Submitted:
21 November 2023
Posted:
22 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Staphylococcal Strains and Growth Media
2.3. Minimum inhibitory Concentrations (MICs) Determination
2.4. Evaluation of the combined Antistaphylococcal Effect
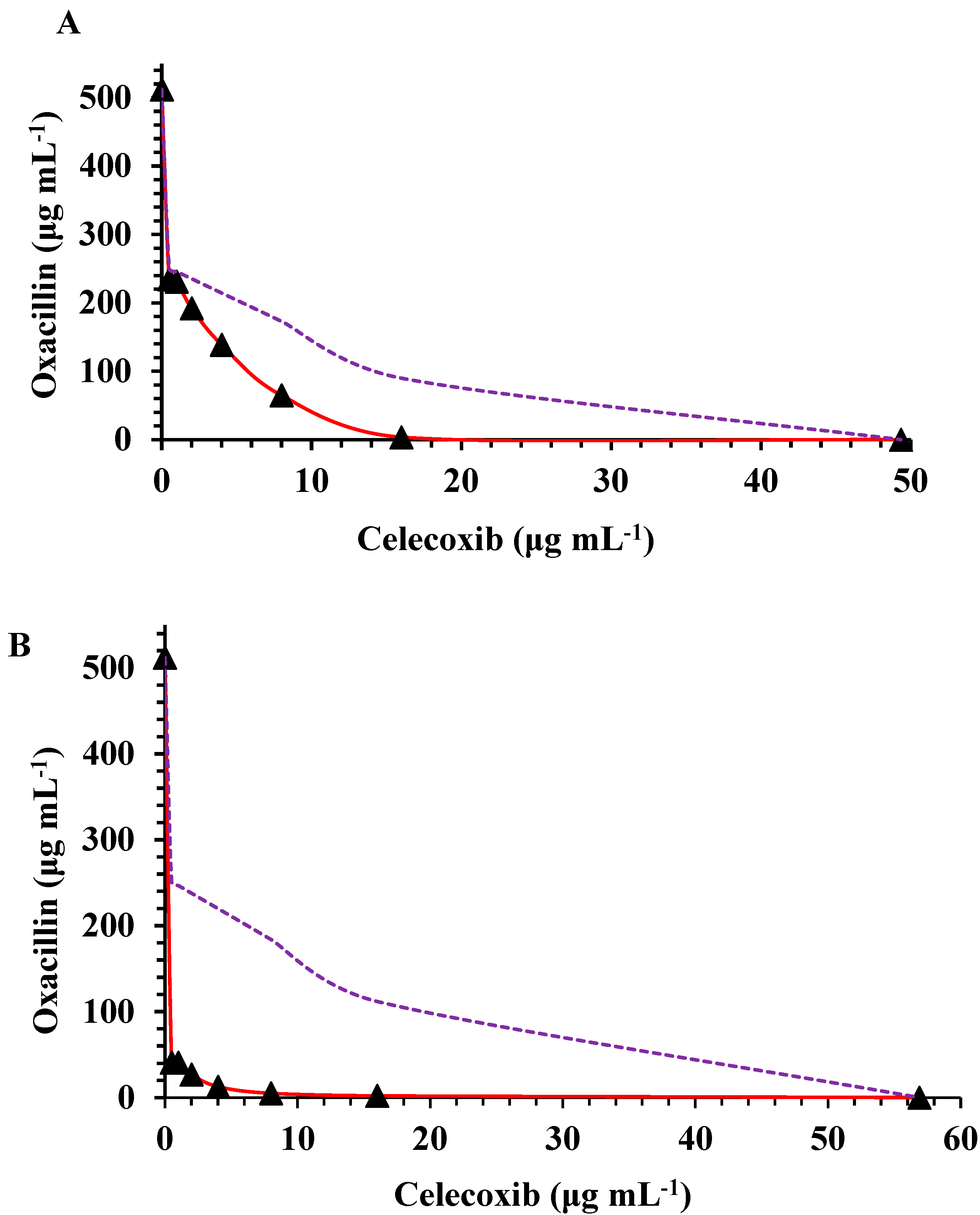
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakr, A.; Bregeon, F.; Mege, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus nasal colonisation: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, E.; Goetz, C.; Droppa-Almeida, D.; Chamberland, S.; Jacques, M.; Malouin, F. Secondary Staphylococcus aureus intramammary colonisation is reduced by non-aureus staphylococci exoproducts. Microbes Infect. 2022, 24, 104879. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, N.; Ryan, J.E.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018, 31, e00084–17. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro surveill. 2010, 15, pii–19688. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S. Management of bone and joint infections due to Staphylococcus aureus. Intern. Med. J. 2005, 35, S79–S96. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.D.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: microbial pathogenesis, immunity, and clinical management. Nat. Rev. Microbial. 2022, 20, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Minion, J.; Skinner, S.; Wong, A. Disseminated Exophiala dermatitidis causing septic arthritis and osteomyelitis. BMC Infect. Dis. 2018, 18, 255. [Google Scholar] [CrossRef] [PubMed]
- Sommer, T.; Karsy, M.; Driscoll, M.J.; Jensen, R.L. Varicella-zoster virus infection and osteomyelitis of the skull. World Neurosurg. 2018, 115, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbial. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Marriott, I. Apoptosis-associated uncoupling of bone formation and resorption in osteomyelitis. Front. Cell. Infect. Microbiol. 2013, 3, 67718. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.; Tarkowski, A. Staphylococcus aureus-induced inflammation and bone destruction in experimental models of septic arthritis. J. Periodontal. Res. 1999, 34, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L. Novel therapeutic interventions towards improved management of septic arthritis. BMC. Musculoskelet. Disord. 2021, 22, 530. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, J.; El Samad, Y.; Brunschweiler, B.; Grados, F.; Dehamchia-Rehailia, N.; Sejourne, A.; Schmit, J.L.; Gabrion, A.; Fardellone, P.; Paccou, J. Morbimortality in adult patients with septic arthritis: a three- year hospital-based study. BMC. Infect. Dis. 2016, 16, 239. [Google Scholar] [CrossRef]
- Huang, J.F.; Wu, Q.N.; Zheng, X.Q.; Sun, X.L.; Wu, C.Y.; Wang, X.B.; Wu, C.W.; Wang, B.; Wang, X.Y.; Bergman, M.; et al. The characteristics and mortality of osteoporosis, osteomyelitis, or rheumatoid arthritis in the diabetes population: a retrospective study. Int J Endocrinol. 2020, 2020, 8821978. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.; Baertl, S.; Alt, V.; Rupp, M. What is the burden of osteomyelitis in Germany? an analysis of inpatient data from 2008 through 2018. BMC Infect. Dis. 2021, 21, 550. [Google Scholar] [CrossRef] [PubMed]
- Minguez, S.; Molinos, S.; Mateo, L.; Gimenez, M.; Mateu, L.; Cabello, J.; Olive, A. Septic arthritis due to methicillin-resistant Staphylococcus aureus in adults. Reumatol Clin. 2015, 11, 381–386. [Google Scholar] [CrossRef]
- Abram, S.G.F.; Alvand, A.; Judge, A.; Beard, D.J.; Price, A.J. Mortality and adverse joint outcomes following septic arthritis of the native knee: a longitudinal cohort study of patients receiving arthroscopic washout. Lancet Infect. Dis. 2020, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet. 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Stake, S.; Scully, R.; Swenson, S.; Lee, D.; Lee, R.; Sparks, A.; Pandarinath, R. Repeat irrigation and debridement for patients with acute septic knee arthritis: incidence and risk factors. J Clin Orthop Trauma. 2020, 11, S177–S183. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.R.; Vowden, P. Wound debridement part 2: sharp techniques. J.Wound Care. 1999, 8, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus osteomyelitis: bone, bugs, and surgery. Infect. Immun. 2020, 88, e00932–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Roberts, M.; Al-Kassas, R. Implantable drug delivery systems for the treatment of osteomyelitis. Drug Dev Ind Pharm. 2022, 48, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.L.; Oliveira, P.R.; Carvalho, V. C.; Cimerman, S.; Savio, E. Recommendations for the treatment of osteomyelitis. Braz J Infect Dis. 2014, 18, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, J.C.; Winston, L.G. Clinical failures of appropriately treated methicillin-resistant Staphylococcus aureus infections. J. Infect. 2008, 57, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Marinho, D.S.; Huff, G.; Ferreira, B.L.; Castro, H.; Rodrigues, C.R.; de Sousa, V.P.; Cabral, L.M. The study of vancomycin use and its adverse reactions associated to patients of a Brazilian university hospital. BMC Res. Notes. 2011, 4, 236. [Google Scholar] [CrossRef] [PubMed]
- Congedi, S.; Minotti, C.; Giaquinto, C.; Da Dalt, L.; Dona, D. Acute infectious osteomyelitis in children: new treatment strategies for an old enemy. World J Pediatr. 2020, 16, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Wieland, B.W.; Marcantoni, J.R.; Bommarito, K.M.; Warren, D.K.; Marschall, J. A retrospective comparison of ceftriaxone versus oxacillin for osteoarticular infections due to methicillin-susceptible Staphylococcus aureus. Clin. Infect. Dis. 2012, 54, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.R.; Bradley, J.S.; Chatterjee, A.; Copley, L.A.; Robinson, J.; Kronman, M.P.; Arrieta, A.; Fowler, S.L.; Harrison, C.; Carrillo-Marquez, M.A.; et al. Clinical practice guideline by the pediatric infectious diseases society and the infectious diseases society of America: 2021 guideline on diagnosis and management of acute hematogenous osteomyelitis in pediatrics. J. Pediatric. Infect. Dis. Soc. 2021, 10, 801–844. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.M.; Turnidge, J.D.; Sentry, A. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from sentry antimicrobial surveillance programme, 1998-1999. Antimicrob. Agents Chemother. 2002, 46, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Helito, C.P.; Zanon, B.B.; Miyahara, H.D.E.S.; Pecora, J.R.; Lima, A.L.; Oliveira, P.R.; Vicente, J.R.; Demange, M.K.; Camanho, G.L. Clinical and epidemiological differences between septic arthritis of the knee and hip caused by oxacillin-sensitive and-resistant Staphylococcus aureus. Clinics (Sao Paulo). 2015, 70, 30–33. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today. 2016, 21, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Moller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin, and amoxicillin–clavulanic acid: properties, indications, and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Domingos, O.D.S.; Alcantara, B.G.V.; Santos, M.F.C.; Maiolini, T.C.S.; Dias, D.F.; Baldim, J.L.; Lago, J.H.G.; Soares, M.G.; Chagas-Paula, D.A. Anti-inflammatory derivatives with dual mechanism of action from the metabolomic screening of Poincianella pluviosa. Molecules. 2019, 24, 4375. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical pharmacology of corticosteroids. Respir Care. 2018, 63, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Otlu, S.; Celebi, O.; Aksu, P.; Saglam, A.G.; Dogan, A.N.C.; Mutlu, N. An investigation of antibacterial effects of steroids. Turkish J. Vet. Anim. Sci. 2017, 41, 22. [Google Scholar] [CrossRef]
- Chiu, H.C.; Lee, S.L.; Kapuriya, N.; Wang, D.; Chen, Y.R.; Yu, S.L.; Kulp, S.K.; Teng, L.J.; Chen, C.S. Development of novel antibacterial agents against methicillin-resistant Staphylococcus aureus. Bioorg Med. Chem. 2012, 20, 4653–4660. [Google Scholar] [CrossRef]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing celecoxib as a topical antimicrobial agent. Front. Microbiol. 2015, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qu, X.; Tang, H.; Wang, Y.; Yang, H.; Yuan, W.; Yue, B. Diclofenac resensitises methicillin-resistant Staphylococcus aureus to β-lactams and prevents implant infections. Adv. Sci. 2021, 8, 2100681. [Google Scholar] [CrossRef] [PubMed]
- Kivitz, A.J.; Espinoza, L.R.; Sherrer, Y.R.; Liu-Dumaw, M.; West, C.R.A. Comparison of the efficacy and safety of celecoxib 200 mg and celecoxib 400 mg once daily in treating the signs and symptoms of psoriatic arthritis. Semin. Arthritis Rheum. 2007, 37, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin Med (Lond). 2021, 21, 131–134. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.A. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the class study: a randomised controlled trial. celecoxib long-term arthritis safety study. JAMA. 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Krasselt, M.; Baerwald, C.; Petros, S.; Seifert, O. Mortality of sepsis in patients with rheumatoid arthritis: a single-center retrospective analysis and comparison with a control group. J. Intensive Care Med. 2021, 36, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.C.; Barbulescu, A.L.; Firulescu, S.C.; Chisalau, A.B.; Parvanescu, C.D.; Ciurea, P.L.; Sandu, R.E.; Turcu-Stiolica, A.; Boldeanu, M.V.; Vintila, E.M.; et al. Staphylococcus aureus-induced septic arthritis of the ankle related to malum perforans in a diabetes patient. Rom J Morphol Embryol. 2021, 62, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Rondevaldova, J.; Hummelova, J.; Tauchen, J.; Kokoska, L. In vitro antistaphylococcal synergistic effect of isoflavone metabolite demethyltexasin with amoxicillin and oxacillin. Microb. Drug Resist. 2018, 24, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically-8th edition: Approved Standard M7-A8. CLSI, Wayne, PA, USA. 2009. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: how to develop a stronger in vitro proof-of-concept. J Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Turnidge, J.D.; Washington, J.A. Antimicrobial agents and susceptibility testing: dilution and disc diffusion testing methods. In Murray PR, Baron EJ, Pfaller MA et al ed. Manual of clinical microbiology, 7th ed. Washington DC: ASM Press. 1999; 1531–1533. [Google Scholar]
- Frankova, A.; Vistejnova, L.; Merinas-Amo, T.; Leheckova, Z.; Doskocil, I.; Wong Soon, J.; Kudera, T.; Laupua, F.; Alonso-Moraga, A.; Kokoska, L. In vitro antibacterial activity of extracts from Samoan medicinal plants and their effect on proliferation and migration of human fibroblasts. J Ethnopharmacol. 2021, 264, 113220. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E-test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Fidelix, T.S.; Macedo, C.R.; Maxwell, L.J.; Fernandes Moca Trevisani, V. Diacerein for osteoarthritis. Cochrane Database Syst. Rev. 2014, 10, CD005117. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, K.; Bruyere, O.; Cooper, C. Diacerein: benefits, risks, and place in the management of osteoarthritis. an opinion-based report from the ESCEO. Drugs Aging. 2016; 33, 75–85. [Google Scholar] [CrossRef]
- Nguon, S.; Novy, P.; Kokoska, L. Potentiation of the in vitro antistaphylococcal effect of oxacillin and tetracycline by the anti-inflammatory drug diacetyl rhein. Chemotherapy. 2013, 59, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Yue, J.; Sun, S.; Lv, Q.; Jian, S.; Xie, Y.; Han, L.; Zhang, F.; Dai, Y.; et al. In vitro antimicrobial activity of diacerein on 76 isolates of gram-positive cocci from bacterial keratitis patients and in vivo study of diacerein eye drops on Staphylococcus aureus keratitis in mice. Antimicrob. Agents Chemother. 2019, 63, e01874–18. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Alhashimi, M.; Mayhoub, A.; Mohammad, H.; Seleem, M.N. Repurposing fenamic acid drugs to combat multidrug resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2020, 64, e02206–19. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Y.; Whittell, L.R.; Jergic, S.; Liu, M.; Harry, E.; Dixon, N.E.; Kelso, M.J.; Beck, J.L.; Oakley, A.J. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem. Biol. 2014, 21, 481–487. [Google Scholar] [CrossRef]
- Etienne, F.; Resnick, L.; Sagher, D.; Brot, N.; Weissbach, H. Reduction of sulindac to its active metabolite, sulindac sulfide: assay and role of the methionine sulfoxide reductase system. Biochem. Biophys. Res. Commun. 2003, 312, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Shirin, H.; Moss, S.F.; Kancherla., S.; Kancherla, K.; Holt, P.R.; Weinstein, I.B.; Sordillo, E.M. Nonsteroidal anti-inflammatory drugs have bacteriostatic and bactericidal activity against Helicobacter pylori. J. Gastroenterol. Hepatol. 2006, 21, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Annamanedi, M.; Kalle, A.M. Celecoxib sensitises Staphylococcus aureus to antibiotics in macrophages by modulating SIRT1. PLoS one. 2014, 9, e99285. [Google Scholar] [CrossRef] [PubMed]
- Annamanedi, M.; Varma, G.Y.N.; Anuradha, K.; Kalle, A.M. Celecoxib enhances the efficacy of low-dose antibiotic treatment against polymicrobial sepsis in mice and clinical Isolates of ESKAPE pathogens. Front. Microbiol. 2017, 8, 805. [Google Scholar] [CrossRef]
- DeMarco, C.E.; Cushing, LA.; Frempong-Manso, E.; Seo, S.M.; Jaravaza, T.A.; Kaatz, G.W. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3235–3239. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Kalle, A.M.; Rizvi, A. Inhibition of bacterial multidrug resistance by celecoxib, a cyclooxygenase-2 inhibitor. Antimicrob. Agents Chemother. 2011, 50, 439–442. [Google Scholar] [CrossRef]
- Worthington, R.L.; Melander, C. Combination approaches to combat multi-drug resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gonga, L.; Thorn, C.F.; Bertagnolli, M.M.; Grosser, T.; Altman, R.B.; Klein, T.E. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacognet. Genomics. 2012, 22, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Lee, C.H.; Chien, C.C.; Chen, I.L. Impact of teicoplanin maintenance dose and MIC values on the clinical outcomes of patients treated for methicillin-resistant Staphylococcus aureus bacteremia. Infect. Drug Resist. 2018, 11, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Mori, Y.; Yamada, G.; Cammack, I.; Shinohara, T.; Matsuzaka, S.; Hoshi, T. Listeria monocytogenes ankle osteomyelitis in a patient with rheumatoid arthritis on adalimumab: a report and literature review of Listeria monocytogenes osteomyelitis. Intern. Med. 2021, 60, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Zheng, C.; Zheng, C.; Wang, B.; Shen, P.; Xie, Z.; Qu, Y. Efficacy of pre-emptive use of cyclooxygenase-2 inhibitors for total knee arthroplasty: a mini-review. Arthroplasty. 2019, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- FDA. Centre for drug evaluation and research: application number NDA 20-998. Published 1998. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20998AP_clinphrmr_P1.pdf (accessed on 3 August 2023).
- Sidney, L.E.; Heathman, T.R.; Britchford, E.R.; Abed, A.; Rahman, C.V.; Buttery, L.D. Investigation of localized delivery of diclofenac sodium from poly (D, L-lactic acid-co-glycolic acid)/poly (ethylene glycol) scaffolds using an in vitro osteoblast inflammation model. Tissue Eng. Part A. 2015, 21, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Heraeus Medical. Palacos R+G: high-viscosity, radiopaque bone cement containing gentamicin. Published 2020. Available online: https://www.heraeus.com/media/media/hme/doc_hme/products_us/PALACOS_RG_IFU_US.pdf (accessed on 15 August 2023).
| Compound | Minimum inhibitory concentration (mg/mL) | |||||||||
| Standard ATCCa strains | Clinical isolates | |||||||||
| 25923 | 29213 | 33591b | 33592b | 43300b | BAA 976b | MRSA1b | MRSA2b | MRSA3b | MRSA4b | |
| Acetylsalicylic acid | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Acemetacin | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Ampyrone | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Celecoxib | 64 | 64 | 64 | 64 | 64 | 64 | 32 | 64 | 64 | 64 |
| Cortisone | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Diacerein | 128 | 128 | 128 | 64 | 128 | 128 | 128 | 128 | 64 | 128 |
| Diclofenac sodium | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Diflunisal | 512 | 512 | 512 | 512 | 512 | n.a | 512 | 512 | n.a | n.a |
| Ethenzamide | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Felbinac | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Ibuprofen | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Mefenamic acid | 512 | 512 | 512 | 512 | 512 | n.a | n.a | 512 | 512 | n.a |
| Nabumetone | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Propyphenazone | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Sulindac sulfide | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 |
| Sulindac | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| Tolfenamic acid | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 512 | 256 | 128 |
| Oxacillinc | 0.25 | 0.5 | 512 | 512 | 256 | 64 | 512 | 256 | 256 | 256 |
| S. aureus | MICsa alone | CX concentrations (indicated in bold) in combination with OX at the concentration indicated in the MIC column (mg/mL) and related to FICIb | ||||||||||||
| OXc | CXd | 16 | 8 | 4 | 2 | 1 | 0.5 | |||||||
| MIC | FICI | MIC | FICI | MIC | FICI | MIC | FICI | MIC | FICI | MIC | FICI | |||
| ATCCe 29213 | 0.5 | 60.444 | 0.225 | 0.716 | 0.189 | 0.510 | 0.166 | 0.399 | 0.194 | 0.421 | 0.180 | 0.377 | 0.180 | 0.369 |
| ATCC 25923 | 0.25 | 56.888 | 0.230 | 1.204 | 0.263 | 1.196 | 0.166 | 0.736 | 0.152 | 0.646 | 0.125 | 0.517 | 0.090 | 0.369 |
| ATCC 33591 | 512 | 49.332 | 3.555 | 0.331 | 64.888 | 0.288 | 138.890 | 0.352 | 192.000 | 0.415 | 231.110 | 0.471 | 234.670 | 0.468 |
| ATCC 33592 | 512 | 56.888 | 2.222 | 0.285 | 5.110 | 0.150 | 12.777 | 0.095 | 26.888 | 0.087 | 40.888 | 0.097 | 40.888 | 0.088 |
| ATCC 43300 | 227.56 | 53.333 | 2.000 | 0.308 | 44.222 | 0.344 | 88.000 | 0.461 | 156.444 | 0.725 | 156.444 | 0.706 | - | - |
| BAA 976 | 64 | 64 | 55.999 | 1.124 | 39.999 | 0.749 | 42.666 | 0.729 | 32.000 | 0.531 | 32.000 | 0.515 | 32.000 | 0.507 |
| MRSA1f | 512 | 53.333 | 199.560 | 0.689 | 216.111 | 0.572 | 231.111 | 0.526 | 202.666 | 0.433 | 181.333 | 0.372 | 181.333 | 0.363 |
| MRSA2 | 256 | 64 | 114.000 | 0.695 | 213.333 | 0.958 | 241.777 | 1.006 | 241.777 | 0.975 | 241.777 | 0.960 | 241.777 | 0.952 |
| MRSA3 | 256 | 64 | 88.666 | 0.596 | 135.111 | 0.652 | 142.222 | 0.618 | 156.444 | 0.642 | 156.444 | 0.626 | 156.444 | 0.618 |
| MRSA4 | 256 | 64 | 55.555 | 0.467 | 117.333 | 0.583 | 142.222 | 0.618 | 156.444 | 0.642 | 156.444 | 0.626 | 156.444 | 0.618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





