.; Maseme, M.R.; Langner, E.H.G. Ti(IV)-Exchanged Nano-ZIF-8 and Nano-ZIF-67 for Enhanced Photocatalytic Oxidation of Hydroquinone. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2664–2678. [Google Scholar] [CrossRef]
- Ammawath, W.; Man, Y.C.; Baharin, B.; Rahman, R.A. A new method for determination of tert-butylhydroquinone (TBHQ) in RBD palm olein with FTIR spectroscopy. J. Food Lipids 2004, 11, 266–277. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-hydroquinone based determination of electron and superoxide radical formed in heterogeneous photocatalytic systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





