Submitted:
21 November 2023
Posted:
22 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Preparation of Microrobots
2.1. Electrochemical Depositions
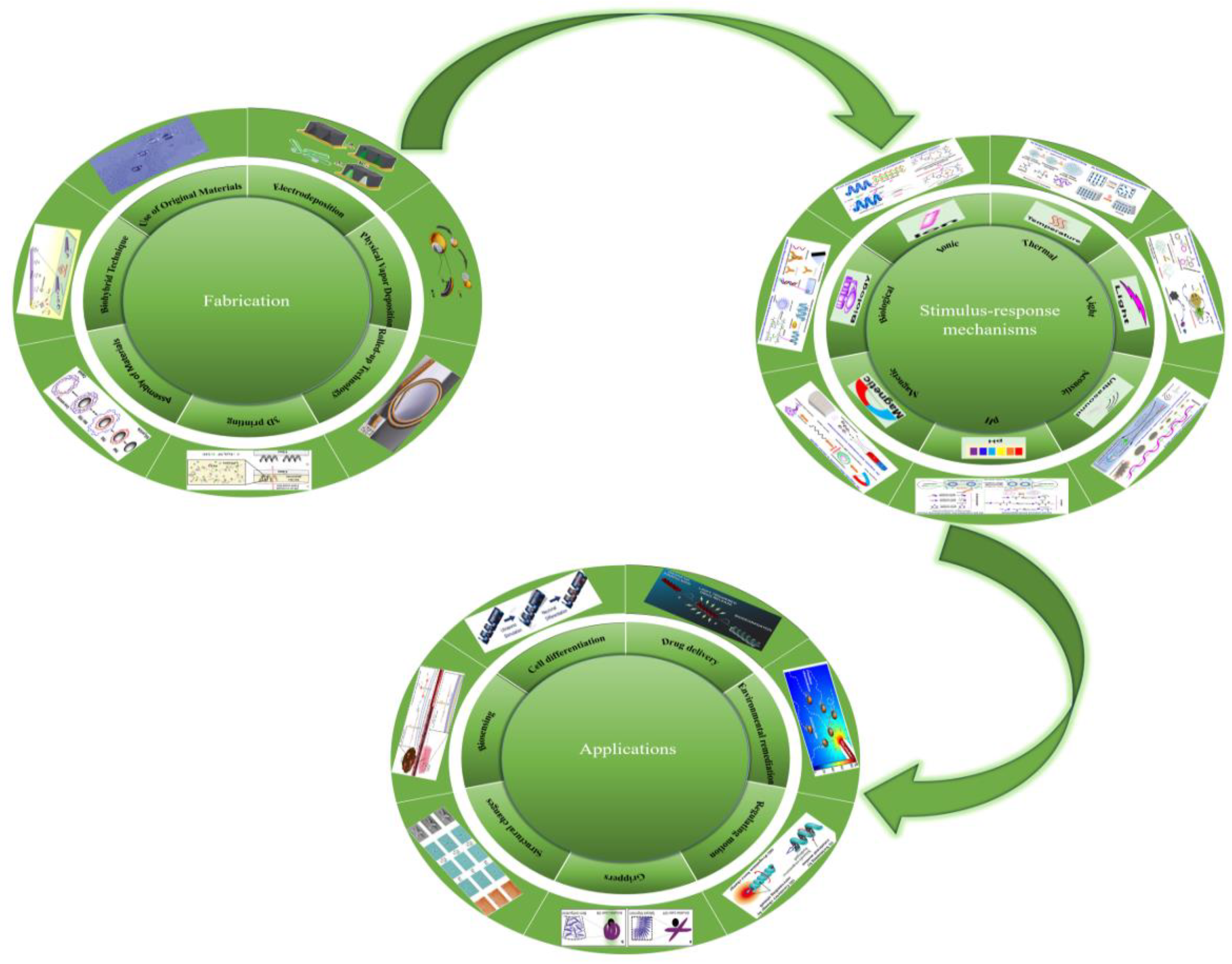
2.1.1. Membrane Template-Assisted Electrodeposition
2.1.1.1. Electrodeposited Nanowires
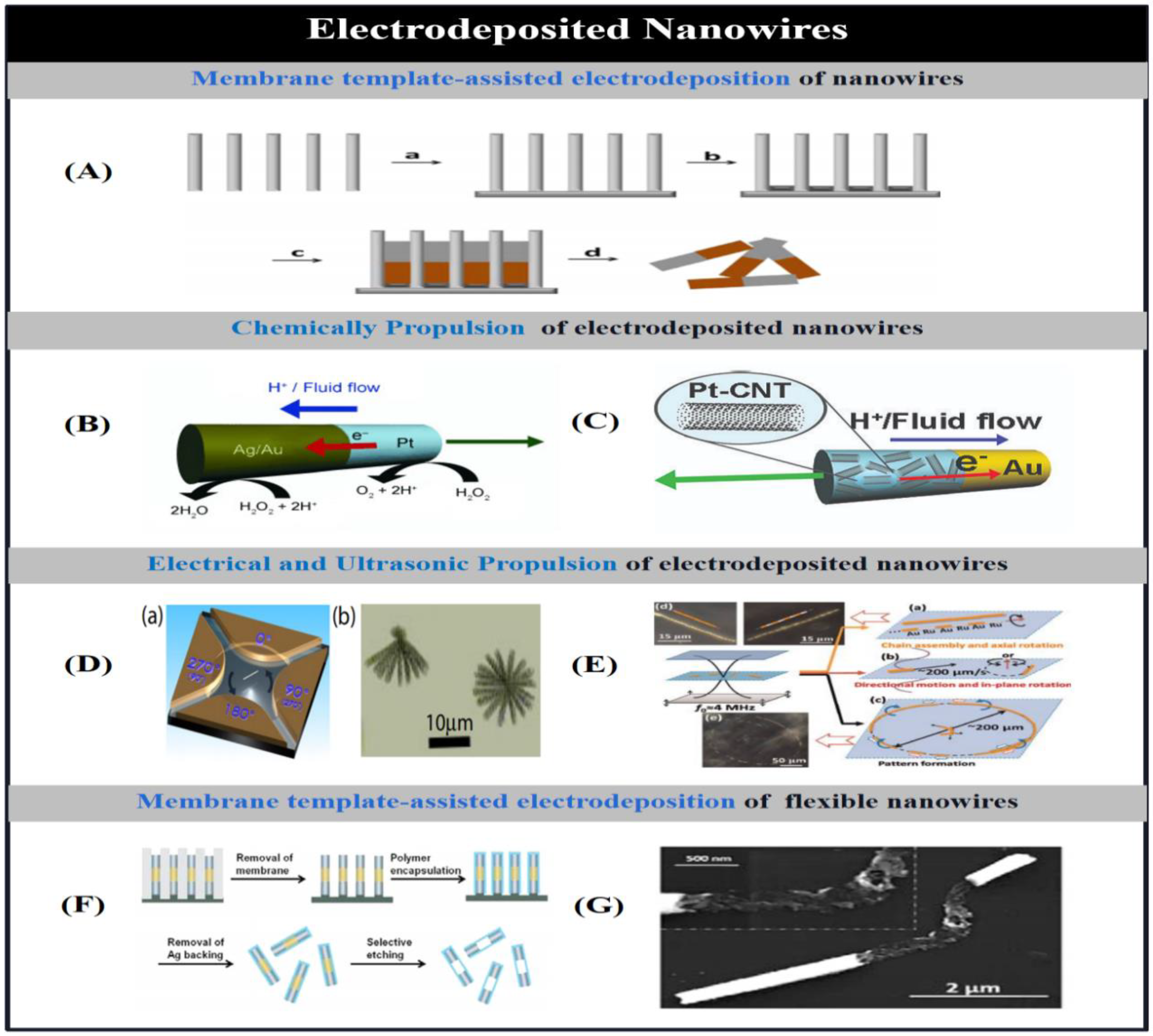
2.1.1.2. Electrodeposited Micro/Nanotubes
2.1.1.3. Electrodeposited Helical Micromotors.
2.1.2. Electrochemical Deposition Based on Other Templates
2.1.3. Asymmetric Bipolar Electrodeposition
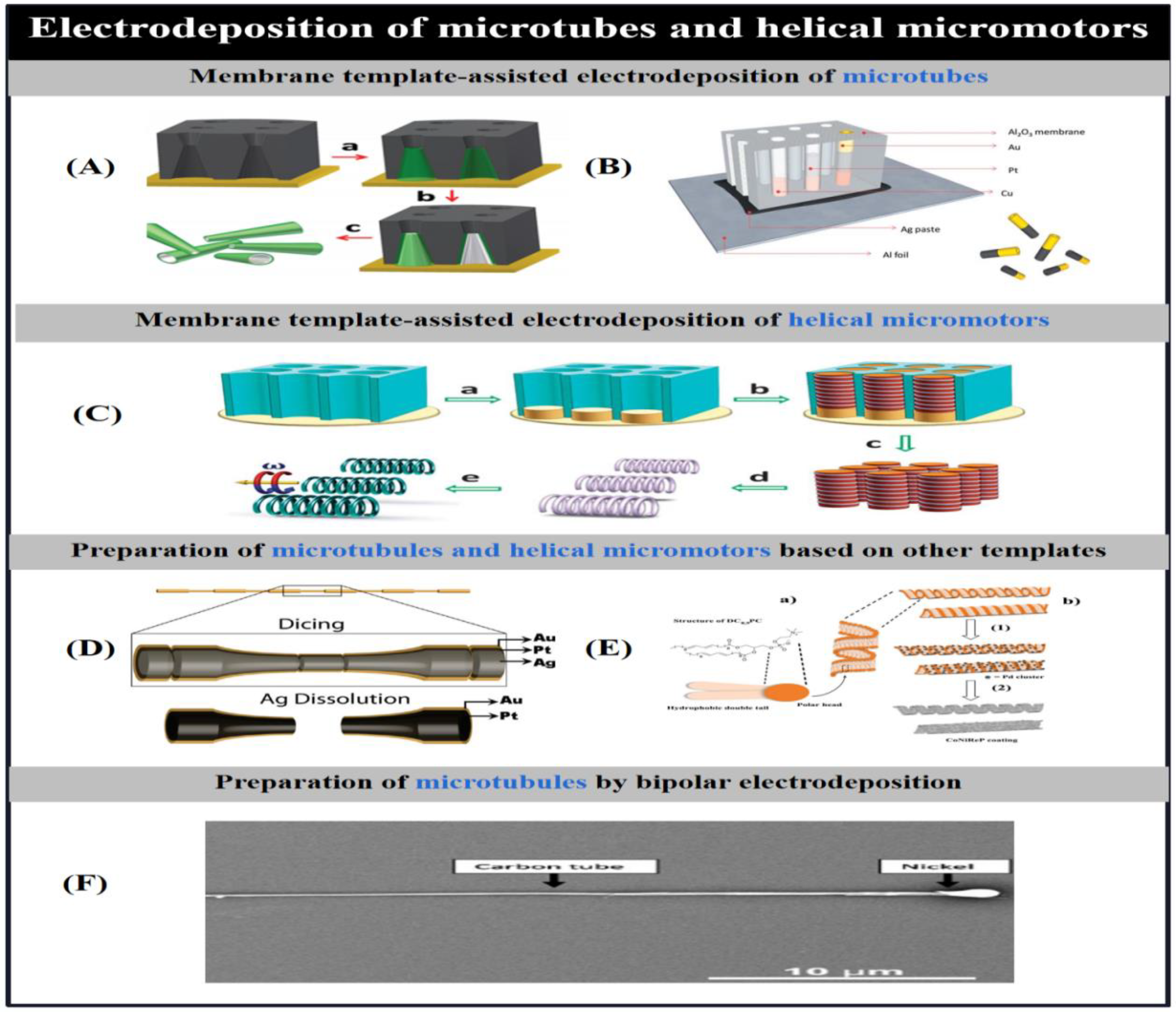
2.2. Physical Vapor Deposition
2.2.1. Conventional Physical Vapor Deposition
2.2.2. Glancing Angle Deposition
2.2.2.1. Helical Micro/Nanomotors by GLAD
2.2.2.2. Janus Micro/Nanomotors by GLAD
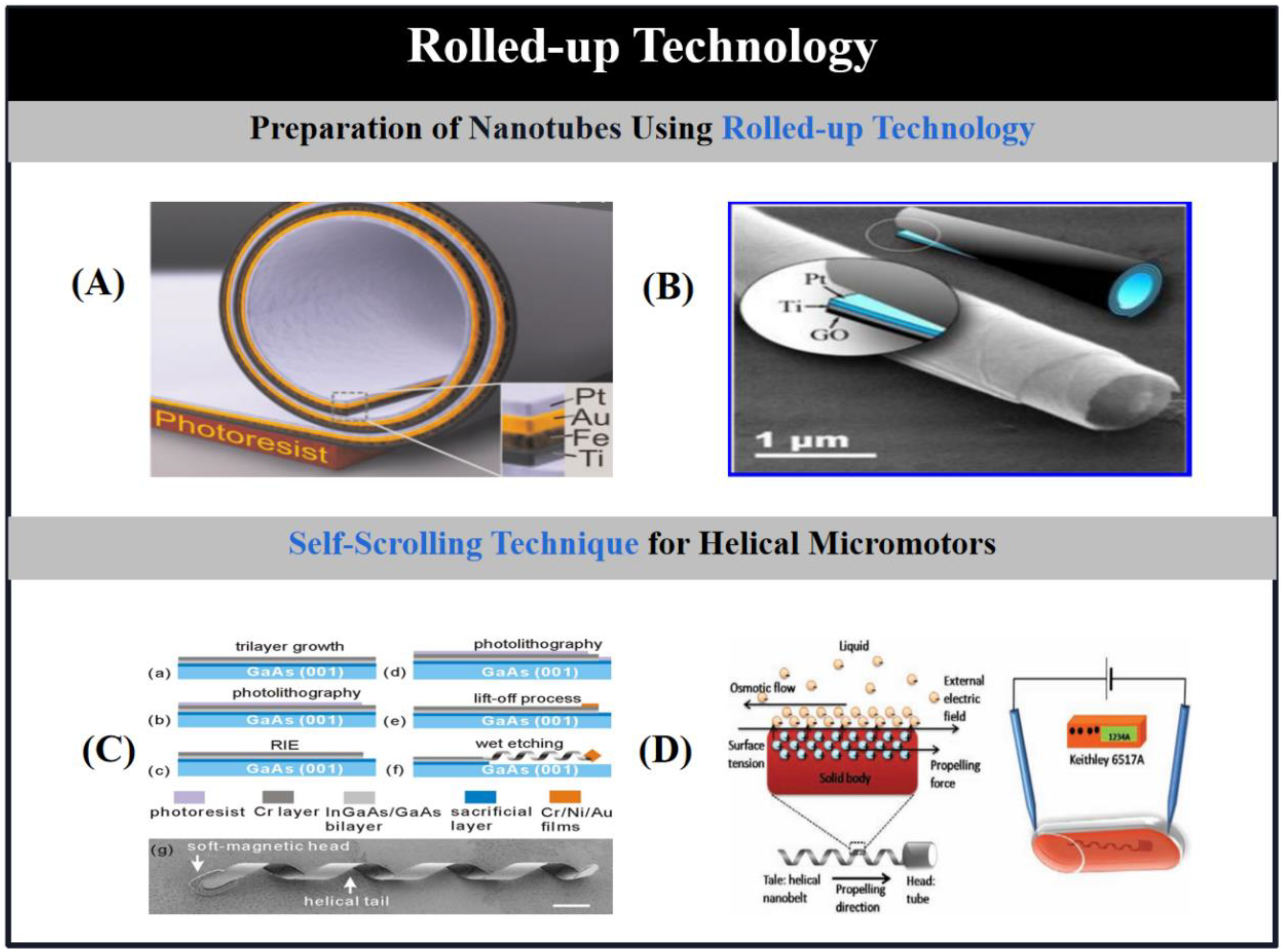
2.3. Rolled-Up Technology
2.3.1. Preparation of Nanotubes Using Rolled-Up Technology
2.3.2. Self-Scrolling Technique for Helical Micromotors.
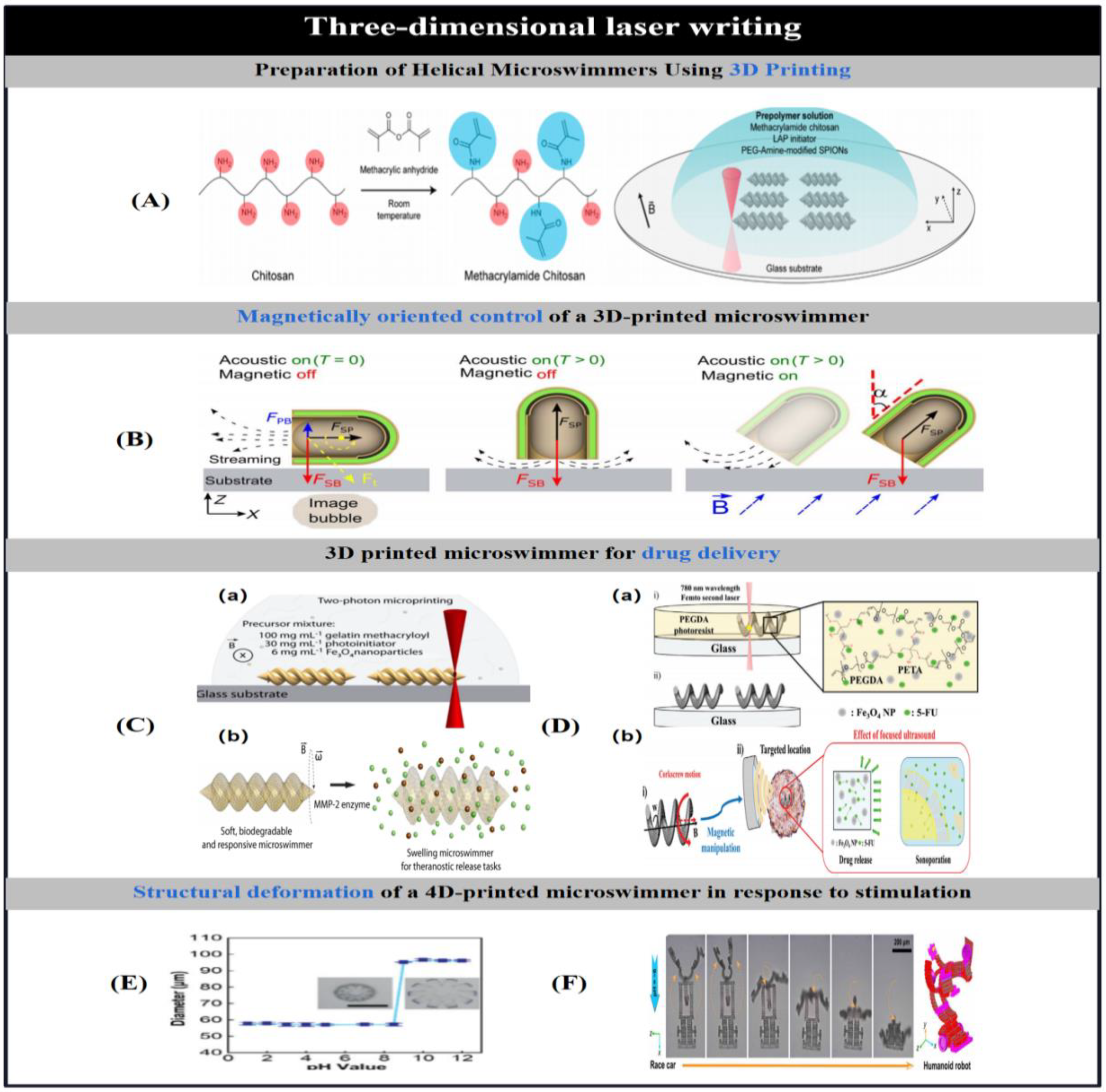
2.4. Three-Dimensional Laser Writing
2.4.1. 3D Printing
2.4.2. 4D Printing
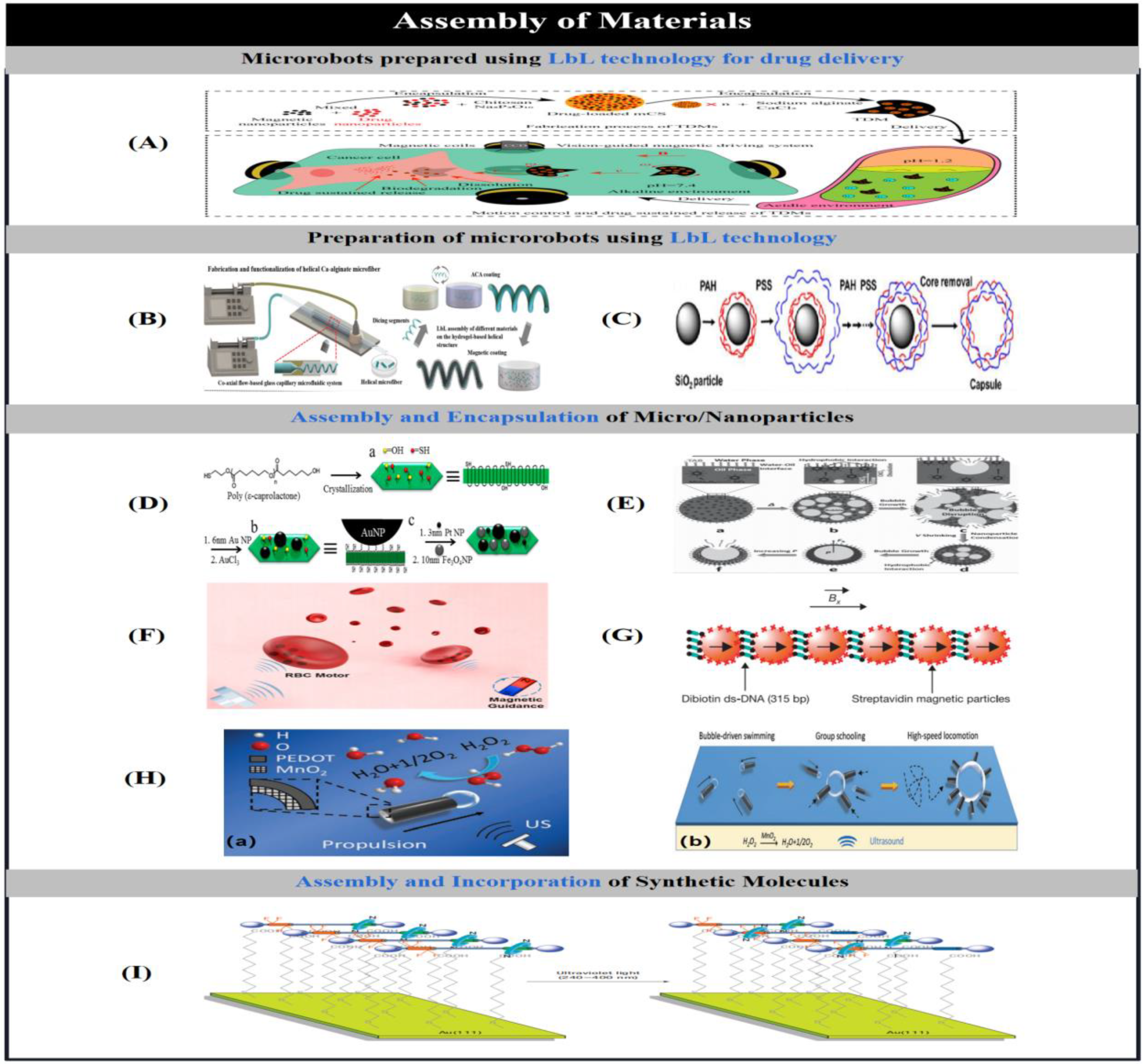
2.5. Assembly of Materials
2.5.1. Layer-by-Layer Assembly
2.5.2. Assembly and Encapsulation of Micro/Nanoparticles
2.5.3. Assembly and Incorporation of Synthetic Molecules
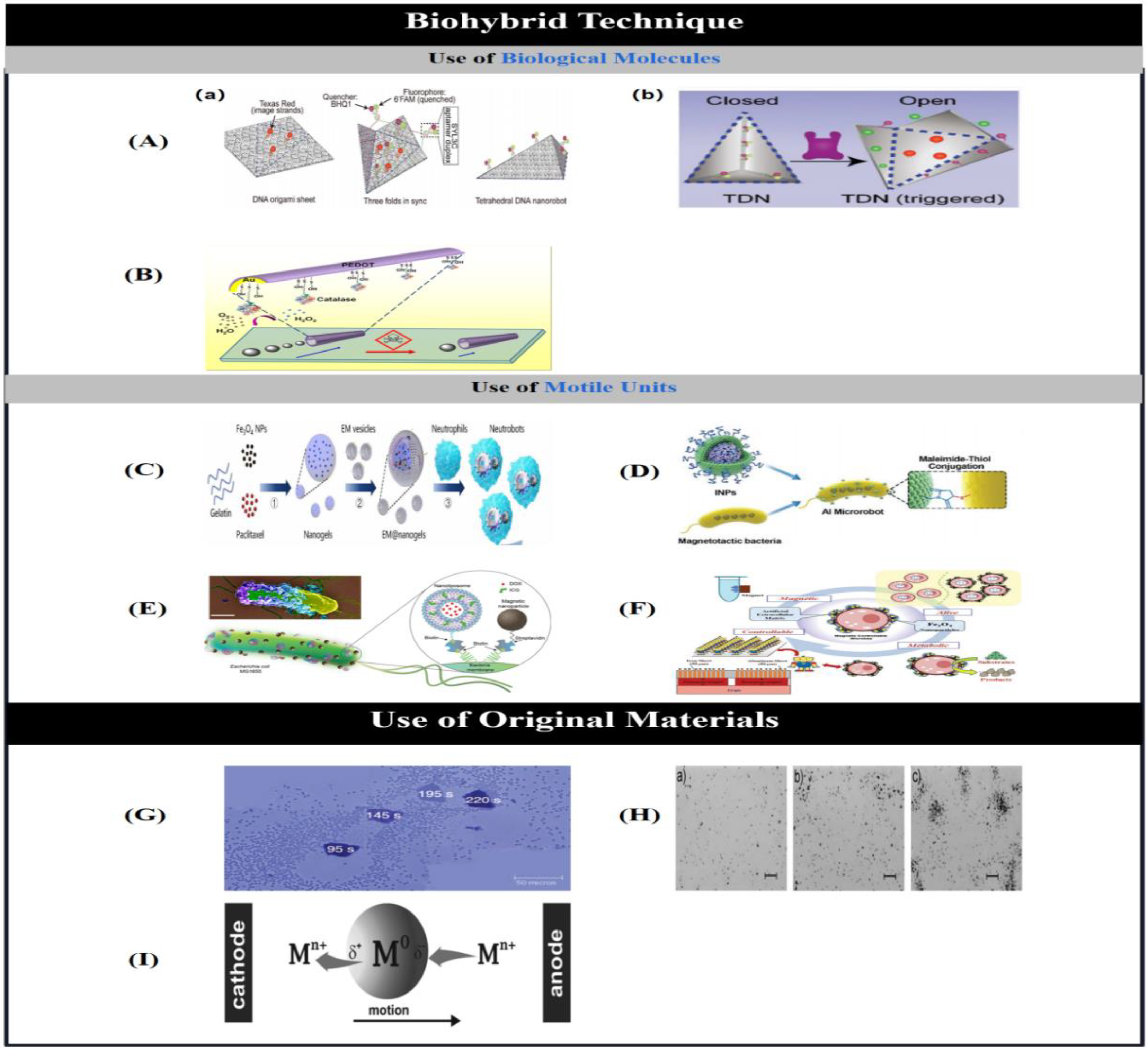
2.6. Biohybrid Technique
2.6.1. Use of Biological Molecules.
2.6.2. Use of Motile Units
2.7. Use of Original Materials
3. Stimulus-response mechanisms and applications of micro/nanobots
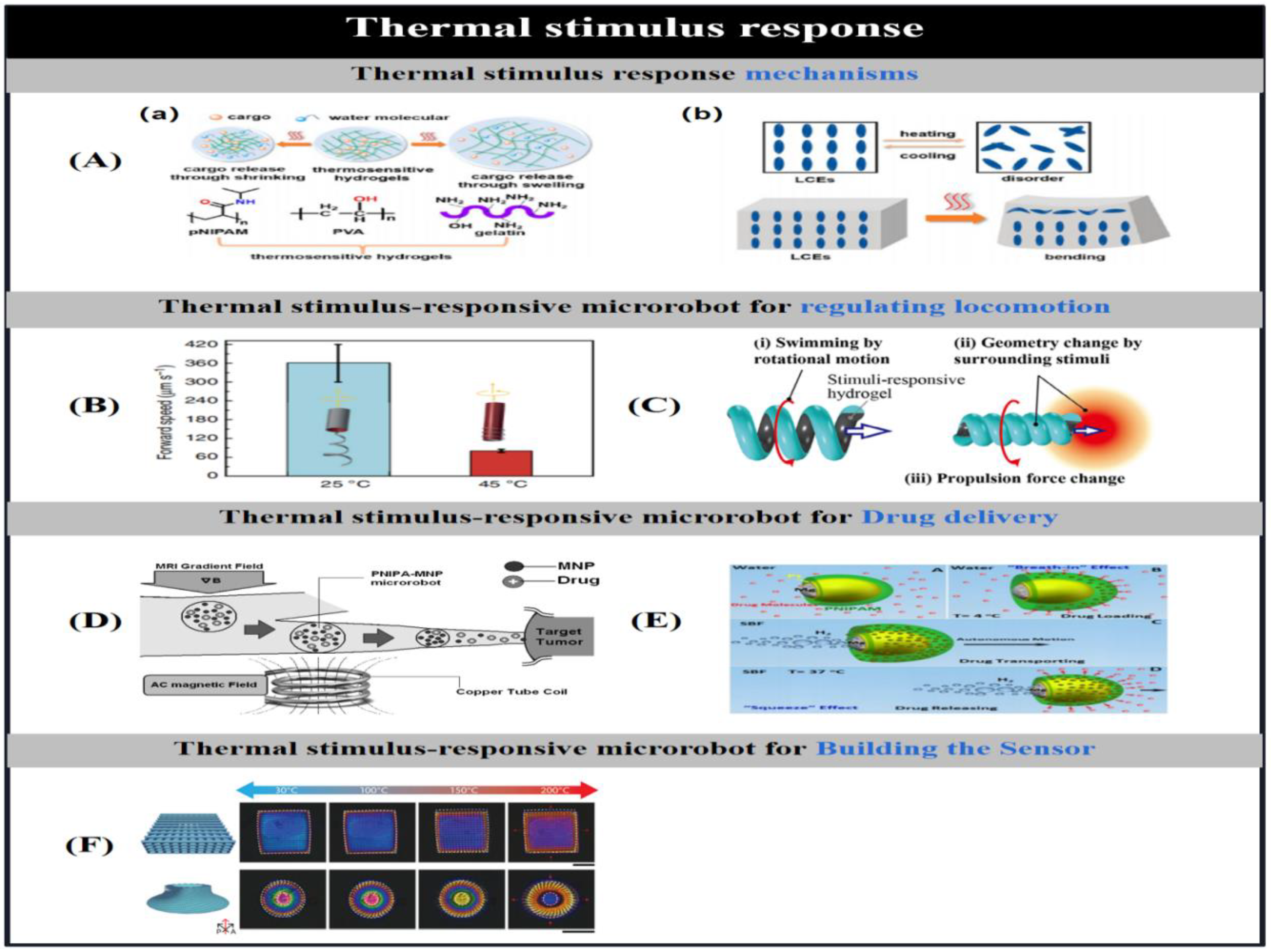
3.1. Thermal Stimulus Response Mechanisms
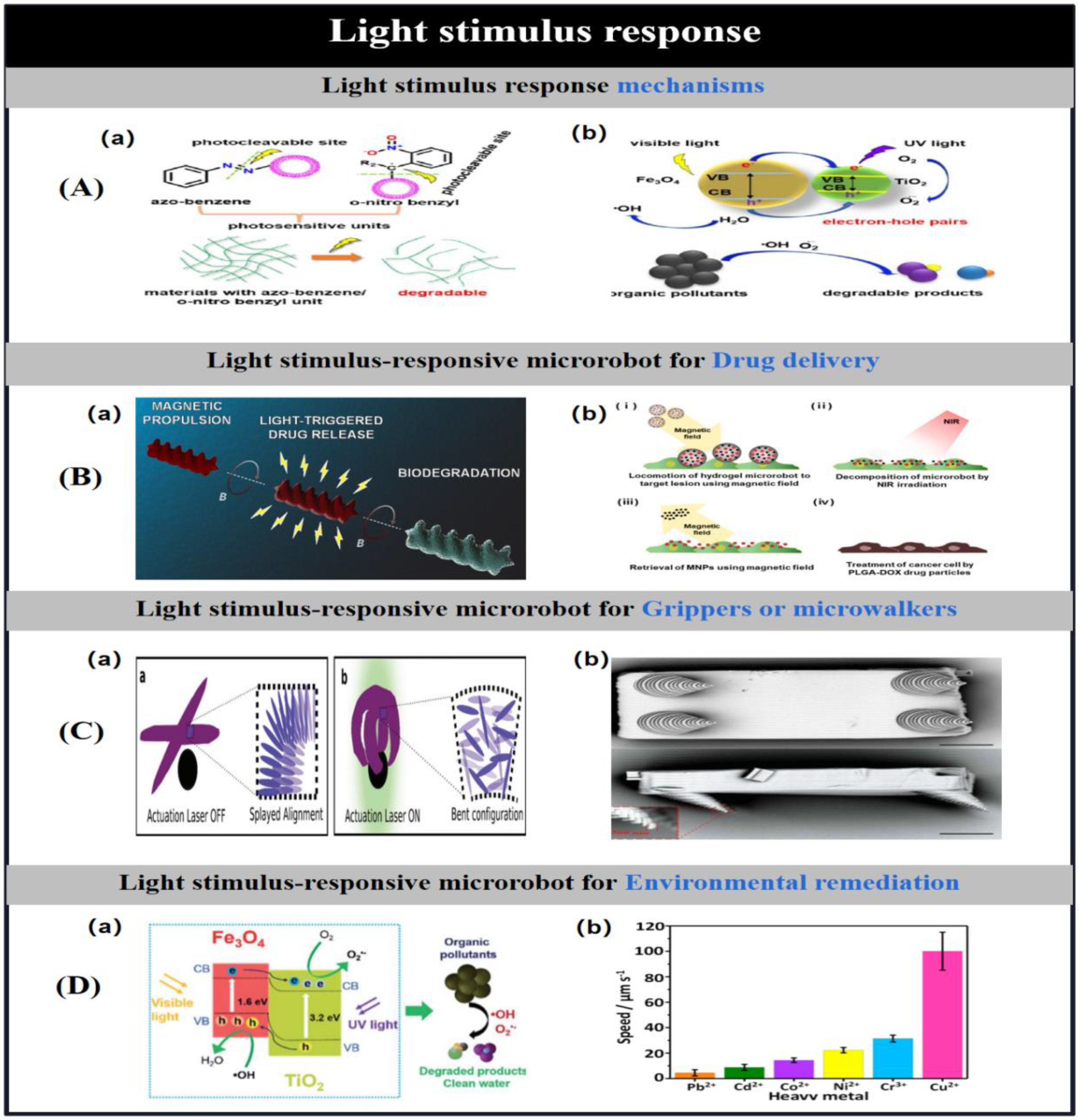
3.2. Light Stimulus Response Mechanisms
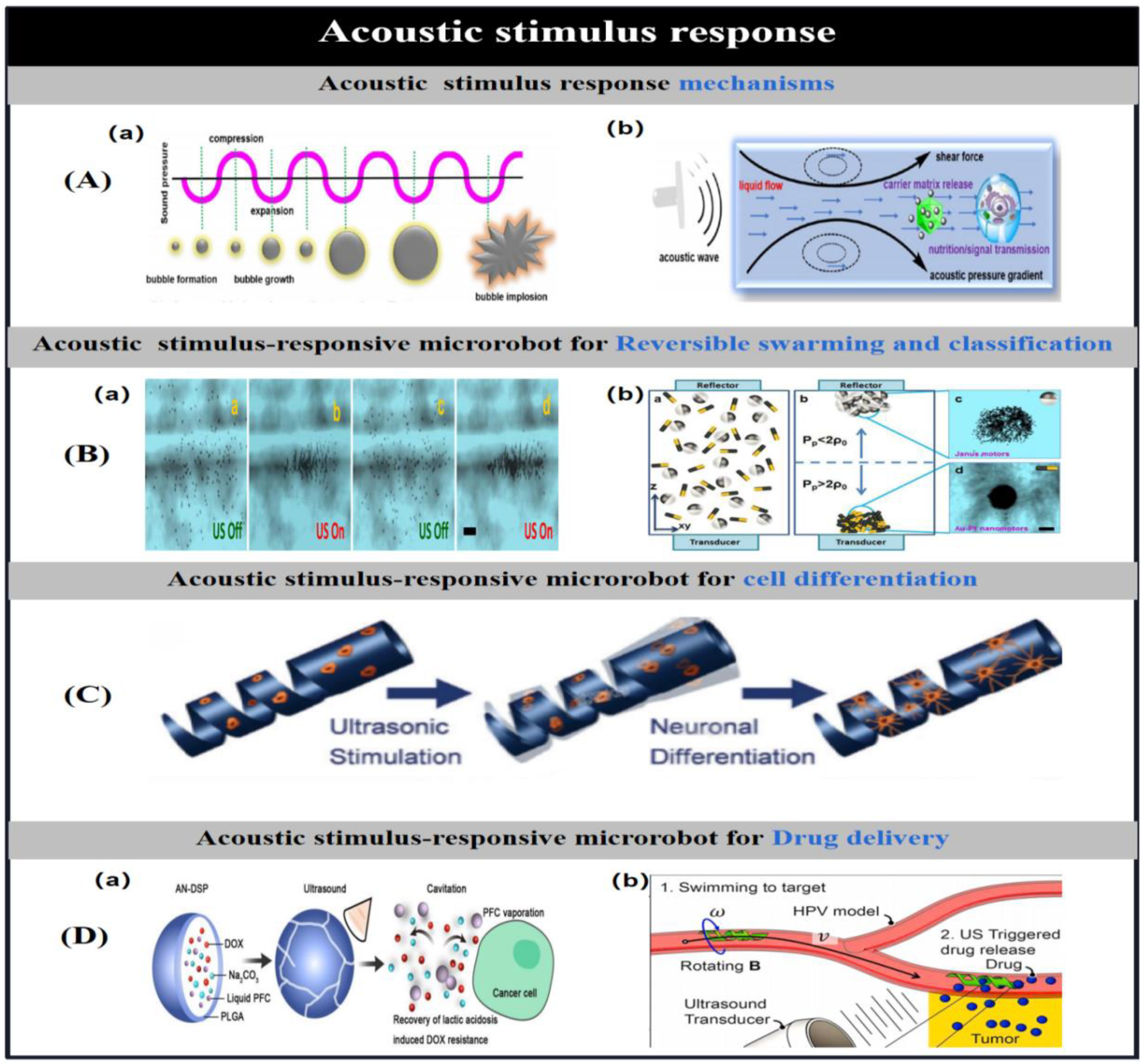
3.3. Acoustic Stimulus Response Mechanisms
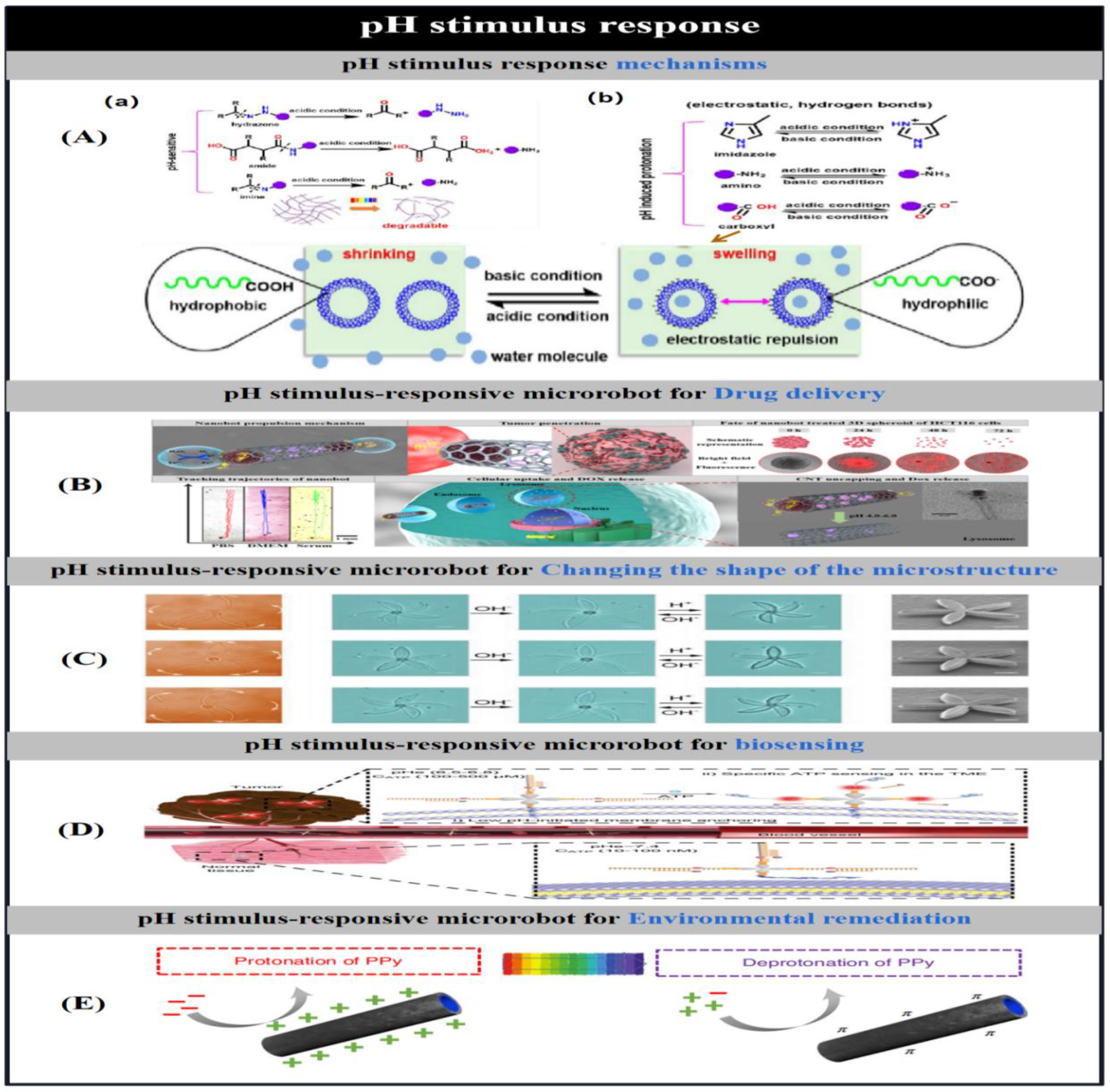
3.4. pH Stimulus Response Mechanisms
3.5. Magnetic Stimulation Response Mechanisms
3.6. Biological Stimulus Response Mechanisms
3.7. Ionic Stimulus Response Mechanisms
3.8. Multi-Stimulus Response Mechanism
4. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou ,Y.;Ye ,M.; Hu, C,; et al. Stimuli-Responsive Functional Micro-/Nanorobots: A Review[J]. ACS nano, 2023.
- Meng, X.; Xu, Y.; Lu, Q.; Sun, L.; An, X.; Zhang, J.; Chen, J.; Gao, Y.; Zhang, Y.; Ning, X. Ultrasound-responsive alkaline nanorobots for the treatment of lactic acidosis-mediated doxorubicin resistance. Nanoscale 2020, 12(25), 13801–13810. [Google Scholar] [CrossRef]
- Park, J.; Kim, J. Y.; Pane, S.; Nelson, B. J.; Choi, H. Acoustically Mediated Controlled Drug Release and Targeted Therapy with Degradable 3D Porous Magnetic Microrobots. Adv. Healthc. Mater. 2021, 10(2). No. 2001096. [Google Scholar] [CrossRef]
- Darmawan, B. A.; Lee, S. B.; Nguyen, V. D.; Go, G.; Nguyen, K.T.; Lee, H.-S.; Nan, M.; Hong, A.; Kim, C.-S.; Li, H.; Bang, D.; Park, J.-O.; Choi, E. Self-folded microrobot for active drug delivery and rapid ultrasound-triggered drug release. Sens. Actuators B Chem. 2020, 324. No. 128752. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Liu, J.-H.; Dong, M.; Müller, L.; Chatzipirpiridis, G.; Hu, C.; Terzopoulou, A.; Torlakcik, H.; Wang, X.; Mushtaq, F.; Puigmartí-Luis, J.; Shen, Q.-D.; Nelson, B. J.; Pané, S. Magnetically driven piezoelectric soft microswimmers for neuron-like cell delivery and neuronal differentiation. Mater. Horizons 2019, 6(7), 1512–1516. [Google Scholar] [CrossRef]
- Lu, X.; Shen, H.; Wei, Y.; Ge, H.; Wang, J.; Peng, H.; Liu, W. Ultrafast Growth and Locomotion of Dandelion-Like Microswarms with Tubular Micromotors. Small 2020, 16(38). No. 2003678. [Google Scholar] [CrossRef]
- Xu, T.; Soto, F.; Gao, W.; Dong, R.; Garcia-Gradilla, V.; Magana, E.; Zhang, X.; Wang, J. Reversible swarming and separation of self-propelled chemically powered nanomotors under acoustic fields. J. Am. Chem. Soc. 2015, 137(6), 2163–2166. [Google Scholar] [CrossRef]
- Ren L, Nama N, McNeill J M, et al. 3D steerable, acoustically powered microswimmers for single-particle manipulation[J]. Science advances, 2019, 5(10), eaax3084.
- Dong, M.; Wang, X.; Chen, X. Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X. H.; Xiao, X.; PuigmartíLuis, J.; Choi, H.; Pêgo, A. P.; Shen, Q. D.; Nelson, B. J.; Pané, S. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30(17). No. 1910323. [Google Scholar] [CrossRef]
- Novelino, L. S.; Ze, Q.; Wu, S.; Paulino, G. H.; Zhao, R. Untethered control of functional origami microrobots with distributed actuation. Proc. Natl. Acad. Sci. U.S.A. 2020, 117(39), 24096–24101. [Google Scholar] [CrossRef]
- Yang, F.; Mou, F.; Jiang, Y.; Luo, M.; Xu, L.; Ma, H.; Guan, J. Flexible Guidance of Microengines by Dynamic Topographical Pathways in Ferrofluids. ACS Nano 2018, 12(7), 6668–6676. [Google Scholar] [CrossRef]
- Mou, F.; Pan, D.; Chen, C.; Gao, Y.; Xu, L.; Guan, J. Magnetically Modulated Pot-Like MnFe2O4 Micromotors: Nano-particle Assembly Fabrication and their Capability for Direct Oil Removal. Adv. Funct. Mater. 2015, 25(39), 6173–6181. [Google Scholar] [CrossRef]
- Mushtaq, F.; Chen, X.; Torlakcik, H.; Steuer, C.; Hoop, M.; iringil, E. C.; Marti, X.; Limburg, G.; Stipp, P.; Nelson, B. J.; Pane, S. Magnetoelectrically Driven Catalytic Degradation of Organics. Adv.Mater. 2019, 31(28). No. 1901378. [Google Scholar] [CrossRef]
- Ge, M.; Xu, D.; Chen, Z.; Wei, C.; Zhang, Y.; Yang, C.; Chen, Y.; Lin, H.; Shi, J. Magnetostrictive-Piezoelectric-Triggered Nano-catalytic Tumor Therapy. Nano Lett. 2021, 21(16), 6764–6772. [Google Scholar] [CrossRef]
- Shi, X.; Shi, Z.; Wang, D.; Ullah, M. W.; Yang, G. Microbial Cells with a Fe3O4 Doped Hydrogel Extracellular Matrix: Manipulation of Living Cells by Magnetic Stimulus. Macromol. Biosci. 2016, 16(10), 1506–1514. [Google Scholar] [CrossRef]
- Zeng H, Wasylczyk P, Parmeggiani C, et al. Light-fueled microscopic walkers[J]. Advanced Materials, 2015, 27(26), 3883-3887.
- Song, X.; Chen, Z.; Zhang, X.; Xiong, J.; Jiang, T.; Wang, Z.; Geng, X.; Cheang, U. K. Magnetic tri-bead microrobot assisted near-infrared triggered combined photothermal and chemotherapy of cancer cells. Sci. Rep. 2021, 11(1), 7907. [Google Scholar] [CrossRef]
- Martella, D.; Nocentini, S.; Nuzhdin, D.; Parmeggiani, C.; Wiersma, D. S. Photonic Microhand with Autonomous Action. Adv.Mater. 2017, 29(42). No. 1704047. [Google Scholar] [CrossRef]
- Mushtaq, F.; Chen, X.; Staufert, S.; Torlakcik, H.; Wang, X.; Hoop, M.; Gerber, A.; Li, X.; Cai, J.; Nelson, B. J.; Pané, S. On-the-fly catalytic degradation of organic pollutants using magneto-photoresponsive bacteria-templated microcleaners. J. Mater. Chem. A 2019, 7(43), 24847–24856. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Yasa, O.; Yasa, I. C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-Triggered Drug Release from 3D-Printed Magnetic Chitosan Microswimmers. ACS Nano 2018, 12(9), 9617–9625. [Google Scholar] [CrossRef]
- Fusco, S.; Huang, H. W.; Peyer, K. E.; Peters, C.; Haberli, M.; Ulbers, A.; Spyrogianni, A.; Pellicer, E.; Sort, J.; Pratsinis, S. E.; Nelson, B. J.; Sakar, M. S.; Pane, S. Shape-switching microrobots for medical applications: the influence of shape in drug delivery and locomotion. ACS Appl. Mater. Interfaces 2015, 7(12), 6803–6811. [Google Scholar] [CrossRef]
- Das, S.; Hunter, E. E.; DeLateur, N. A.; Steager, E. B.; Weiss, R.; Kumar, V. Cellular expression through morphogen delivery by light activated magnetic microrobots. J. Microbio. Robot 2019, 15(2), 79–90. [Google Scholar] [CrossRef]
- Lee, H.; Choi, H.; Lee, M.; Park, S. Preliminary study on alginate/NIPAM hydrogel-based soft microrobot for controlled drug delivery using electromagnetic actuation and near-infrared stimulus. Biomed. Microdevices 2018, 20(4), 103. [Google Scholar] [CrossRef]
- Nguyen, K. T.; Go, G.; Jin, Z.; Darmawan, B. A.; Yoo, A.; Kim, S.; Nan, M.; Lee, S. B.; Kang, B.; Kim, C. S.; Li, H.; Bang, D.; Park, J.O.; Choi, E. A Magnetically Guided Self-Rolled Microrobot for Targeted Drug Delivery, Real-Time X-Ray Imaging, and Microrobot Retrieval. Adv. Healthcare Mater. 2021, 10(6). No. 2001681. [Google Scholar] [CrossRef]
- Xing, J.; Yin, T.; Li, S.; Xu, T.; Ma, A.; Chen, Z.; Luo, Y.; Lai, Z.; Lv, Y.; Pan, H.; Liang, R.; Wu, X.; Zheng, M.; Cai, L. Sequential Magneto-Actuated and Optics-Triggered Biomicrorobots for Targeted Cancer Therapy. Adv. Funct. Mater. 2021, 31(11). No. 2008262. [Google Scholar] [CrossRef]
- Du, X.; Cui, H.; Sun, B.; Wang, J.; Zhao, Q.; Xia, K.; Wu, T.; Humayun, M. S. Photothermally Triggered Shape-AdapTable 3D Flexible Electronics. Adv. Mater. Technol. 2017, 2(10). No. 1700120. [Google Scholar] [CrossRef]
- Kim, D.-i.; Lee, H.; Kwon, S.-h.; Choi, H.; Park, S. Magnetic nano-particles retrievable biodegradable hydrogel microrobot. Sens.Actuators B Chem. 2019, 289, 65–77. [Google Scholar] [CrossRef]
- Villa, K.; Manzanares Palenzuela, C. L.; Sofer, Z.; Matejkova, S.; Pumera, M. Metal-Free Visible-Light Photoactivated C3N4 Bubble-Propelled Tubular Micromotors with Inherent Fluorescence and On/Off Capabilities. ACS Nano 2018, 12(12), 12482–12491. [Google Scholar] [CrossRef]
- Ceylan H, Yasa I C, Yasa O, et al. 3D-printed biodegradable microswimmer for theranostic cargo delivery and release[J]. ACS nano, 2019, 13(3), 3353-3362.
- Terzopoulou, A.; Wang, X.; Chen, X. Z.; Palacios-Corella, M.; Pujante, C.; Herrero-Martin, J.; Qin, X. H.; Sort, J.; deMello, A. J.; Nelson, B. J.; Puigmarti-Luis, J.; Pane, S. Biodegradable Metal-Organic Framework-Based Microrobots (MOFBOTs). Adv. Healthcare Mater. 2020, 9(20). No. 2001031. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.-H.; Hu, C.; Terzopoulou, A.; Chen, X.-Z.; Huang, T.-Y.; Maniura-Weber, K.; Pané, S.; Nelson, B. J. 3D Printed Enzymatically Biodegradable Soft Helical Microswimmers. Adv. Funct. Mater. 2018, 28(45). No. 1804107. [Google Scholar]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot 2021, 6, No. eaaz9519. [Google Scholar] [CrossRef]
- Al-Fandi, M.; Alshraiedeh, N.; Oweis, R.; Alshdaifat, H.; Al-Mahaseneh, O.; Al-Tall, K.; Alawneh, R. Novel Selective Detection Method of Tumor Angiogenesis Factors Using Living Nano-Robots. Sensors (Basel) 2017, 17(7), 1580. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Shi, Q.; Maffeo, C.; Kojima, M.; Dong, L.; Aksimentiev, A.; Huang, Q.; Fukuda, T.; Arai, T. A tetrahedral DNA nanorobot with conformational change in response to molecular trigger. Nanoscale 2021, 13(37), 15552–15559. [Google Scholar]
- Xiao, X.; Xu, Z.; Wang, W.; Sun, S.; Qiao, Y.; Jiang, L.; Yan, Y.; Huang, J. Enzyme-Responsive Molecular Assemblies Based on Host-Guest Chemistry. Langmuir 2021, 37(27), 8348–8355. [Google Scholar]
- Xu L, Mou F, Gong H, et al. Light-driven micro/nanomotors: from fundamentals to applications[J]. Chemical Society Reviews, 2017, 46(22), 6905-6926.
- Zhao G, Seah T H, Pumera M. External-energy-independent polymer capsule motors and their cooperative behaviors[J]. Chemistry–A European Journal, 2011, 17(43), 12020-12026.
- Lee T C, Alarcón-Correa M, Miksch C, et al. Self-propelling nanomotors in the presence of strong Brownian forces[J]. Nano letters, 2014, 14(5), 2407-2412.
- Wilson D A, Nolte R J M, Van Hest J C M. Autonomous movement of platinum-loaded stomatocytes[J]. Nature chemistry, 2012, 4(4), 268-274.
- Liu R, Sen A. Autonomous nanomotor based on copper–platinum segmented nanobattery[J]. Journal of the American Chemical Society, 2011, 133(50), 20064-20067.
- Mou, F.; Kong, L.; Chen, C.; Chen, Z.; Xu, L.; Guan, J. Light-Controlled Propulsion, Aggregation and Separation of Water-FuelledTiO2/Pt Janus Submicromotors and Their “on-the-Fly” Photo-catalytic Activities. Nanoscale 2016, 8, 4976–4983. [Google Scholar] [CrossRef]
- Zhou, D.; Ren, L.; Li, Y. C.; Xu, P.; Gao, Y.; Zhang, G.; Wang, W.; Mallouk, T. E.; Li, L. Visible Light-Driven, Magnetically Steerable Gold/Iron Oxide Nanomotors. Chem. Commun. 2017, 53, 11465–11468. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Zhan, X.; Dai, B.; Zheng, J.; Liu, J.; Tang, J. A Silicon Nanowire as a Spectrally Tunable Light-Driven Nanomotor. Adv. Mater. 2017, 29, 1701451. [Google Scholar] [CrossRef]
- Wong, F.; Sen, A. Progress toward Light-Harvesting Self- Electrophoretic Motors: Highly Efficient Bimetallic Nanomotors and Micropumps in Halogen Media. ACS Nano 2016, 10, 7172–7179. [Google Scholar] [CrossRef]
- Wang, X.; Sridhar, V.; Guo, S.; Talebi, N.; Miguel-López, A.; Hahn, K.; van Aken, P. A.; Sánchez, S. Fuel-Free Nanocap-Like Motors Actuated under Visible Light. Adv. Funct. Mater. 2018, 28, 1705862. [Google Scholar] [CrossRef]
- Baraban L, Makarov D, Streubel R, et al. Catalytic Janus motors on microfluidic chip: deterministic motion for targeted cargo delivery[J]. ACS nano, 2012, 6(4), 3383-3389.
- Brown A, Poon W. Ionic effects in self-propelled Pt-coated Janus swimmers[J]. Soft matter, 2014, 10(22), 4016-4027.
- Zhang J, Yao Y, Sheng L, et al. Self-Fueled Motors: Self-Fueled Biomimetic Liquid Metal Mollusk (Adv. Mater. 16/2015)[J]. Advanced Materials, 2015, 27(16), 2550-2550.
- Srivastava S K, Guix M, Schmidt O G. Wastewater mediated activation of micromotors for efficient water cleaning[J]. Nano letters, 2016, 16(1), 817-821.
- Gao W, Pei A, Wang J. Water-driven micromotors[J]. ACS nano, 2012, 6(9), 8432-8438.
- Soler L, Magdanz V, Fomin V M, et al. Self-propelled micromotors for cleaning polluted water[J]. ACS nano, 2013, 7(11), 9611-9620.
- Fiedler, C.; Ulbricht, C.; Truglas, T.; Wielend, D.; Bednorz, M.; Groiss, H.; Bruggemann, O.; Teasdale, I.; Salinas, Y. Reversible Speed Regulation of Self-Propelled Janus Micromotors via Thermoresponsive Bottle-Brush Polymers. Chemistry 2021, 27(10), 3262–3267. [Google Scholar]
- Wei G, Allen P, Renfeng D, Joseph W, et al. Catalytic Iridium-Based Janus Micromotors Powered By Ultralow Levels Of Chemical Fuels[J], Journal of the American Chemical Society, 2014, 136(6), 2276-2279.
- Gao W, Uygun A, Wang J. Hydrogen-bubble-propelled zinc-based microrockets in strongly acidic media[J]. Journal of the American Chemical Society, 2012, 134(2), 897-900.
- Gao W, D’Agostino M, Garcia-Gradilla V, et al. Multi-fuel driven janus micromotors[J]. Small, 2013, 9(3), 467-471.
- Gao, W.; Feng, X.; Pei, A.; Gu, Y.; Li, J.; Wang, J. Seawater-Driven Magnesium Based Janus Micromotors for EnvironmentalRemediation. Nanoscale 2013, 5, 4696–4700. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Yang, X.; Yang, L.; Shen, Y.; Shang, W. Multi-functionalized micro-helical capsule robots with superior loading and releasing capabilities. J. Mater. Chem. B 2021, 9(5), 1441–1451. [Google Scholar] [CrossRef]
- Li J, Angsantikul P, Liu W, et al. Micromotors spontaneously neutralize gastric acid for pH-responsive payload release[J]. Angewandte Chemie International Edition, 2017, 56(8), 2156-2161.
- Iacovacci V, Blanc A, Huang H, et al. High-resolution SPECT imaging of stimuli-responsive soft microrobots[J]. Small, 2019, 15(34), 1900709.
- Tabatabaei, S. N.; Lapointe, J.; Martel, S. Shrinkable Hydrogel-Based Magnetic Microrobots for Interventions in the Vascular Network. Adv. Robotics 2011, 25(8), 1049–1067. [Google Scholar]
- Mou, F.; Chen, C.; Zhong, Q.; Yin, Y.; Ma, H.; Guan, J. Autonomous motion and temperature-controlled drug delivery of Mg/Pt-poly(N-isopropylacrylamide) Janus micromotors driven by simulated body fluid and blood plasma. ACS Appl. Mater. Interfaces 2014, 6(12), 9897–9903. [Google Scholar]
- Dekanovsky, L.; Khezri, B.; Rottnerova, Z.; Novotny, F.; Plutnar, J.; Pumera, M. Chemically programmable microrobots weaving a web from hormones. Nature Machine Intelligence 2020, 2(11), 711–718. [Google Scholar]
- Chen, W.; Sun, M.; Fan, X.; Xie, H. Magnetic/pH-sensitive double-layer microrobots for drug delivery and sustained release. Appl. Mater. Today 2020, 19, No. 100583. [Google Scholar] [CrossRef]
- Li, H.; Go, G.; Ko, S. Y.; Park, J.-O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft microrobot for targeted drug delivery. Smart Mater. Struct. 2016, 25(2). No. 027001. [Google Scholar]
- Wang, X.; Chen, X. Z.; Alcantara, C. C. J.; Sevim, S.; Hoop, M.; Terzopoulou, A.; de Marco, C.; Hu, C.; de Mello, A. J.; Falcaro, P.; Furukawa, S.; Nelson, B. J.; Puigmarti-Luis, J.; Pane, S. MOFBOTS:Metal-Organic-Framework-Based Biomedical Microrobots. Adv.Mater. 2019, 31(27). No. 1901592. [Google Scholar]
- Chengzhi, H.; Riederer, K.; Klemmer, M.; Pane, S.; Nelson, B.J. Electrosynthesis of Magnetoresponsive Microrobot for Targeted Drug Delivery using Calcium Alginate. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE, 2016; pp 2111−2114. [CrossRef]
- Di, Z.; Zhao, J.; Chu, H.; Xue, W.; Zhao, Y.; Li, L. An Acidic-Microenvironment-Driven DNA Nanomachine Enables Specific ATP Imaging in the Extracellular Milieu of Tumor. Adv. Mater. 2019, 31(33). No. 1901885. [Google Scholar]
- Liu, Y. M.; Wang, W.; Zheng, W. C.; Ju, X. J.; Xie, R.; Zerrouki, D.; Deng, N. N.; Chu, L. Y. Hydrogel-based microactuators with remote-controlled locomotion and fast Pb2+-response for micromanipulation. ACS Appl. Mater. Interfaces 2013, 5(15), 7219–7226. [Google Scholar]
- Mou, F.; Xie, Q.; Liu, J.; Che, S.; Bahmane, L.; You, M.; Guan, J. ZnO-based micromotors fueled by CO2: the first example of self-reorientation-induced biomimetic chemotaxis. Natl. Sci. Rev. 2021, 8(11). No. nwab066. [Google Scholar]
- Wang H, Pumera M. Fabrication of micro/nanoscale motors[J]. Chemical reviews, 2015, 115(16), 8704-8735.
- Paxton WF, Kistler KC, Olmeda CC, Sen A, St Angelo SK, Cao Y, Mallouk TE, Lammert PE, Crespi VH. Catalytic nanomotors: autonomous movement of striped nanorods. J Am Chem Soc. 2004 Oct 20;126(41):13424-31.
- Fan, D. L.; Zhu, F. Q.; Cammarata, R. C.; Chien, C. L. Manipulation of Nanowire in Suspension by AC Electric Fields. Appl. Phys. Lett. 2004, 85, 4175. [Google Scholar] [CrossRef]
- Fan, D. L.; Zhu, F. Q.; Cammarata, R. C.; Chien, C. L. Controllable High-Speed Rotation of Nanowires. Phys. Rev. Lett. 2005, 94, 247208. [Google Scholar] [CrossRef]
- Gao, W.; Sattayasamitsathit, S.; Manesh, K. M.; Weihs, D.; Wang, J. Magnetically Powered Flexible Metal Nanowire Motors. J. Am. Chem. Soc. 2010, 132, 14403–14405. [Google Scholar] [CrossRef]
- Zhao G, Ambrosi A, Pumera M. Self-propelled nanojets via template electrodeposition[J]. Nanoscale, 2013, 5(4): 1319-1324.
- Tanase M, Bauer L A, Hultgren A, et al. Magnetic alignment of fluorescent nanowires[J]. Nano Letters, 2001, 1(3): 155-158.
- Gao W, Sattayasamitsathit S, Orozco J, et al. Highly efficient catalytic microengines: template electrosynthesis of polyaniline/platinum microtubes[J]. Journal of the American Chemical Society, 2011, 133(31): 11862-11864.
- Zhang, L.; Petit, T.; Lu, Y.; Kratochvil, B. E.; Peyer, K. E.; Pei, R.; Lou, J.; Nelson, B. J. Controlled Propulsion and Cargo Transport of Rotating Nickel Nanowires near a Patterned Solid Surface. ACS Nano 2010, 4, 6228–6234. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Bidoz S, Arsenault A C, Manners I, et al. Synthetic self-propelled nanorotors[J]. Chemical Communications, 2005 (4): 441-443.
- Ambulo C P, Burroughs J J, Boothby J M, et al. Four-dimensional printing of liquid crystal elastomers[J]. ACS applied materials & interfaces, 2017, 9(42), 37332-37339.
- Demirok, U. K.; Laocharoensuk, R.; Manesh, K. M.; Wang, J. Ultrafast Catalytic Alloy Nanomotors. Angew. Chem., Int. Ed. 2008, 47, 9349–9351. [Google Scholar]
- Laocharoensuk R, Burdick J, Wang J. Carbon-nanotube-induced acceleration of catalytic nanomotors[J]. ACS nano, 2008, 2(5), 1069-1075.
- Zacharia, N. S.; Sadeq, Z. S.; Ozin, G. A. Enhanced Speed of Bimetallic Nanorod Motors by Surface Roughening. Chem. Commun. 2009, 5856–5858.
- Suk T C, Vesselin N P, Dimiter N P, Orlin D V, et al. Remotely powered self-propelling particles and micropumps based on miniature diodes[J], Nature materials, 2007, 6(3), 235.0-240.
- Wang, W.; Castro, L. A.; Hoyos, M.; Mallouk, T. E. Autonomous Motion of Metallic Microrods Propelled by Ultrasound. ACS Nano 2012, 6, 6122–6132. [Google Scholar] [CrossRef] [PubMed]
- Nadal, F.; Lauga, E. Asymmetric Steady Streaming as aMechanism for Acoustic Propulsion of Rigid Bodies. Phys. Fluids 2014, 26, 082001. [Google Scholar] [CrossRef]
- Garcia-Gradilla, V.; Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Functionalized Ultrasound-Propelled Magnetically Guided Nano-motors: Toward Practical Biomedical Applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef] [PubMed]
- Mair L O, Evans B, Hall A R, et al. Highly controllable near-surface swimming of magnetic Janus nanorods: application to payload capture and manipulation[J]. Journal of Physics D: Applied Physics, 2011, 44(12), 125001.
- Abbott J J, Peyer K E, Lagomarsino M C, et al. How should microrobots swim?[J]. The international journal of Robotics Research, 2009, 28(11-12), 1434-1447.
- Mirkovic, T.; Foo, M. L.; Arsenault, A. C.; Fournier-Bidoz, S.; Zacharia, N. S.; Ozin, G. A. Hinged Nanorods Made Using a Chemical Approach to Flexible Nanostructures. Nat. Nanotechnol. 2007, 2, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Gutman, E.; Stucki, N.; Seitz, B. F.; Wendel-García, P. D.; Newton, T.; Pokki, J.; Ergeneman, O.; Pané, S.; Or, Y.; Nelson, B. J. Undulatory Locomotion of Magentic MultilinkNanoswimmers. Nano Lett. 2015, 15, 4829–4833. [Google Scholar] [CrossRef] [PubMed]
- Walter F P, Paul T B, Timothy R K, Yang W, Thomas E M, Ayusman S, et al. Catalytically Induced Electrokinetics For Motors And Micropumps[J], Journal of the American Chemical Society, 2006, 128(46), 14881-14888.
- Sanchez S, Ananth A N, Fomin V M, et al. Superfast motion of catalytic microjet engines at physiological temperature[J]. Journal of the American Chemical Society, 2011, 133(38), 14860-14863.
- Vicario, J.; Eelkema, R.; Browne, W. R.; Meetsma, A.; La Crois, R. M.; Feringa, B. L. Catalytic Molecular Motors: Fuelling Autonomous Movement by a Surface Bound Synthetic Manganese Catalase. Chem. Commun. 2005, 3936−3938.
- Solovev A A, Xi W, Gracias D H, et al. Self-propelled nanotools[J]. Acs Nano, 2012, 6(2), 1751-1756.
- Fattah Z, Loget G, Lapeyre V, et al. Straightforward single-step generation of microswimmers by bipolar electrochemistry[J]. Electrochimica acta, 2011, 56(28), 10562-10566.
- Solovev A A, Mei Y, Bermúdez Ureña E, et al. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles[J]. Small, 2009, 5(14), 1688-1692.
- Solovev A A, Sanchez S, Pumera M, et al. Magnetic control of tubular catalytic microrobots for the transport, assembly, and delivery of micro-objects[J]. Advanced Functional Materials, 2010, 20(15), 2430-2435.
- Wei G, Sirilak S, Aysegul U, Allen P, Adam P, Joseph W, et al. Polymer-based tubular microrobots: role of composition and preparation.[J], Nanoscale, 2012, 4(7), 2447-2453.
- Zhao, G.; Pumera, M. Concentric Bimetallic Microjets byElectrodeposition. RSC Adv. 2013, 3, 3963–3966. [Google Scholar] [CrossRef]
- Liu, L.; Yoo, S.-H.; Lee, S. A.; Park, S. Wet-ChemicalSynthesis of Palladium Nanosprings. Nano Lett. 2011, 11, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Li J, Sattayasamitsathit S, Dong R, et al. Template electrosynthesis of tailored-made helical nanoswimmers[J]. Nanoscale, 2014, 6(16), 9415-9420.
- Manesh, K. M.; Cardona, M.; Yuan, R.; Clark, M.; Kagan, D.; Balasubramanian, S.; Wang, J. Template-Assisted Fabrication of Salt-Independent Catalytic Tubular Microengines. ACS Nano 2010, 4, 1799–1804. [Google Scholar] [CrossRef]
- Schuerle, S.; Pane, S.; Pellicer, E.; Sort, J.; Baro, M. D.; Nelson, B. J. Helical and Tubular Lipid Microstructures That Are Electroless-Coated with CoNiReP for Wireless Magnetic Manipulation. Small 2012, 8, 1498–1502. [Google Scholar] [CrossRef]
- Loget G, Roche J, Kuhn A. True bulk synthesis of Janus objects by bipolar electrochemistry[J]. Advanced materials, 2012, 37(24), 5111-5116.
- Loget, G.; Larcade, G.; Lapeyre, V.; Garrigue, P.; Warakulwit, C.; Limtrakul, J.; Delville, M.-H.; Ravaine, V.; Kuhn, A. Single Point Electrodeposition of Nickel for the Dissymmetric Decoration of Carbon Tubes. Electrochim. Acta 2010, 55, 8116–8120. [Google Scholar] [CrossRef]
- Gao, W.; Feng, X.; Pei, A.; Kane, C. R.; Tam, R.; Hennessy, C.; Wang, J. Bioinspired Helical Microswimmers Based on Vascular Plants. Nano Lett. 2014, 14, 305–310. [Google Scholar] [CrossRef]
- Baraban, L.; Streubel, R.; Makarov, D.; Han, L.; Karnaushenko, D.; Schmidt, O. G.; Cuniberti, G. Fuel-Free Locomotion of Janus Motors: Magnetically Induced Thermophoresis. ACS Nano 2013, 7, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Banholzer, M. J.; Xu, X.; Huang, L.; Mirkin, C. A. Rational Design and Synthesis of Catalytically Driven Nanorotors. J.Am. Chem. Soc. 2007, 129, 14870–14871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fei, S.; Byun, Y.-M.; Lammert, P. E.; Crespi, V. H.; Sen, A.; Mallouk, T. E. Dynamic Interactions between Fast Microscale Rotors. J. Am. Chem. Soc. 2009, 131, 9926–9927. [Google Scholar] [CrossRef]
- Paxton, W. F.; Sundararajan, S.; Mallouk, T. E.; Sen, A. Chemical Locomotion. Angew. Chem., Int. Ed. 2006, 45, 5420–5429. [Google Scholar] [CrossRef]
- Micheletto, R.; Fukuda, H.; Ohtsu, M. A Simple Method for the Production of a Two-Dimensional, Ordered Array of Small Latex Particles. Langmuir 1995, 11, 3333–3336. [Google Scholar] [CrossRef]
- Hong, Y.; Velegol, D.; Chaturvedi, N.; Sen, A. Biomimetic Behavior of Synthetic Particles: From Microscopic Randomness to Macroscopic Control. Phys. Chem. Chem. Phys. 2010, 12, 1423–1435. [Google Scholar] [CrossRef]
- Wheat P M, Marine N A, Moran J L, et al. Rapid fabrication of bimetallic spherical motors[J]. Langmuir, 2010, 26(16), 13052-13055.
- Valadares, L. F.; Tao, Y. G.; Zacharia, N. S.; Kitaev, V.; Galembeck, F.; Kapral, R.; Ozin, G. A. Catalytic Nanomotors: Self-Propelled Sphere Dimers. Small 2010, 6, 565–572. [Google Scholar] [CrossRef]
- Zhao G, Pumera M. Geometric asymmetry driven Janus micromotors[J]. Nanoscale 2014, 6(19), 11177-11180.
- Tierno, P.; Albalat, R.; Sagues, F. Autonomously Moving Catalytic Microellipsoids Dynamically Guided by External MagneticFields. Small 2010, 6, 1749–1752. [Google Scholar] [CrossRef]
- Fangzhi M, Chuanrui C, Huiru M, Yixia Y, Qingzhi W, Jianguo G, et al. Self-propelled micromotors driven by the magnesium-water reaction and their hemolytic properties.[J], Angewandte Chemie, 2013, 52(28), 7208-7212.
- Zhao Y, Ye D, Wang G C, et al. Designing nanostructures by glancing angle deposition[C]//Nanotubes and Nanowires. SPIE, 2003, 5219: 59-73.
- Ghosh, A.; Fischer, P. Controlled Propulsion of Artificial Magnetic Nanostructured Propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef]
- Schamel, D.; Mark, A. G.; Gibbs, J. G.; Miksch, C.; Morozov, K. I.; Leshansky, A. M.; Fischer, P. Nanopropellers and Their Actuation in Complex Viscoelastic Media. ACS Nano 2014, 8, 8794–8801. [Google Scholar] [CrossRef]
- Ghosh A, Paria D, Rangarajan G, et al. Velocity fluctuations in helical propulsion: How small can a propeller be[J]. The journal of physical chemistry letters, 2014, 5(1), 62-68.
- Walker D, Kubler M, Morozov K I, et al. Optimal length of low Reynolds number nanopropellers[J]. Nano letters, 2015, 15(7), 4412-4416.
- Schamel, D.; Pfeifer, M.; Gibbs, J. G.; Miksch, B.; Mark, A.G.; Fischer, P. Chiral Colloidal Molecules and Observation of the Propeller Effect. J. Am. Chem. Soc. 2013, 135, 12353–12359. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J. G.; Fragnito, N. A.; Zhao, Y. Asymmetric Pt/Au Coated Catalytic Micromotors Fabricated by Dynamic Shadowing Growth. Appl. Phys. Lett. 2010, 97, 253107. [Google Scholar] [CrossRef]
- Yuping He, Jinsong Wu, Yiping Zhao. Designing Catalytic Nanomotors By Dynamic Shadowing Growth[J], NANO LETTERS, 2007, 7(5), 1369-1375.
- J. G. Gibbs, Y.-P. Zhao. Design and characterization of rotational multicomponent catalytic nanomotors.[J], Small, 2009, 5(20), 2304-2308.
- Huang, W.; Manjare, M.; Zhao, Y. Catalytic Nanoshell Micromotors. J. Phys. Chem. C 2013, 117, 21590–21596. [Google Scholar] [CrossRef]
- Yongfeng M, Alexander A S, Samuel S, Oliver G S, et al. Rolled-up nanotech on polymers: from basic perception to self-propelled catalytic microengines.[J], Chemical Society reviews, 2011, 40(5), 2109-2119.
- Harazim S M, Xi W, Schmidt C K, et al. Fabrication and applications of large arrays of multifunctional rolled-up SiO/SiO 2 microtubes[J]. Journal of Materials Chemistry, 2012, 22(7), 2878-2884.
- Kun Y, Manoj M, Christopher A B, Bo Y, Tina T S, Yiping Z, et al. Nanostructured Scrolls from Graphene Oxide for Microjet Engines.[J], Journal of Physical Chemistry Letters, 2012, 3(16), 2204-2208.
- Hong Wang, James Guo Sheng Moo, Martin Pumera. Tissue cell assisted fabrication of tubular catalytic platinum microengines.[J], Nanoscale, 2014, 6(19), 11359.0-11363.0.
- Magdanz V, Stoychev G, Ionov L, et al. Stimuli-responsive microjets with reconfigurable shape[J]. Angewandte Chemie, 2014, 126(10), 2711-2715.
- Bell D J, Leutenegger S, Hammar K M, et al. Flagella-like propulsion for microrobots using a nanocoil and a rotating electromagnetic field[C]//Proceedings 2007 IEEE international conference on robotics and automation. IEEE, 2007: 1128-1133.
- Zhang L, Abbott J J, Dong L, et al. Artificial bacterial flagella: Fabrication and magnetic control[J]. Applied Physics Letters, 2009, 94(6): 064107.
- Li Z, Jake J A, Lixin D, Kathrin E P, Bradley E K, Haixin Z, Christos B, Bradley J N, et al. Characterizing the swimming properties of artificial bacterial flagella.[J], Nano Letters, 2009, 9(10), 3663.0-3667.0.
- Hwang, G.; Braive, R.; Couraud, L.; Cavanna, A.; Abdelkarim, O.; Robert-Philip, I.; Beveratos, A.; Sagnes, L.; Haliyo, S.; Regnier, S. ́ Electro-Osmotic Propulsion of Helical Nanobelt Swimmers. Int. J. Rob. Res. 2011, 30, 806–819. [Google Scholar] [CrossRef]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K. K.; FrancoObregon, A.; Nelson, B. J. Magnetic Helical Micromachines: Fabrication, Controlled Swimming, and Cargo Transport. Adv. Mater. 2012, 24, 811–816. [Google Scholar] [CrossRef]
- Zeeshan, M. A.; Grisch, R.; Pellicer, E.; Sivaraman, K. M.; Peyer, K. E.; Sort, J.; Ozkale, B.; Sakar, M. S.; Nelson, B. J.; Pane, S. Hybrid Helical Magnetic Microrobots Obtained by 3D Template-Assisted Electrodeposition. Small 2014, 10, 1284–1288. [Google Scholar] [CrossRef]
- Kim S, Qiu F, Kim S, et al. Fabrication and characterization of magnetic microrobots for three-dimensional cell culture and targeted transportation[J]. Advanced Materials 2013, 25(41): 5863-5868.
- Kümmel F, Ten Hagen B, Wittkowski R, et al. Circular motion of asymmetric self-propelling particles[J]. Physical review letters, 2013, 110(19), 198302.
- Ten Hagen B, Kümmel F, Wittkowski R, et al. Gravitaxis of asymmetric self-propelled colloidal particles[J]. Nature communications, 2014, 5(1), 4829.
- Kim Y, Yuk H, Zhao R, et al. Printing ferromagnetic domains for untethered fast-transforming soft materials[J]. Nature, 2018, 558(7709), 274-279.
- De Marco C, Alcântara C C J, Kim S, et al. Indirect 3D and 4D printing of soft robotic microstructures[J]. Advanced Materials Technologies, 2019, 4(9), 1900332.
- Cvetkovic C, Raman R, Chan V, et al. Three-dimensionally printed biological machines powered by skeletal muscle[J]. Proceedings of the National Academy of Sciences, 2014, 111(28), 10125-10130.
- Frutiger D R, Vollmers K, Kratochvil B E, et al. Small, fast, and under control: wireless resonant magnetic micro-agents[J]. The International Journal of Robotics Research, 2010, 29(5), 613-636.
- Jin D, Chen Q, Huang T Y, et al. Four-dimensional direct laser writing of reconfigurable compound micromachines[J]. Materials Today 2020, 32, 19-25.
- Yuk H, Lu B, Lin S, et al. 3D printing of conducting polymers[J]. Nature communications, 2020, 11(1), 1604.
- Ge Q, Dunn C K, Qi H J, et al. Active origami by 4D printing[J]. Smart materials and structures, 2014, 23(9), 094007.
- Huang, T.-Y.; Huang, H.-W.; Jin, D. D.; Chen, Q. Y.; Huang, J. Y.; Zhang, L.; Duan, H. L. Four-dimensional micro-building blocks. Sci. Adv. 2020, 6, No. eaav8219. [Google Scholar] [CrossRef]
- Scarpa, E.; Lemma, E. D.; Fiammengo, R.; Cipolla, M. P.; Pisanello, F.; Rizzi, F.; De Vittorio, M. Microfabrication of pH-responsive 3D hydrogel structures via two-photon polymerization of high-molecular-weight poly(ethylene glycol) diacrylates. Sens. Actuators B Chem. 2019, 279, 418–426. [CrossRef]
- Wu Z, Wu Y, He W, et al. Self-propelled polymer-based multilayer nanorockets for transportation and drug release[J]. Angewandte Chemie International Edition, 2013, 52(27), 7000-7003.
- Wu, Y.; Wu, Z.; Lin, X.; He, Q.; Li, J. Autonomous Movement of Controllable Assembled Janus Capsule Motors. ACS Nano 2012, 6, 10910–10916. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zhou, T.; Zhang, H.; Li, C. Y. Directed Self-Assembly of Nanoparticles for Nanomotors. ACS Nano 2013, 7, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, J.; Gao, W.; Xu, T.; Christianson, C.; Gao, W.; Galarnyk, M.; He, Q.; Zhang, L.; Wang, J. Turning Erythrocytes into Functional Micromotors. ACS Nano 2014, 8, 12041–12048. [Google Scholar] [CrossRef]
- Dreyfus, R.; Baudry, J.; Roper, M. L.; Fermigier, M.; Stone, H. A.; Bibette, J. Microscopic Artificial Swimmers. Nature 2005, 437, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Palacci, J.; Sacanna, S.; Steinberg, A. P.; Pine, D. J.; Chaikin, P. M. Living Crystal of Light-Activated Colloidal Surfers. Science 2013, 339, 936–940. [Google Scholar] [CrossRef]
- Vicario J, Walko M, Meetsma A, et al. Fine tuning of the rotary motion by structural modification in light-driven unidirectional molecular motors[J]. Journal of the American Chemical Society, 2006, 128(15), 5127-5135.
- Zhang, H.; Duan, W.; Liu, L.; Sen, A. Depolymerization-Powered Autonomous Motors Using Biocompatible Fuel. J. Am. Chem. Soc. 2013, 135, 15734–15737. [Google Scholar] [CrossRef]
- Abid, J. P.; Frigoli, M.; Pansu, R.; Szeftel, J.; Zyss, J.; Larpent, C.; Brasselet, S. Light-Driven Directed Motion of Azobenzene-Coated Polymer Nanoparticles in an Aqueous Medium. Langmuir 2011, 27, 7967–7971. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Oh, S.; Nakagawa, M. Light-Driven Motion of Liquids on a Photoresponsive Surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef]
- Berna J, Leigh D A, Lubomska M, et al. Macroscopic transport by synthetic molecular machines[J]. Nature materials, 2005, 4(9), 704-710.
- Vicario, J.; Katsonis, N.; Ramon, B. S.; Bastiaansen, C. W. M.; Broer, D. J.; Feringa, B. L. Nanomotor Rotates Microscale Objects. Nature 2006, 440, 163. [Google Scholar]
- Camacho-Lopez, M.; Finkelmann, H.; Palffy-Muhoray, P.; Shelley, M. Fast Liquid-Crystal Elastomer Swims into the Dark. Nat. Mater. 2004, 3, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Sengupta S, Patra D, Ortiz-Rivera I, et al. Self-powered enzyme micropumps[J]. Nature chemistry, 2014, 6(5), 415-422.
- Sanchez, S.; Solovev, A. A.; Mei, Y.; Schmidt, O. G. Dynamics of Biocatalytic Microengines Mediated by Variable Friction Control. J. Am. Chem. Soc. 2010, 132, 13144–13145. [Google Scholar] [CrossRef] [PubMed]
- Orozco J, García-Gradilla V, D’Agostino M, et al. Artificial enzyme-powered microfish for water-quality testing[J]. ACS nano, 2013, 7(1), 818-824.
- Magdanz V, Sanchez S, Schmidt O G. Development of a sperm-flagella driven micro-bio-robot[J]. Advanced materials, 2013, 25(45), 6581-6588.
- Williams B J, Anand S V, Rajagopalan J, et al. A self-propelled biohybrid swimmer at low Reynolds number[J]. Nature communications, 2014, 5(1), 3081.
- Wang, X.; Cai, J.; Sun, L.; Zhang, S.; Gong; Li, X.; Yue, S.; Feng, L.; Zhang, D. Facile Fabrication of Magnetic Microrobots Based on Spirulina Templates for Targeted Delivery and Synergistic Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11(5), 4745–4756. [Google Scholar]
- Akolpoglu, M. B.; Alapan, Y.; Dogan, N. O.; Baltaci, S. F.; Yasa, O.; Aybar Tural, G.; Sitti, M. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci. Adv. 2022, 8, No. eabo6163. [Google Scholar] [CrossRef]
- Hong, Y.; Diaz, M.; Cordova-Figueroa, U. M.; Sen, A. Light-Driven Titanium-Dioxide-Based Reversible Microfireworks and Micromotor/Micropump Systems. Adv. Funct. Mater. 2010, 20, 1568–1576. [Google Scholar] [CrossRef]
- Ibele, M.; Mallouk, T. E.; Sen, A. Schooling Behavior of Light-Powered Autonomous Micromotors in Water. Angew. Chem., Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Wentao Duan, Ran Liu, Ayusman Sen. Transition between collective behaviors of micromotors in response to different stimuli.[J], Journal of the American Chemical Society, 2013, 135(4): 1280-1283.
- Loget, G.; Kuhn, A. Propulsion of Microobjects by Dynamic Bipolar Self-Regeneration. J. Am. Chem. Soc. 2010, 132, 15918–15919. [Google Scholar] [CrossRef] [PubMed]
- Loget, G.; Kuhn, A. Electric Field-Induced Chemical Locomotion of Conducting Objects. Nat. Commun. 2011, 2, 535. [Google Scholar] [CrossRef] [PubMed]
- Bouffier L, Kuhn A. Design of a wireless electrochemical valve[J]. Nanoscale, 2013, 5(4): 1305-1309.
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation[J]. Nature reviews Molecular cell biology, 2013, 14(10): 630-642.(暂时放置).
- Galajda, P. t.; Ormos, P. l. Complex Micromachines Produced and Driven by Light. Appl. Phys. Lett. 2001, 78, 249. [Google Scholar] [CrossRef]
- Huang H W, Sakar M S, Petruska A J, et al. Soft micromachines with programmable motility and morphology[J]. Nature communications, 2016, 7(1), 12263.
- del Pozo, M.; Delaney, C.; Pilz da Cunha, M.; Debije, M. G.; Florea, L.; Schenning, A. P. H. J. Temperature-Responsive 4D Liquid Crystal Microactuators Fabricated by Direct Laser Writing by Two-Photon Polymerization. Small Struct. 2022, 3. No. 2100158. [Google Scholar] [CrossRef]
- Yoshida, K.; Onoe, H. Soft Spiral-Shaped Microswimmers for Autonomous Swimming Control by Detecting Surrounding Environments. Adv. Intell. Syst. 2020, 2(9). No. 2000095. [Google Scholar]
- Andhari, S. S.; Wavhale, R. D.; Dhobale, K. D.; Tawade, B. V.; Chate, G. P.; Patil, Y. N.; Khandare, J. J.; Banerjee, S. S. Self-Propelling Targeted Magneto-Nanobots for Deep Tumor Penetration and pH-Responsive Intracellular Drug Delivery. Sci. Rep. 2020, 10(1), 4703. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Z.; Jin, D.; Zhang, C.; Sun, R.; Li, Z.; Hu, K.; Ni, J.; Cai, Z.; Pan, D.; Wang, X.; Zhu, W.; Li, J.; Wu, D.; Zhang, L.; Chu, J. Botanical-Inspired 4D Printing of Hydrogel at the Microscale. Adv.Funct. Mater. 2020, 30(4). No. 1907377. [Google Scholar] [CrossRef]
- Maura Power, S. A.; Shanel, S.; Yang, G.-Z. Towards hybrid microrobots using pH- and photo-responsive hydrogels for cancer targeting and drug delivery. 2017 IEEE International Conference on Robotics and Automation (ICRA); IEEE, 2017; pp 6002−6007. [CrossRef]
- Wang, D.; Gao, C.; Wang, W.; Sun, M.; Guo, B.; Xie, H.; He, Q. Shape-Transformable, Fusible Rodlike Swimming Liquid Metal Nanomachine. ACS Nano 2018, 12(10), 10212–10220. [Google Scholar] [CrossRef]
- Sridhar, V.; Podjaski, F.; Alapan, Y.; Kroger, J.; Grunenberg, L.; Kishore, V.; Lotsch, B. V.; Sitti, M. Light-driven carbon nitride microswimmers with propulsion in biological and ionic media and responsive on-demand drug delivery. Sci. Robot. 2022, 7. No. eabm1421. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H.; Dong, L.; Shi, Q.; Li, J.; Sun, T.; Huang, Q.; Fukuda, T. Ionic shape-morphing microrobotic end-effectors for environmentally adaptive targeting, releasing, and sampling. Nat.Commun. 2021, 12(1), 411. [Google Scholar] [CrossRef] [PubMed]
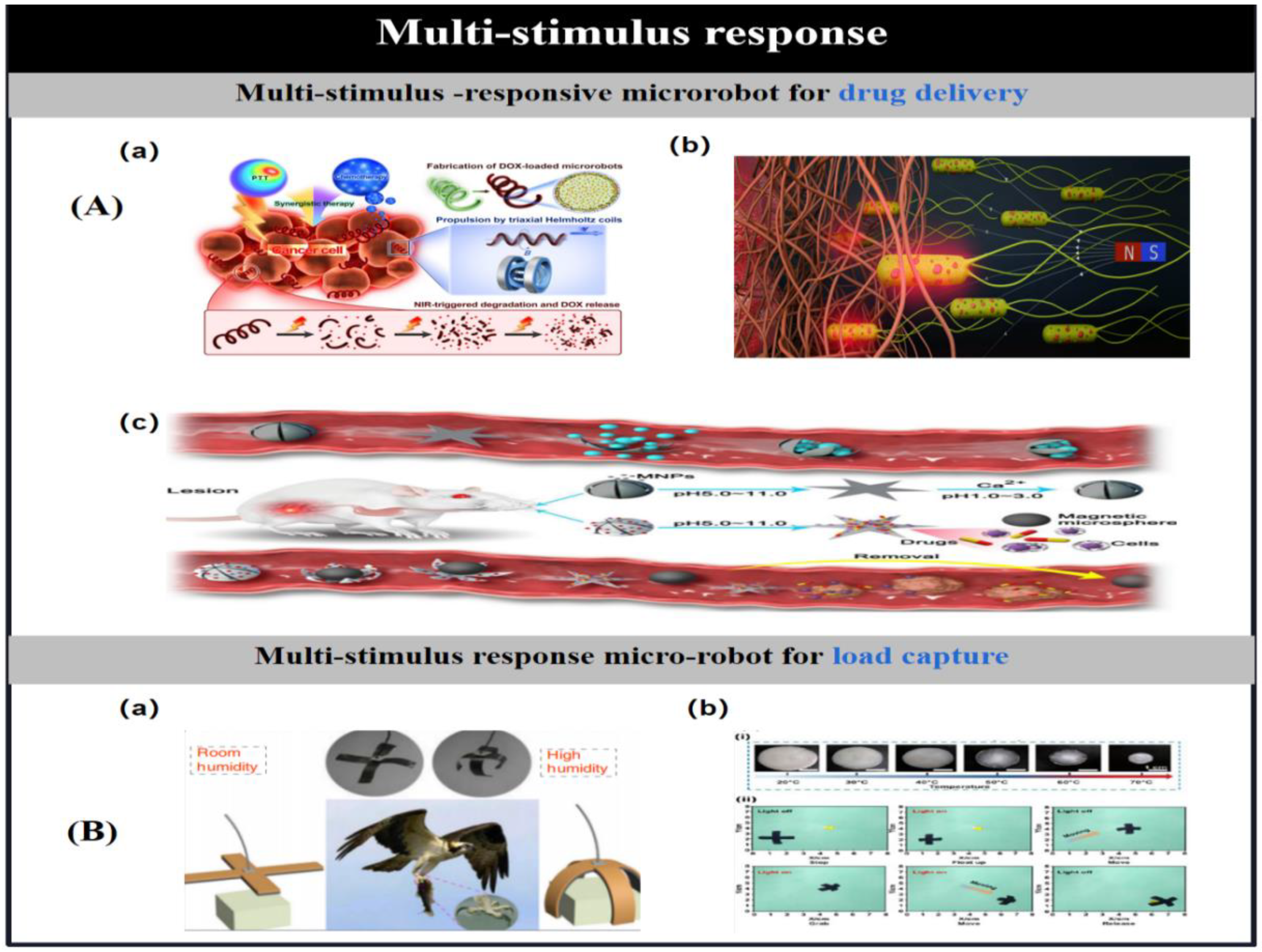
- Dong, Y.; Wang, J.; Guo, X.; Yang, S.; Ozen, M. O.; Chen, P.; Liu, X.; Du, W.; Xiao, F.; Demirci, U.; Liu, B. F. Multi-stimuli-responsive programmable biomimetic actuator. Nat. Commun. 2019, 10(1), 4087. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Chen, L.; Duan, H.; Chen, Y.; He, M.; Wang, Z. 3D printed ultra-fast photothermal responsive shape memory hydrogel for microrobots. Int. J. Extrem. Manuf. 2022, 4(1). No. 015302. [Google Scholar] [CrossRef]

| Preparation method | Type of propulsion | Types | Propulsion mechanism | Ref | |
| electrodeposition | membrane template- assisted Electrode-position |
chemical | nanowires | self-electrophoresis | [71,79] |
| chemical | nanotubes | bubble recoil | [75,77] | ||
| magnetic | helical | magnetic forces/torques | [104,107] | ||
| magnetic | flexible nanowires | magnetic forces/torques | [74,78,90] | ||
| ultrasound | nanowires | acoustic pressure difffference | [85] | ||
| electric | metallic nanowires | Dielectrophoretic force | [72,73] | ||
| electric | metallic microobjects | dynamic bipolar self-regeneration | [175] | ||
| asymmetric bipolar electrodeposition | chemical | Janus | bubble recoil | [105] | |
| physical vapor deposition | Physical Vapor Deposition | chemical | Janus | self-diffffusiophoresis,self-electrophoresis, Bubble Propulsion, Surface tension gradients |
[38,46,50,114] |
| magnetic | Janus | magnetically induced thermophoresis | [108] | ||
| light | Janus | diffffusiophoresis induced by light | [113,141] | ||
| Glancing angle deposition | magnetic | helical | magnetic forces/torques | [120,121] | |
| Rolled-up Technology | Rolled-up | chemical | nanotubes | bubble recoil | [97] |
| ultrasound | perflfluorocarbon-loaded microbullets |
acoustic droplet vaporization | [178] | ||
| Self-Scrolling | magnetic | helical | magnetic forces/torques | [135] | |
| electric | helical | electroosmotic flflow | [137] | ||
| 3D direct laser writing | magnetic | helical | magnetic forces/torques | [138,139] | |
| light | micromotors with anisotropicgeometry | optical momentum conversion (OMC) | [179] | ||
| Assembly of Materials | Layer-by-Layer Assembly | chemical | nanotubes | bubble recoil | [152] |
| magnetic | chain of magnetic particles | magnetic forces/torques | [156] | ||
| chemical | Janus | bubble recoil | [153] | ||
| Assembly and Encapsulation | chemical | Janus | self-diffffusiophoresis | [39,154] | |
| ultrasound | magnetic nanoparticle- loaded red blood cells |
asymmetric distribution of encapsulated magnetic nanoparticles |
[155] | ||
| light | Janus | diffffusiophoresis induced by light | [157] | ||
| Assembly and Incorporation | light | micromotors based on photoresponsive surfaces |
surface free energy gradient generated by photoisomerization of molecules |
[160,161] | |
| light | liquid crystal films/liquid crystal elastomers | photoisomerization of molecular motor induced deformation | [163,164] | ||
| chemical | Janus | surface tension gradients/bubble recoil | [94,159] | ||
| BiohybridTechnique | Biological Molecules | biohybrid | micro/nanomotors based on enzymes | enzyme-catalyzed reactions | [166] |
| Motile Units | biohybrid | micro/nanomotors based on motile units | intact motile cells | [168] | |
| Use of Original Materials | light | particles of photoresponsive inorganic materials | photoinduced self-diffffusiophoresis | [172,173] | |
| electric | conducting microobjects | bipolar chemistry induced asymmetric bubble generation | [176] | ||
| Stimuli | composition | Shape | Response | Application | Ref | |
| temperature | NIPAM/AAM/PEGDA/9mTc [Tc] | tubular(100 μm diameter ) | swelling/shrinking | Tracking, imaging | [59] | |
| NIPAM/MNPs | round | swelling/shrinking | Treating Cancer | [60] | ||
| NIPAM/PEDGA/MNPs/Fe3O4 | flagellated | swelling/shrinking | conditioning movement | [180] | ||
| Mg/Pt-NIPAM | spherical(50 um diameter ) | swelling/shrinking | Drug delivery | [61] | ||
| LC | hexagonal(3 μm height; 20 μm width) | swelling/shrinking | temperature sensor | [181] | ||
| NIPAM/AAc/NaAlg | spiral(300 μm inner diameter) | swelling/shrinking | Speed and direction adjustment | [182] | ||
| light | IP-DIP/LCE | fourlegged(100 um× 50 um × 10 um) | shape transformation | Motion Modulation | [16] | |
| IP-DIP/LCE | four fingers(200 um× 200 um × 20 um) | bending deformation | Particle Capture | [18] | ||
| Spirulina platensis/Fe3O4/TiO2 | spiral (2.8 μm diameter; 50−80 μm length) | photocatalysis degradation | Removal of organic contaminants | [19] | ||
| chitosan | double helix (6μm diameter; 20 μm length) |
photocleavage | Drug delivery | [20] | ||
| biotin/NH2−Fe3O4/streptavidin | tri-bead(10 μm in length) | photocleavage | cancer treatment | [17] | ||
| NIPAM-AAM/PEGDA/SiO2-coatedFe2O3/GO nanosheet | tubular(270 μm inner diameter; 580 μm outer diameter) |
swelling/shrinking | Drug delivery | [21] | ||
| NIPAM/alginate/MNPs | spring(800 μm diameter; 1600 μm length) | swelling/shrinking | Drug delivery | [23] | ||
| E-dent 400/PDA/MNPS/lipiodol | cylindrical (500 μm diameter) | photothermal effect | Drug delivery | [24] | ||
| Geltin/PVA/MNPs/PLGA | roun(100−250 μm ) | photothermal effect | cancer treatment | [27] | ||
| ultrasound | PLGA/PFC | Spherical( 150nmdiameter) | cavitation effect | Drug resistance resulting from the induction of lactic acidosis by tumour tissue. | [2] | |
| PEGDA/PETA | helix(40 μm diameter; 120 μm length) | acoustic streaming effect | Effect of various drug release patterns on the therapeutic effectiveness of cancer cells. | [3] | ||
| E-dent 400/NdFeB | helix | acoustic streaming effect | Reduced stimulus response time for rapid drug release | [4] | ||
| P(VDF-TrFE)/CFO | helix(250 μm inner diameter) | acoustic streaming effect | Neuron-like cell trafficking and cell differentiation | [5] | ||
| PEDOT/MnO2 | tubular(5 μm diameter; 15 μm length) | cavitation effect | Dynamic assembly, swarming | [6] | ||
| Au−Pt | tubular(200 nm diameter; 2 μm length) | acoustic streaming effect | Dynamic assembly, swarming | [7] | ||
| pH | Mg/Au/EUDRAGITU L100- 55 Cy5/Apt/Lip | round(20 μm diameter) | Consumption of local protons | Stomach acid neutralisation, drug release, cancer treatment | [58] | |
| chitosan/sodium alginate/Fe3O4 | thumbtack-like | Dissolved under alkaline conditions | Drug delivery | [63] | ||
| PHEMA/PEGDA/Fe3O4 | Eight arms(150 μm thickness) | swelling/shrinking, absorption/release of aqueous solutions | Drug delivery | [64] | ||
| IPL-780/PDA/Ni/Ti | helix(10 μm helical diameter) | pH-induced bond hydrolysis | Drug delivery | [65] | ||
| CoNi/alginate | cylindrical | swelling/shrinking, absorption/release of aqueous solutions | Drug delivery | [66] | ||
| PPy/Fe3O4/Pt | (tubular(12.4 μm length; 4.4 μm width) | Charge change-affinity regulation-aggregation of estrogen fibres | Removal of estrogenic contaminants from water | [62] | ||
| Cy5/Apt/Lip | coin | Acid Driven - Specific Targeting | Biosensory imaging (ATP) | [67] | ||
| AAc/NIPAM/PVP | leaf | Expansion,contraction, torsion | Multi-degree-of-freedom shape transformation | [184] | ||
| PEGDA/glycerol/CEA | pyramid/dome | distortion by swelling | shape shift | [151] | ||
| EMK/AAc/NIPAM/DPEPA | hollow sphere(60 μm diameter) | welling/shrinking | shape shift | [147] | ||
| EMK/AAc/NIPAM/DPEPA | humanoid-robot(300 um× 400 um× 400 um) | Module Assembly | Vehicle-human shape shifting. | [150] | ||
| magnetic field | GelMA/CFO/BFO | helix(100 μm length) | magnetoelectric effect | Inducing neuron-like cell differentiation | [9] | |
| NdFeB/silicone | origami | Magnetic control | Instant shape locking while moving without constraints | [10] | ||
| MnFe2O4/oleic acid | pot-like hollow | Hydrophobic interactions - tight magnetic shell layer | Bubble jet to remove oil droplets | [12] | ||
| IP-Dip/Ni/Au | half-capsule(7.5 μm length; 5 μm diameter; 500 nm thickness) |
paramagnetic effect | Direction of Motion Adjustment | [8] | ||
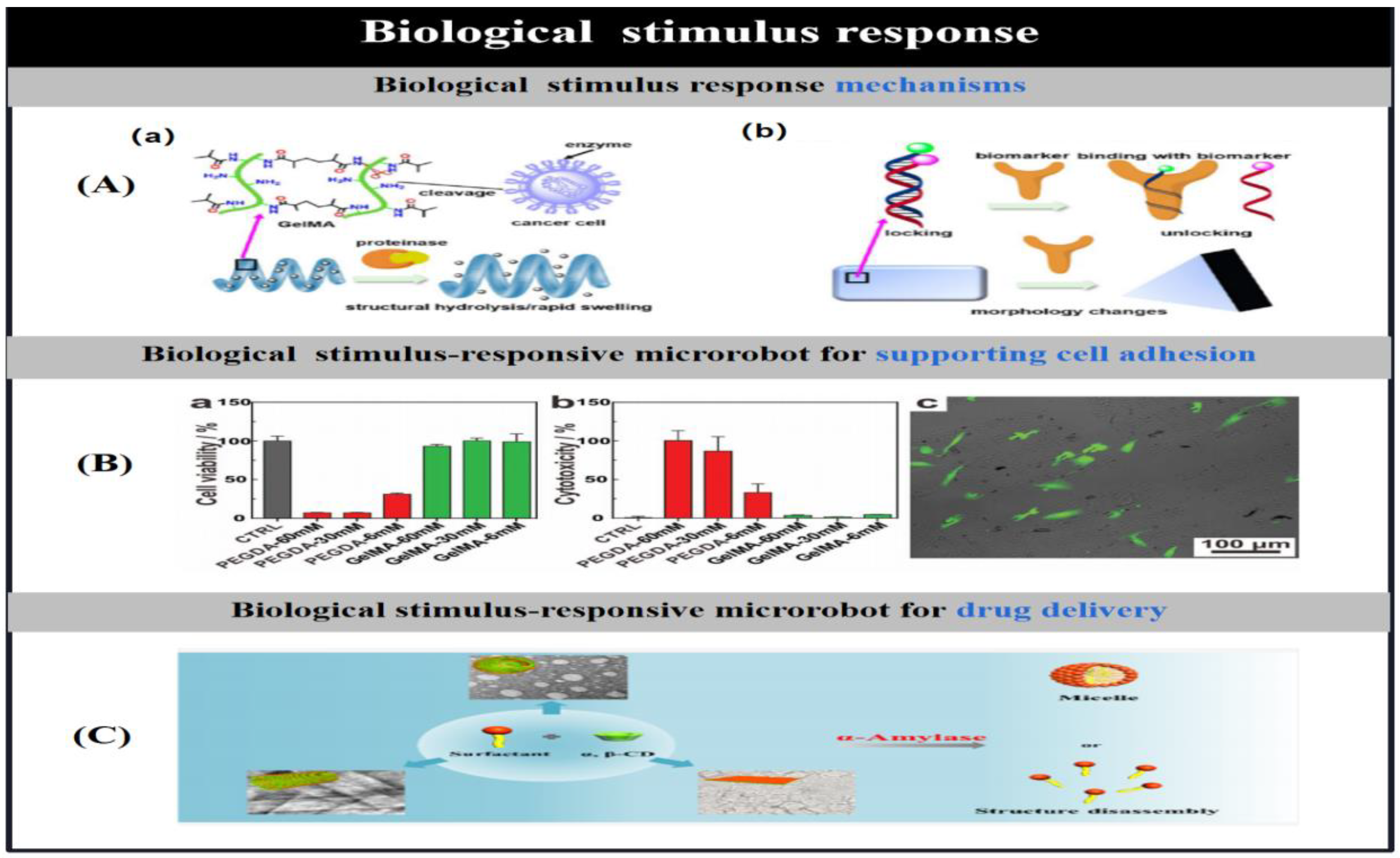
| bio-stimulation | enzyme | gelatin methacryloyl/ poly(ethylene glycol) amine/Fe3O4 |
double helical (20 μm length; 6 μm diameter) |
bond hydrolysis-swelling | Drug delivery | [29] |
| GelMA/Fe@ZIF-8 | helix | bond hydrolysis | Drug delivery | [30] | ||
| GelMA/PEGDA/Fe3O4 | helix(30 μm length) | bond hydrolysis | cell culture | [31] | ||
| inflammatory factors |
gelatin/Fe3O4/neutrophil | Neurophil(105 nm diameter) | chemotaxis | Crossing the blood-brain barrier to release drugs | [32] | |
| angiogenic factor VEGF | E. coli bacteria | spherical | specific binding | Early Cancer Diagnosis | [33] | |
| biomarker (EpCAM) | DNA | tetrahedral | Controlled conformational changes | Early Cancer Diagnosis | [34] | |
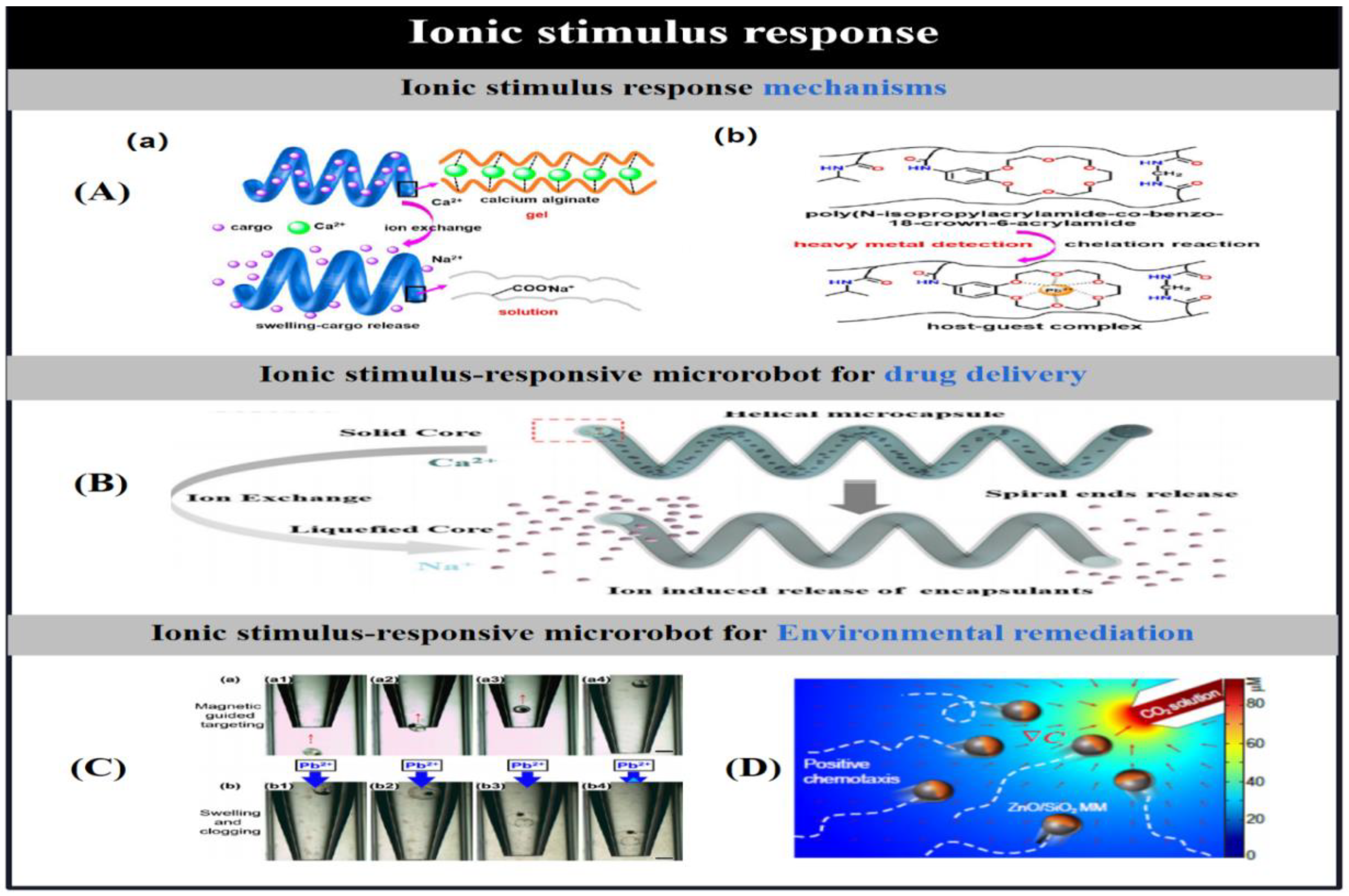
| ion | alginate/chitosan/Fe3O4 | helical | ion exchange | Drug delivery | [57] | |
| NIPAM/BC18A6m/MNPs | spherical | chelation sensing Pb2+ ion | Heavy metal detection (Pb2+) | [68] | ||
| ZnO/SiO2 | Janus(2.5 μm diameter) | Continuous corrosion by H+ | Detection of CO2 | [69] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





