Submitted:
21 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
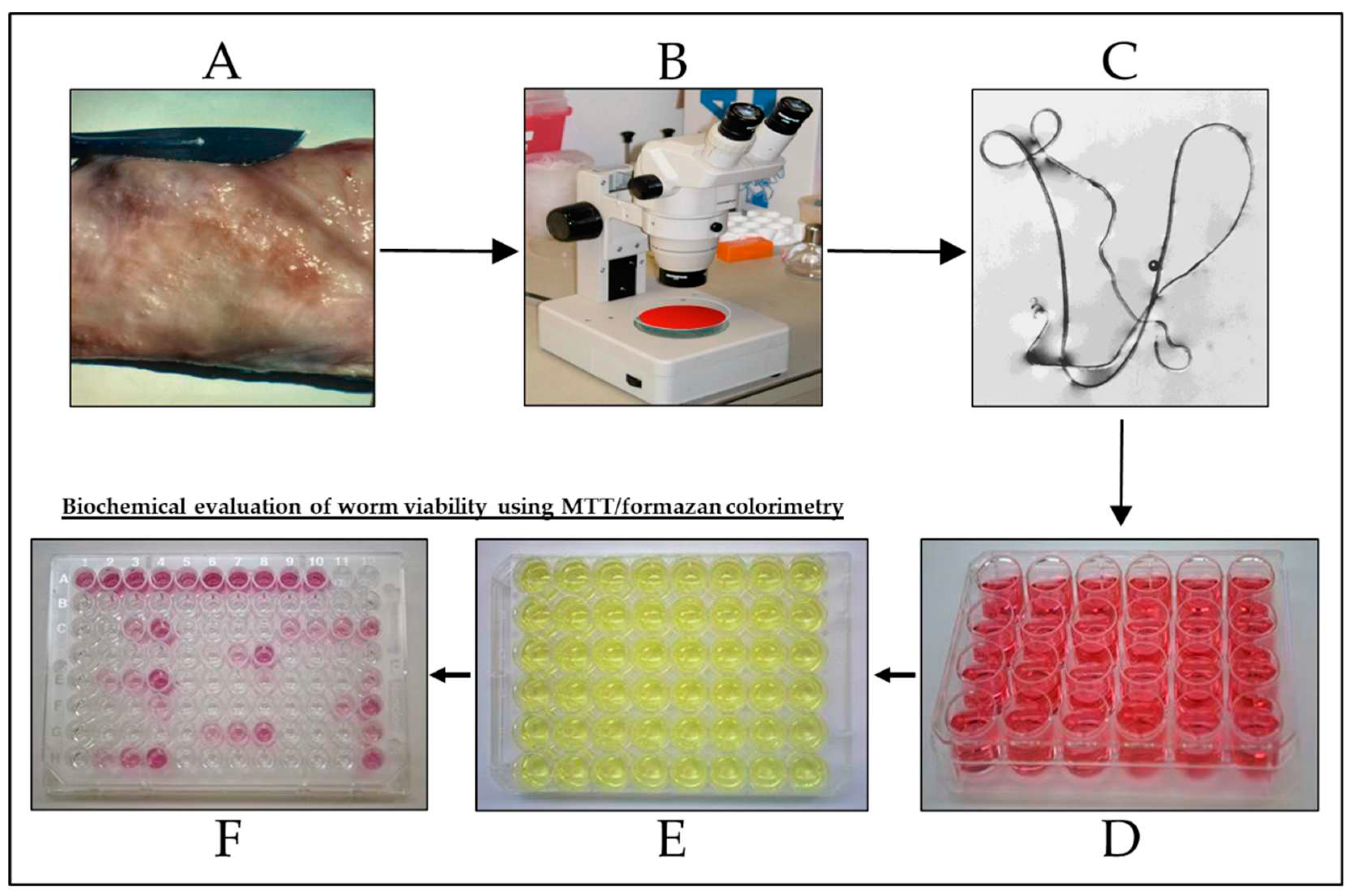
2.1. Parasites - Onchocerca gutturosa
2.2. Origin of drugs tested
2.3. In vitro drug activity against O. gutturosa adult worms as described by Townson et al. [13]
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brattig, N.W.; Cheke, R.A.; Garms, R. Onchocerciasis (river blindness)-more than a century of research and control. Acta Trop. 2021, 218, 105677. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 15 October 2023).
- Duke, B.O.; Zea-Flores, G.; Muñoz, B. The embryogenesis of Onchocerca volvulus over the first year after a single dose of ivermectin. Trop Med Parasitol, 1991. 42(3): p. 175-80.
- Basáñez, M.-G.; Pion, S.D.; Boakes, E.; Filipe, J.A.; Churcher, T.S.; Boussinesq, M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect. Dis. 2008, 8, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Plaisier, A.; van Oortmarssen, G.; Remme, J.; Habbema, J. The reproductive lifespan of Onchocerca volvulus in West African savanna. Acta Trop. 1991, 48, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Gryseels, B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev, 2000. 13(2): p. 207-22.
- von Samson-Himmelstjerna, G.; Harder, A.; Sangster, N. C.; Coles, G. C. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology, 2005. 130(Pt 3): p. 343-7.
- Prichard, R.K.; Geary, T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Osei-Atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Phenotypic Evidence of Emerging Ivermectin Resistance in Onchocerca volvulus. PLOS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef]
- Nana-Djeunga, H.C.; Bourguinat, C.; Pion, S.D.; Bopda, J.; Kengne-Ouafo, J.A.; Njiokou, F.; Prichard, R.K.; Wanji, S.; Kamgno, J.; Boussinesq, M. Reproductive Status of Onchocerca volvulus after Ivermectin Treatment in an Ivermectin-Naïve and a Frequently Treated Population from Cameroon. PLOS Neglected Trop. Dis. 2014, 8, e2824. [Google Scholar] [CrossRef]
- Milton, P.; Hamley, J.I.D.; Walker, M.; Basáñez, M.-G. Moxidectin: an oral treatment for human onchocerciasis. Expert Rev. Anti-infective Ther. 2020, 18, 1067–1081. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 2. Oral anti-infective drugs and drug combinations for off-label use. Parasites Vectors 2023, 16, 1–22. [Google Scholar] [CrossRef]
- Townson, S.; Ramirez, B.; Fakorede, F.; Mouries, M.-A.; Nwaka, S. Challenges in drug discovery for novel antifilarials. Expert Opin. Drug Discov. 2007, 2, S63–S73. [Google Scholar] [CrossRef]
- Townson, S.; Connelly, C.; Muller, R. Optimization of culture conditions for the maintenance of Onchocerca gutturosa adult worms in vitro. J. Helminthol. 1986, 60, 323–330. [Google Scholar] [CrossRef]
- Tagboto, S.; Orish, V. Drug development for onchocerciasis-the past, the present and the future. Front. Trop. Dis. 2022, 3. [Google Scholar] [CrossRef]
- Ehrens, A.; Hoerauf, A.; Hübner, M.P. Current perspective of new anti-Wolbachial and direct-acting macrofilaricidal drugs as treatment strategies for human filariasis. GMS Infect Dis, 2022. 10: p. Doc02.
- Panic, G.; Vargas, M.; Scandale, I.; Keiser, J. Activity Profile of an FDA-Approved Compound Library against Schistosoma mansoni. PLOS Neglected Trop. Dis. 2015, 9, e0003962. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Halliday, A.; Gentil, K.; Hoerauf, A.; Pearlman, E.; Taylor, M.J. Onchocerciasis: the Role of Wolbachia Bacterial Endosymbionts in Parasite Biology, Disease Pathogenesis, and Treatment. Clin. Microbiol. Rev. 2011, 24, 459–468. [Google Scholar] [CrossRef]
- Hoerauf, A. Filariasis: new drugs and new opportunities for lymphatic filariasis and onchocerciasis. Curr. Opin. Infect. Dis. 2008, 21, 673–681. [Google Scholar] [CrossRef]
- Johnston, K.L.; Ford, L.; Umareddy, I.; Townson, S.; Specht, S.; Pfarr, K.; Hoerauf, A.; Altmeyer, R.; Taylor, M.J. Repurposing of approved drugs from the human pharmacopoeia to target Wolbachia endosymbionts of onchocerciasis and lymphatic filariasis. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 278–286. [Google Scholar] [CrossRef]
- Johnston, K.L.; Cook, D. A. N.; Berry, N. G.; David Hong, W.; Clare, R. H.; Goddard, M.; Ford, L.; Nixon, G. L.; O'Neill, P. M.; Ward, S. A. Identification and prioritization of novel anti-Wolbachia chemotypes from screening a 10,000-compound diversity library. Sci Adv, 2017. 3(9): p. eaao1551.
- Townson, S.; Tagboto, S.; McGarry, H.F.; Egerton, G.L.; Taylor, M.J. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 2006, 5, 4. [Google Scholar] [CrossRef]
- Fenollar, F.; Maurin, M.; Raoult, D. Wolbachia pipientis Growth Kinetics and Susceptibilities to 13 Antibiotics Determined by Immunofluorescence Staining and Real-Time PCR. Antimicrob. Agents Chemother. 2003, 47, 1665–1671. [Google Scholar] [CrossRef]
- AAljayyoussi, G.; Tyrer, H.E.; Ford, L.; Sjoberg, H.; Pionnier, N.; Waterhouse, D.; Davies, J.; Gamble, J.; Metuge, H.; Cook, D.A.N.; et al. Short-Course, High-Dose Rifampicin Achieves Wolbachia Depletion Predictive of Curative Outcomes in Preclinical Models of Lymphatic Filariasis and Onchocerciasis. Sci. Rep. 2017, 7, 210. [Google Scholar] [CrossRef]
- Wanji, S.; Hoerauf, A.; Klarmann-Schulz, U. ISRCTN38954299 - The efficacy of rifampicin plus albendazole against river blindness (onchocerciasis) in Cameroon. Available online: https://www.isrctn.com/ISRCTN38954299 (accessed on 5 November 2023).
- Townson, S.; Freeman, A.; Harris, A.; Harder, A. Activity of the cyclooctadepsipeptide emodepside against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi.. Am J Trop Med Hyg, 2005. 73(6; Suppl. 1): p. abstract 28076.
- Hübner, M.P.; Townson, S.; Gokool, S.; Tagboto, S.; Maclean, M.J.; Verocai, G.G.; Wolstenholme, A.J.; Frohberger, S.J.; Hoerauf, A.; Specht, S.; et al. Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 27–35. [Google Scholar] [CrossRef]
- Kruecken, J.; Holden-Dye, L.; Keiser, J.; Prichard, R.K.; Townson, S.; Makepeace, B.L.; Huebner, M.P.; Hahnel, S.R.; Scandale, I.; Harder, A.; et al. Development of emodepside as a possible adulticidal treatment for human onchocerciasis-The fruit of a successful industrial-academic collaboration. PLOS Pathog. 2021, 17, e1009682. [Google Scholar] [CrossRef]
- Gillon, J.; Dennison, J.; Berg, F.v.D.; Delhomme, S.; Cheeseman, K.D.; Rossi, C.P.; Wourgaft, N.S.; Specht, S.; Pedrique, B.; Monnot, F.; et al. Safety, tolerability and pharmacokinetics of emodepside, a potential novel treatment for onchocerciasis (river blindness), in healthy male subjects. Br. J. Clin. Pharmacol. 2021, 87, 3949–3960. [Google Scholar] [CrossRef]
- DNDi. Available online: https://dndi.org/research-development/portfolio/emodepside/ (accessed on 15 October 2023).
- Hübner, M.P.; Martin, C.; Specht, S.; Koschel, M.; Dubben, B.; Frohberger, S.J.; Ehrens, A.; Fendler, M.; Struever, D.; Mitre, E.; et al. Oxfendazole mediates macrofilaricidal efficacy against the filarial nematode Litomosoides sigmodontis in vivo and inhibits Onchocerca spec. motility in vitro. PLOS Neglected Trop. Dis. 2020, 14, e0008427. [Google Scholar] [CrossRef]
- Risch, F.; Koschel, M.; Lenz, B.; Specht, S.; Hoerauf, A.; Hübner, M. P.; Scandale, I. Comparison of the macrofilaricidal efficacy of oxfendazole and its isomers againt the rodent filarial Litomosoides sigmodontis. Frontiers in Trop Dis, 2022. 3:982421.
- Risch, F.; Scheunemann, J.F.; Reichwald, J.J.; Lenz, B.; Ehrens, A.; Gal, J.; Fercoq, F.; Koschel, M.; Fendler, M.; Hoerauf, A.; et al. The efficacy of the benzimidazoles oxfendazole and flubendazole against Litomosoides sigmodontis is dependent on the adaptive and innate immune system. Front. Microbiol. 2023, 14, 1213143. [Google Scholar] [CrossRef]
- Bach, T.; Galbiati, S.; Kennedy, J.K.; Deye, G.; Nomicos, E.Y.H.; Codd, E.E.; Garcia, H.H.; Horton, J.; Gilman, R.H.; Gonzalez, A.E.; et al. Pharmacokinetics, Safety, and Tolerability of Oxfendazole in Healthy Adults in an Open-Label Phase 1 Multiple Ascending Dose and Food Effect Study. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- eWHORM. Available online: https://ewhorm.org/ (accessed on 5 November 2023).
- Townson, S.; Connelly, C.; Dobinson, A.; Muller, R. Drug activity against Onchocerca gutturosa males in vitro: a model for chemotherapeutic research on onchocerciasis. J. Helminthol. 1987, 61, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Strote, G.; Wieland, S.; Darge, K.; Comley, J. C. In vitro assessment of the activity of anthelmintic compounds on adults of Onchocerca volvulus. Acta Leiden, 1990. 59(1-2): p. 285-96.
- Dominguez-Vazquez, A.; Taylor, H.R.; Greene, B.M.; Ruvalcaba-Macias, A.M.; Rivas-Alcala, A.R.; Murphy, R.P.; Beltran-Hernandez, F. Comparison of flubendazole and diethylcarbamazine in treatment of onchocerciasis. Lancet 1983, 1, 139–143. [Google Scholar] [CrossRef]
- Mackenzie, C.D.; Geary, T.G. Flubendazole: a candidate macrofilaricide for lymphatic filariasis and onchocerciasis field programs. Expert Rev. Anti-infective Ther. 2011, 9, 497–501. [Google Scholar] [CrossRef]
- Hübner, M.P.; Ehrens, A.; Koschel, M.; Dubben, B.; Lenz, F.; Frohberger, S.J.; Specht, S.; Quirynen, L.; Lachau-Durand, S.; Tekle, F.; et al. Macrofilaricidal efficacy of single and repeated oral and subcutaneous doses of flubendazole in Litomosoides sigmodontis infected jirds. PLOS Neglected Trop. Dis. 2019, 13, e0006320. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Kale, O. Small-scale trials of six drugs against Onchocerca volvulus. Tropenmed Parasitol, 1978. 29(2): p. 163-7.
- Kura, K.; Milton, P.; Hamley, J. I. D.; Walker, M.; Bakajika, D. K.; Kanza, E. M.; Opoku, N. O.; Howard, H.; Nigo, M. M.; Asare, S. Can mass drug administration of moxidectin accelerate onchocerciasis elimination in Africa? Philos Trans R Soc Lond B Biol Sci, 2023. 378(1887): p. 20220277.
- Baggish, A.L.; Hill, D.R. Antiparasitic Agent Atovaquone. Antimicrob. Agents Chemother. 2002, 46, 1163–1173. [Google Scholar] [CrossRef]
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Sci. Rep. 2020, 10, 17872. [Google Scholar] [CrossRef] [PubMed]
| Bioactivity | Drug |
|---|---|
|
Antibacterial |
Carbadox; Cefaclor; Cefamandole nafate; Cefoperazone; Cefoxitin sodium; Cefsulodin sodium; Ceftibuten; Cefuroxime sodium; Chlorhexidine dihydrochloride; Chloroxylenol; Demeclocycline hydrochloride; Doxycycline hydrochloride; Furazolidone; Gramicidin; Lasalocid sodium; Merbromin; Methacycline hydrochloride; Methenamine; Minocycline hydrochloride; Nitrofurantoin; Nitroxoline; Ofloxacin; Oxytetracycline; Rifampcin; Rifaximin; Sulfaquinoxaline sodium; Teicoplanin |
|
Anticancer |
Azaserine; Bleomycin; Daunorubicin; Doxorubicin; Epirubicin hydrochloride; Isotretinon; Lomustine; Mitoxantrone hydrochloride; Tretinoin |
|
Antihypertensive/ vasodilator |
Dipyridamole; Guanethidine; Losartan; Nicardipine hydrochloride; Nicotinyl alcohol tartrate; Nifedipine |
|
Antiinfective |
Benzethonium chloride; Broxyquinoline; Dequalinium chloride; Methylbenzethonium chloride; Nitrofurazone; Oxyquinoline hemisulfate; Phenylethyl alcohol; Resorcinol monoacetate |
|
Antiinflammatory/ antihistamine |
Dexamethasone acetate; Doxylamine succinate; Meloxicam sodium; Prednisolone tebutate; Sulfasalazine |
|
Antiparasitic |
Amitraz; Atovaquone; Candicidin; Clorsulon; Diethylcarbamazine citrate; Flubendazole; Hexetidine; Homidium bromide; Iodoquinol; Levamisole hydrochloride; Moxidectin; Primaquine phosphate; Pyrantel pamoate; |
|
Antiviral |
Oseltamivir phosphate; Valganciclovir hydrochloride |
|
Neurological |
Acepromazine maleate; Almotriptan; Ampyzine sulfate; Apomorphine hydrochloride; Armodafinil; Bupropion; Chlorpromazine; Danazol; Desipramine hydrochloride; Dopamine hydrochloride; Isradipine (also antihypertensive/vasodilator) ; Methsuximide; Methylphenidate hydrochloride; Olanzapine; Oxidopamine hydrochloride; Penfluridol; Rivastigmine tartrate; Selegiline hydrochloride; Zaleplon |
|
Various |
Alendronate sodium; Anisindione; Ascorbyl palmitate; Bromhexine hydrochloride; Butacaine; β-Carotene; Clopidogrel sulfate; Dienestrol; Dioxybenzone; Docusate sodium; Fluorescein; Mangafodipir trisodium; Methylergonovine maleate; Propoxycaine hydrochloride; Riboflavin; Sennoside A; Tetrahydrozoline hydrochloride |
| DRUG | Mot Redn | MTT Redn | ||
|---|---|---|---|---|
| % | p-value | % | p-value | |
| IMMITICIDE (positive control) | 100.00 | - | 91.09 | - |
| ACEPROMAZINE MALEATE | 53.57 | <0.0001 | 57.67 | <0.001 |
| AMITRAZ | 89.29 | <0.0001 | 77.23 | <0.0001 |
| AMPYZINE SULFATE | 78.57 | <0.0001 | 76.98 | <0.0001 |
| APOMORPHINE HYDROCHLORIDE | 78.57 | <0.0001 | 76.49 | <0.0001 |
| ARMODAFINIL | 82.14 | <0.0001 | 84.65 | <0.0001 |
| ASCORBYL PALMITATE | 57.14 | <0.0001 | 67.08 | <0.001 |
| BLEOMYCIN | 53.57 | <0.0001 | 53.22 | <0.01 |
| BROMHEXINE HYDROCHLORIDE | 57.14 | <0.0001 | 56.93 | <0.01 |
| BROXYQUINOLINE | 82.14 | <0.0001 | 82.18 | <0.0001 |
| CANDICIDIN | 67.86 | <0.0001 | 72.03 | <0.001 |
| CARBADOX | 60.71 | <0.0001 | 66.83 | <0.001 |
| CEFSULODIN SODIUM | 50.00 | <0.0001 | 21.04 | 0.18 |
| CEFTIBUTEN | 75.00 | <0.0001 | 71.53 | <0.001 |
| CEFUROXIME SODIUM | 92.86 | <0.0001 | 79.70 | <0.0001 |
| CHLORPROMAZINE | 82.14 | <0.0001 | 79.46 | <0.0001 |
| CLOPIDOGREL SULFATE | 28.57 | <0.01 | 53.47 | <0.01 |
| DEMECLOCYCLINE HYDROCHLORIDE | 82.14 | <0.0001 | 76.24 | <0.001 |
| DEXAMETHASONE ACETATE | 78.57 | <0.0001 | 61.14 | <0.001 |
| DIENESTROL | 78.57 | <0.0001 | 75.74 | <0.0001 |
| DOCUSATE SODIUM | 75.00 | <0.0001 | 66.58 | <0.001 |
| DOPAMINE HYDROCHLORIDE | 82.14 | <0.0001 | 69.55 | <0.001 |
| DOXORUBICIN | 60.71 | <0.0001 | 64.36 | <0.001 |
| DOXYCYCLINE HYDROCHLORIDE | 53.57 | <0.0001 | 37.13 | <0.05 |
| EPIRUBICIN HYDROCHLORIDE | 75.00 | <0.0001 | 77.48 | <0.0001 |
| FLUORESCEIN | 50.00 | <0.001 | 45.54 | <0.01 |
| GUANETHIDINE | 78.57 | <0.0001 | 79.95 | <0.0001 |
| MANGAFODIPIR TRISODIUM | 89.29 | <0.0001 | 76.49 | <0.0001 |
| METHENAMINE | 92.86 | <0.0001 | 78.47 | <0.0001 |
| METHSUXIMIDE | 71.43 | <0.0001 | 66.34 | <0.001 |
| METHYLERGONOVINE MALEATE | 57.14 | <0.0001 | 55.45 | <0.001 |
| METHYLPHENIDATE HYDROCHLORIDE | 75.00 | <0.0001 | 88.12 | <0.0001 |
| MINOCYCLINE HYDROCHLORIDE | 78.57 | <0.0001 | 72.52 | <0.001 |
| MOXIDECTIN | 78.57 | <0.0001 | 71.78 | <0.001 |
| NICOTINYL ALCOHOL TARTRATE | 67.86 | <0.0001 | 70.30 | <0.001 |
| NIFEDIPINE | 92.86 | <0.0001 | 82.92 | <0.0001 |
| PREDNISOLONE TEBUTATE | 96.43 | <0.0001 | 82.18 | <0.0001 |
| PRIMAQUINE PHOSPHATE | 92.86 | <0.0001 | 84.16 | <0.0001 |
| PROPOXYCAINE HYDROCHLORIDE | 60.71 | <0.0001 | 60.15 | <0.001 |
| RIBOFLAVIN | 60.71 | <0.0001 | 56.19 | <0.01 |
| RIFAMPIN | 57.14 | <0.0001 | 56.44 | <0.01 |
| RIVASTIGMINE TARTRATE | 92.86 | <0.0001 | 81.93 | <0.001 |
| SENNOSIDE A | 53.57 | <0.0001 | 52.23 | <0.01 |
| TETRAHYDROZOLINE HYDROCHLORIDE | 53.57 | <0.001 | 53.22 | <0.01 |
| VALGANCICLOVIR HYDROCHLORIDE | 50.00 | <0.0001 | 53.71 | <0.01 |
| Drug (Molecular weight/bioactivity) | Molecular Structure | Trial 1 | Trial 2 | |||
|---|---|---|---|---|---|---|
| Mot Redn (%) | MTT Redn (%) | p-value | Mot Redn (%) | MTT Redn (%) | ||
| IMMITICIDE, positive control (501.34/Anthelmintic) |
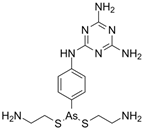 |
100.00 | 91.09 | - | 100.00 | Range 77.19- 98.88 |
| ATOVAQUONE (366.85/Antimalarial) |
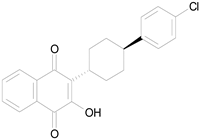 |
100.00 | 90.10 | <0.0001 | 100.00 | 74.42 |
| BENZETHONIUM CHLORIDE (448.09/Antiinfective) |
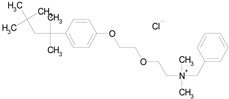 |
100.00 | 88.12 | <0.0001 | 100.00 | 71.91 |
| CHLORHEXIDINE DIHYDROCHLORIDE (578.38/Antibacterial) | 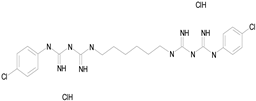 |
100.00 | 90.10 | <0.0001 | 100.00 | 100.00 |
| GRAMICIDIN, gramicidin A shown (1882.34/Antibacterial) |
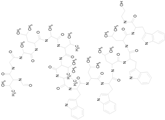 |
100.00 | 76.24 | <0.0001 | 100.00 | 84.27 |
| IODOQUINOL (396.96/Antiamoebic) |
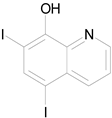 |
100.00 | 80.20 | <0.0001 | 100.00 | 87.64 |
| ISRADIPINE (371.40/Calcium channel blocker) |
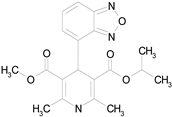 |
100.00 | 87.13 | <0.0001 | 100.00 | 78.95 |
| LASALOCID SODIUM (612.79/Antibacterial) |
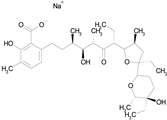 |
100.00 | 89.11 | <0.0001 | 73.33 | 90.70 |
| LEVAMISOLE HYDROCHLORIDE (240.76/Anthelmintic) |
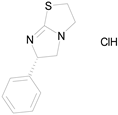 |
100.00 | 92.08 | <0.0001 | 100.00 | 55.81 |
| LOSARTAN (422.92/Antihypertensive) |
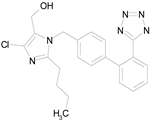 |
100.00 | 88.12 | <0.0001 | 100.00 | 82.56 |
| MELOXICAM SODIUM (373.39/Antiinflammatory) |
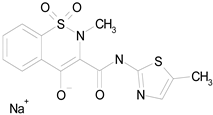 |
100.00 | 89.11 | <0.0001 | 100.00 | 47.67 |
|
METHYLBENZETHONIUM CHLORIDE (462.12/Antiinfective) |
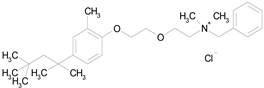 |
100.00 | 91.09 | <0.0001 | 100.00 | 67.44 |
| MITOXANTRONE HYDROCHLORIDE (517.41/Antineoplastic) |
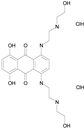 |
100.00 | 91.09 | <0.0001 | 100.00 | 70.93 |
| NITROFURANTOIN (238.16/Antibacterial) |
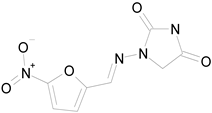 |
100.00 | 75.25 | <0.0001 | 100.00 | 92.70 |
| NITROFURAZONE (198.14/Antibacterial) |
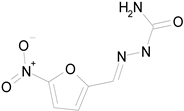 |
100.00 | 85.15 | <0.0001 | 100.00 | 95.51 |
| OXYQUINOLINE HEMISULFATE (243.24/Antiinfective) |
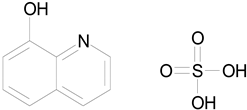 |
100.00 | 86.14 | <0.0001 | 100.00 | 91.57 |
| PYRANTEL PAMOATE (594.69/Anthelmintic) |
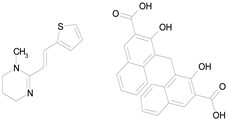 |
100.00 | 85.15 | <0.0001 | 100.00 | 90.45 |
| RIFAXIMIN (785.90/Antibacterial) |
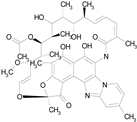 |
100.00 | 91.09 | <0.0001 | 100.00 | 68.60 |
| CEFACLOR (367.81/Antibacterial) |
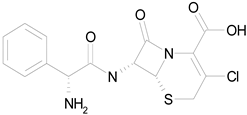 |
nd | nd | nd | 100.00 | 96.51 |
| DEQUALINIUM CHLORIDE (527.59/Antiinfective) |
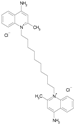 |
nd | nd | nd | 100.00 | 61.40 |
| HEXETIDINE (339.61/Antifungal) |
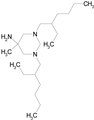 |
nd | nd | nd | 100.00 | 88.60 |
| HOMIDIUM BROMIDE (394.32/Antiprotozoal) |
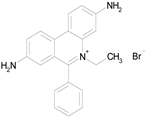 |
nd | nd | nd | 100.00 | 81.58 |
| NITROXOLINE (190.16/Antibacterial) |
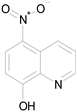 |
nd | nd | nd | 100.00 | 82.46 |
| PENFLURIDOL (523.98/Antipsychotic) |
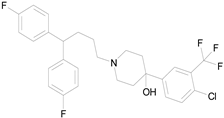 |
nd | nd | nd | 100.00 | 64.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





