1. Introduction
Polymers are present in numerous agroformulations as wetting agents and adjuvants to improve spreading and bioactivity of active ingredients [
1]. These surface-active polymer adjuvants may have direct benefits to plant health, particularly when the crop is under stress. Climate change is imposing a greater frequency of nonpredictable drought, temperature extremes, and increased soil salinity on crops. Novel methods to protect plants against abiotic stresses are needed [
2,
3].
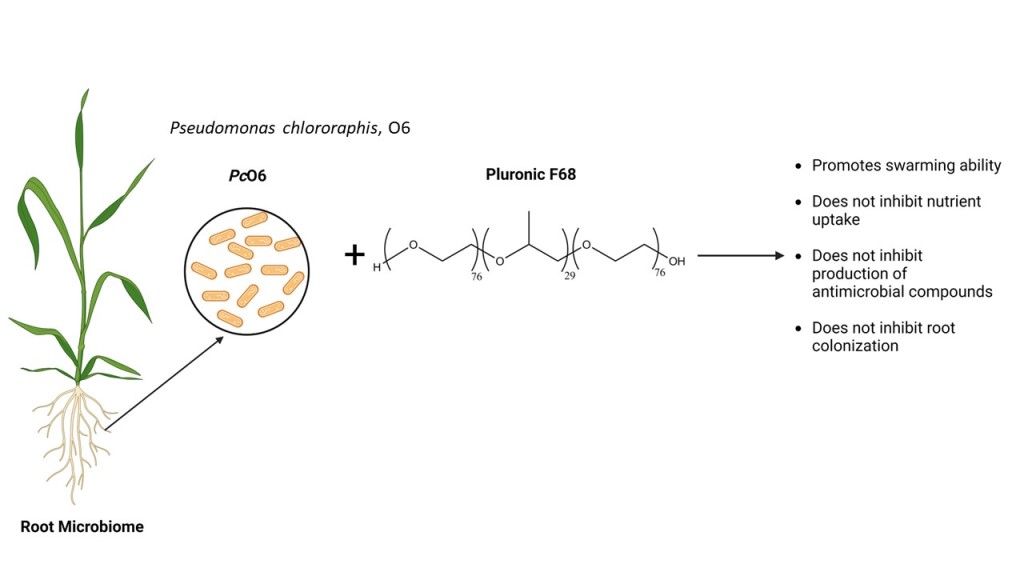
The use of Pluronic F68 may provide adjuvant functionality while introducing membrane-protective activity. Pluronic F68 protects eukaryotic cell membranes from shear stress and bubble-facilitated ruptures in bioreactors and inserts into damaged cell membranes to “heal” permeabilized bilayer limiting electrolyte loss from the cells [
4,
5,
6,
7,
8,
9]. Pluronic 68 is an 8,400 D triblock copolymer which is 80% hydrophilic and a molecular structure of HO-[PEO]
76-[PPO]
29-[PEO]
76-OH, where PEO is polyethylene oxide and PPO is polypropylene oxide (
Figure 1). Of the numerous Pluronics that differ in MW and PEO/PPO ratios, F68 already has FDA approval for human use [
10]. Field studies with F68 demonstrate enhanced shoot regeneration in some plants and cryoprotection of frozen plant tissues in others [
11,
12,
13,
14,
15,
16]. Initial studies from our group confirm that F68 at concentrations up to 10 g/L has no phytotoxicity on wheat seedlings [
17].
Any agricultural formulation application should not harm the association of plants with beneficial microbiomes. The plant’s microbiome is essential for plant’s welfare, including greater tolerance to both abiotic and biotic stress [
18,
19]. The Gram-negative strain,
Pseudomonas chlororaphis O6 (
PcO6) isolated from field-grown wheat roots has probiotic effects promoting protection from pathogenic challenge and stresses due to drought and salinity [
20,
21]. The ability of
PcO6 to directly inhibit pathogen growth and induce systemic resistance to pathogens is in part due to production of the metabolites, the phenazines. Phenazines are antimicrobial and are one of the triggers of induced plant resistance [
22,
23,
24]. Pluronics exhibit a range of MWs and PEO/PPO ratios (
Supplemental Table 1). Previous work found that certain Pluronics enhanced, and others decreased phenazine production in isolate
PcO6, although they all promoted pseudomonad swarming motility, consistent with their surfactant activity [
25]. Phenazines and other surfactants interact positively in the inhibition of pythium species as pathogens [
26]. Indeed, a nonionic detergent caused lysis of plant pathogenic
Pythium and
Phytophthora species zoospores but were without activity on their mycelia [
27]. This background information stimulated our studies directed at understanding the potential interactions between the probiotic pseudomonad,
PcO6, and F68.
The studies reported in this paper examined effects on growth and production of phenazines by PcO6 to examine for inhibition or stimulation. The ability of the polymer to alter swarming motility of the bacterium on the surface of 0.5% nutrient agar was studied. F68 was fluorescently labelled to determine whether F68 was associated with bacterial cells and whether labelling was sensitive to washing. Potential cryoprotectant effects of F68 for the prokaryote were determined by assessment of culturability after freeze/thaw cycles at -20 °C in cell suspensions amended with F68.
2. Materials and Methods
2.1. Effects of F68 on growth of PcO6 in liquid culture
Stocks of
PcO6 were maintained frozen at -80 °C in 15% sterile glycerol. Stocks were thawed and inoculated onto Lysogeny Broth (LB) or minimal medium (MM) 2% agar plates with growth at 22 C for 30 h. To determine whether F68 showed dose-dependent toxicity for
PcO6, cultures were prepared with a starting inoculum of 10
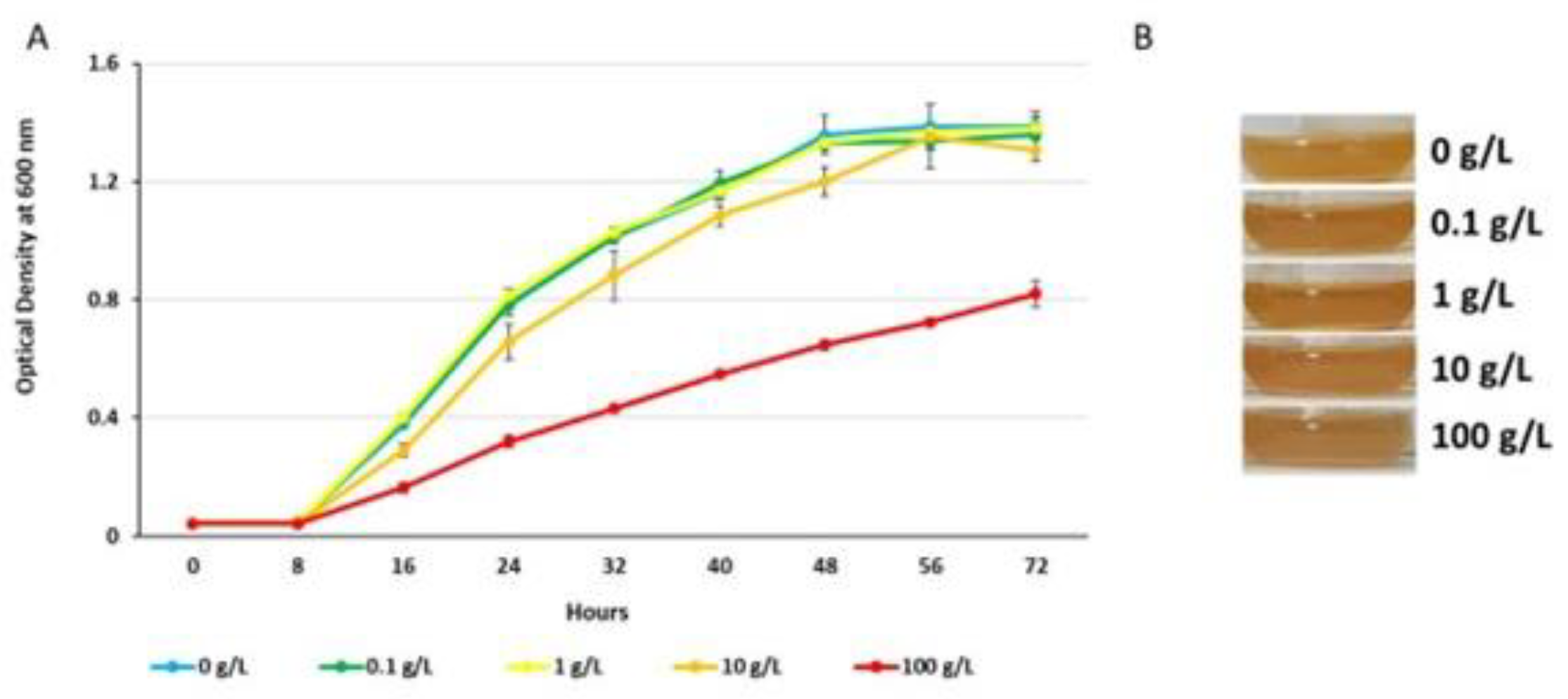
5 colony forming units per mL (CFU/mL) in LB broth containing defined doses of F68 (0 to 100 g/L) with shaking at 125 rpm at 22 °C. Aliquots were withdrawn from the culture and optical density measured at 600 nm using a BioTek Synergy HTX Multimode Reader (Agilent Technologies; Santa Clara, CA) as a measure of cell density. Images of the color of the cultured cells were taken at late stationary phase to record the production of the orange pigmented phenazines typical of this strain when grown on LB medium [
36].
To show whether F68 was utilized as a carbon source by PcO6, cultures were established in a defined minimal medium (MM) containing phosphate salts as a pH 7.1 buffer (10.5 g/L dibasic; 4.5 g/L monobasic K phosphates), ammonium ions as a N source from ammonium sulfate (0.125 g/L), and magnesium sulfate heptahydrate (0.125 g/L) as both a S source and Mg ion source. This medium was used without C sources (the no-C control) or with the addition of F68 (1.72 g/L). Additional comparative treatments were included with amendments of an osmolyte glycine betaine (GB) (1.88 g/L), or the mix of 0.86 g/L F68 with 0.94 g/L GB. Three flasks for each treatment were prepared. Growth was measured after shaking at 125 rpm for 120 h at 22 °C when cultures were into late stationary phase. Images of the cultures were taken to record whether the phenazine pigments were produced. Serial dilutions were prepared in sterile distilled water and aliquots plated onto LB agar. Colonies were counted after 48 h and CFU/ml for the original cultures calculated.
2.2. F68 Effects on PcO6 swarming mobility
The effect of F68 as a surfactant on the swarming motility of
PcO6 cells was determined on MM containing 20 g sucrose/L as the C source and 0.5% agar [
28]. The agar was amended with defined concentrations of F68 (0, 0.0001 g/L, 0.001 g/L, 0.01 g/L, 0.1 g/L, 1 g/L, and 10 g/L). Above 10 g/L, the F68 formed micelles and the agar would not set. The agar plate was inoculated in the center with 2 µL applications of
PcO6 cells at 104 CFU/mL. The
PcO6 cells were previously cultured for 48 h on unamended MM broth. The diameters of the colonies were measured after 48 h incubation at 22 °C and their morphologies were noted in images. All chemicals were from Fischer Scientific.
2.3. Labelling of PcO6 cells with fF68
Fluorescein-labelled F68 (fF68) was prepared by the method described in Cartwright et al. (2022). Late logarithmic phase PcO6 cells grown in MM broth were exposed in 1 ml aliquots (1-2 x108 CFU/mL) to 0.1 mM fF68 or 0.1 mM unlabeled F68 as a control for 30 min. Another control used cells without any treatment. The cells were observed for fluorescence directly or after washing. For washing, cells were pelleted by centrifugation at 10,000 g for 10 minutes and suspended twice in 1 ml MM before final suspension in 1 ml MM. The fluorescence of the cell was observed using a Nikon TE-2000 microscope with a FITC filter set for excitation at 488 nm and emission at 516 nm. The integration time was 1 sec. Cells from three replicates for each treatment were observed with five fields of view for each sample.
2.4. Cryoprotection by F68 for PcO6 cell suspensions
Cells of PcO6, grown to the late logarithmic phase in a 22 °C incubator with shaking at 125 rpm, were pelleted by centrifugation at 10,000 g for 10 minutes in four sterile 50 mL tubes. The cell pellet was resuspended in sterile double distilled water before centrifugation and removal of the wash solutions. The cells in each tube were suspended in either sterile 15% glycerol, 0.1% w/v F68, 1% w/v F68, or double distilled water. The mixtures were shaken to generate homogenous suspensions before 1 ml aliquots were transferred to sterile Eppendorf tubes. The initial concentrations were 3.6 ± 0.5 x 108 culturable cells per ml, based on culturability on LB agar medium. All tubes were placed into a temperature-cycling freezer that cycled between -20°C for 48 hours and 20°C for 15 minutes. At defined times one tube for each of the four suspensions was removed and allowed to thaw at room temperature. These samples were serially diluted in sterile water and samples plated onto LB solidified with 2% agar plates, in triplicate. Colonies were counted after 2 d incubation to determine culturable cell densities in the samples.
3. Results
3.1. Growth of PcO6 in liquid cultures, and its phenazine production, are not altered by the presence of F68
On rich LB medium,
PcO6 growth was not altered with concentrations of F68 at 0.1 and 1 g/L from the growth rate without F68 (
Figure 2 A). Growth rate was decreased by 10 g/L F68 and more extensively with 100 g/L (
Figure 2 A). In the LB medium, the presence of F68 did not alter the potential of
PcO6 to produce orange - colored phenazines (
Figure 2 B).
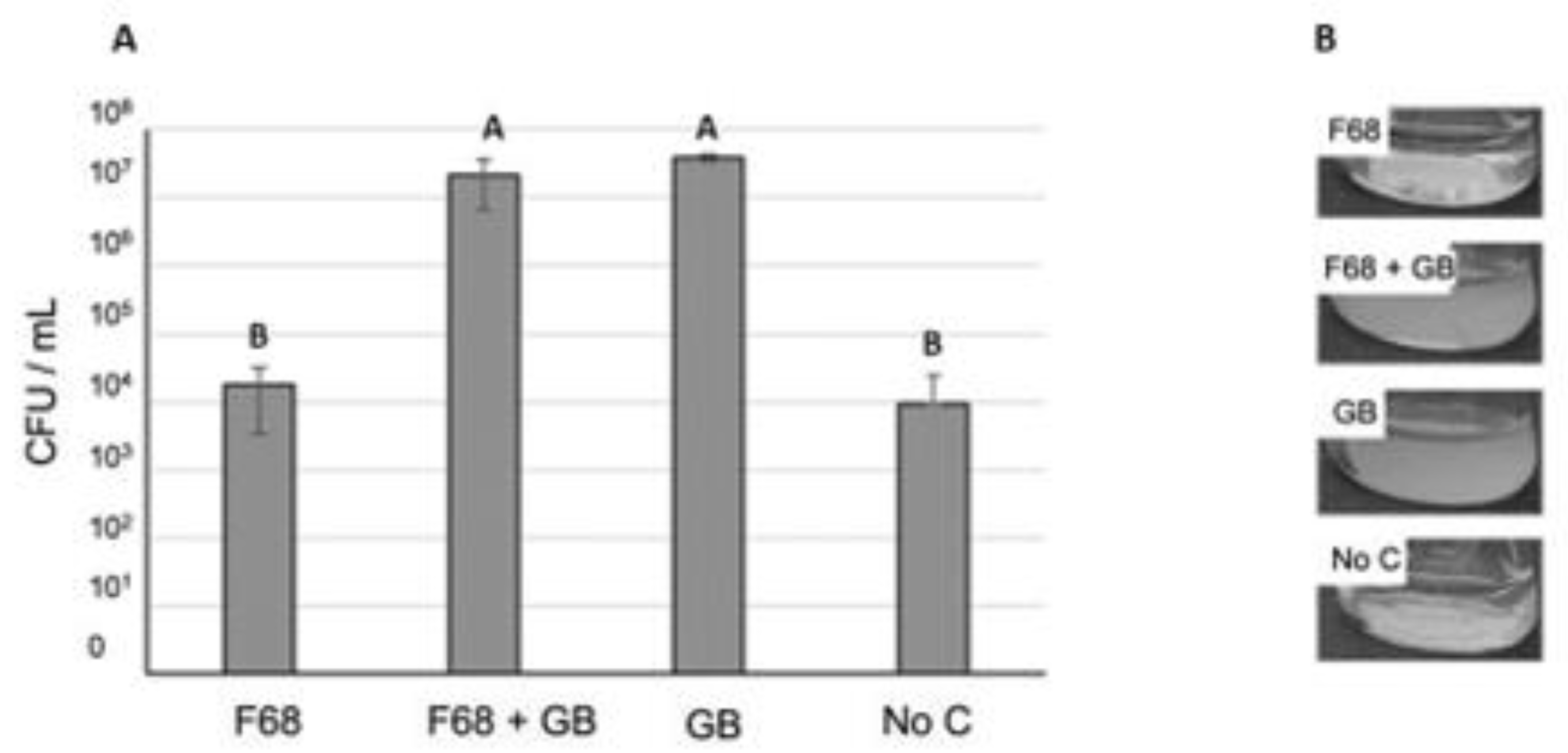
When F68 was added to a defined medium lacking a C source, no growth of
PcO6 was observed (
Figure 3). The CFU/ml at 120 h after inoculation for the medium with only F68 as C source was equal to that of medium inoculated with
PcO6 but lacking any C source. This assessment shows that the F68 was not toxic to the pseudomonad cells. Cell density increases were observed when an osmoprotectant, glycine betaine (GB) [
29,
30], was added into the defined medium, and co-addition of F68 to the GB cultures did not affect the final CFU/ml values. No additions to this defined medium caused the
PcO6 cells to generate orange - colored phenazines.
3.2. F68 enhances swarming behavior of PcO6
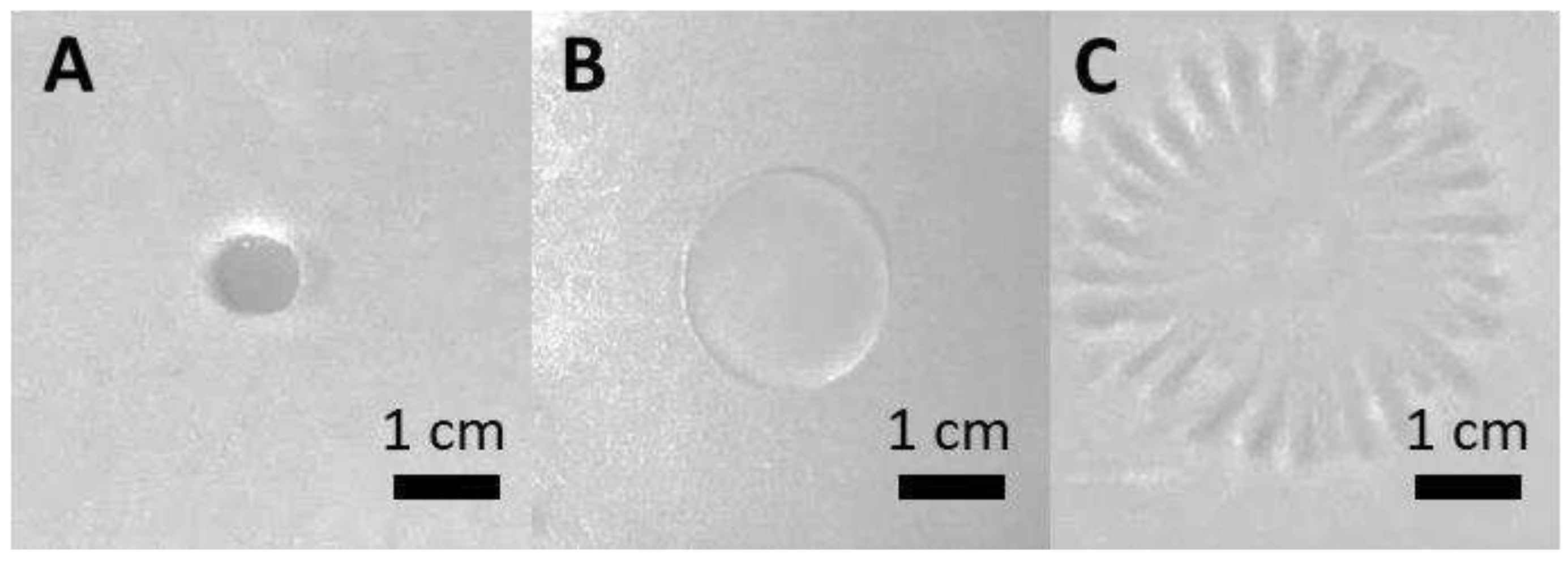
A positive effect of F68 was observed on swarming for
PcO6. Inocula (20 µl aliquots) added to MM 0.5 % agar plates with sucrose (20 g/L) as the C source grew in 48 h to colonies of approximately 0.8 cm diameter. The diameter of the colony and its tight edged morphology was not affected by addition of 0.0001 g/L F68 (
Figure 4 A). With 0.1 g/L F68 the colony diameter increased (
Figure 4 B) but retained a tight-edge morphology so that the colony was still circular in shape. However, with 1 g/L F68 the colony diameter increased, and a dendritic growth morphology was observed (
Figure 4 C). This change in morphology occurs after the transition point in the relationship between surfactant activity and concentration of F68 (
Supplemental Figure 1). At concentrations of F68 lower than 0.001% there is a rapid change in surface activity but between 0.01% and 0.1% the change in surface activity with concentration levels (
Supplemental Figure 1).
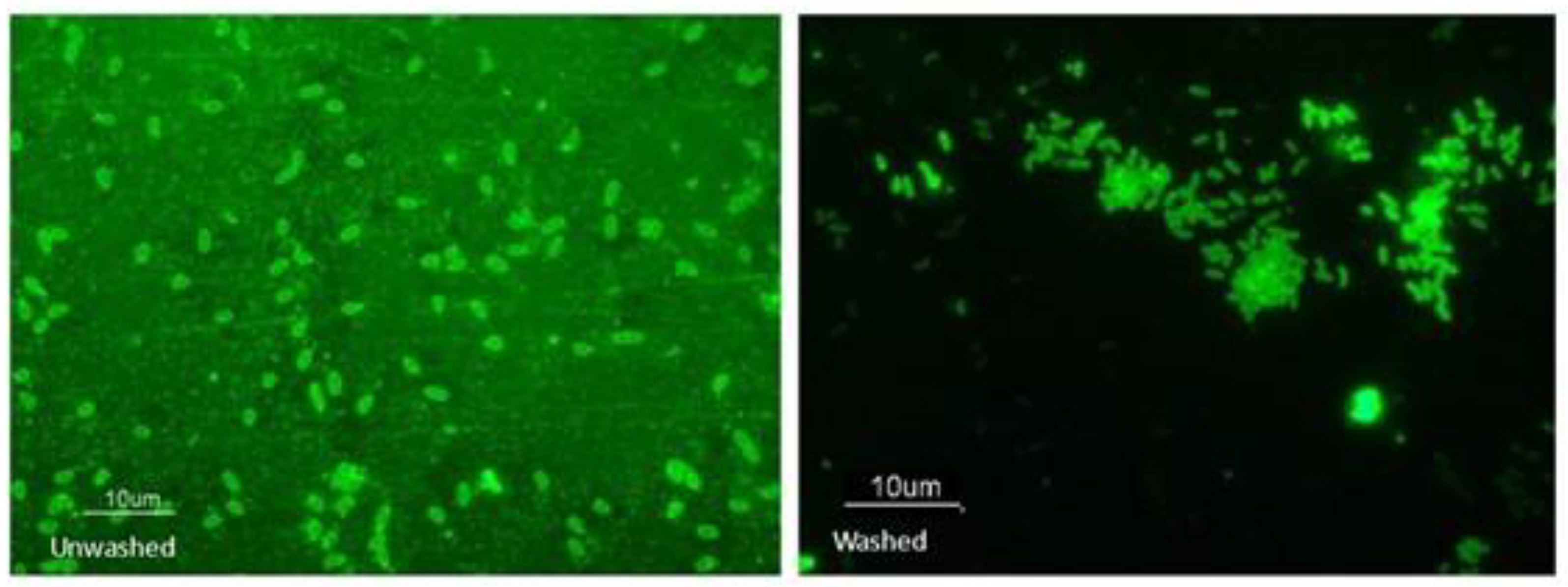
3.3. PcO6 cells become fluorescent when exposed to fF68
To explore further how F68 influences
PcO6 cells, the Pluronic was labelled with fluorescein, producing a fluorescent complex, fF68. Exposure of
PcO6, grown to early stationary phase in rich LB in shake culture, to fF68 for 3 h resulted in cells with outlines that were brightly fluorescent as shown in
Figure 5. Two control treatments were examined; first the cells alone did not show fluorescence (
Supplemental Figure 2) and second of
PcO6 cells with nonlabelled F68 for 3 h (
Supplemental Figure 2) did not induce fluorescence. The fluorescence of
PcO6 caused by treatment with fF68 was stable to wash in minimal medium.
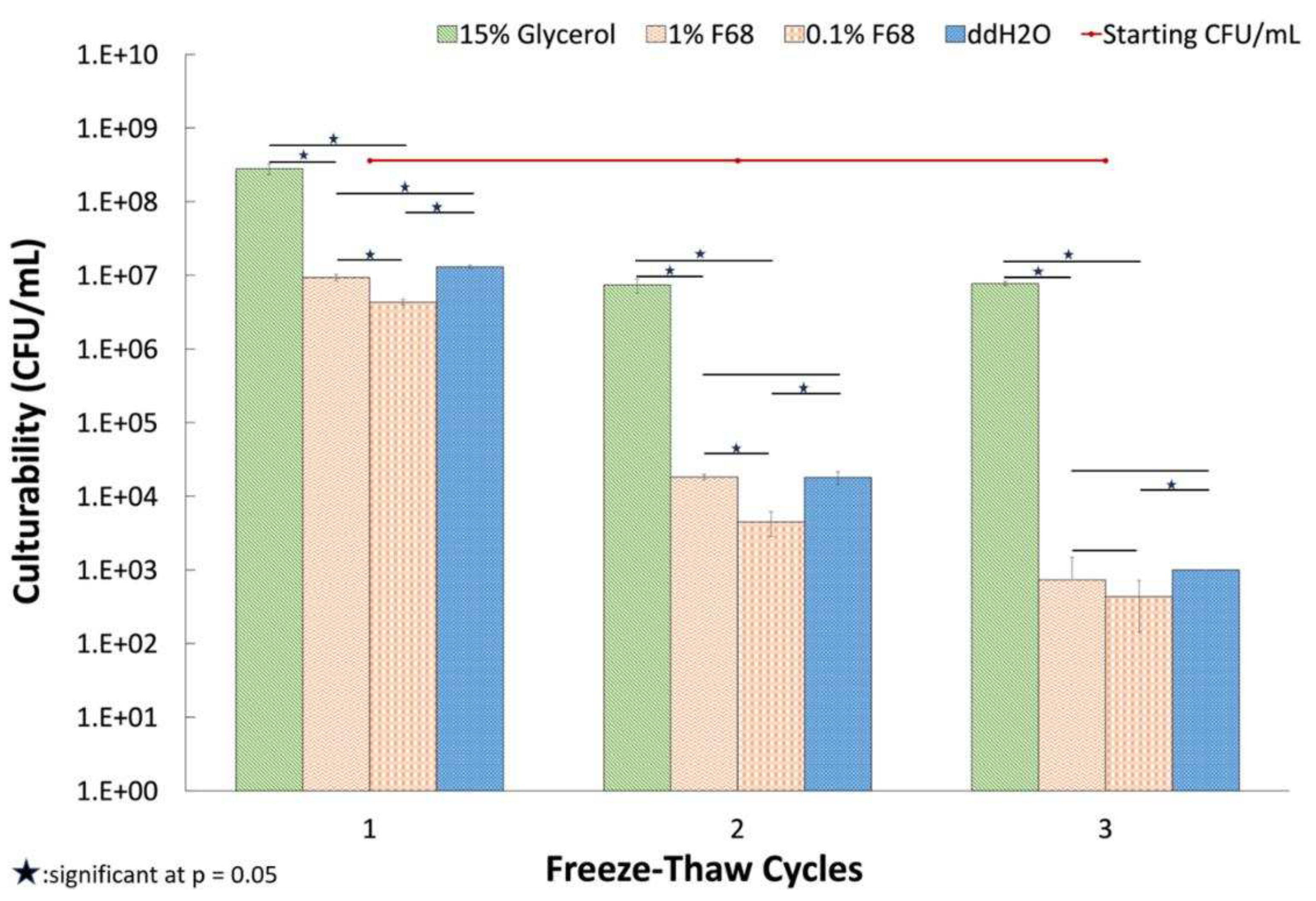
3.4. Suspension in F68 does not provide cryoprotection to PcO6
Suspensions of
PcO6 cells, with starting CFU/mL of 3.2 ± 0.5 x10
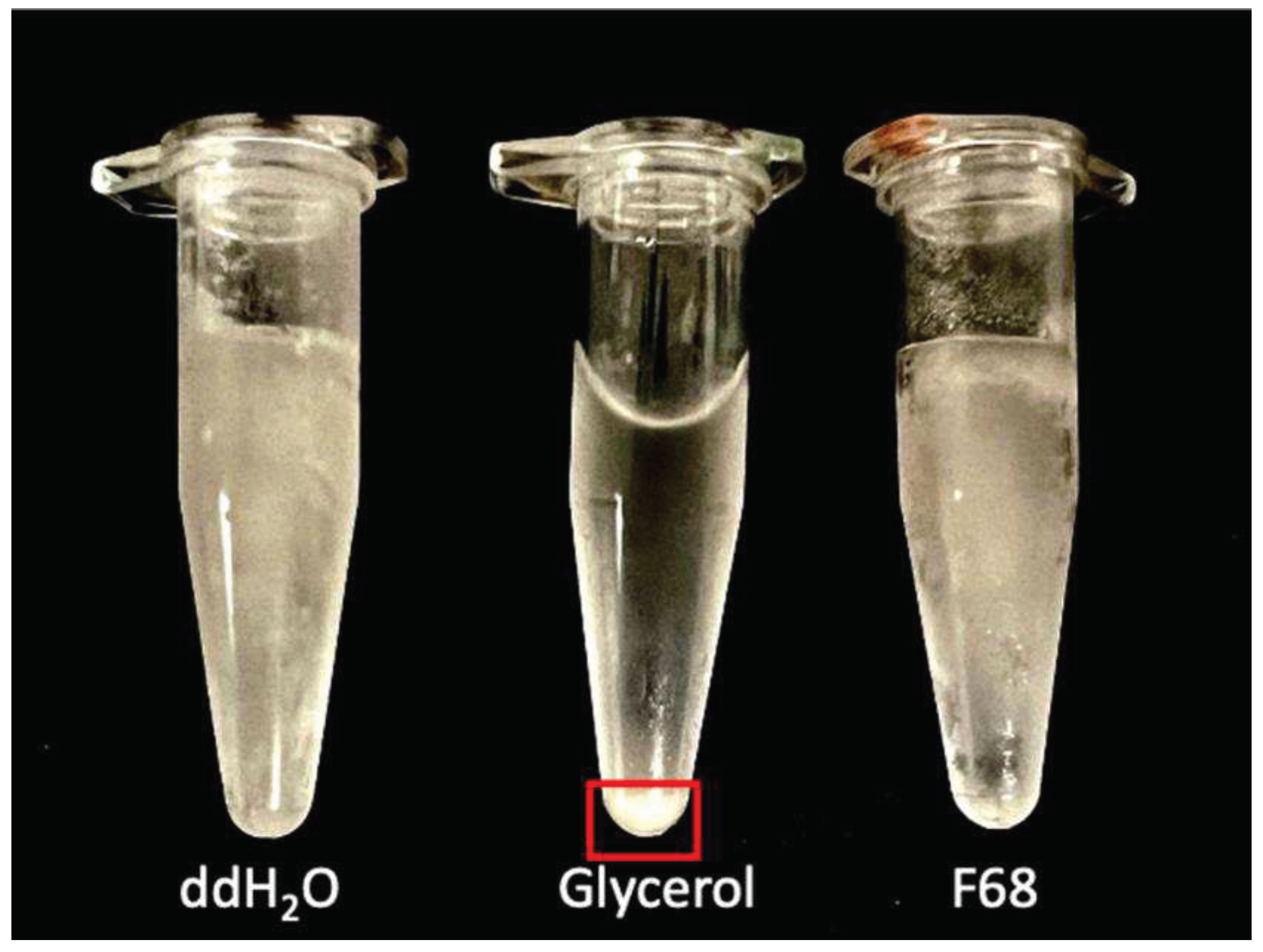
8, showed decreased culturability with the increasing number of freeze thaw cycles from the storage temperature of -20 °C (Figure. 6). Loss in culturability was least for the cell suspensions in glycerol. The suspensions in sterile water showed a greater loss in culturability, losses that were not improved by suspensions in 0.1 or 1% F68 when sampled after one, two and three thaw periods. Suspension in 1% F68 gave statistically higher (p=0.05) culturable cells for the first two cycles but not the third cycle. By the third freeze thaw cycle, the survival of culturable cells was statistically less in 0.1% F68 than in the suspensions in water and 1% F68. During thaw after this third cycle period, the glycerol suspensions thawed faster than samples in water or F68 as shown in
Figure 7 where ice is still visible for the suspensions in F68 and water but not for the glycerol suspension. Additionally, a pellet was formed with the glycerol suspension (Figure. 7) whereas thawing of the water- and F68- samples gave optically dense suspensions typical of suspended cells. This pellet in the glycerol sample, when resuspended before the plating of the sample, produced the suspensions with the culturable cell number shown in
Figure 7. In some of the published cryoprotection studies (
Supplemental Table 2), F68 was active when added during the thawing process [
15]. However, when 1% F68 was added to suspensions of cells frozen at -20 °C and without any previous thaw in either 15% glycerol or water, no consistent effect, either protective or detrimental, was observed in thrice repeated studies (data not shown).
4. Discussion
F68 was compatible with the growth of the plant probiotic
PcO6 on both rich and minimal defined media confirming its classification as an FDA-approved adjuvant. These findings agreed with a previous publication where cell densities of
PcO6 grown in LB or MM liquid shake cultures were not affected by 0.5% (v/v) Pluronics P104, P108, P123 or the reverse Pluronic 25R2 [
25]. Each of these Pluronics have different proportions of PPE and PPO as shown in
Supplemental Table 1 although all are surfactant active.
F68 also did not alter the production of phenazines from
PcO6 unlike the increases during growth on LB medium with 0.5% amendments seen with 25R2 and the decreases with P104 and P123; the null effect of F68 paralleled that of F108 [
25]. These responses suggest that the presence of F68 in formulations would not alter the production of phenazines, that are important for biocontrol-active pseudomonads [
23]. Like all the Pluronics examined in previous studies by Housley et al. (2009), the surface activity of F68 promoted spreading of colonies of
PcO6 on a soft agar surface. Such activity may enhance colonization of the root surface, a factor essential for plant protection by biocontrol pseudomonads [
31]. The finding that
PcO6 does not utilize F68 as a C source for growth would add to its longevity in the rhizosphere if it were present in applied agricultural formulations.
PcO6 cells appeared to sorb a coating of F68, as determined by fluorescence imaging of cells exposed to fF68. This label was stable by washing but currently it is not resolved whether any of the Pluronic is internalized by the bacterial cells. Labelling of organelles of fF68 within root cells has been observed [
17]. It is possible internalization is restricted by the complexity of the bacterial surface layers. For the pseudomonad there exists an extracellular polymeric layer, the outer lipopolysaccharide layer that surrounds the periplasmic space and then the plasma membrane. The bacterial plasma membrane is full of active components, including porins, transporters, the housing complexes and signal transduction sites for flagella, environmental sensors, as well as all the structures of the electron transport chain. It is possible this layered complexity in the bacterial membranes limits membrane healing effects proposed for F68 action with eukaryotic membranes [
7,
8,
9].
We were surprised by the finding that F68 did not act as a cryoprotectant for the
PcO6 cells. As explained above, this result may be due to the complexity of the multiple layers that enclose the cytoplasm of the Gram-negative cell preventing healing of ice-crystal damaged membranes. It is unlikely that these results are due to the concentrations of F68 examined being too low; the values were within the range found in published literature (
Supplemental Table 2). Indeed, 1% (w/v) F68 contains 7 x 10
17 molecules F68/ml. Consequently 2 x 10
9 molecules of F68 would be available for each
PcO6 cell in our studies using suspensions at 3.2 x 10
8 CFU/ml. Further calculations, based on the size of
PcO6 cells and an F68 molecule, propose that 4 x 10
3 molecules of F68 would be needed to fully cover a single cell of
PcO6. The concentration of F68 in the 1% treatments thus exceeds the amount required for complete
PcO6 cell coverage; these calculations support the observation of overall cell fluorescence after exposure to fF68.
Supplemental Table 2 provides several examples where cryoprotection for eukaryotic cells is observed. Agricultural formulations with a cryogenic-protective role would be valuable under the pressure of climate instability where field soil temperatures fluctuate into freezing conditions. For instance, In Cache Valley Utah USA, where colonized field-grown, winter wheat roots were the source of
PcO6 [
20], the winter conditions can result in the soil being frozen to depths of 20 cm multiple times creating several freeze/thaw cycles [
32]. This freezing depth could subject
PcO6 cells to freezing in the soil or when attached to the roots of winter wheat. Studies with another biocontrol-active pseudomonad,
P. fluorescens Pf5, indicate that the RpoS regulon provides protection against freeze damage and a comparative RpoS regulon active in stress response is present in
PcO6 [
33,
34]. The RpoS regulon could account for the survival through the freeze/thaw cycles of the
PcO6 cells suspended only in water. The studies also support the cryoprotection offered by suspending the cells in 15% glycerol, where loss in culturability was restricted to loss of 1 log unit after three free/thaw cycles.
5. Conclusions
In summary, the findings support the adjuvant classification of F68. This Pluronic did not limit growth of a plant probiotic PcO6 or alter its production of the important biocontrol trait of phenazine biosynthesis. Altered swarming might boost colonization of the bacterium on plant surfaces. The surfactant activity of F68 could be valuable for its inclusion as an adjuvant in formulations for crop applications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. The materials are Supplementary Tables 1 and 2; Supplementary Figures 1 and 2.
Author Contributions
The following statements should be used “Conceptualization, DWB and AJA; methodology, ARS,AC,MZ,AG,AJA,DWB; formal analysis, ARS, AC,MZ,AG.; investigation, ARS, AC, MZ, AG.; resources, AJA, DWB.; data curation, ARS,AC,MZ,AG,AJA,DWB.; writing—original draft preparation, ARS,AJA, DWB.; writing—review and editing, ARS,AC,MZ,AG,AJA,DWB.; visualization, ARS, AC, MZ, AJA, DWB; supervision, AJA, DWB; project administration, AJA,DWB; funding acquisition, AJA,DWB. All authors have read and agreed to the published version of the manuscript.”.
Funding
This work was supported by grants from the Utah Agricultural Station Projects 1581 and 1746 and the National Science Foundation Research Experience for Undergraduate Program, 1950299.
Data Availability Statement
All of the data from these studies are available upon request to the authors of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hazen, J.L. Adjuvants—Terminology, Classification, and Chemistry1. wete 2000, 14, 773–784. [Google Scholar] [CrossRef]
- Bertrand, A.; Castonguay, Y. Plant Adaptations to Overwintering Stresses and Implications of Climate Change. Can. J. Bot. 2003, 81, 1145–1152. [Google Scholar] [CrossRef]
- Willick, I.R.; Tanino, K.K.; Gusta, L.V. The Impact of Global Climate Change on the Freezing Tolerance of Winter Cereals in Western Canada. Journal of Agronomy and Crop Science 2021, 207, 88–99. [Google Scholar] [CrossRef]
- Murhammer, D.W.; Goochee, C.F. Sparged Animal Cell Bioreactors: Mechanism of Cell Damage and Pluronic F-68 Protection. Biotechnol Prog 1990, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Erickson, L.E.; Yiin, T.-Y.; Glasgow, L.A. A Review of the Effects of Shear and Interfacial Phenomena on Cell Viability. Critical Reviews in Biotechnology 1993, 13, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Palomares, L.A.; González, M.; Ramírez, O.T. Evidence of Pluronic F-68 Direct Interaction with Insect Cells: Impact on Shear Protection, Recombinant Protein, and Baculovirus Production*. Enzyme Microb Technol 2000, 26, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; River, L.P.; Pan, F.S.; Ji, L.; Wollmann, R.L. Surfactant-Induced Sealing of Electropermeabilized Skeletal Muscle Membranes in Vivo. Proc Natl Acad Sci U S A 1992, 89, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Moloughney, J.G.; Weisleder, N. Poloxamer 188 (P188) as a Membrane Resealing Reagent in Biomedical Applications. Recent Pat Biotechnol 2012, 6, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Houang, E.M.; Bates, F.S.; Sham, Y.Y.; Metzger, J.M. All-Atom Molecular Dynamics-Based Analysis of Membrane-Stabilizing Copolymer Interactions with Lipid Bilayers Probed under Constant Surface Tensions. J Phys Chem B 2017, 121, 10657–10664. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Gill, M.I.S.; Cancino, G.O.; Anthony, P.; Davey, M.R.; Power, J.B.; Lowe, K.C. Pluronic F-68 Enhanced Shoot Regeneration in Micropropagated Citrus Rootstock and Passiflora Species. Acta Biotechnologica 2003, 23, 349–358. [Google Scholar] [CrossRef]
- Kumar, V.; Laouar, L.; Davey, M.R.; Mulligan, B.J.; Lowe, K.C. Pluronic F-68 Stimulates Growth of Solanum Dulcamara in Culture. Journal of Experimental Botany 1992, 43, 487–493. [Google Scholar] [CrossRef]
- Kok, A.D.-X.; Wan Abdullah, W.M.A.N.; Tan, N.-P.; Ong-Abdullah, J.; Sekeli, R.; Wee, C.-Y.; Lai, K.-S. Growth Promoting Effects of Pluronic F-68 on Callus Proliferation of Recalcitrant Rice Cultivar. 3 Biotech 2020, 10, 116. [Google Scholar] [CrossRef]
- Kok, A.D.-X.; Mohd Yusoff, N.F.; Sekeli, R.; Wee, C.-Y.; Lamasudin, D.U.; Ong-Abdullah, J.; Lai, K.-S. Pluronic F-68 Improves Callus Proliferation of Recalcitrant Rice Cultivar via Enhanced Carbon and Nitrogen Metabolism and Nutrients Uptake. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef]
- Anthony, P.; Jelodar, N.B.; Lowe, K.C.; Power, J.B.; Davey, M.R. Pluronic F-68 Increases the Post-Thaw Growth of Cryopreserved Plant Cells. Cryobiology 1996, 33, 508–514. [Google Scholar] [CrossRef]
- Lowe, K.C.; Anthony, P.; Davey, M.R.; Power, J.B. Beneficial Effects of Pluronic F-68 and Artificial Oxygen Carriers on the Post-Thaw Recovery of Cryopreserved Plant Cells. Artificial Cells, Blood Substitutes, and Biotechnology 2001, 29, 297–316. [Google Scholar] [CrossRef]
- Cartwright, A.; Anderson, A.J.; Britt, D.W. Pluronic F68-Capped SiO2 Nanoparticles Are Compatible as Delivery Vehicles to Roots and Shoots. MRS Advances 2022, 7, 327–332. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Berendsen, R.L.; de Jonge, R.; Stringlis, I.A.; Van Dijken, A.J.H.; Van Pelt, J.A.; Van Wees, S.C.M.; Yu, K.; Zamioudis, C.; Bakker, P.A.H.M. Pseudomonas Simiae WCS417: Star Track of a Model Beneficial Rhizobacterium. Plant Soil 2021, 461, 245–263. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology (Basel) 2022, 11, 437. [Google Scholar] [CrossRef]
- Radtke, C.; Cook, W.S.; Anderson, A. Factors Affecting Antagonism of the Growth of Phanerochaete Chrysosporium by Bacteria Isolated from Soils. Appl Microbiol Biotechnol 1994, 41, 274–280. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-Butanediol, a Bacterial Volatile Produced by Pseudomonas chlororaphis O6, Is Involved in Induction of Systemic Tolerance to Drought in Arabidopsis Thaliana. Mol Plant Microbe Interact 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Pierson, L.S., III; Pierson, E.A. Phenazine Antibiotic Production in Pseudomonas Aureofaciens: Role in Rhizosphere Ecology and Pathogen Suppression. FEMS Microbiology Letters 1996, 136, 101–108. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phenazines in Plant-Beneficial Pseudomonas Spp.: Biosynthesis, Regulation, Function and Genomics. Environ Microbiol 2018, 20, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Han, S.H.; Zdor, R.E.; Anderson, A.J.; Spencer, M.; Yang, K.Y.; Kim, Y.H.; Lee, M.C.; Cho, B.H.; Kim, Y.C. Inhibition of Seed Germination and Induction of Systemic Disease Resistance by Pseudomonas chlororaphis O6 Requires Phenazine Production Regulated by the Global Regulator, gacS. J Microbiol Biotechnol 2007, 17, 586–593. [Google Scholar]
- Housley, L.; Anderson, T.; Sontag, N.; Han, S.-H.; Britt, D.W.; Anderson, A.J. Pluronics’ Influence on Pseudomonad Biofilm and Phenazine Production. FEMS Microbiology Letters 2009, 293, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Perneel, M.; D’hondt, L.; De Maeyer, K.; Adiobo, A.; Rabaey, K.; Höfte, M. Phenazines and Biosurfactants Interact in the Biological Control of Soil-Borne Diseases Caused by Pythium Spp. Environ Microbiol 2008, 10, 778–788. [Google Scholar] [CrossRef]
- Stanghellini, M.E. Inhibitory and Lytic Effects of a Nonionic Surfactant on Various Asexual Stages in the Life Cycle of Pythium and Phytophthora Species. Phytopathology 1987, 77, 112. [Google Scholar] [CrossRef]
- Kearns, D.B. A Field Guide to Bacterial Swarming Motility. Nat Rev Microbiol 2010, 8, 634–644. [Google Scholar] [CrossRef]
- Smith, L.T.; Pocard, J.A.; Bernard, T.; Le Rudulier, D. Osmotic Control of Glycine Betaine Biosynthesis and Degradation in Rhizobium meliloti. J Bacteriol 1988, 170, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants Under Abiotic Stresses. Frontiers in Plant Science 2019, 10. [Google Scholar] [CrossRef]
- de Weger, L.A.; van der Bij, A.J.; Dekkers, L.C.; Simons, M.; Wijffelman, C.A.; Lugtenberg, B.J.J. Colonization of the Rhizosphere of Crop Plants by Plant-Beneficial Pseudomonads. FEMS Microbiology Ecology 1995, 17, 221–227. [Google Scholar] [CrossRef]
- Cane, J. Global Warming, Advancing Bloom and Evidence for Pollinator Plasticity from Long-Term Bee Emergence Monitoring. Insects 2021, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, V.O.; Loper, J.E. The Sigma Factor RpoS Is Required for Stress Tolerance and Environmental Fitness of Pseudomonas fluorescens Pf-5. Microbiology (Reading) 2005, 151, 3001–3009. [Google Scholar] [CrossRef]
- Oh, S.A.; Kim, J.S.; Han, S.H.; Park, J.Y.; Dimkpa, C.; Edlund, C.; Anderson, A.J.; Kim, Y.C. The GacS-Regulated Sigma Factor RpoS Governs Production of Several Factors Involved in Biocontrol Activity of the Rhizobacterium Pseudomonas chlororaphis O6. Can J Microbiol 2013, 59, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Glauser, S.C.; Talbot, T.R. Some Studies on Freezing and Thawing Human Erythrocytes. Am J Med Sci 1956, 231, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ashwood-Smith, M.J.; Voss, W.A.G.; Warby, C. Cryoprotection of Mammalian Cells in Tissue Culture with Pluronic Polyols. Cryobiology 1973, 10, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lei, J.; Zhang, M.; Wu, F.; Ren, M.; Yang, J.; Wu, Q.; Shi, X. Optimization of Preservation Methods Provides Insights into Photosynthetic Picoeukaryotes in Lakes. Microbiol Spectr 2022, 10, e0255721. [Google Scholar] [CrossRef]
- Doğan, A.; Yalvaç, M.E.; Yılmaz, A.; Rizvanov, A.; Şahin, F. Effect of F68 on Cryopreservation of Mesenchymal Stem Cells Derived from Human Tooth Germ. Appl Biochem Biotechnol 2013, 171, 1819–1831. [Google Scholar] [CrossRef]
- González-Hernández, Y.; Fischer, R.W. Serum-Free Culturing of Mammalian Cells - Adaptation to and Cryopreservation in Fully Defined Media. ALTEX - Alternatives to animal experimentation 2007, 24, 110–116. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).