Submitted:
21 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
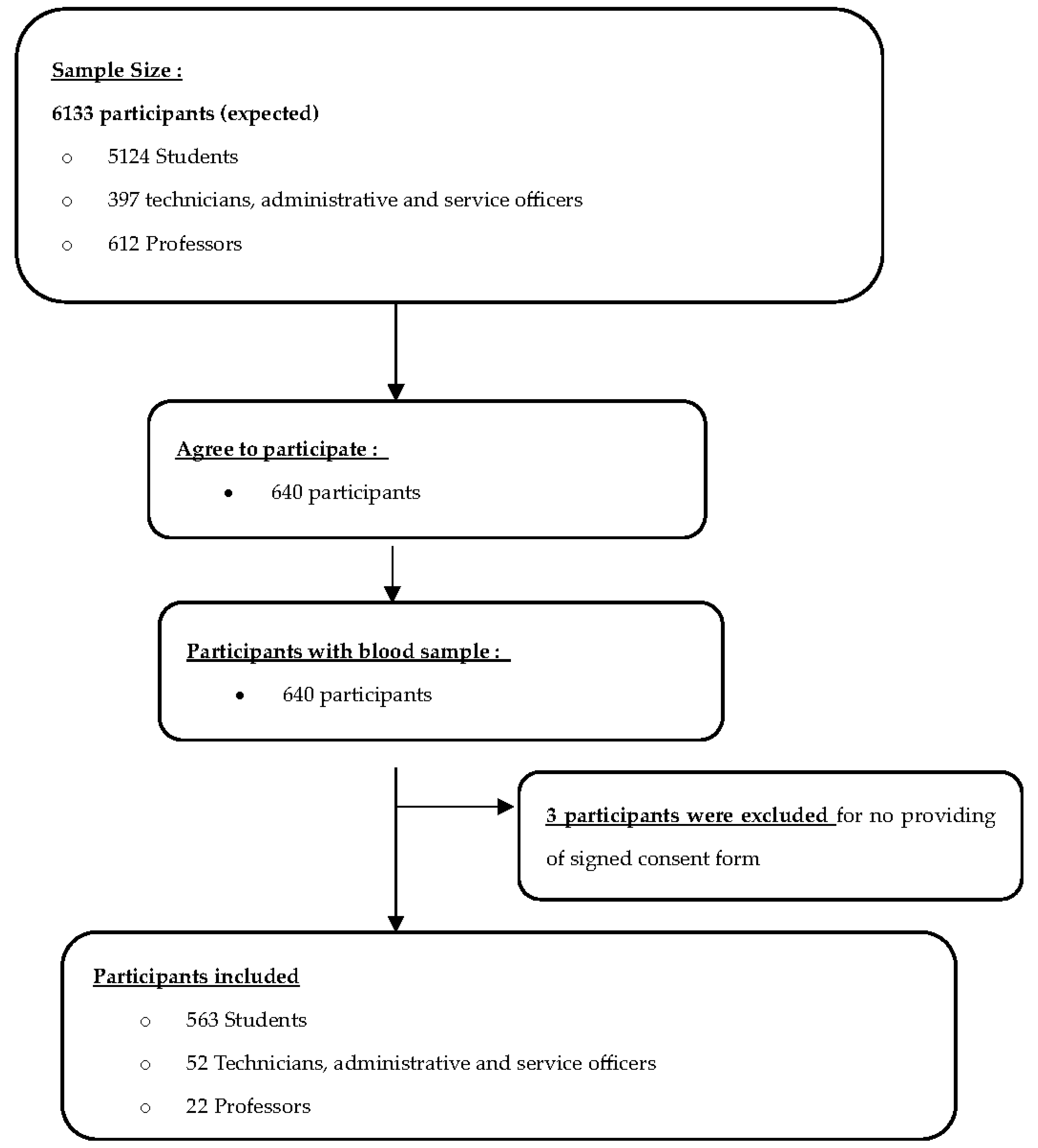
2.1. Study Design and Population
2.2. Sample Size Calculation
2.3. Questionnaire
2.4. Blood Collection and SARS-CoV-2 Antibodies Detection
2.5. Statistical Analyses
2.6. Ethics Statement
3. Results
3.1. Baseline characteristics of participants
3.2. Seroprevalence of IgM et IgG SARS-CoV-2 antibodies
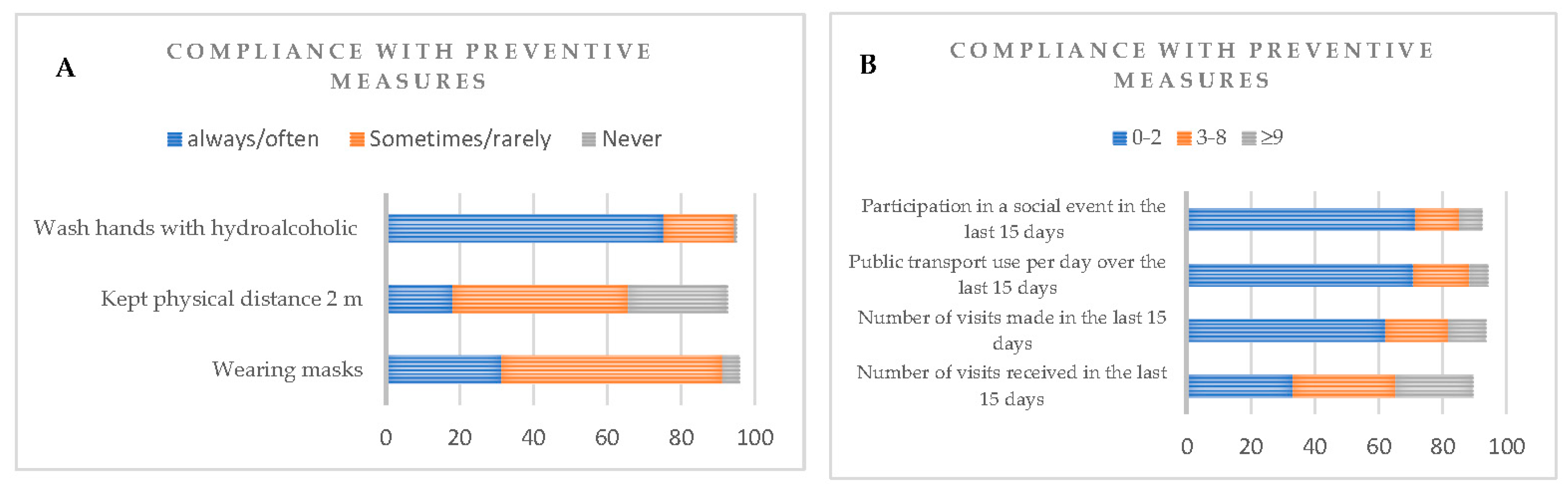
3.3. Compliance with preventive measures and antibodies’ seroprevalence
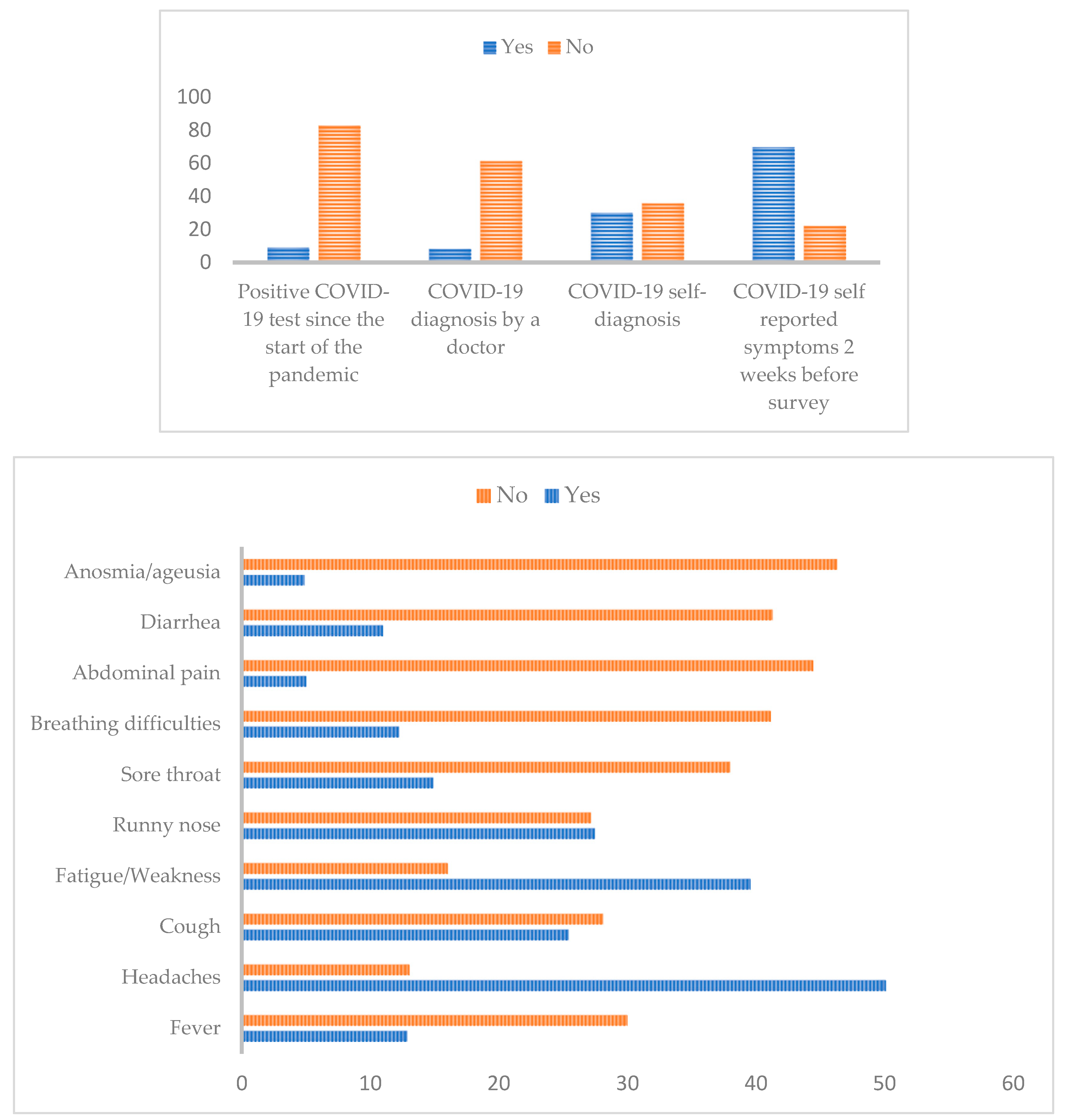
3.4. Diagnosis and Clinical symptoms related to COVID-19 and antibodies’ seroprevalence
3.5. Risk factors associated with SARS-CoV-2 IgM and IgG seropositivity among PHS community
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Total, N (%) | IgM | IgG | ||||
|---|---|---|---|---|---|---|---|
| Seronegative N ( %) | Seropositive N (%) |
p-value | Seronegative N (%) |
Seropositive N (%) |
p-value | ||
| Chronic Condition | |||||||
| Asthma | 0.30 | 0.78 | |||||
| Yes | 90 (14.13) | 81 (90) | 9 (10) | 10 (11.11) | 80 (88.89) | ||
| No | 547 (85.87) | 512 (93.6) | 35 (6.40) | 51 (9.32) | 496 (90.38) | ||
| Cardiac disease | 1 | 1 | |||||
| Yes | 5 (0.78) | 5 (100) | 0 (0) | 0 (0) | 5 (100) | ||
| No | 632 (99.22) | 588 (93.04) | 44 (6.96) | 61 (9.65) | 571 (90.35) | ||
| Rheumatism | 0.94 | 0.61 | |||||
| Yes | 23 (3.61) | 22 (95.65) | 1 (4.35) | 1 (4.35) | 22 (95.65) | ||
| No | 614 (96.39) | 571 (93) | 43 (7) | 60 (9.77) | 554 (90.23) | ||
| Hypertension | 1 | 1 | |||||
| Yes | 12 (1.89) | 11 (91.67) | 1 (8.33) | 1 (8.33) | 11 (91.67) | ||
| No | 625 (98.11) | 582 (93.12) | 43 (6.88) | 60 (9.6) | 565 (90.4) | ||
| Diabetes | 1 | 1 | |||||
| Yes | 5 (0.78) | 5 (100) | 0 (0) | 0 (0) | 5 (100) | ||
| No | 632 (99.22) | 588 (93.04) | 44 (6.96) | 61 (9.65) | 571 (90.35) | ||
| HIV | 1 | 1 | |||||
| Yes | 4 (0.63) | 4 (100) | 0 (0) | 0 (0) | 4 (100) | ||
| No | 633 (99.37) | 589 (93.05) | 44 (6.95) | 61 (9.64) | 572 (90.36) | ||
| Sickle cell disease | 1 | 0.37 | |||||
| Yes | 16 (2.51) | 15 (93.75) | 1 (6.25) | 0 (0) | 16 (100) | ||
| No | 621 (97.49) | 578 (93.08) | 43 (6.92) | 61 (9.82) | 560 (90.18) | ||
| Allergy | 1 | 1 | |||||
| Yes | 39 (6.12) | 36 (92.31) | 3 (7.69) | 4 (10.26) | 35 (89.74) | ||
| No | 598 (93.88) | 557 (93.15) | 41 (6.85) | 57 (8.53) | 541 (90.47) | ||
| Spasmophilia | 1 | 1 | |||||
| Yes | 7 (1.1) | 7 (100) | 0 (0) | 1 (14.29) | 6 (85.71) | ||
| No | 630 (98.90) | 586 (93.02) | 44 (6.98) | 60 (9.52) | 570 (90.48) | ||
| Sinusitis | 0.81 | 0.094 | |||||
| Yes | 10 (1.57) | 10 (100) | 0 (0) | 3 (30) | 7 (70) | ||
| No | 627 (98.43) | 583 (92.98) | 44 (7.02) | 58 (9.25) | 569 (90.75) | ||
| Hepatitis B | 0.44 | 0.52 | |||||
| Yes | 12 (1.89) | 10 (83.33) | 2 (16.67) | 0 (0) | 12 (100) | ||
| No | 625 (98.11) | 583 (93.28) | 42 (6.72) | 61 (9.76) | 564 (90.24) | ||
Appendix B
| Total, N (%) | IgM | IgG | |||||
|---|---|---|---|---|---|---|---|
| Seronegative n ( %) |
Seropositive n ( %) |
p- value | Seronegative n ( %) |
Seropositive n ( %) |
p- value | ||
| Wearing masks | 0.17 | 0.73 | |||||
| Always/Often | 201 (31.39) | 183 (91.04) | 18 (8.96) | 19 (9.45) | 182 (90.55) | ||
| Sometimes/Rarely | 380 (59.81) | 355 (93.42) | 25 (6.58) | 38 (10) | 342 (90) | ||
| Never | 30 (4.71) | 30 (100) | 0 (0) | 4 (13.33) | 26 (86.67) | ||
| Kept a physical distance of 2 m | 0.35 | 0.33 | |||||
| Always/Often | 116 (18.21) | 110 (94.83) | 6 (5.17) | 8 (6.90) | 108 (93.10) | ||
| Sometimes/Rarely | 303 (47.56) | 277 (91.42) | 26 (8.58) | 31 (10.23) | 272 (89.77) | ||
| Never | 171 (26.84) | 161 (94.15) | 10 (5.85) | 21 (12.28) | 150 (87.72) | ||
|
Wash hands with hydroalcoholic or with soap and water |
0.105 | 0.599 | |||||
| Always/Often | 480 (75.35) | 451 (93.96) | 29 (6.04) | 51 (10.62) | 429 (89.38) | ||
| Sometimes/Rarely | 122 (19.15) | 108 (88.52) | 14 (11.48) | 10 (8.20) | 112 (91.80) | ||
| Never | 4 (0.63) | 4 (100) | 0 (0) | 0 (0) | 4 (100) | ||
| Number of visits received in the last 15 days | 0.583 | 0.971 | |||||
| 0-2 | 219 (34.38) | 205 (93.61) | 14 (6.39) | 23 (10.50) | 196 (89.50) | ||
| 3-8 | 204 (32.02) | 188 (92.16) | 16 (7.84) | 21 (10.29) | 183 (89.71) | ||
| ≥9 | 154 (24.17) | 146 (94.80) | 8 (5.20) | 15 (9.74) | 139 (90.26) | ||
| Number of visits made in the last 15 days | 0.008* | 0.946 | |||||
| 0-2 | 396 (62.17) | 373 (94.20) | 23 (5.81) | 41 (10.35) | 355 (89.65) | ||
| 3-8 | 125 (19.62) | 109 (87.20) | 16 (12.80) | 12 (9.60) | 113 (90.40) | ||
| ≥9 | 75 (11.78) | 73 (97.33) | 2 (2.67) | 7 (9.33) | 68 (90.67) | ||
| Public transport use per day over the last 15 days | 0.878 | 0.668 | |||||
| 0-2 | 451 (70.8) | 419 (92.9) | 32 (7.1) | 44 (9.75) | 407 (90.25) | ||
| 3-5 | 113 (17.74) | 105 (92.92) | 8 (7.1) | 14 (12.39) | 99 (87.61) | ||
| ≥9 | 36 (5.65) | 33 (91.67) | 3 (8.33) | 3 (8.33) | 33 (91.67) | ||
| Participation in a social event in the last 15 days | 0.589 | 0.289 | |||||
| 0-2 | 456 (71.58) | 423 (92.76) | 33 (7.24) | 51 (11.18) | 405 (88.82) | ||
| 3-8 | 87 (13.66) | 79 (90.80) | 8 (9.20) | 5 (5.75) | 82 (94.25) | ||
| ≥9 | 46 (7.22) | 44 (95.65) | 2 (4.35) | 4 (8.70) | 42 (91.30) | ||
Appendix C
| IgM | IgG | |||
|---|---|---|---|---|
| Variable | Univariate OR (95% CI) |
p- value | Univariate OR (95% CI) |
p- value |
| Allergy | 1.132 [0.26 – 3.32] | 0.842 | 0.922 [0,35 - 3,16] | 0.881 |
| Asthma | 1.625 [0.71 – 3.37] | 0.216 | 0.822 [0.42 – 1.78] | 0.594 |
| Cardiac disease | ||||
| Rheumatism | 0.603 [0.03 – 2.98] | 0.625 | 2.383 [0.49 -43.02] | 0.399 |
| Hypertension | 1.230 [0.06 – 6.55] | 0.844 | 1.169 [0,22 - 21,53] | 0.882 |
| Sickle cell disease | 0.896 [0.05 – 4.6] | 0,916 | ||
| Sinusitis | - | - | 0.238 [0,064 - 1,13] | 0.041 * |
| Diabetes | - | - | ||
| HIV | - | - | ||
| Spasmophilia | - | - | 0.632 [0.10 – 12.03] | 0.673 |
Appendix D
| IgM | IgG | |||
|---|---|---|---|---|
| Variable | Univariate OR (95% CI) |
p- value | Univariate OR (95% CI) |
p- value |
| Wearing masks | 0.796 [0,5893 - 1,073] | 0.134 | 0.966 [0.74 – 1.25] | 0.793 |
| Failure to respect the 2m distance | 0.980 [0,7380 - 1,278] | 0.886 | 1.229 [0.96 – 1.59] | 0.108 |
| Number of visits received in the last 14 days | 0.901 [0.701 – 1.15] | 0.404 | 1.002 [0.82 – 1.23] | 0.984 |
| Number of visits made in the last 14 days | ||||
| Wash hands with hydroalcoholic | 0.854 [0.62 – 1.17] | 0.327 | 0.891 [0.68 – 1.17] | 0.405 |
| Public transport use per day over the last 15 days | 1.043 [0.75 – 1.40] | 0.793 | 1.072 [0.82 – 1.441] | 0.630 |
| Participation in a social event in the last 15 days | 0.952 [0.71 – 1.24] | 0.734 | 1.094 [0.86 – 1.420] | 0.473 |
| Positive COVID-19 test since the start of the pandemic | 1.657 [0.60 – 3.87] | 0.278 | 0.909 [0.398 – 2.45] | 0.834 |
| COVID-19 diagosis by a doctor | 0.264 [0.014 – 1.28] | 0.95 | 0.576 [0.263 – 1.40] | 0.191 |
| COVID-19 self-diagosis | 0.467 [0.21 – 0.97] | 0.051 | 0.742 [0.39 - 1.41] | 0.360 |
| Treatment | 1.269 [0.36 - 8.072] | 0.742 | ||
| COVID-19 symptoms 2 weeks before survey | 0.880 [0.44 – 1.88] | 0.727 | 0.885 [0.45 - 1.65] | 0.713 |
| Fever | 0.889 [0.28 – 2.45] | 0.829 | 1.908 [0.80 - 5.306] | 0.174 |
| Cough | 1.222 [0.52 – 2.89] | 0.643 | 1.321 [0.68 – 2.59] | 0.409 |
| Fatigue | 0.608 [0.26 – 1.50] | 0.262 | 2.829 [1.44 – 5.55] | 0.0023 * |
| Headaches | 0.765 [0.33 – 2] | 0.556 | 0.838 [0.35 - 1.79] | 0.669 |
| Sore throat | 1.299 [ 0.51 – 3.06] | 0.5 | 0.999 [0.488 – 2.18] | 0.998 |
| Runny nose | 0.889 [0.38 – 2.11] | 0.807 | 1.323 [0.697 – 2.54] | 0.394 |
| Breathing difficulties | 0.654 [0.186 – 1.79] | 0.451 | 1.850 [0.80 – 5.046] | 0.183 |
| Abdominal pain | 0.831 [0.13 – 3.03] | 0.809 | 2.327 [0.66 – 14.74] | 0.261 |
| Diarrhea | 0.357 [0.056 – 1.27] | 0.172 | 1.382 [0.62 – 3.52] | 0.460 |
| Anosmia/ageusia | 0.899 [0.14 – 3.28] | 0.890 | 2.209 [0.63 – 14.00] | 0.291 |
References
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Yesudhas, D.; Srivastava, A.; Gromiha, M.M. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection 2021, 49, 199–213. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General’s opening remarks at the media briefing on COVID19 -March 2020.
- https://www.worldometers.info/coronavirus/. (accessed on 12 November 2023).
- Mofijur, M.; Fattah, I.M.R.; Alam, M.A.; Ong, H.C.; Rahman, S.M.A.; Najafi, G.; Ahmed, S.F.; Uddin, M.A.; Mahlia, T.M.I. Impact of COVID-19 on the social, economic, environmental and energy domains: Lessons learnt from a global pandemic. Sustain. Prod. Consum. 2021, 26, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Zareipour, M.; Baljani, E.; Moradali, M.R. Social Consequences of the COVID-19 Pandemic. A Systematic Review. Invest. Educ. Enferm. 2022, 40. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, H.; Zhang, R. Effects of Pandemic Outbreak on Economies: Evidence From Business History Context. Front. Public. Health 2021, 9, 632043. [Google Scholar] [CrossRef] [PubMed]
- World Bank Open Data. Data. 2021. Available online: https://data.worldbank.org/.
- Mok, K.H. Impact of COVID-19 on Higher Education: Critical Reflections. High. Educ. Policy 2022, 35, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Radhin, V.; Ka, N.; Benson, N.; Mathew, A.J. Effect of pandemic based online education on teaching and learning system. Int. J. Educ. Dev. 2021, 85, 102444. [Google Scholar] [CrossRef] [PubMed]
- Diouara, A.A.M.; Lo, S.; Nguer, C.M.; Senghor, A.; Diop Ndiaye, H.; Manga, N.M.; Danfakha, F.; Diallo, S.; Faye Dieme, M.E.; Thiam, O.; et al. Hepatitis E Virus Seroprevalence and Associated Risk Factors in Pregnant Women Attending Antenatal Consultations in Senegal. Viruses 2022, 14, 1742. [Google Scholar] [CrossRef] [PubMed]
- https://www.worldometers.info/coronavirus/country/senegal/. (accessed on 12 November 2023).
- Diallo, A.I.; Faye, A.; Tine, J.A.D.; Ba, M.F.; Gaye, I.; Bonnet, E.; Traore, Z.; Ridde, V. Factors associated with the acceptability of government measures to address COVID-19 in Senegal. Rev. Epidemiol. Sante Publique 2022, 70, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.F.; Ridde, V.; Diallo, A.I.; Tine, J.A.D.; Kane, B.; Gaye, I.; Traore, Z.; Bonnet, E.; Faye, A. Acceptability of contact management and care of simple cases of COVID-19 at home: a cross-sectional study in Senegal. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- https://www.worldbank.org/en/results/2021/08/31/world-bank-financing-helps-to-support-senegal-in-the-fight-against-covid-191. (accessed on 13 May 2023).
- Zhou, Y.; Rahman, M.M.; Khanam, R. The impact of the government response on pandemic control in the long run-A dynamic empirical analysis based on COVID-19. PLoS One 2022, 17, e0267232. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.; Enria, D.; Giesecke, J.; Heymann, D.L.; Ihekweazu, C.; Kobinger, G.; Lane, H.C.; Memish, Z.A.; Oh, M.D.; Sall, A.A.; et al. Living with the COVID-19 pandemic: act now with the tools we have. Lancet 2020, 396, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Torretta, S.; Zuccotti, G.; Cristofaro, V.; Ettori, J.; Solimeno, L.; Battilocchi, L.; D'Onghia, A.; Bonsembiante, A.; Pignataro, L.; et al. Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature. Ear Nose Throat J. 2021, 100, 131S–138S. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Lee, N.J.; Woo, S.H.; Kim, J.M.; Kim, H.M.; Jo, H.J.; Park, Y.E.; Han, M.G. Validation of real-time RT-PCR for detection of SARS-CoV-2 in the early stages of the COVID-19 outbreak in the Republic of Korea. Sci. Rep. 2021, 11, 14817. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Islam, M.M.; Ali, M.H.; Mukerjee, N.; Maitra, S.; Kamal, M.A.; Ghosh, A.; Castrosanto, M.A.; Alexiou, A.; Ashraf, G.M.; et al. COVID-19 diagnostic methods in developing countries. Environ. Sci. Pollut. Res. Int. 2022, 29, 51384–51397. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Wang, J.H.; Hsueh, P.R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int. J. Infect. Dis. 2020, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Sepidarkish, M.; Leeflang, M.M.G.; Riahi, S.M. , Nourollahpour Shiadeh, M.; Esfandyari, S.; Mokdad, A.H., Hotez, P.J., Gasser, R.B. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 331–340. [Google Scholar] [CrossRef]
- Hou, H.; Wang, T.; Zhang, B.; Luo, Y.; Mao, L.; Wang, F.; Wu, S.; Sun, Z. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology 2020, 9, e01136. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Widdowson, M.A.; Mulholland, K. Estimating the Percentage of a Population Infected with SARS-CoV-2 Using the Number of Reported Deaths: A Policy Planning Tool. Pathogens 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Cascini, F.; Failla, G.; Gobbi, C.; Pallini, E.; Hui, J.; Luxi, W.; Villani, L.; Quentin, W.; Boccia, S.; Ricciardi, W. A cross-country comparison of Covid-19 containment measures and their effects on the epidemic curves. BMC Public. Health 2022, 22, 1765. [Google Scholar] [CrossRef] [PubMed]
- Vusirikala, A.; Whitaker, H.; Jones, S.; Tessier, E.; Borrow, R.; Linley, E.; Hoschler, K.; Baawuah, F.; Ahmad, S.; et al. Seroprevalence of SARS-CoV-2 antibodies in university students: Cross-sectional study, December 2020, England. J Infect 2021, 83, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Tsitsilonis, O.E.; Paraskevis, D.; Lianidou, E.; Pierros, V.; Akalestos, A.; Kastritis, E.; Moutsatsou, P.; Scorilas, A.; Sphicopoulos, T.; Terpos, E.; et al. Seroprevalence of Antibodies against SARS-CoV-2 among the Personnel and Students of the National and Kapodistrian University of Athens, Greece: A Preliminary Report. Life 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.R.K.; Srinivasan, S.; Rodriguez, S.; Rydzak, N.; Herzog, C.M.; Gontu, A.; Bharti, N.; Small, M.; Rogers, C.J.; Schade, M.M. et al; et al. SARS-CoV-2 Seroprevalence in a University Community: A Longitudinal Study of the Impact of Student Return to Campus on Infection Risk Among Community Members. medRxiv.
- Migliara, G.; Renzi, E.; Baccolini, V.; Cerri, A.; Donia, P.; Massimi, A.; Marzuillo, C.; De Vito, C.; Casini, L.; et al. Predictors of SARS-CoV-2 Infection in University Students: A Case-Control Study. Int. J. Environ. Res. Public. Health 2022, 19, 14376. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kilcoyne, A.; Ucer, C. Non-infectious status indicated by detectable IgG antibody to SARS-CoV-2. Br. Dent. J. 2020, 229, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Assaid, N.; Arich, S.; Charoute, H.; Akarid, K.; Anouar Sadat, M.; Maaroufi, A.; Ezzikouri, S.; Sarih, M. Kinetics of SARS-CoV-2 IgM and IgG Antibodies 3 Months after COVID-19 Onset in Moroccan Patients. Am. J. Trop. Med. Hyg. 2023, 108, 145–154. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Okba, N.M.A.; Igloi, Z.; Bogers, S.; Embregts, C.W.E.; Laksono, B.M.; Leijten, L.; Rokx, C.; Rijnders, B.; Rahamat-Langendoen, J.; et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. Commun. 2020, 11, 3436. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Mansour, M.; Leven, E.; Bouvier, N.M.; Patel, G.; Firpo-Betancourt, A.; Mendu, R.; Jhang, J.; Arinsburg, S.; Gitman, M.; et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe 2020, 1, e283–e289. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Haq, M.; Rehman, A.; Haq, N.U. Anti-nucleocapsid IgG antibodies in SARS-CoV-2 recovered health care workers. One year follow-up study. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231187744. [Google Scholar] [CrossRef] [PubMed]
- Haveri, A.; Ekstrom, N.; Solastie, A.; Virta, C.; Osterlund, P.; Isosaari, E.; Nohynek, H.; Palmu, A.A.; Melin, M. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans. Eur. J. Immunol. 2021, 51, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef]
- Seck, S.M.; Mbow, M.; Kane, Y.; Cisse, M.M.; Faye, G.; Kama, A.; Sarr, M.; Nitcheu, P.; Dahaba, M.; Diallo, I.M. et al.; et al. Prevalence of SARS-CoV-2 antibodies in hemodialysis patients in Senegal: a multicenter cross-sectional study. BMC Nephrol. 2021, 22, 384. [Google Scholar] [CrossRef] [PubMed]
- Ahouidi, A.D.; Anderson, M.; Diedhiou, C.K.; Dia, A.; Mbow, M.; Dia, Y.; Mboup, A.; Gaye, A.G.; Manga, N.M.; Cloherty, G.; et al. Seroprevalence of SARS-CoV-2 IgG antibodies in a healthcare setting during the first pandemic wave in Senegal. IJID Reg. 2022, 2, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Talla, C.; Loucoubar, C.; Roka, J.L.; Barry, M.A.; Ndiaye, S.; Diarra, M.; Thiam, M.S.; Faye, O.; Dia, M.; Diop, M.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Senegal: a national population-based cross-sectional survey, between October and November 2020. IJID Reg 2022, 3, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Senegalese Ministry of Health and Social Action., [Final report of the 2nd seroprevalence survey of Covid-19 in Senegal, 2023.
- Diouara, A.A.M.; Thiam, F.; Coundoul, S.; Manga, N.M.; Ndiaye, H.D.; Senghor, A.; Tene, S.D.; Sané, S.; Kane, B.; Sarr, H.; et al. High Exposure to SARS-CoV-2 among Pregnant Women after the First and Second Waves of COVID-19 in Senegal. Preprints. [CrossRef]
- Ndiaye, A.J.S.; Beye, M.; Lo, G.; Kacel, I.; Sow, A.; Leye, N.; Padane, A.; Mboup, A.; Diop-Ndiaye, H.; et al. Genomic Epidemiology of SARS-CoV-2 in Urban Settings in Senegal. Viruses 2023, 15, 1233. [Google Scholar] [CrossRef]
- Manirakiza, A.; Malaka, C.; Longo, J.D.; Yambiyo, B.M.; Diemer, S.H.; Namsenei, J.; Coti-Reckoundji, C.S.G.; Bouhouda, M.; Belizaire, M.R.D.; Roungou, J.B.; et al. Sero-prevalence of anti-SARS-CoV-2 antibodies among communities between July and August 2022 in Bangui, Central African Republic. J Public Health Afr 2023, 14, 2315. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, S.; Herrera, B.B.; Chaplin, B.; Ogunsola, S.; Osibogun, A.; Onawoga, F.; John-Olabode, S.; Akase, I.E.; Nwosu, A.; Hamel, D.J. et al.; et al. High SARS-CoV-2 seroprevalence in Lagos, Nigeria with robust antibody and cellular immune responses. J. Clin. Virol. Plus 2023, 3, 100156. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Wong, P.; Ellingson, M.K.; Lucas, C.; Klein, J.; Israelow, B.; Silva, J.; Oh, J.E.; Mao, T.; Tokuyama, M.; et al. Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. medRxiv.
- Zhang, H.; Tang, Y.; Tao, J. Sex-Related Overactivation of NLRP3 Inflammasome Increases Lethality of the Male COVID-19 Patients. Front. Mol. Biosci. 2021, 8, 671363. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, S.; Hedrich, C.M. SARS-CoV-2 infections in children and young people. Clin. Immunol. 2020, 220, 108588. [Google Scholar] [CrossRef]
- Backhaus, I.; Hermsen, D.; Timm, J.; Boege, F.; Lubke, N.; Degode, T.; Gobels, K.; Dragano, N. SARS-CoV-2 seroprevalence and determinants of infection in young adults: a population-based seroepidemiological study. Public. Health 2022, 207, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Bornstein, M.; Fall, M.; Nianogo, R.; Glik, D.; Massey, P. Cross-sectional study of COVID-19 knowledge, beliefs and prevention behaviours among adults in Senegal. BMJ Open 2022, 12, e057914. [Google Scholar] [CrossRef] [PubMed]
- Jaureguizar, J.; Redondo, I.; Galende, N.; Ozamiz, N. Factors related to compliance with the COVID-19 health regulations among young people. World J. Psychiatry 2021, 11, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Crimmins, E.M. How does age affect personal and social reactions to COVID-19: Results from the national Understanding America Study. PLoS One 2020, 15, e0241950. [Google Scholar] [CrossRef] [PubMed]
- https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-27-october-2022. (accessed on 12 November 2023).
- Prakash, N.; Srivastava, B.; Singh, S.; Sharma, S.; Jain, S. Effectiveness of social distancing interventions in containing COVID-19 incidence: International evidence using Kalman filter. Econ. Hum. Biol. 2022, 44, 101091. [Google Scholar] [CrossRef]
- https://tradingeconomics.com/senegal/coronavirus-vaccination-rate. (accessed on 12 November 2023).
- https://ourworldindata.org/covid-vaccinations. (accessed on 12 November 2023).
- Van Egeren, D.; Stoddard, M.; White, L.F.; Hochberg, N.S.; Rogers, M.S.; Zetter, B.; Joseph-McCarthy, D.; Chakravarty, A. Vaccines Alone Cannot Slow the Evolution of SARS-CoV-2. Vaccines 2023, 11, 853. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J. Infect. Public. Health 2022, 15, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Gower, C.; Andrews, N.; Public Health England Delta Variant Vaccine Effectiveness Study, G. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. Reply. N. Engl. J. Med. 2021, 385, e92. [Google Scholar] [CrossRef]
- Cohen, J.I.; Burbelo, P.D. Reinfection With SARS-CoV-2: Implications for Vaccines. Clin. Infect. Dis. 2021, 73, e4223–e4228. [Google Scholar] [CrossRef]
- Adepoju, P. Closing Africa's wide COVID-19 testing and vaccination gaps. Lancet Microbe 2021, 2, e573. [Google Scholar] [CrossRef] [PubMed]
- https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-booster-percent-pop5. (accessed on 12 November 2023).
- https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. (accessed on 12 November 2023).
- Lampros, A.; Talla, C.; Diarra, M.; Tall, B.; Sagne, S.; Diallo, M.K.; Diop, B.; Oumar, I.; Dia, N.; Sall, A.A. et al.; et al. Shifting Patterns of Influenza Circulation during the COVID-19 Pandemic, Senegal. Emerg. Infect. Dis. 2023, 29, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.C.; Nery, T.; Starke, A.C.; de Bem Alves, A.C.; Speck, A.E.; A, S.A. Post-viral fatigue in COVID-19: A review of symptom assessment methods, mental, cognitive, and physical impairment. Neurosci. Biobehav. Rev. 2022, 142, 104902. [Google Scholar] [CrossRef] [PubMed]
- Silaghi-Dumitrescu, R.; Patrascu, I.; Lehene, M.; Bercea, I. Comorbidities of COVID-19 Patients. Medicina 2023, 59, 1393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.M.; Gomez-Perez, A.M.; Fernandez-Garcia, J.C.; Barahona San Millan, R.; Aguilera Luque, A.; de Hollanda, A.; Jimenez, A.; Jimenez-Murcia, S.; Munguia, L.; Ortega, E.; et al. Coronavirus disease 2019 (COVID-19) and obesity. Impact of obesity and its main comorbidities in the evolution of the disease. Eur Eat Disord Rev 2020, 28, 799–815. [Google Scholar] [CrossRef] [PubMed]
- A NA-R, A.D.A.; Alshalan, A.; Muteb Alshalan, K.; Muharib, R.A.K.; Hamdan, R.A.A.; Talal Alruwaili, A.; Abdulhamid Alanazi, A.; Khalid Alshalan, M.; Fahid, A.A. Epidemiological Characteristics, Pathogenesis and Clinical Implications of Sinusitis in the Era of COVID-19: A Narrative Review. J. Asthma Allergy 2023, 16, 201–211. [Google Scholar]
- Wolff, D.; Nee, S.; Hickey, N.S.; Marschollek, M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection 2021, 49, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Aburto, S.; Cisterna, M.; Acuna, J.; Ruiz, C.; Viscardi, S.; Marquez, J.L.; Villano, I.; Letelier, P.; Guzman, N. Obesity as a Risk Factor for Severe COVID-19 in Hospitalized Patients: Epidemiology and Potential Mechanisms. Healthcare 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Mackey, K.; Ayers, C.K.; Kondo, K.K.; Saha, S.; Advani, S.M.; Young, S.; Spencer, H.; Rusek, M.; Anderson, J.; Veazie, S.; et al. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths : A Systematic Review. Ann. Intern. Med. 2021, 174, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M. Insights into disparities observed with COVID-19. J. Intern. Med. 2021, 289, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Fogh, K.; Eriksen, A.R.R.; Hasselbalch, R.B.; Kristensen, E.S.; Bundgaard, H.; Nielsen, S.D.; Jorgensen, C.S.; Scharff, B.; Erikstrup, C.; Saekmose, S.G.; et al. Seroprevalence of SARS-CoV-2 antibodies in social housing areas in Denmark. BMC Infect. Dis. 2022, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Megasari, N.L.A.; Yamani, L.N.; Juniastuti, J.; Lusida, M.I.; Mori, Y. Seroprevalence of SARS-CoV-2 anti-spike IgG antibody among COVID-19 vaccinated individuals residing in Surabaya, East Java, Indonesia. PeerJ 2023, 11, e16142. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total, N (%) | IgM | IgG | ||||
|---|---|---|---|---|---|---|---|
| Seronegative N (%) |
Seropositive N (%) |
p-value | Seronegative N (%) |
Seropositive N (%) |
p-value | ||
| Overall | 637 (100) | 593 (93.09) | 44 (6.91) | 51 (8) | 576 (92) | ||
| Age (years) | |||||||
| Mean | 23.20 | 23.06 | 25.16 | 22.4 | 23.28 | ||
| Median | 21 | 21 | 21 | 21 | 21 | ||
| Range | 18 - 63 | 18 - 63 | 18 - 59 | 18 - 47 | 18 - 63 | ||
| Age group | <0,001* | 0,98 | |||||
| [18-25] | 546 (85.71) | 516 (94.50) | 30 (5.5) | 53 (9.7) | 493 (92.3) | ||
| (25-35] | 38 (5.96) | 29 (76.31) | 9 (23.69) | 4 (10.53) | 34 (89.47) | ||
| (35-45] | 26 (4.08) | 24 (92.31) | 2 (7.69) | 2 (7.69) | 24 (92.31) | ||
| (45-55] | 12 (1.88) | 12 (100) | 0 (0) | 1 (8.33) | 11 (91.67) | ||
| (55-65] | 6 (0.94) | 4 (66.67) | 2 (33.34) | 0 (0) | 6 (100) | ||
| Gender | 0.16 | 0.89 | |||||
| Women | 395 (62) | 373 (94.43) | 22 (5.57) | 37 (9.36) | 358 (90.63) | ||
| Men | 235 (36.89) | 213 (90.64) | 22 (9.36) | 24 (10.21) | 211 (89.79) | ||
| Occupation | 0.15 | 0.71 | |||||
| Students | 563 (88.38) | 528 (93.78) | 35 (6.22) | 55 (9.77) | 508 (90.23) | ||
| Professors | 22 (3.45) | 19 (86.36) | 3 (13.64) | 1 (9.61) | 21 (90.38) | ||
| TAS Officers | 52 (8.16) | 46 (88.46) | 6 (11.54) | 5 (4.54) | 47 (95.45) | ||
| Level of education | 0.18 | 0.45 | |||||
| Bachelor degree | 450 (70.64) | 423 (94) | 27 (6) | 46 (10.22) | 404 (89.78) | ||
| Master degree | 117 (18.36) | 105 (84.74) | 12 (10.26) | 12 (10.25) | 105 (89.75) | ||
| PhD | 20 (3.14) | 17 (85) | 3 (15) | 1 (5) | 19 (95) | ||
| others | 18 (2.82) | 16 (88.89) | 2 (11.11) | 0 (0) | 18 (100) | ||
| Nationality | 0.29 | 0.30 | |||||
| Senegalese | 605 (94.66) | 565 (93.39) | 40 (6.61) | 56 (9.26) | 549 (90.74) | ||
| Others † | 30 (5.03) | 26 (86.67) | 4 (13.33) | 5 (16.67) | 25 (83.33) | ||
| Ethnic groups | 0.25 | 0.17 | |||||
| Wolof | 153 (24.02) | 145 (94.77) | 8 (5.23) | 14 (9.15) | 139 (90.85) | ||
| Fula | 139 (21.82) | 128 (92.09) | 11 (7.91) | 11 (7.91) | 128 (92.09) | ||
| Serer | 79 (12.40) | 72 (91.14) | 7 (8.86) | 8 (10.13) | 71 (89.87) | ||
| Jola | 16 (2.50) | 14 (87.50) | 2 (12.50) | 0 (0) | 16 (100) | ||
| Malinke | 17 (2.67) | 16 (94.12) | 1 (5.88) | 3 (17.65) | 14 (82.35) | ||
| Soninke | 9 (1.41) | 9 (100) | 0 (0) | 3 (33.33) | 6 (66.67) | ||
| Mauri | 7 (1.1) | 7 (100) | 0 (0) | 0 (0) | 7 (100) | ||
| Others † | 18 (2.82) | 14 (77.78) | 4 (22.22) | 2 (11.12) | 16 (88.88) | ||
| Accommodation type | 0.73 | 0.76 | |||||
| Halls of residence | 138 (21.67) | 127 (92.03) | 11 (7.97) | 12 (8.69) | 126 (91.31) | ||
| Family home | 479 (75.19) | 447 (93.32) | 32 (6.68) | 48 (10.02) | 431 (89.98) | ||
| Family members | 0.54 | 0.32 | |||||
| 1-2 | 37 (5.81) | 36 (97.30) | 1 (2.70) | 2 (5.40) | 35 (94.60) | ||
| 3-5 | 247 (38.77) | 227 (91.90) | 20 (8.10) | 29 (11.75) | 218 (88.25) | ||
| 6-8 | 188 (29.51) | 176 (93.62) | 12 (6.38) | 20 (10.64) | 168 (89.36) | ||
| 9 or plus | 74 (11.61) | 78 (90.70) | 8 (9.30) | 5 (5.81) | 81 (94.19) | ||
| Matrimonial status | 0.062 | 0.53 | |||||
| Single | 558 (87.60) | 524 (93.9) | 34 (6.1) | 54 (9.68) | 504 (90.32) | ||
| Married | 60 (9.42) | 51 (85) | 9 (15) | 7 (11.67) | 53 (88.33) | ||
| Divorced | 2 (0.31) | 2 (100) | 0 (0) | 0 (0) | 2 (100) | ||
| Smoker | 0.08 | 0.16 | |||||
| Yes | 10 (1.88) | 10 (100) | 0 (0) | 0 (0) | 14 (100) | ||
| No | 538 (84.30) | 503 (93.49) | 35 (6.51) | 57 (9.69) | 531 (90.31) | ||
| Stopped > 1 year | 10 (1.41) | 8 (80) | 2 (20) | 0 (0) | 10 (100) | ||
| Alcohol consumer | 0.12 | 0.038 | |||||
| Yes | 21 (3.62) | 19 (90.48) | 2 (9.52) | 1 (4.76) | 20 (95.24) | ||
| No | 554 (86.81) | 516 (93.14) | 38 (6.86) | 51 (9.20) | 503 (90.80) | ||
| Stopped > 1 year | 3 (0.31) | 3 (100) | 0 (0) | 1 (33.34) | 2 (66.66) | ||
| BMI Categorized | 0.089 | 0.55 | |||||
| Underweight | 69 (10.83) | 67 (97.10) | 2 (2.90) | 6 (8.70) | 63 (91.30) | ||
| Normal weight | 240 (37.68) | 224 (93.33) | 16 (6.67) | 28 (11.67) | 212 (88.33) | ||
| Overweight /Obesity | 53 (8.32) | 46 (86.79) | 7 (11.36) | 8 (15.09) | 45 (84.91) | ||
| Blood Group | 0.95 | 0.094 | |||||
| A- | 9 (1.41) | 8 (88.89) | 1 (11.11) | 1 (11.11) | 8 (88.89) | ||
| A+ | 140 (21.98) | 131 (93.57) | 9 (6.43) | 14 (10) | 126 (90) | ||
| AB- | 1 (0.15) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | ||
| AB+ | 25 (3.92) | 24 (96) | 1 (4) | 3 (12) | 22 (88) | ||
| B- | 5 (0.78) | 4 (80) | 1 (20) | 0 (0) | 5 (100) | ||
| B+ | 102 (16.01) | 94 (92.16) | 8 (7.84) | 7 (6.86) | 95 (93.14) | ||
| O- | 24 (3.77) | 22 (91.67) | 2 (8.33) | 1 (4.17) | 23 (95.83) | ||
| O+ | 300 (47.08) | 279 (93) | 21 (7) | 29 (9.67) | 271 (90.33) | ||
| COVID-19 Vaccination | 0.624 | <0.0001 | |||||
| Yes | 221 (35.01) | 203 (91.85) | 18 (8.15) | 7 (3.17) | 214 (96.83) | ||
| No | 365 (57.46) | 340 (95.15) | 25 (6.85) | 49 (13.42) | 316 (86.58) | ||
| Prefer not to say | 51 (7.54) | 50 (98.04) | 1 (1.96) | 5 (9.8) | 46 (90.2) | ||
| Total, N (%) | IgM | IgG | |||||
|---|---|---|---|---|---|---|---|
| Seronegative N, (%) |
Seropositive N, (%) |
p-value | Seronegative N, (%) |
Seropositive N, (%) |
p-value | ||
| Positive COVID-19 test since the start of the pandemic | 0.411 | 0.814 | |||||
| Yes | 57 (8.95) | 51 (89.47) | 6 (10.53) | 6 (10.53) | 51 (89.47) | ||
| No | 528 (82.89) | 493 (93.37) | 35 (6.63) | 51 (9.66) | 477 (90.34) | ||
| COVID-19 diagnosis by a doctor | 0.276 | 0.281 | |||||
| Yes | 52 (8.16) | 51 (98.08) | 1 (1.92) | 8 (15.38) | 44 (84.62) | ||
| No | 390 (61.22) | 363 (93.08) | 27 (6.92) | 37 (9.49) | 353 (90.51) | ||
| COVID-19 self-diagnosis | 0.049 * | 0.45 | |||||
| Yes | 191 (29.98) | 181 (94.76) | 10 (5.24) | 22 (11.52) | 169 (88.48) | ||
| No | 227 (35.64) | 203 (89.43) | 24 (10.57) | 20 (8.81) | 207 (91.19) | ||
| COVID-19 symptoms 2 weeks before survey | 0.871 | 0.835 | |||||
| Yes | 444 (69.70) | 413 (93.02) | 31 (6.98) | 46 (10.36) | 398 (89.64) | ||
| No | 140 (21.98) | 129 (92.14) | 11 (7.86) | 13 (9.29) | 127 (90.71) | ||
| Fever | 1 | 0.242 | |||||
| Yes | 82 (12.87) | 77 (93.90) | 5 (6.10) | 6 (7.32) | 76 (92.68) | ||
| No | 191 (29.98) | 178 (93.19) | 13 (6.81) | 25 (13.09) | 166 (86.91) | ||
| Headaches | 0.727 | 0.817 | |||||
| Yes | 319 (50.08) | 298 (93.42) | 21 (6.58) | 36 (11.28) | 283 (88.72) | ||
| No | 83 (13.03) | 76 (91.57) | 7 (8.43) | 8 (9.64) | 75 (90.36) | ||
| Cough | 0.804 | 0.509 | |||||
| Yes | 162 (25.43) | 150 (92.59) | 12 (7.41) | 17 (10.49) | 145 (89.51) | ||
| No | 179 (28.10) | 168 (93.86) | 11 (6.14) | 24 (13.41) | 155 (86.59) | ||
| Fatigue | 0.372 | 0.0027 * | |||||
| Yes | 252 (39.56) | 238 (94.44) | 14 (5.56) | 20 (7.94) | 232 (92.06) | ||
| No | 102 (16.01) | 93 (91.18) | 9 (8.82) | 20 (19.61) | 82 (80.39) | ||
| Runny nose | 0.977 | 0.489 | |||||
| Yes | 175 (27.47) | 164 (93.71) | 11 (6.29) | 19 (10.86) | 156 (89.14) | ||
| No | 173 (27.16) | 161 (93.06) | 12 (6.94) | 24 (13.87) | 149 (86.13) | ||
| Sore throat | 0.729 | 1 | |||||
| Yes | 95 (14.91) | 87 (91.58) | 8 (8.42) | 11 (11.58) | 84 (88.42) | ||
| No | 242 (37.99) | 226 (93.39) | 16 (6.61) | 28 (11.57) | 214 (88.43) | ||
| Missing | 300 (47.10) | 280 (93.33) | 20 (6.67) | 22 (7.33) | 278 (92.67) | ||
| Breathing difficulties | 0.61 | 0.23 | |||||
| Yes | 78 (12.24) | 74 (94.87) | 4 (5.13) | 6 (7.69) | 72 (92.31) | ||
| No | 262 (41.13) | 242 (92.37) | 20 (7.63) | 35 (13.36) | 227 (86.64) | ||
| Abdominal pain | 0.92 | 0.39 | |||||
| Yes | 32 (5.02) | 30 (93.75) | 2 (6.25) | 2 (6.25) | 30 (93.75) | ||
| No | 283 (44.43) | 262 (93.58) | 21 (7.42) | 38 (13.43) | 245 (86.57) | ||
| Diarrhea | 0.25 | 0.59 | |||||
| Yes | 70 (10.99) | 68 (97.14) | 2 (2.86) | 7 (10) | 63 (90) | ||
| No | 263 (41.29) | 243 (92.39) | 20 (7.61) | 35 (13.31) | 228 (86.69) | ||
| Anosmia/ageusia | 1 | 0.397 | |||||
| Yes | 31 (4.87) | 29 (93.55) | 2 (6.45) | 2 (6.45) | 29 (93.55) | ||
| No | 295 (46.31) | 274 (92.88) | 21 (7.12) | 39 (13.22) | 256 (86.78) | ||
| IgM | IgG | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Univariate OR (95% CI) |
p- value | Multivariate OR (95% CI) |
p-value | Univariate OR (95% CI) |
p- value | Multivariate OR (95% CI) |
p-value |
| Age | 1.034 [0.99 -1.06] | 0.055 | 0.971 [0,74 -1,21] | 0,808 | 1.023 [0.98 – 1.081] | 0.345 | 0.974 [0.89 – 1.071] | 0.573 |
| Gender | 1.75 [0.943 -3.25] | 0.073 | 0.776 [0,10 – 4.6] | 0.791 | 0.906 [0.53 -1.578] | 0.728 | 0.913 [0.52 -1.62 | 0.757 |
| Occupation | 1.652 [0.92 – 2.72] | 0.065 | 1.345[0,06 – 22.03] | 0.834 | 1.26 [0.69 – 2.75] | 0.508 | 2.545 [0.60 – 15.33] | 0.250 |
| Level of education | 1.526 [0.91 – 2.47] | 0.095 | 1.709 [0.31 – 9.47] | 0.532 | 0.973 [0.61 – 1.61] | 0.911 | 0.917 [0.48 – 1.73] | 0.790 |
| Nationality | 0.074 [0.003 – 2.21] | 0.083 | 1.96 [0.64 – 4.93] | 0.186 | 2.286 [0.49 – 7.82] | 0.225 | ||
| Ethnic groups | 0.985 [0.86 – 1.13] | 0.826 | 0.723 [0.49 – 0.96] | 0.046 * | 0.95 [0.84 - 1.07] | 0.426 | 0.967 [0.85 – 1.095] | 0.6 |
| Place of residence | 0.995 [0.72 – 1.23] | 0.740 | 0.562 [0.17 – 1.24] | 0.23 | 0.998 [0.80 – 1.27] | 0.992 | 0.981 [0.74 – 1.32] | 0.894 |
| Accommodation type | 1.21 [0.57 – 2.40] | 0.60 | 4.209 [0.91 – 21.62) | 0.067 | 1.17 [0.62 – 2.37] | 0.643 | 0.924 [0.41 – 2.23] | 0.854 |
| Family members | 1.133 [0.77 – 1.66] | 0.522 | 1.849 [0.78 – 4.78] | 0.175 | 1.138 [0.81 – 1.6] | 0.452 | 1.234 [0.83 – 1.85] | 0.296 |
| Marital status | 2.399 [1.08 – 4.87] | 0.021 * | 4.44 [0.28 – 54.32] | 0.235 | 1.02 [0.47 – 2.68] | 0.956 | 1.135 [0.43 – 3.95] | 0.818 |
| Smoker | 1.004 [0.21 – 2.48] | 0.995 | 2.897 [0.087 – 36.8] | 0.457 | ||||
| Alcohol | 1.064 [0.18 -3.17] | 0.926 | 2.06 [0.066 – 30.84} | 0.622 | 0.835 [0.32 – 3.25] | 0.747 | ||
| BMI Categorized | 2.015 [1.12 – 3.56] | 0.016 * | 1.215 [0.39 – 3.73] | 0.729 | 0.785 [0.49 – 1.29] | 0.329 | 0.718 [0.43 – 1.21] | 0.206 |
| Blood Group | 1.011 [0.89 – 1.152] | 0.861 | 1.049 [0.786 – 1.47] | 0.235 | 1.023 [0.91 – 1.14] | 0.679 | 0.988 [0.86 – 1.24] | 0.842 |
| Vaccination Status | 1.206 [0.63 – 2.25] | 0.560 | 0.428 [0.08 – 1.89] | 0.285 | 4.741 [2.25 – 11.65] | <0.001 * | 2.714 [0.59 – 48.48] | 0.325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





