Submitted:
22 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theory
3. Computation
4. Results and Discussions
4.1. General information of the atomic data
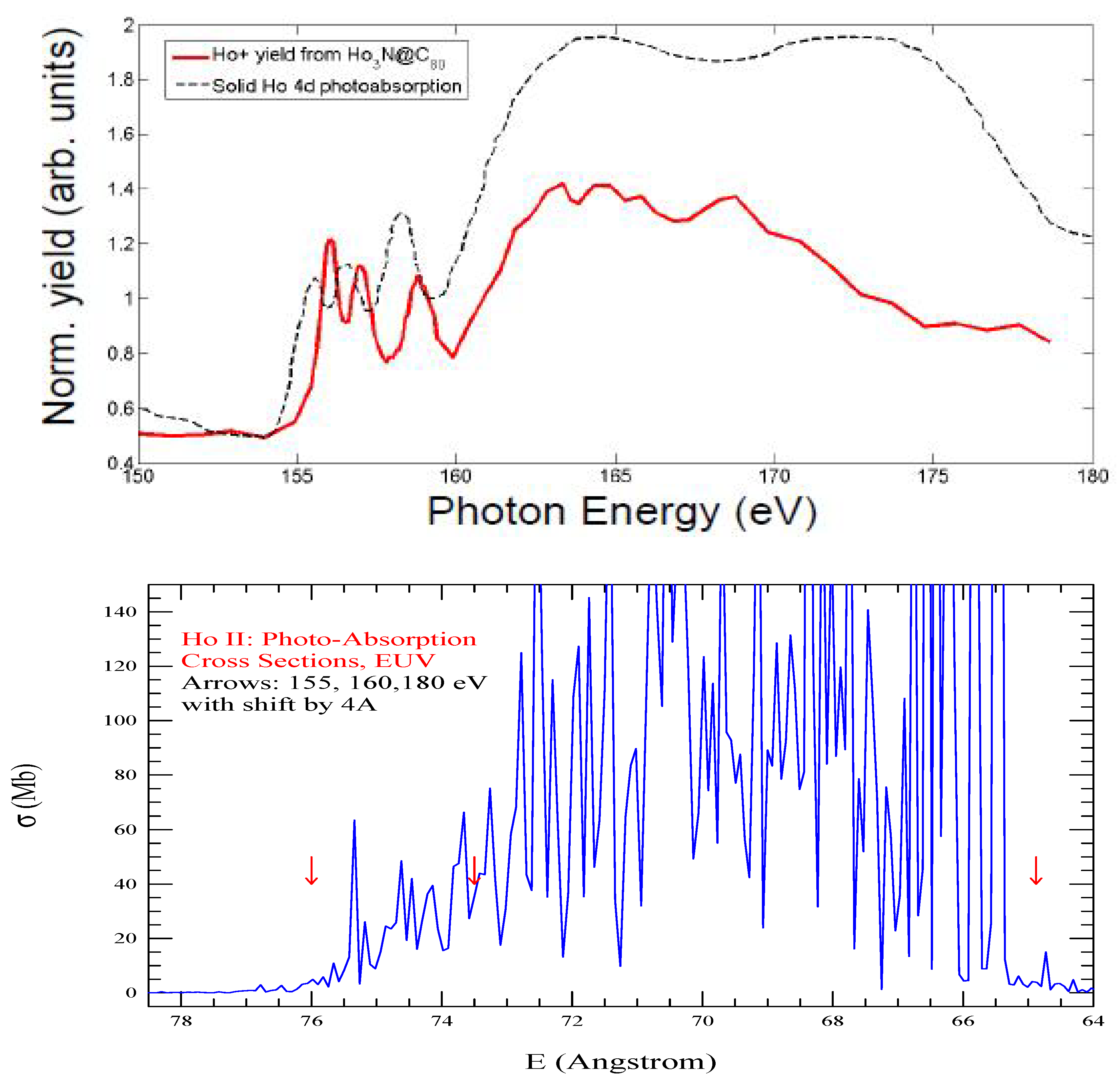
4.2. Features of the lanthanide ions
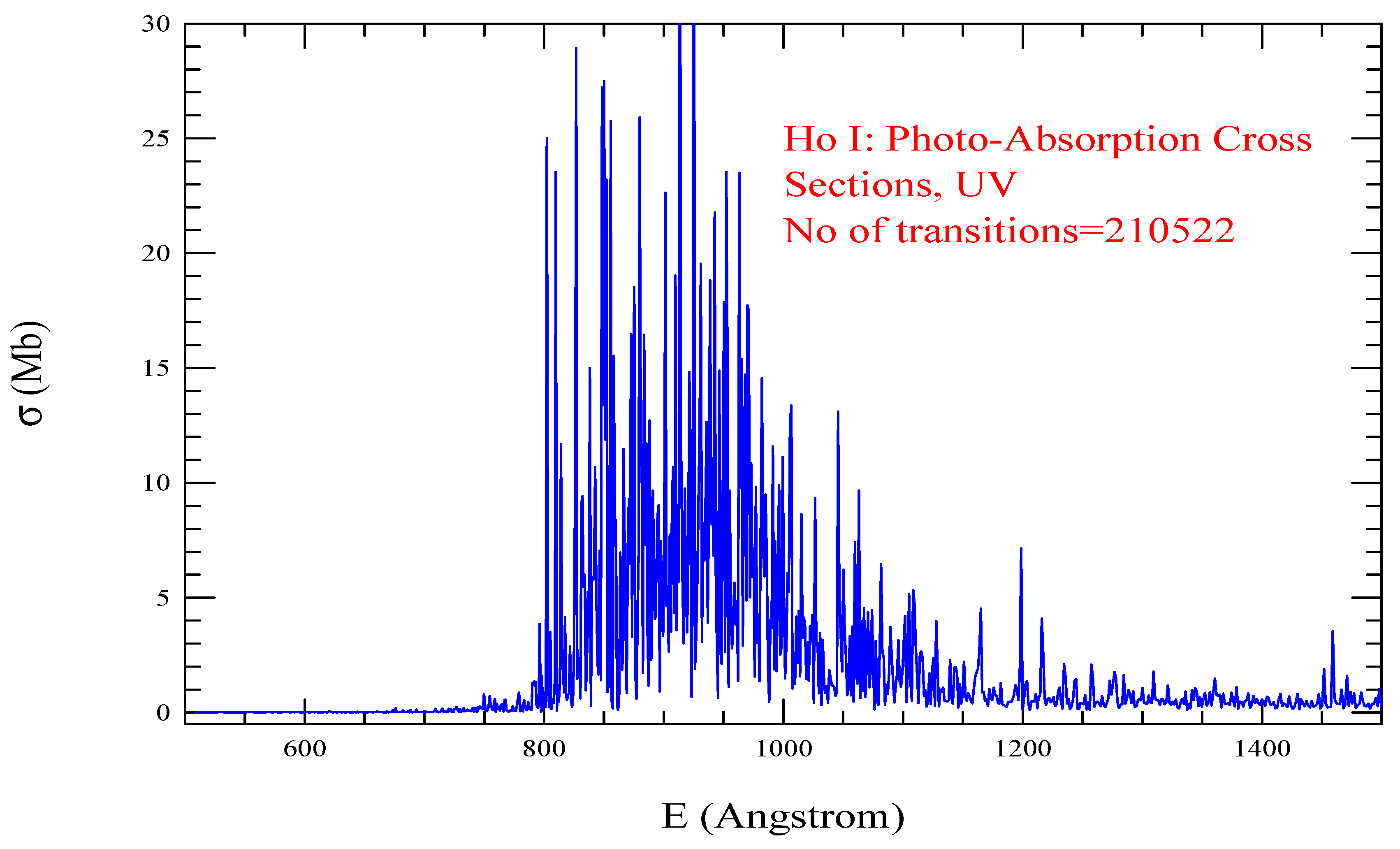
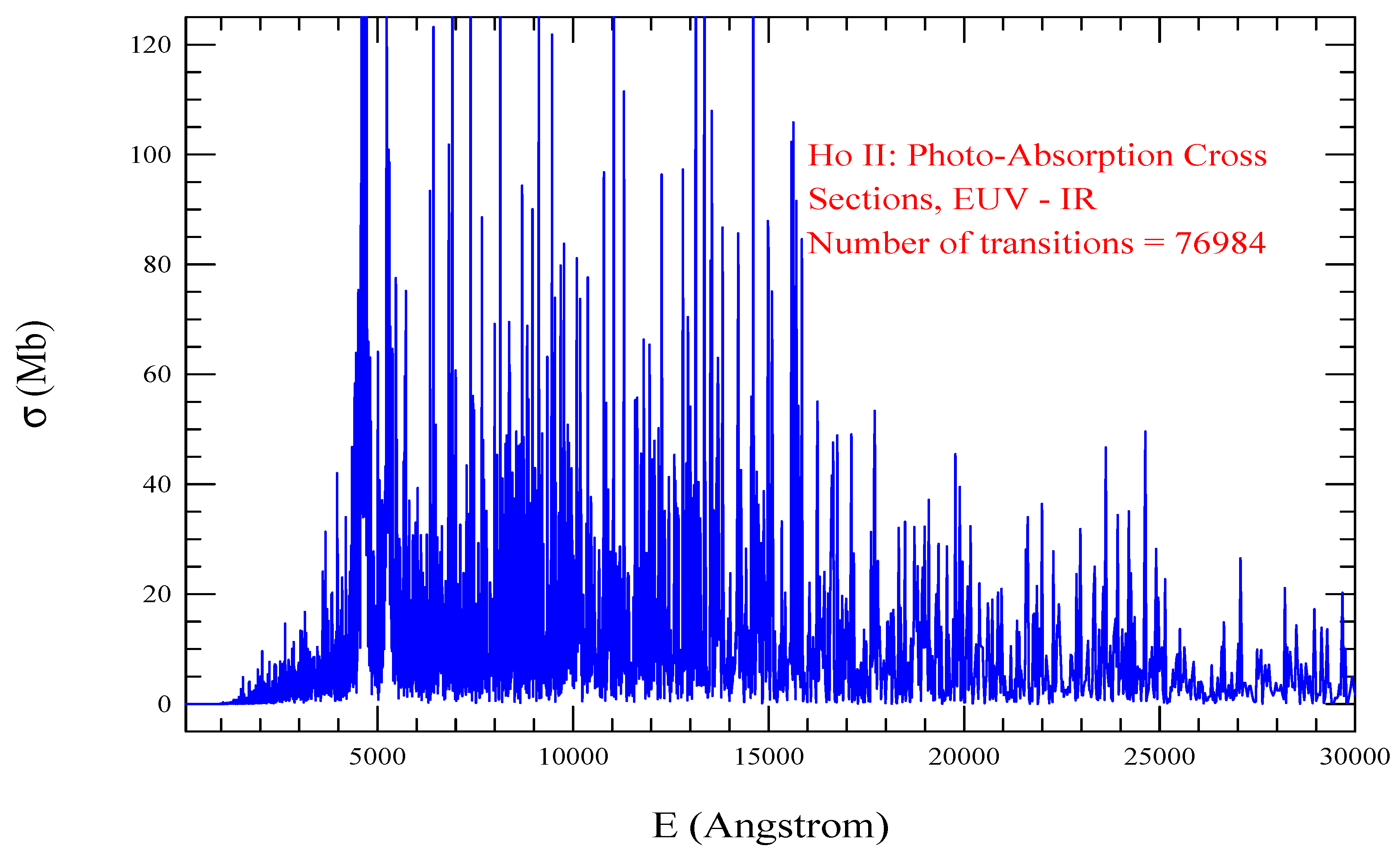
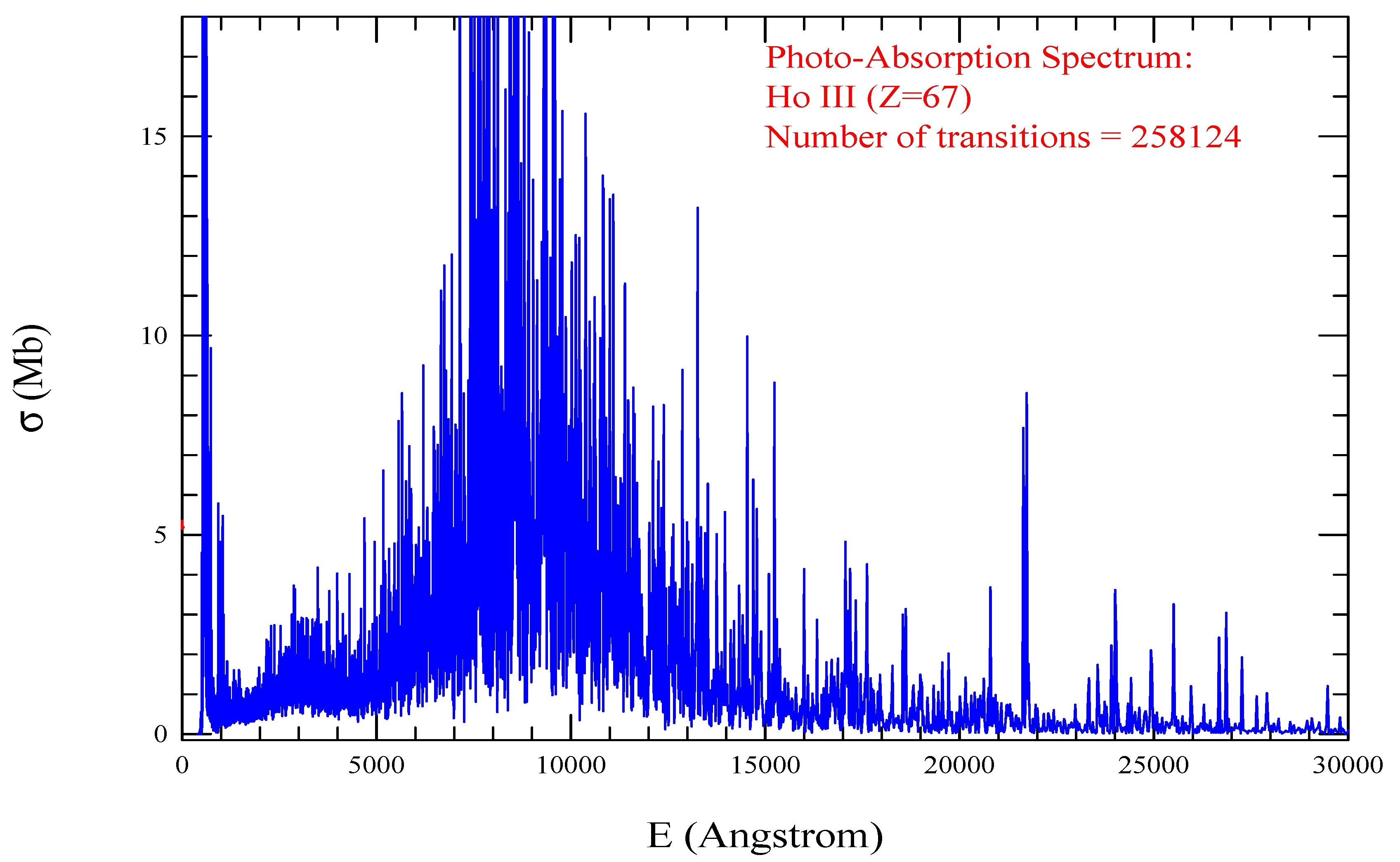
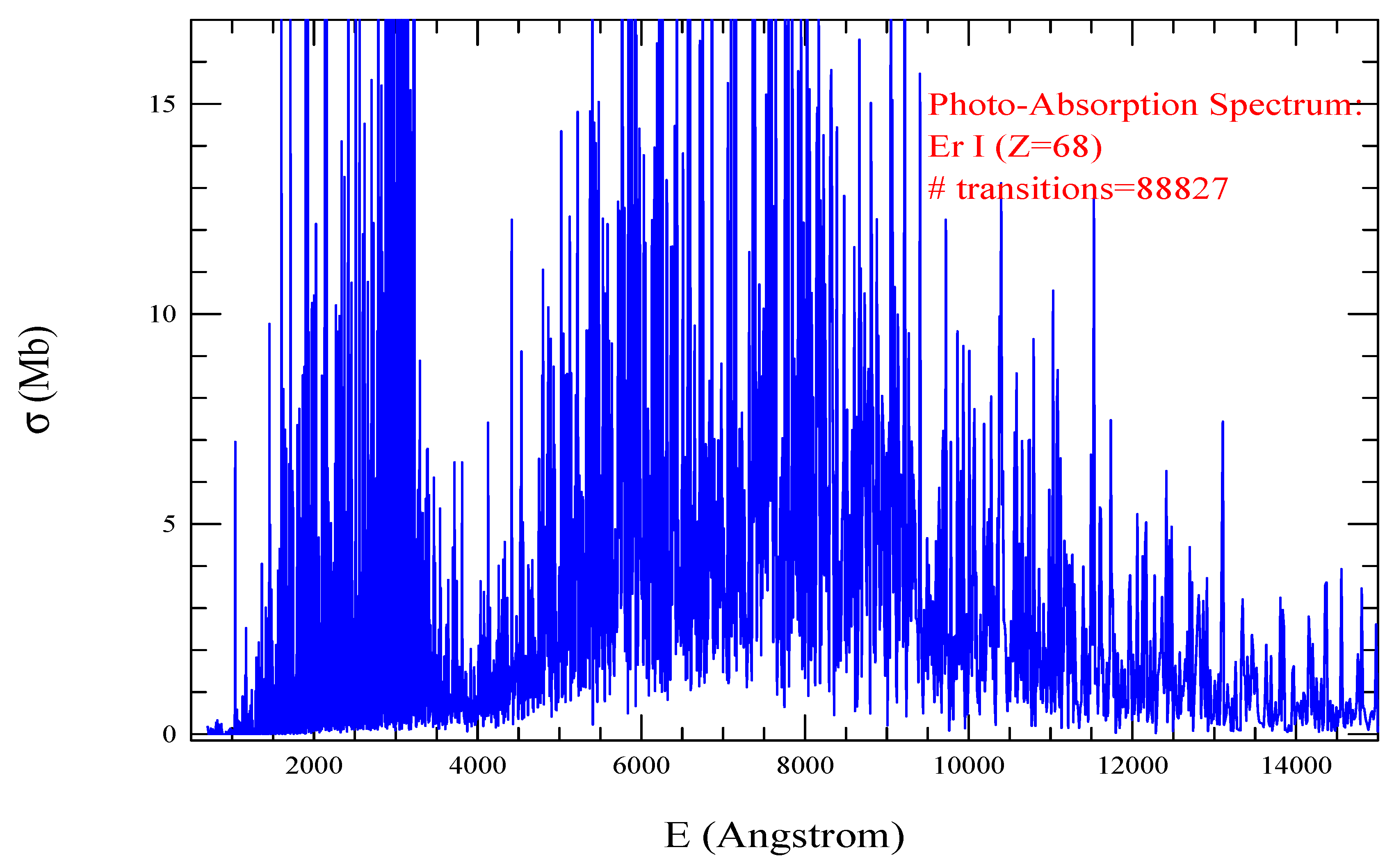
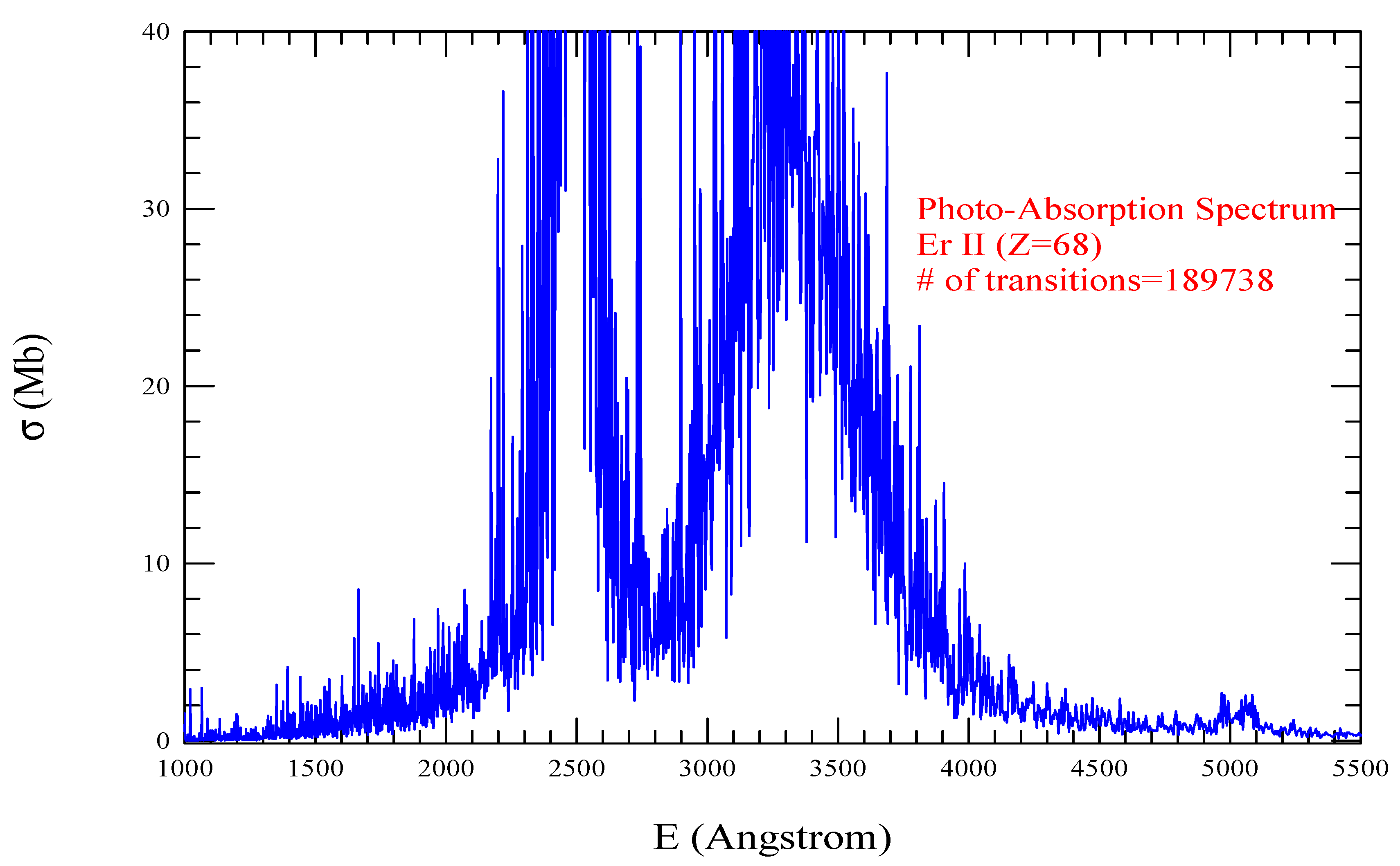
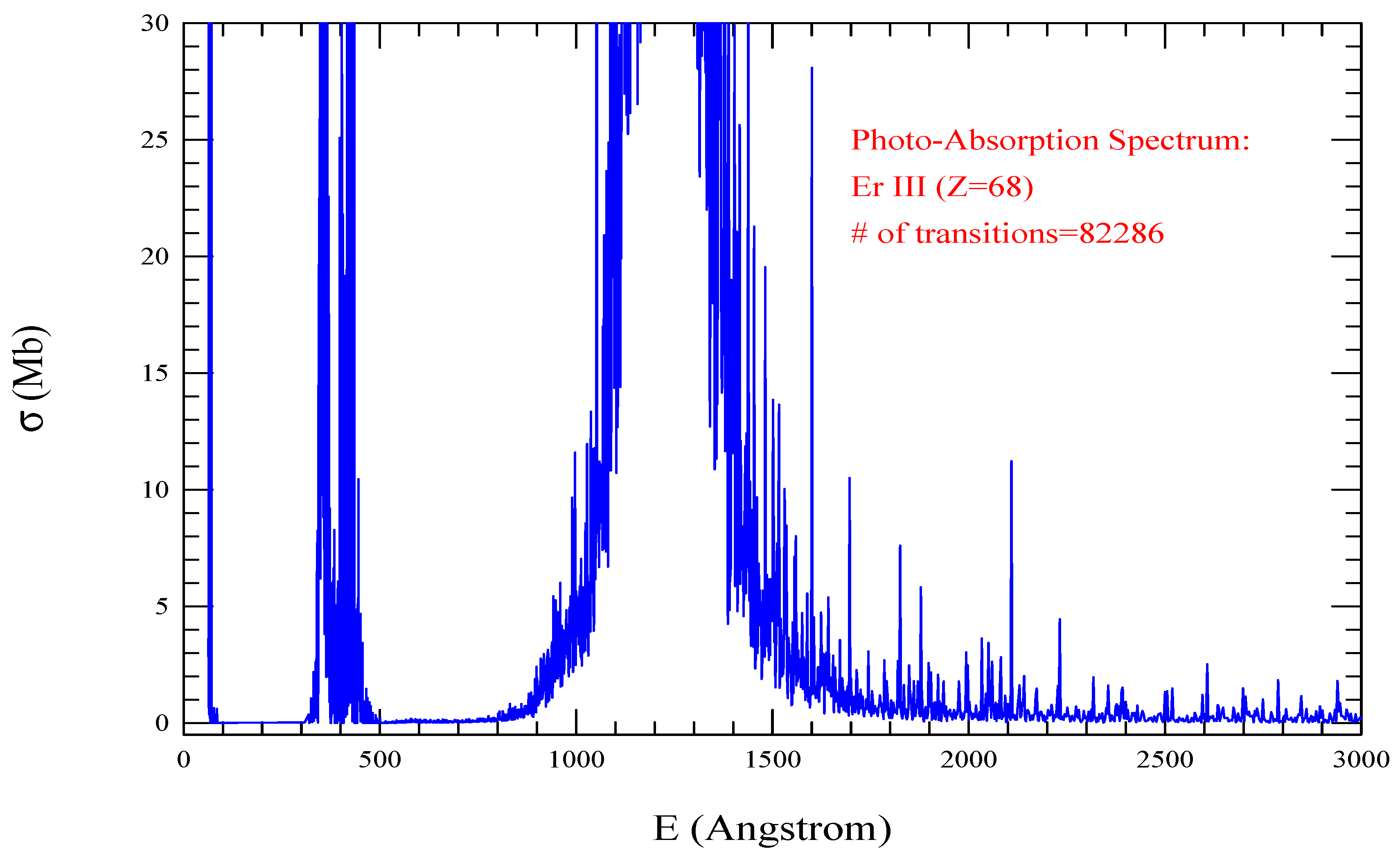
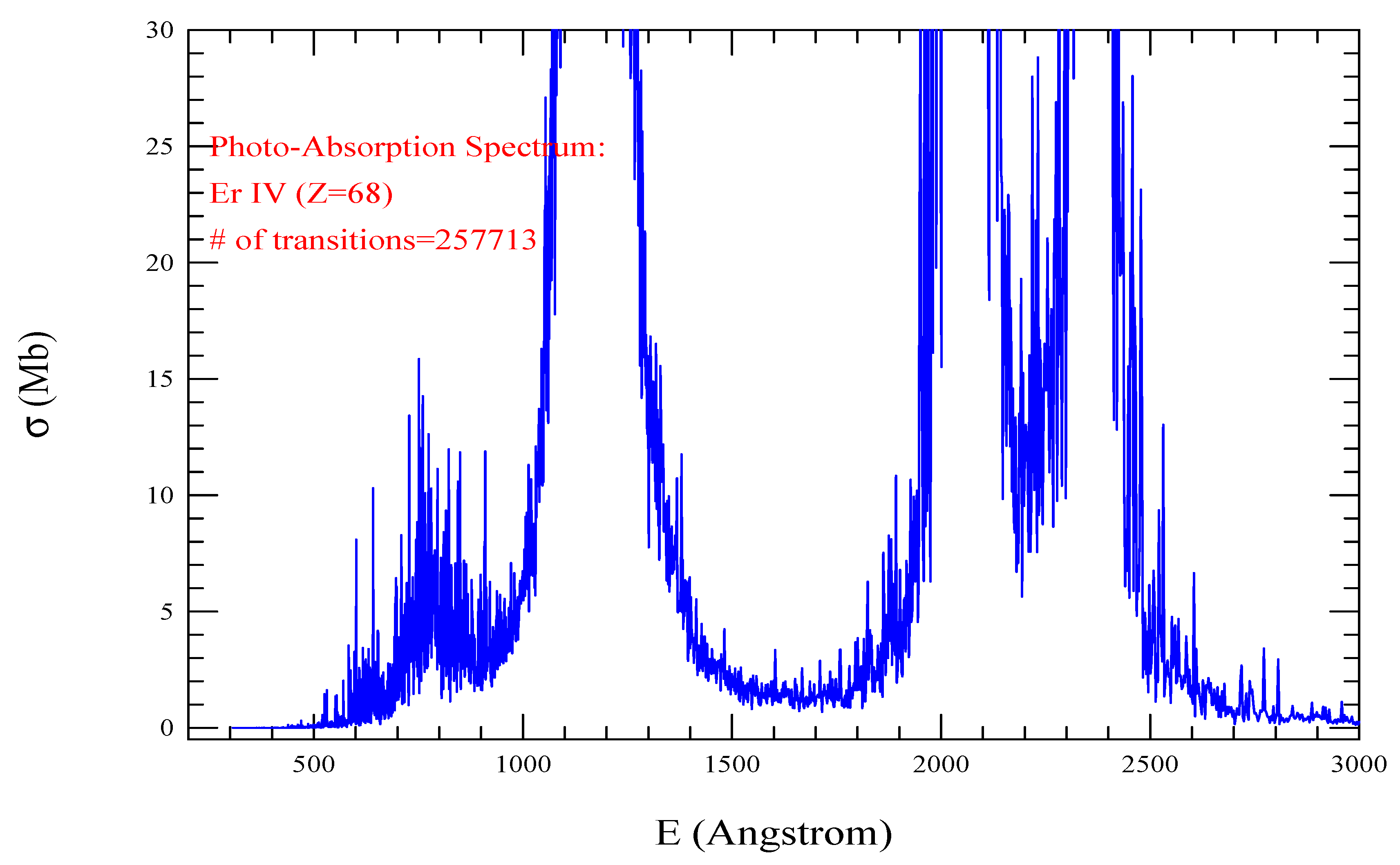

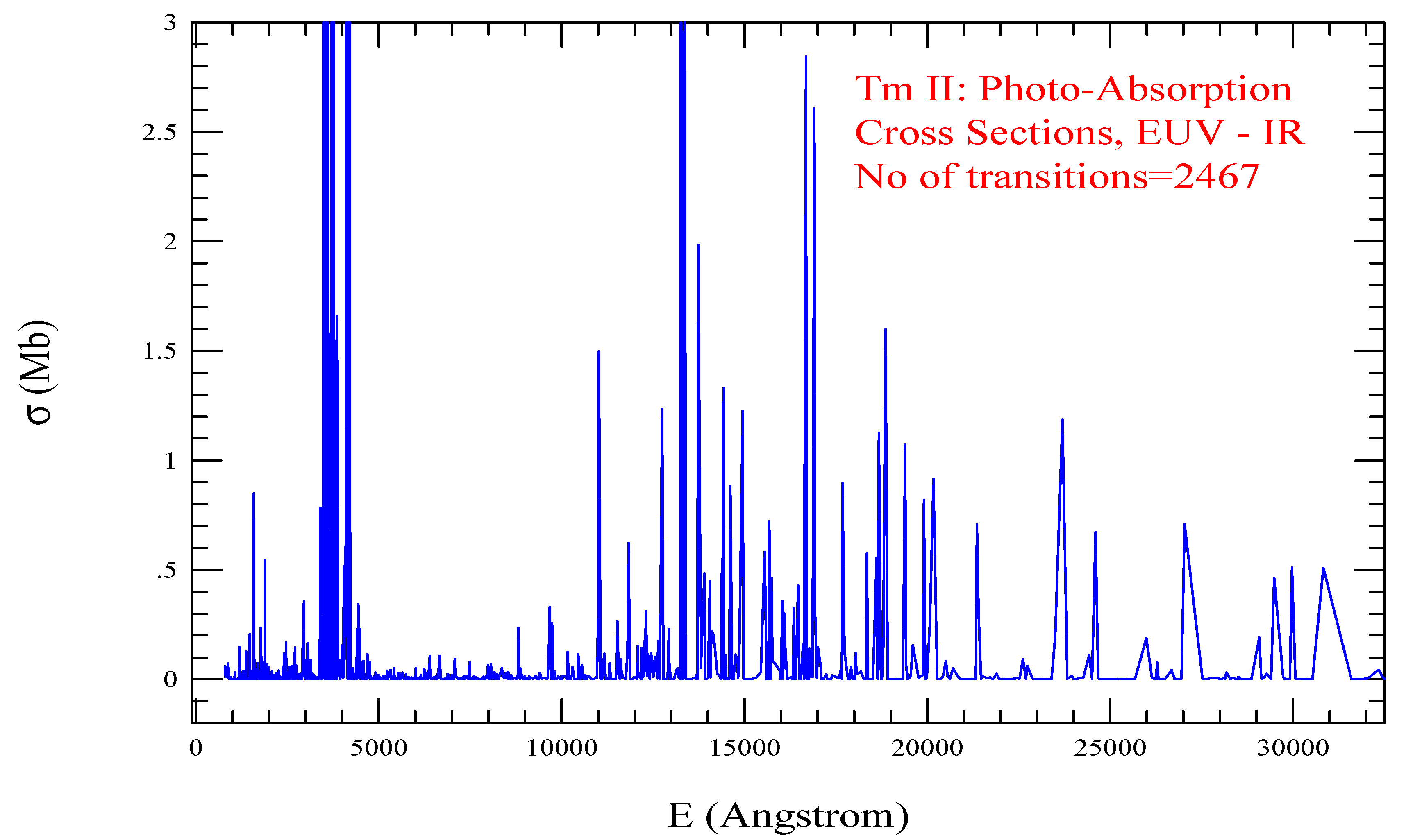
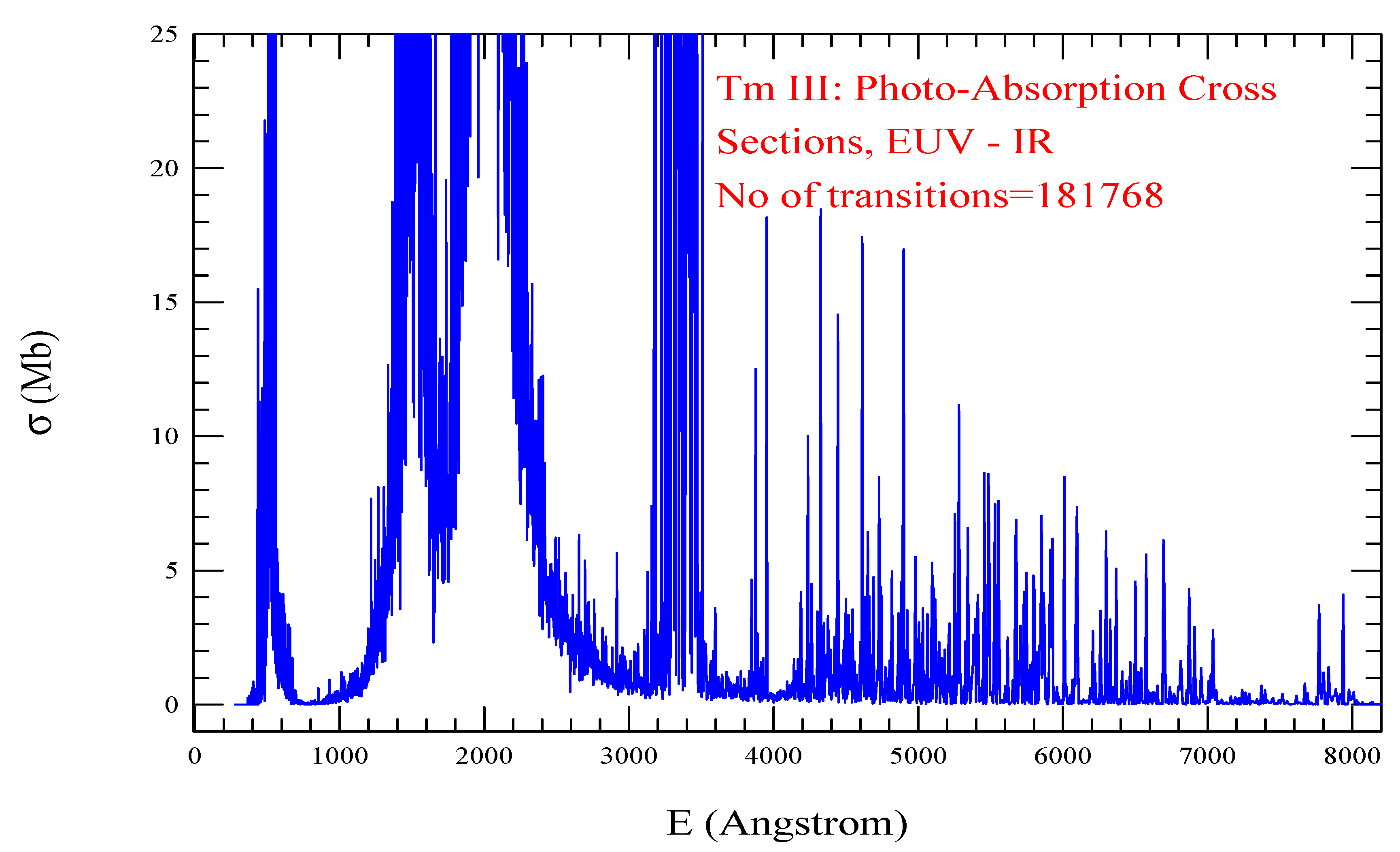
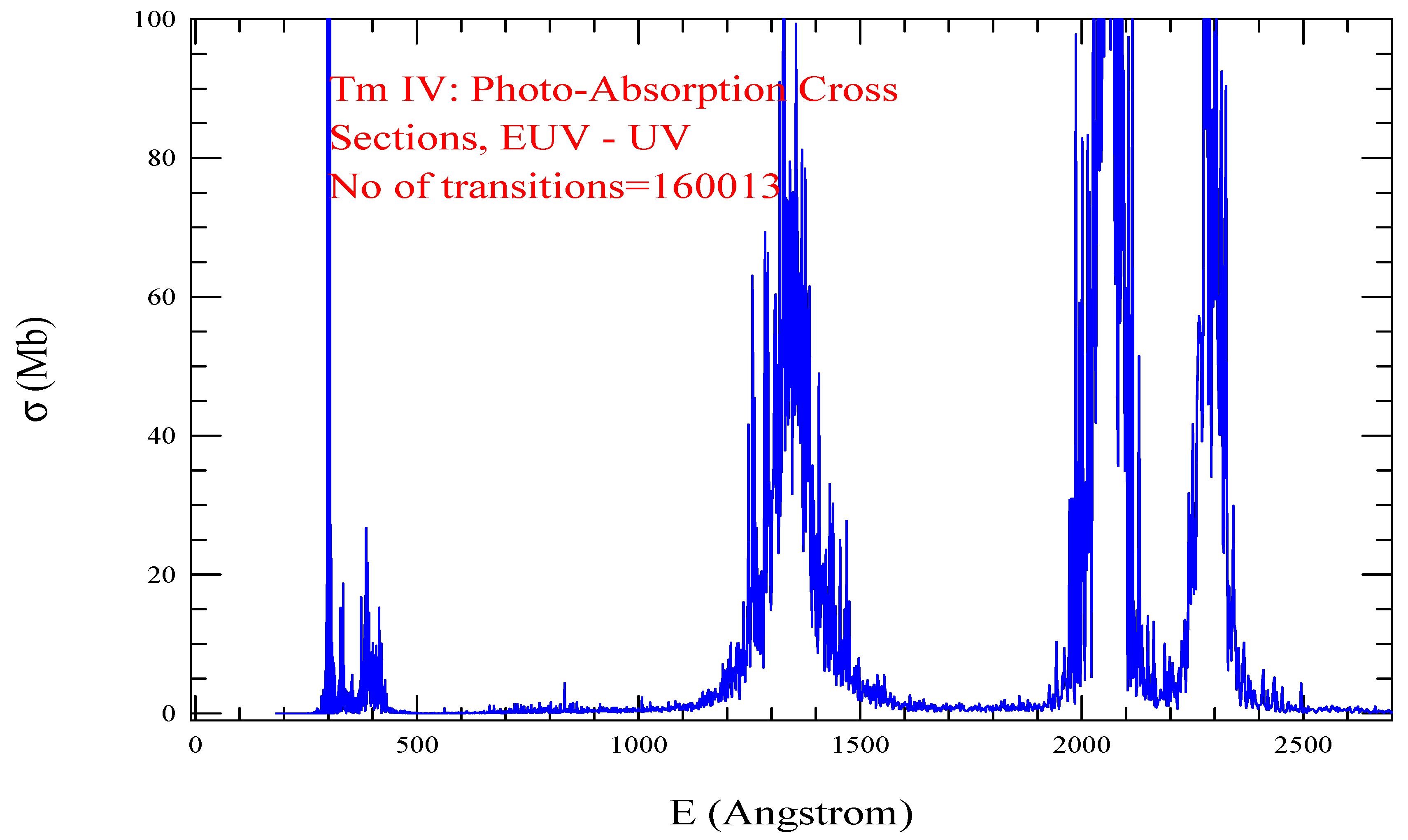
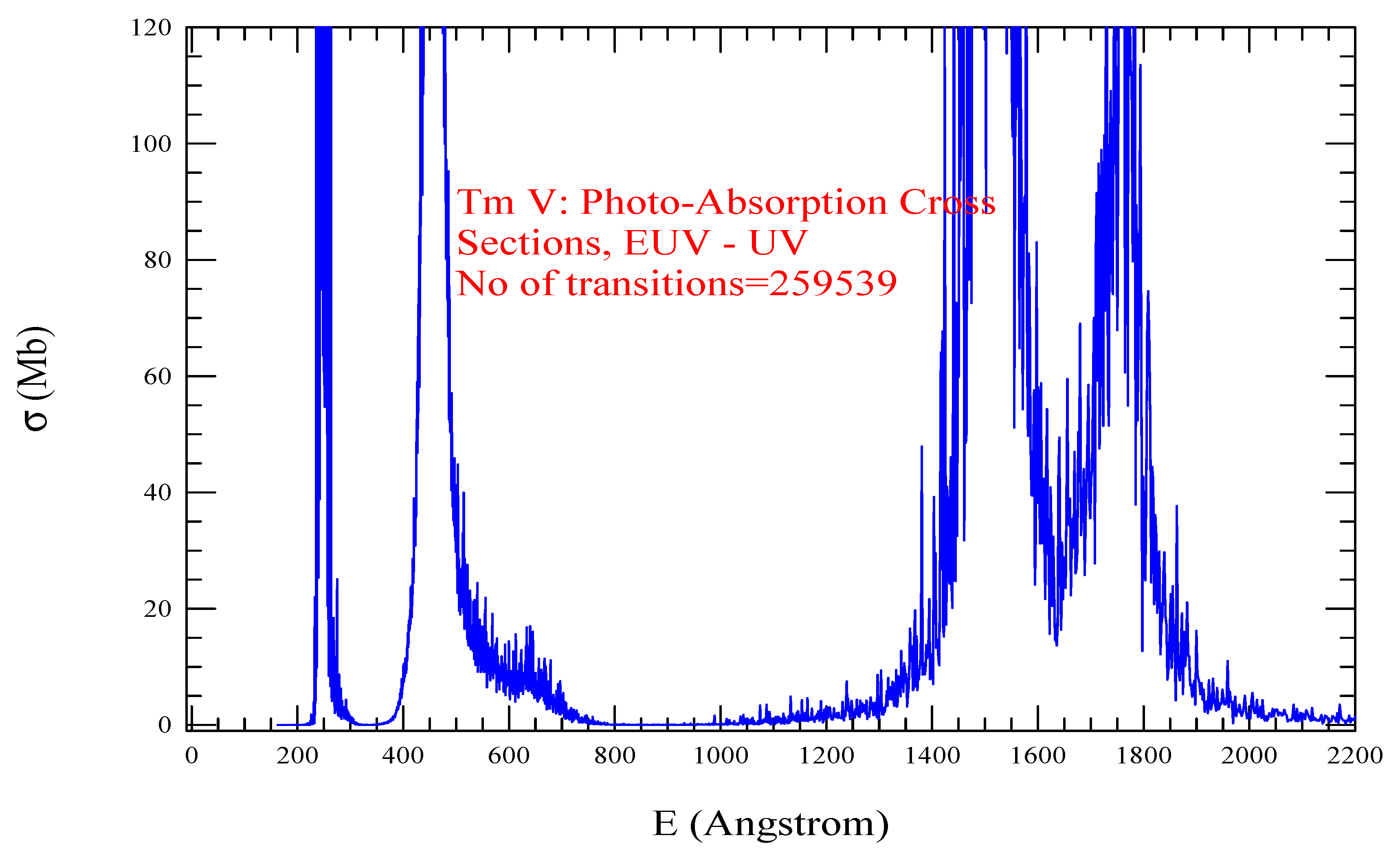
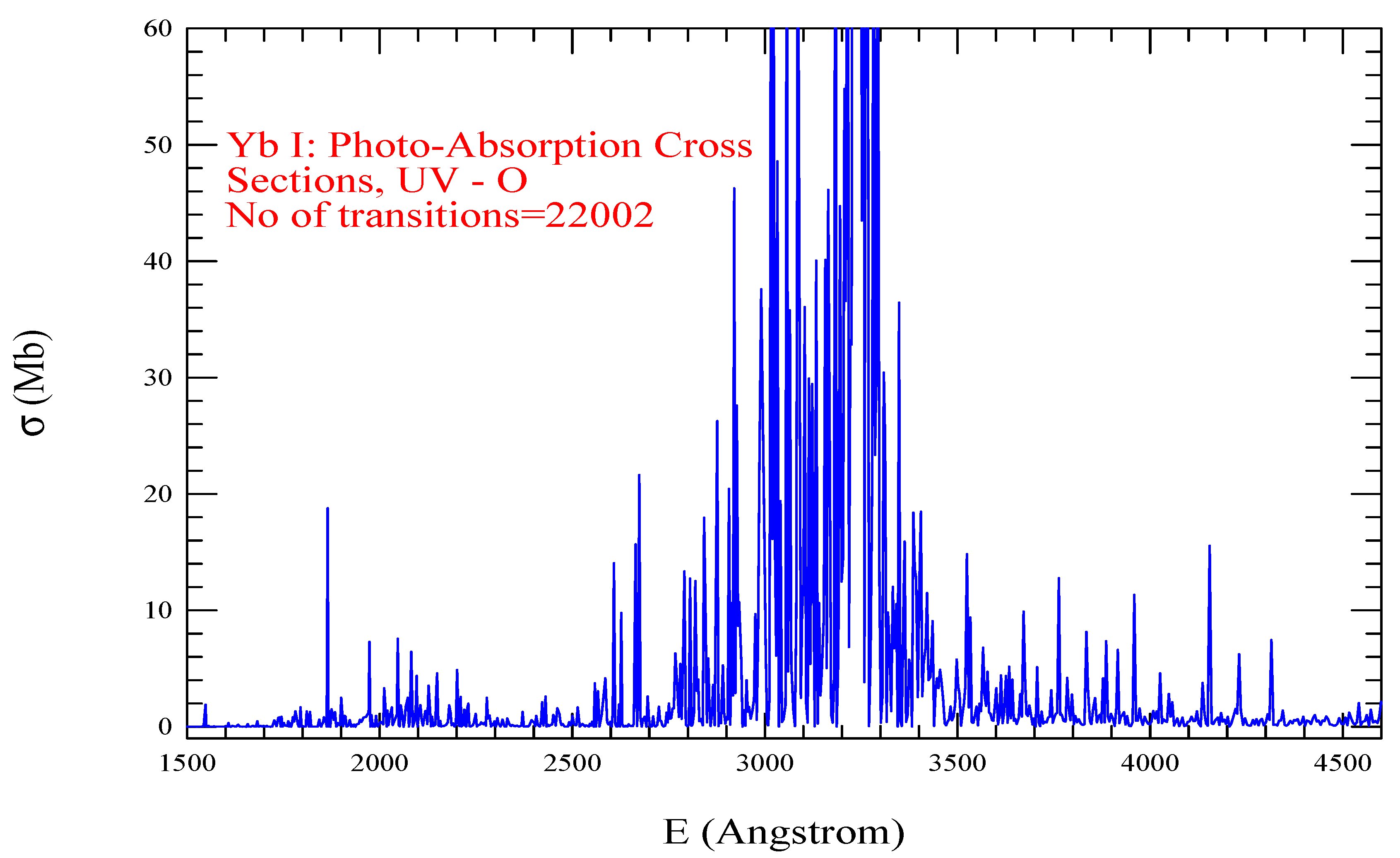
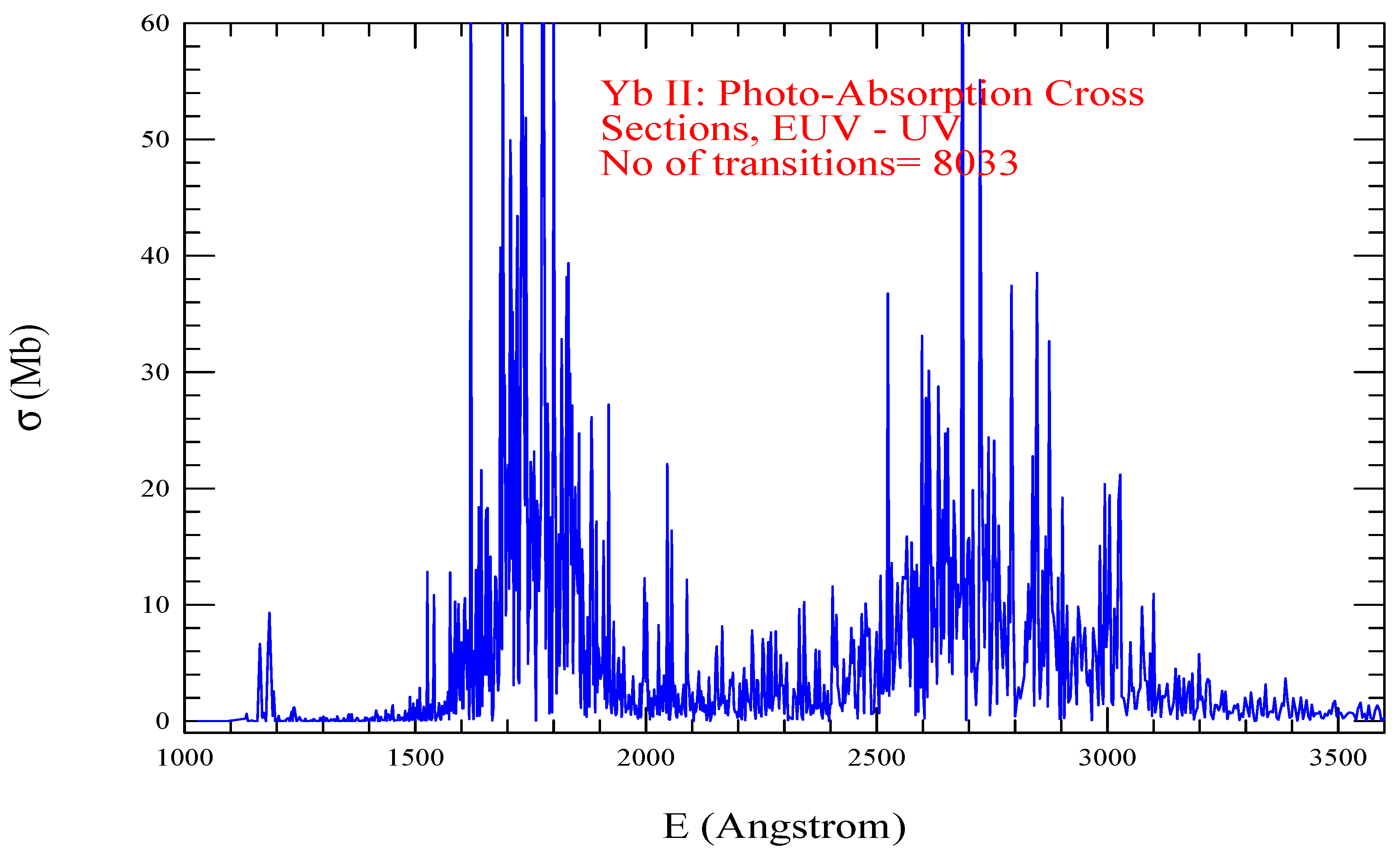
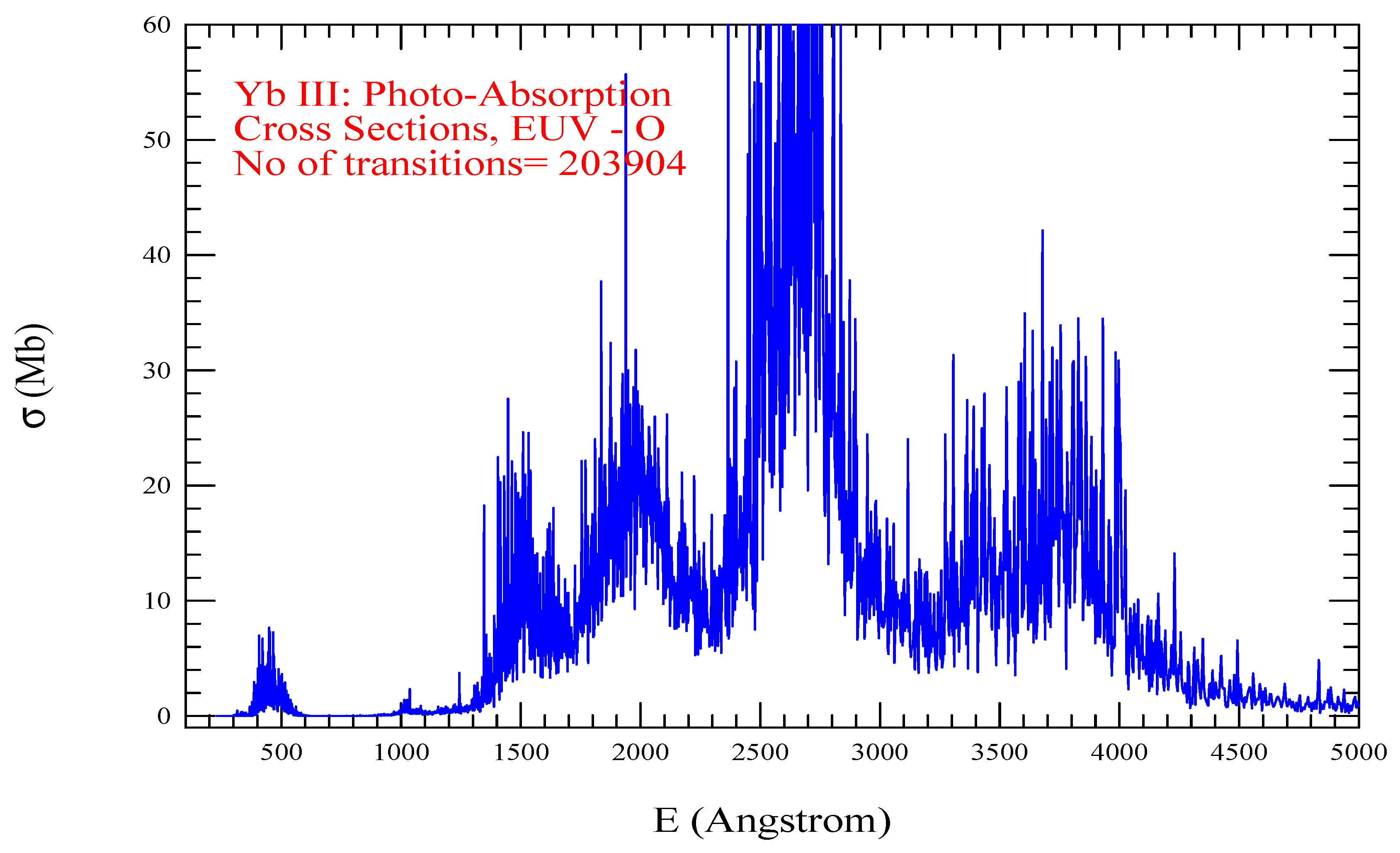
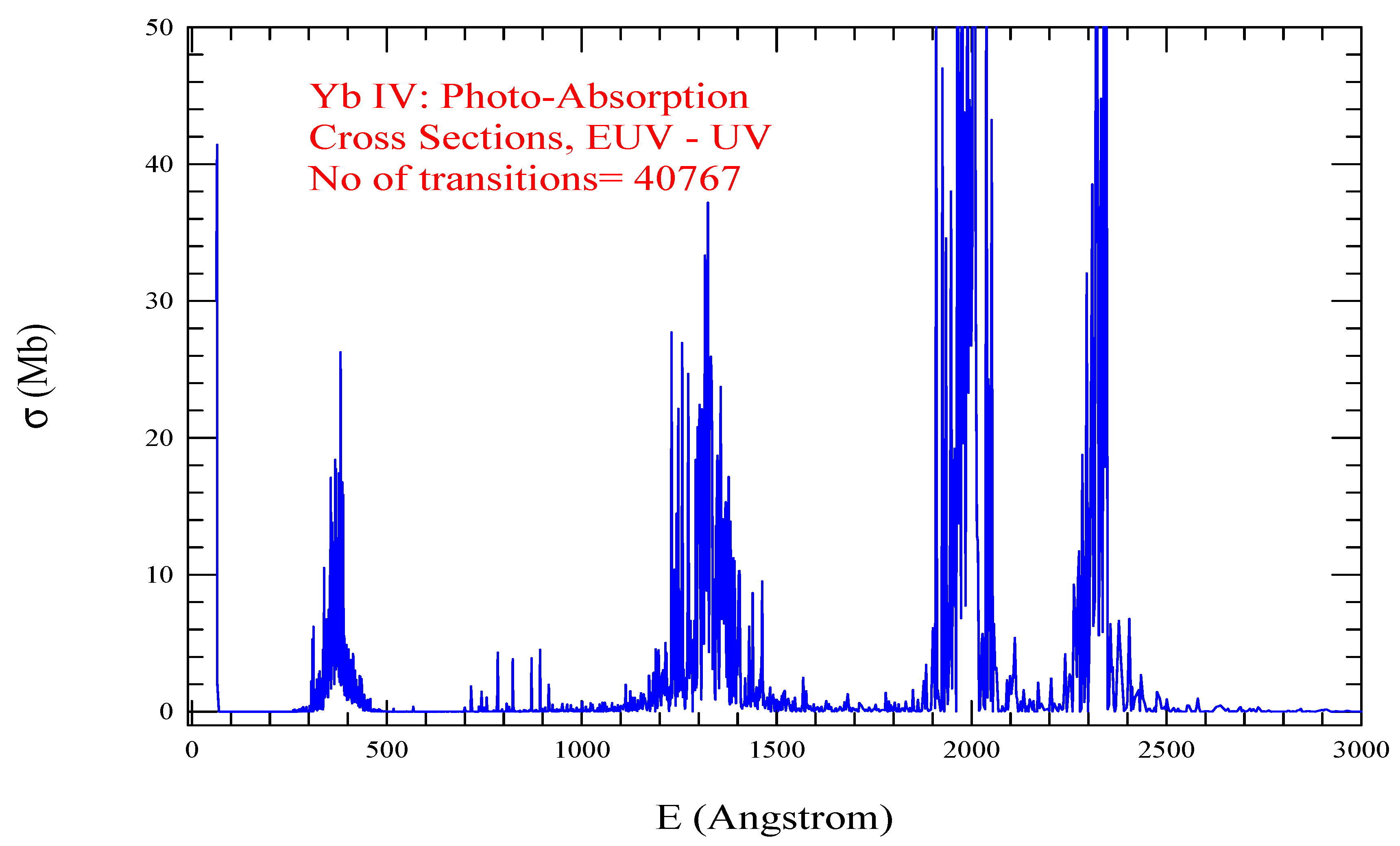
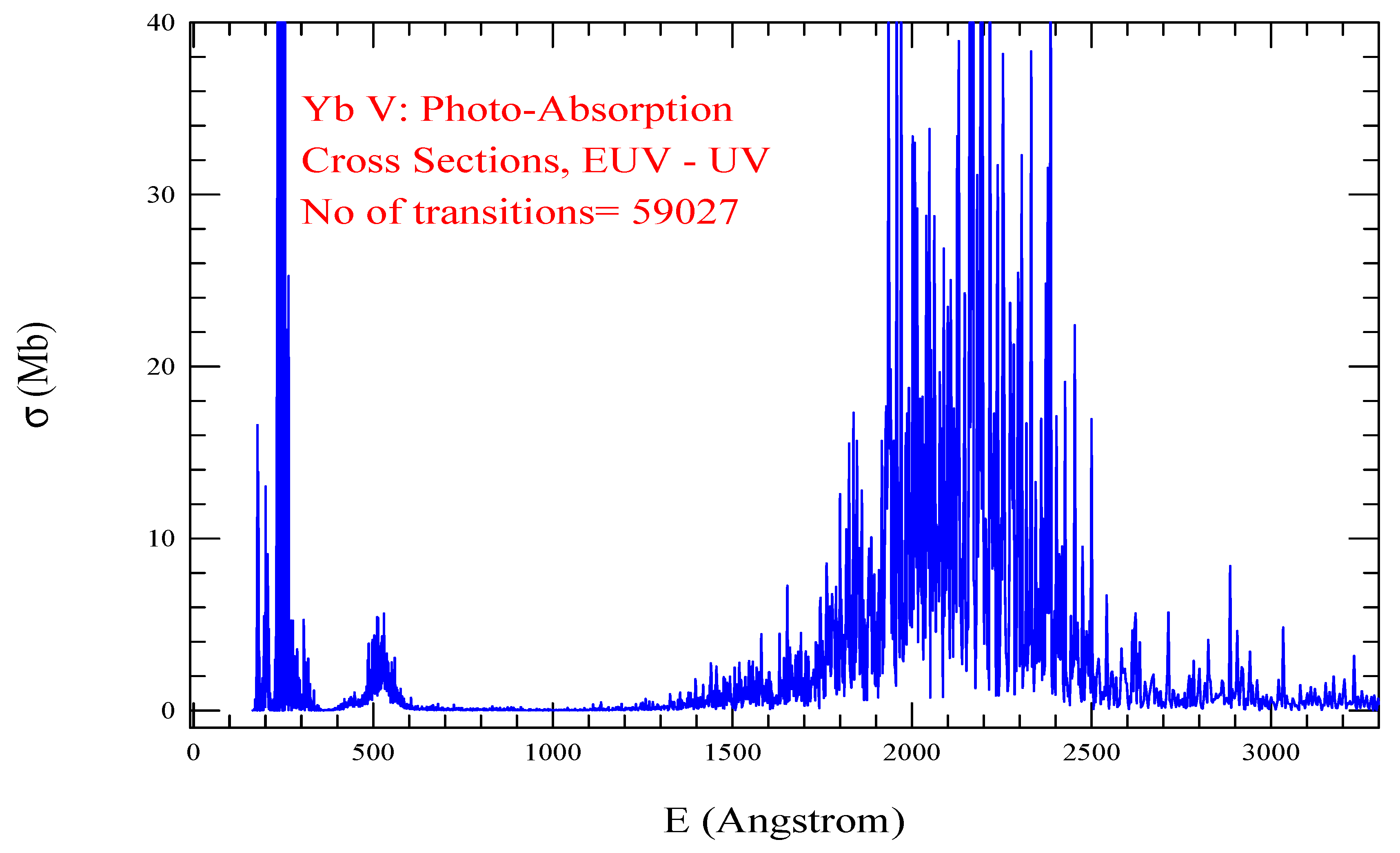
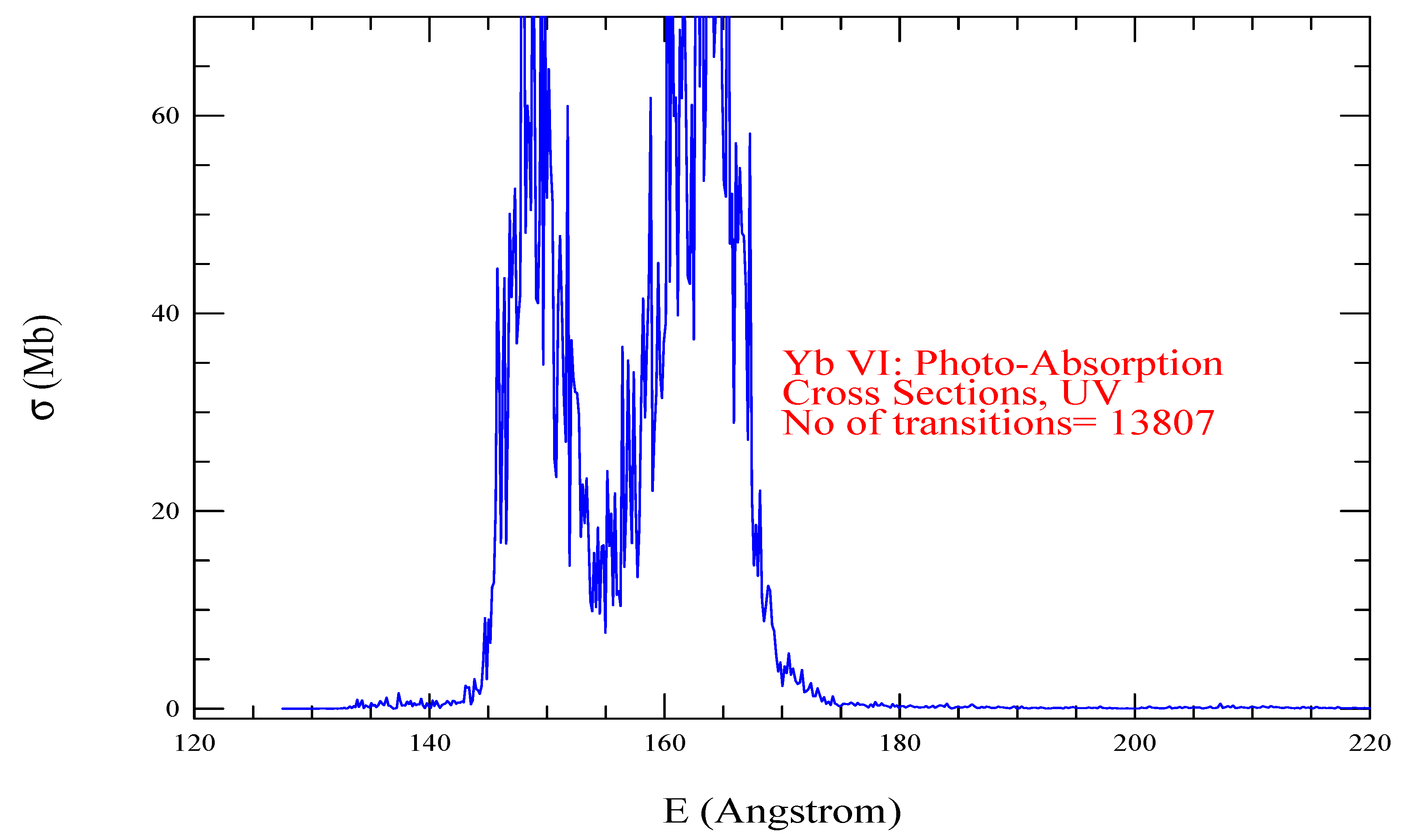
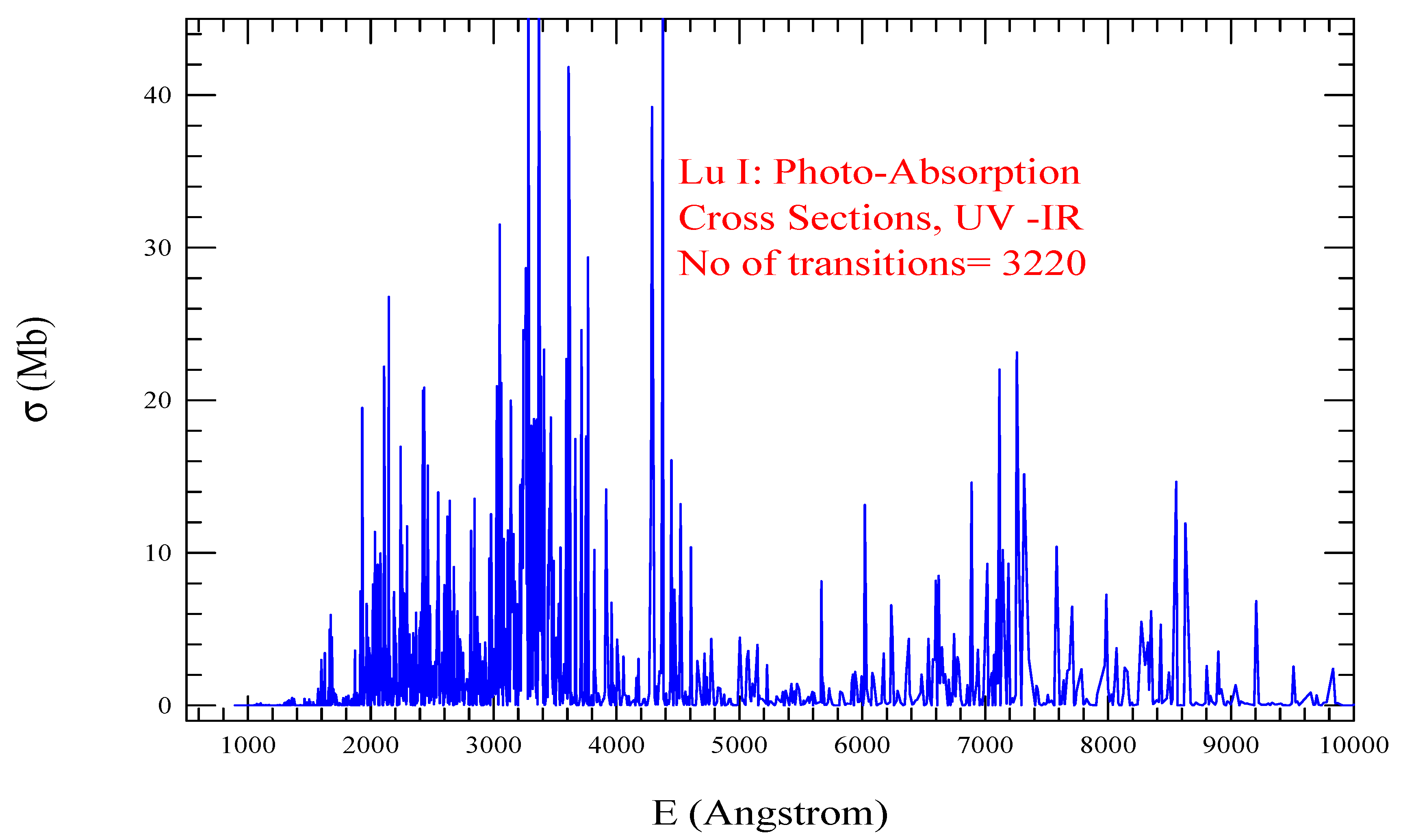
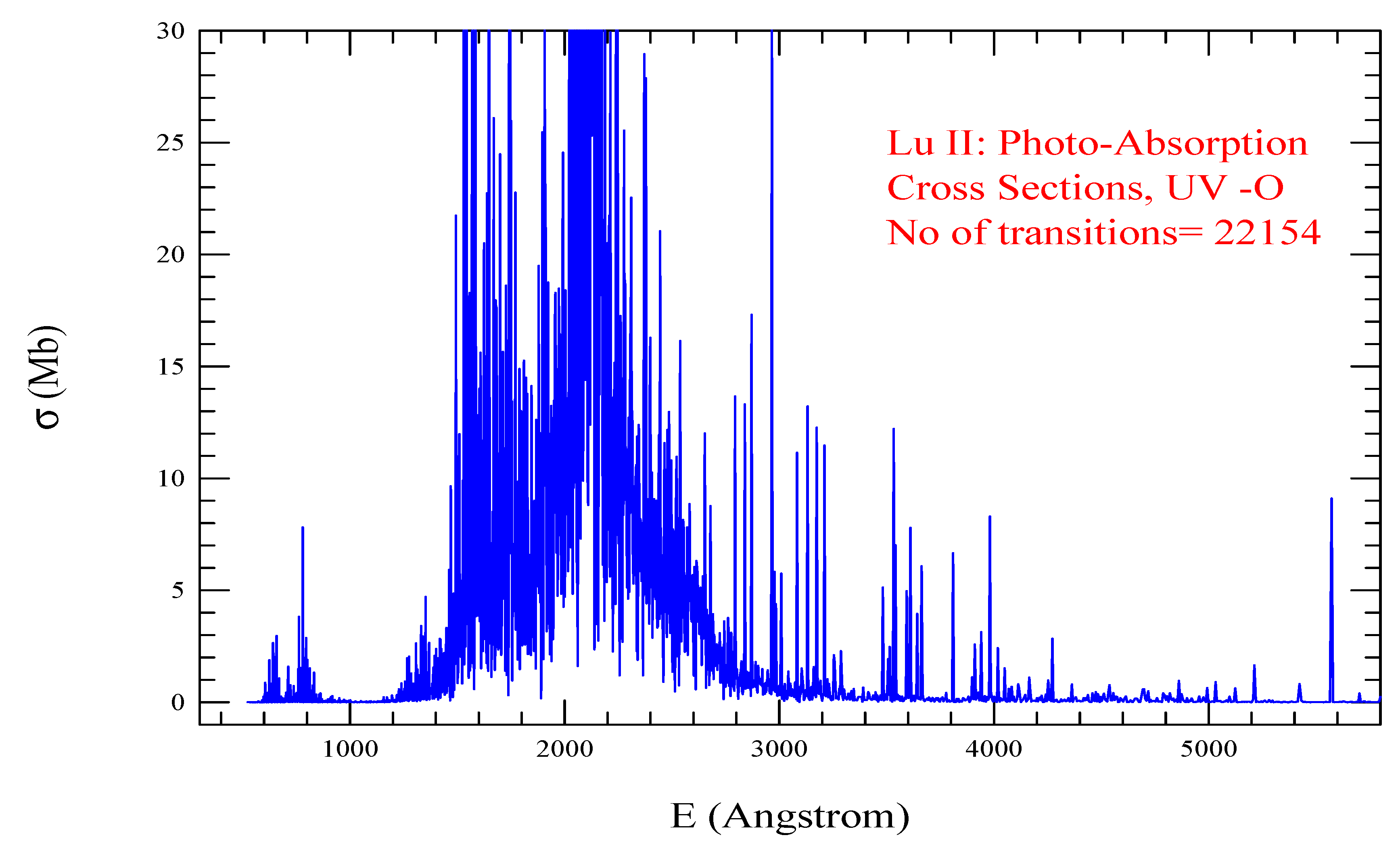
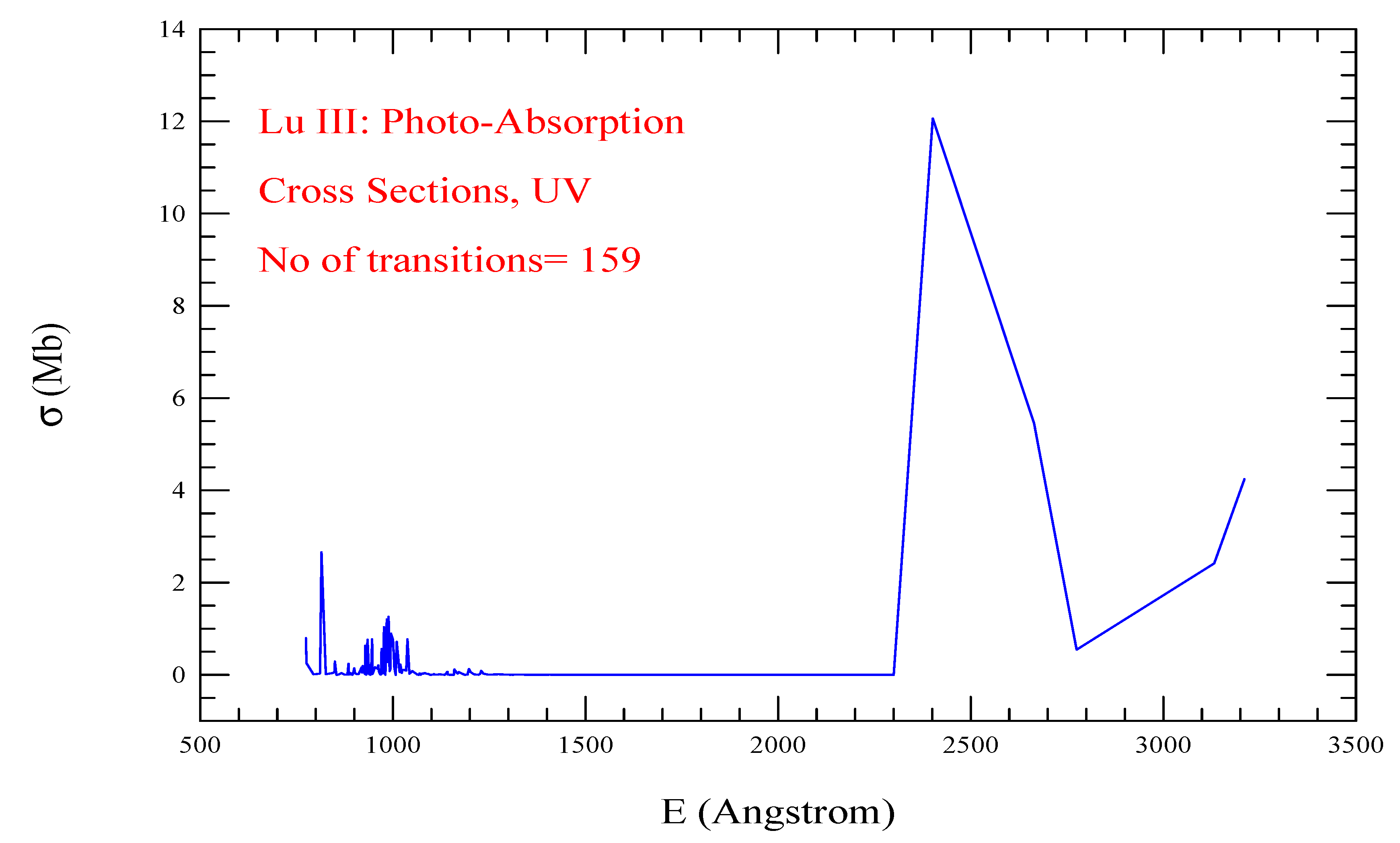
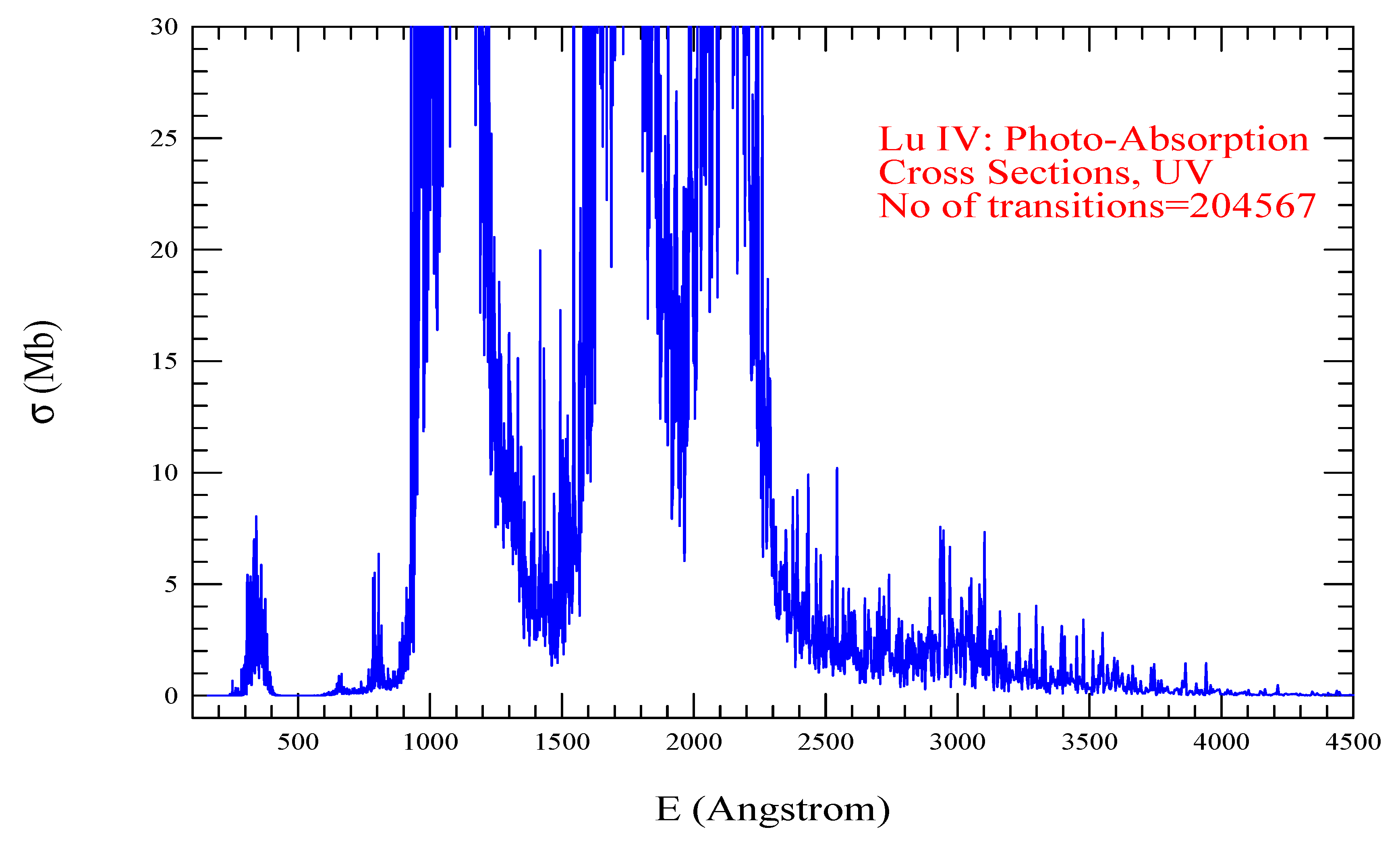
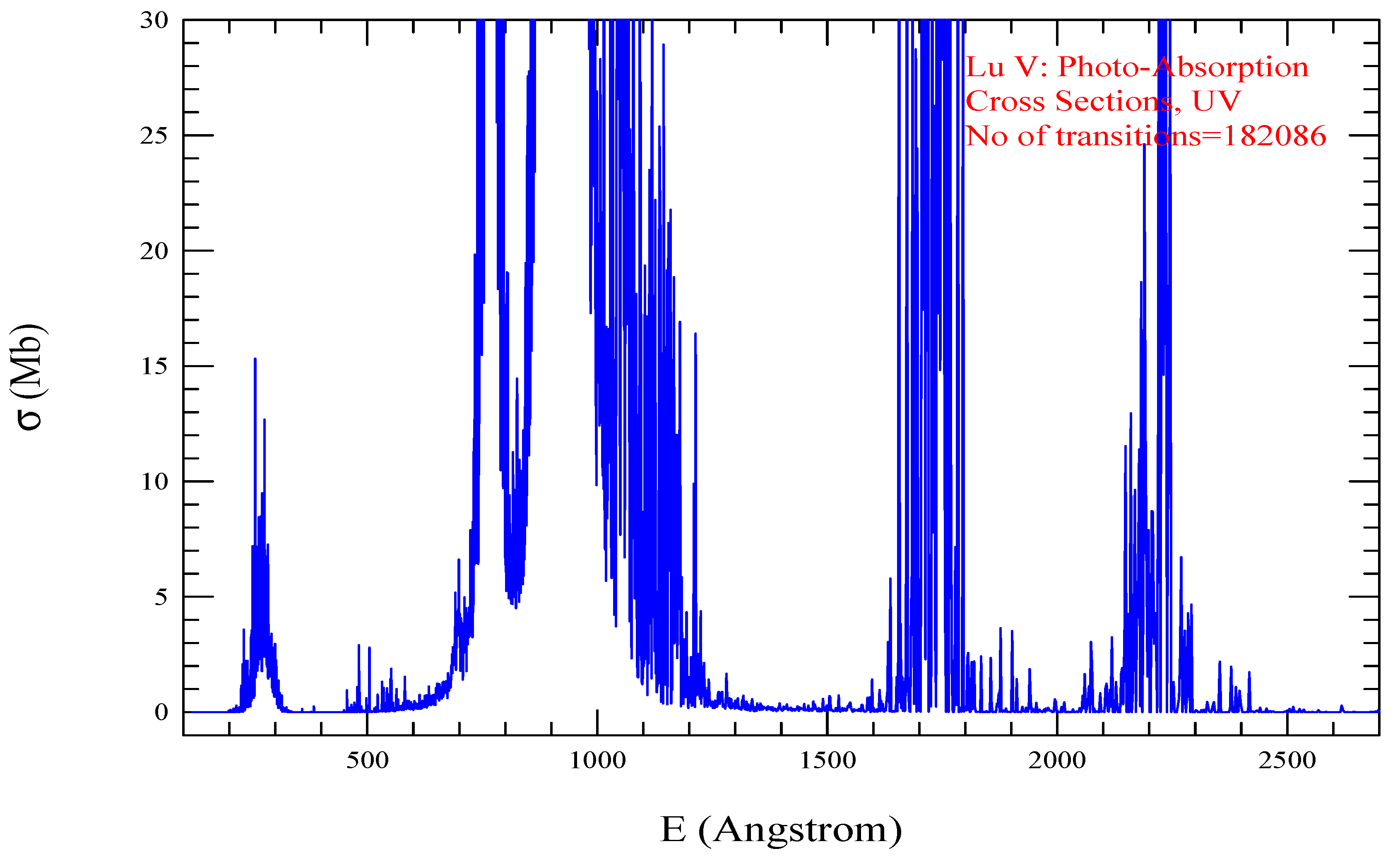
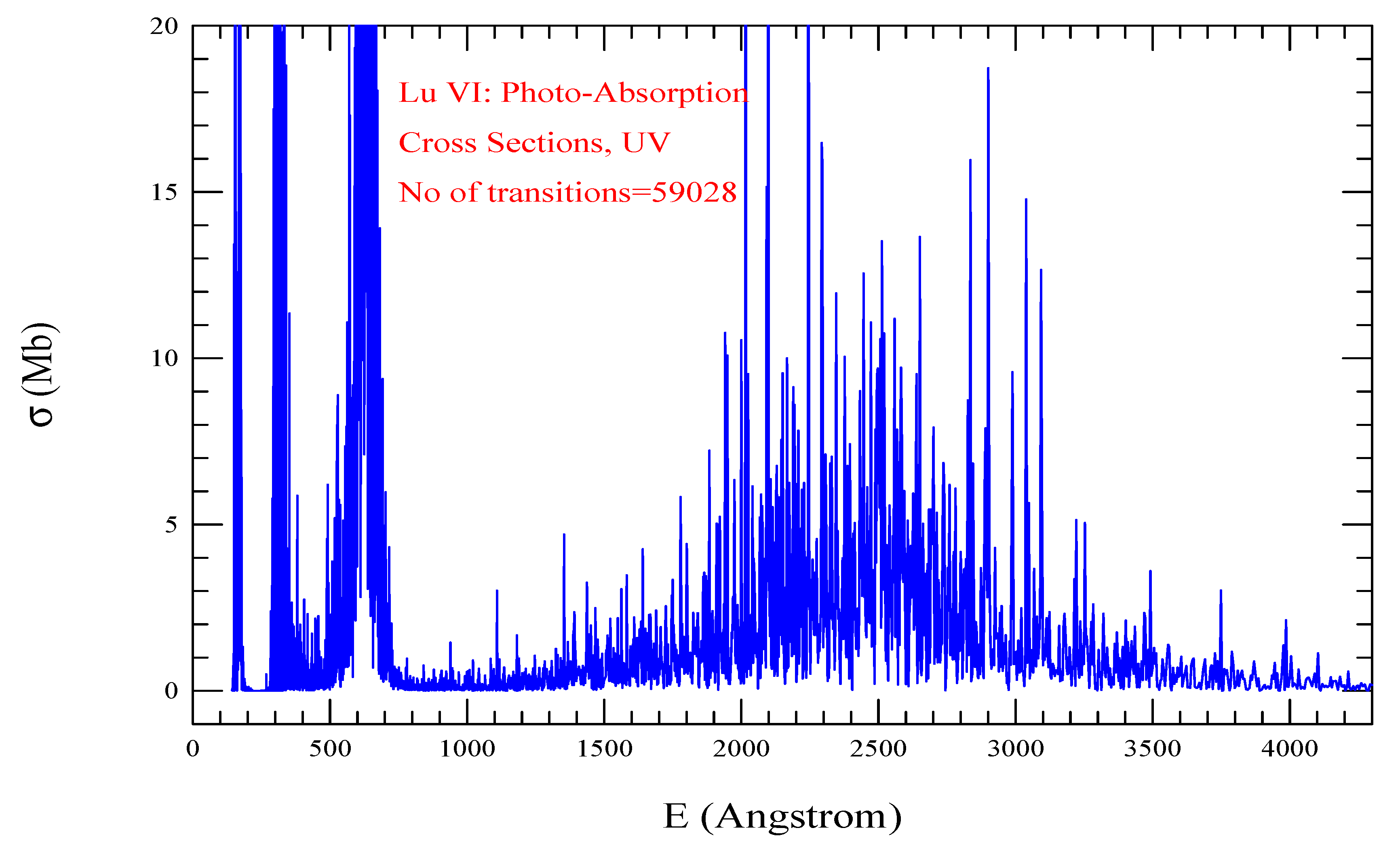
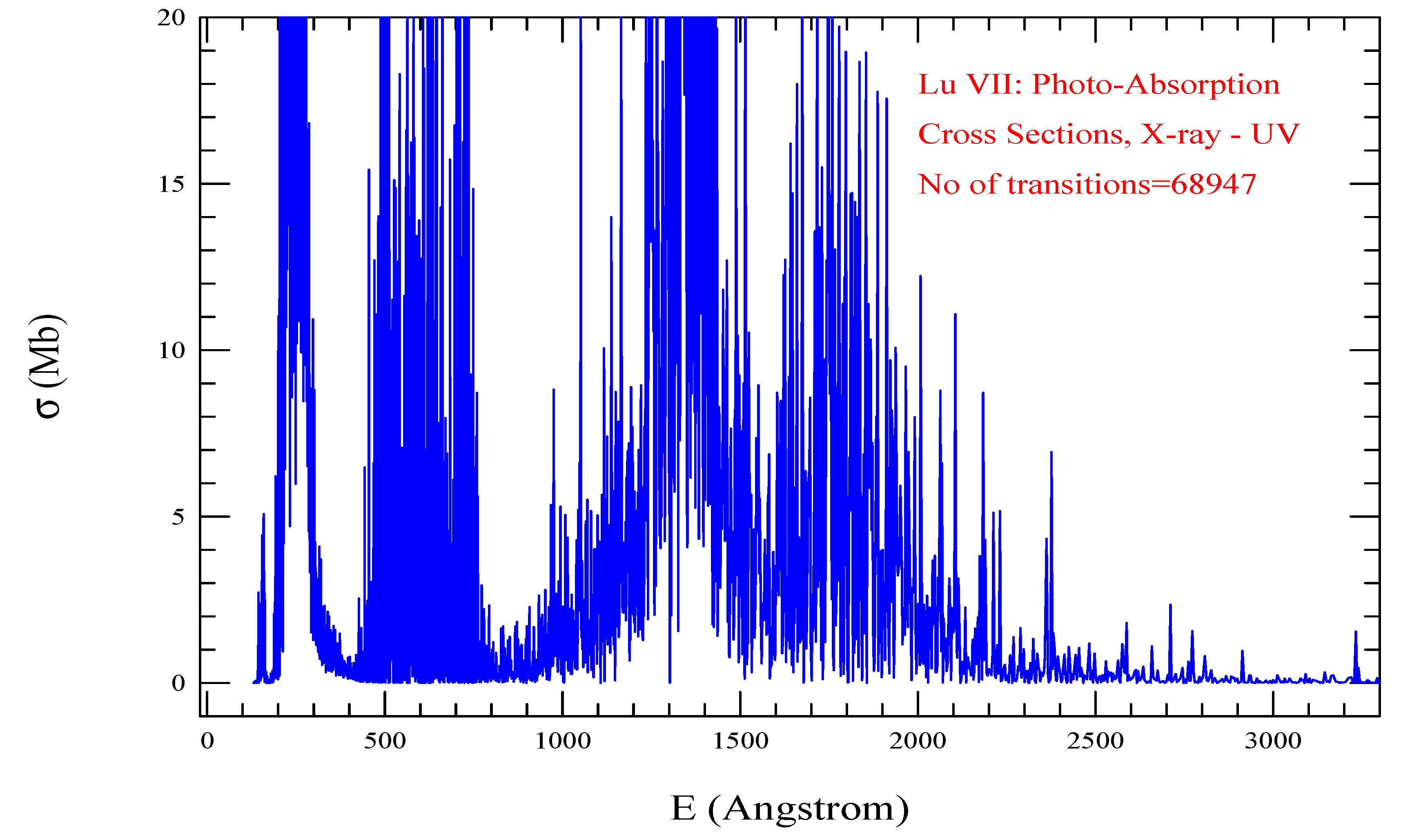
| Transition | ||
|---|---|---|
| NIST | SS | |
| Ho I | ||
| 3.73e+07 | 2.77E+07 | - |
| 1.62e+08 | 1.00E+08 | - |
| Ho II | ||
| 6.35e+07 | 3.09E+07 | |
| 4.87e+07 | 4.64E+07 | |
| Ho III: No A-value is available | ||
| Er I | ||
| 1.16e+08 | 2.49E+08 | |
| 7.28e+07 | 7.26E+07 | |
| Er II | ||
| 2.0e+07 | 4.67E+07 | |
| 1.4e+07 | 1.01E+07 | |
| Er III, Er IV: No A-value is available | ||
| Tm I | ||
| 5.3e+06 | 3.33E+06 | |
| 1.47e+07 | 1.81E+07 | |
| Tm II | ||
| 1.06E+08 | 7.29E+07 | |
| 1.57E+07 | 2.19E+07 | |
| Tm III, IV, V: No A-value is available | ||
| Yb I | ||
| 1.00E+08 | 1.66E+08 | |
| 6.83E+07 | 9.12E+07 | |
| Yb II | ||
| 6.83E+07 | 9.21E+07 | |
| 1.92e+08 | 1.66E+08 | |
| Yb III, IV, V, VI: No A-value is available | ||
| Lu I | ||
| 7.90e+06 | 4.53E+06 | |
| 1.85e+08 | 3.10E+08 | |
| Lu II | ||
| 4.53e+08 | 3.97E+08 | |
| 7.14e+07 | 5.43E+07 | |
| Lu III, IV, V, VI, VII: No A-value is available | ||
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- B.P. Abbott et al, Phys. Rev. Lett 2017, 161101.
- E. Pian et al. Nature 2017, 67, 551.
- D. Kasen, J. Barnes, N.R. Badnell. Astrophys J 2013, 25, 774.
- M. Tanaka and K. Hotokezaka. Astrophys. J 2013, 113, 775.
- M Tanaka, D Kato, G Gaigalas and K Kawaguchi. MNRAS 2020, 496, 1369–1369.
- Tanaka M. et al. ApJ 2018, 852, 109.
- L Radziutė, Gediminas Gaigalas, Daiji Kato, Pavel Rynkun, and M Tanaka. ApJ Suppl. SerApJ Suppl. Ser 2020, 248, 17.
- Fontes C. J., Fryer C. L., Hungerford A. L., Wollaeger R. T., Korobkin O. MNRAS 2020, 493, 4143.
- Cowan, R. 1981, The Theory of Atomic Structure and Spectra (Berkeley, CA: Univ. California Press).
- Johnson, J. Science 2019, 363, 474–478. [CrossRef] [PubMed]
- C. Kobayashi, A.I. Karakas, M. Lugaro. Astrophy J 2020, 900, 179.
- W.C. Martin, Romuald Zalubas, and Lucy Hagan, in Nat. Stand. Ref. Data Ser., NSRDS-NBS 60, 422 pp. (Nat. Bur. Stand., U.S., 1978). [CrossRef]
- Kramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team (2020), NIST Atomic Spectra Database (ver. 5.8), Online: https://physics.nist.gov/PhysRefData/ASD/levels_form.htm.
- T. A. Carlson, C. W. T. A. Carlson, C. W. Nestor, Jr., N. Wasserman, and J. D. McDowell, At. Data Nucl. Data Tables 1970, 2, 63–99. [Google Scholar]
- W. F. Meggers, C. H. Corliss, and B. F. Scribner, Nat. Bur. Stand., Monograph 145, U.S., 600 pp. (1975). [CrossRef]
- D. C. Morton. Astrophys. J., Suppl. Ser. Erratum: 132, 411 (2001). 2000, 130, 403–436.
- V. A. Komarovskii. Opt. Spektrosk. (Russ.) [Opt. Spectrosc. 71(4), 322 (1991)]. 1991, 71, 559–592.
- "Atomic transition probabilities for Tm I and Tm II, M. E. Wickliffe and J. E. Lawler. J. Opt. Soc. Am. B 1997, 14, 737–753. [CrossRef]
- J. Sugar, W. F. Meggers, and P. Camus. J. Res. Natl. Bur. Stand. (U.S.), Sect. A 1973, 77, 1–43.
- N. P. Penkin and V. A. Komarovskii. J. Quant. Spectrosc. Radiat. Transfer 1976, 16, 217–252.
- J. A. Fedchak, E. A. Den Hartog, J. E. Lawler, P. Palmeri, P. Quinet, and E. Biémont. Astrophys. J. 2000, 542, 1109–1118.
- R. Obaid, H. Xiong, U. Ablikim, S. Augustin, K. Schnorr, A. Battistoni, T. Wolf, A.M. Carroll, R. Bilodeau, T. Osipov, D. Rolles, N. Berah, Proc. APS Division of At. Mol. Opt. Phys. (DAMOP) Annual Meeting, Abstract: B9.00007, Providence RI, May 23-27 (2016).
- Pradhan A.K., Nahar S.N. Atomic Astrophysics and Spectroscopy (Cambridge University press, 2011).
- Nahar SN, Eissner W, Chen GX, Pradhan AK. Atomic data from the Iron Project - LIII. Relativistic allowed and forbidden transition probabilities for Fe XVII. A&A 2003, 408, 789–801.
- Eissner W, Jones M, Nussbaumer H .Techniques for the calculation of atomic structures and radiative data including relativistic corrections. Comput. Phys. Commun. 1974, 8, 270–306. [CrossRef]
- Nahar S.N. Astron. Astrophys. 2003, 413, 779.
- Nahar S.N., Proceedings of the 4th international conference on MTPR-10, Modern Trends in Physics Research, Sharm El Sheikh, Egypt, December 12-16, 2010 (Editor: Lotfia El Nadi, World Scientific, 2013), p. 275-285.
- Nahar S.N. Database NORAD-Atomic-Data for atomic processes in plasma. Atoms 8, issue 4, 68 (2020). The data are available at http://norad.astronomy.ohio-state.edu.
| Ho I (10 orbitals filled), =1019566 | |
|---|---|
| Configurations: | (1), (2), (3) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), | |
| 1.0 (5s), 0.94 (5p), 1.0 (4d), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Ho II (9-0orbitals filled), = 408070 | |
| Configurations: | (1), (2), (3). (4) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.0 (4p), | |
| 1.0 (5s), 1.00 (5p), 1.0 (4d), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Ho III (11 orbitals filled), = 1309895 | |
| Configurations: | (1), (2), (3), (4) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.2 (4p), | |
| 0.925 (5s), 1.50 (4d), 1.00 (5p), 0.95 (4f), 1.20 (6s),1.25 (6p), 1.0 (5d) | |
| Er I (11 orbitals filled), = 206202 | |
| Configurations: | (1), (2), (3), (4), (5) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.05 (4p), 1.0 (5s), | |
| 1.00 (5p), 1.00 (4d), 1.05 (4f), 1.09 (6s),1.0 (6p), 1.0 (5d) | |
| Er II (11 orbitals filled), = 897374 | |
| Configurations: | (1), (2), (3), (4), (5), (6) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.0 (4p), 1.2 (5s), | |
| 0.955 (5p), 1.02 (4d), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Er III (9 orbitals filled), = 409161 | |
| Configurations: | (1), (2), (3), (4), |
| (5), (6), (7) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.0 (4p), 1.8 (5s), | |
| 1.17 (4d), 1.01 (5p), 1.0 (4f), 1.2 (6s),1.0 (6p), 1.0 (5d) | |
| Er IV (11 orbitals filled), = 1309955 | |
| Configurations: | (1), (2), (3), (4) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.0 (5s), | |
| 1.07 (4d), 1.0 (5p), 1.0 (4f), 1.0 (5d),1.0 (6s), 1.0 (6p) | |
| Tm I (11 orbitals filled), = 118759 | |
| Configurations: | (1), (2), (3), (4), (5), (6), |
| (7),(8), (9), (10), (11) | |
| 1.30 (1s), 1.25 (2s), 1.22 (2p), 1.1 (3s), 1.12 (3p), 1.1262 (3d), 1.002 (4s), 1.0606 (4p), | |
| 0.9 (5s), 1.05436 (5p), 0.97512 (4d), 1.0 (4f), 0.718 (6s), 1.16173 (6p), 1.1096 (5d) | |
| Tm II (11 orbitals filled), = 34184 | |
| Configurations: | (1), (2), (3), (4), (5), (6), |
| (7), (8), (9) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.01 (3d), 0.92 (4s), 0.80 (4p), | |
| 1.53 (5s), 0.9 (5p), 1.004 (4d), 1.02 (4f), 1.014 (6s),0.9 (6p), 0.95 (5d) | |
| Tm III (10 orbitals filled), = 849878 | |
| Configurations: | (1), (2), (3), (4), (5), (6) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.00 (4p), 1.0 (5s), | |
| 1.12 (5p), 1.0 (4d), 0.97 (4f), 0.98 (6s),1.00 (6p), 0.98 (5d) | |
| Tm IV (10 orbitals filled), = 1096164 | |
| Configurations: | (1), (2), (3), (4), (5), (6), |
| (7), (8), (9) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.0 (5s), | |
| 1.0 (4d), 1.03 (5p), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Tm V (10 orbitals filled), = 801717 | |
| Configurations: | (1), (2), (3), (4) |
| 1.3 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.0 (5s), | |
| 1.0 (4d), 1.0 (5p), 1.0 (4f),1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Yb I (11 orbitals filled), = 109127 | |
| Configurations: | (1), (2), (3), (4), (5), (6), |
| (7), (8), (9), (10) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.03 (3d), 1.05 (4s), 1.02 (4p), | |
| 0.935 (5s), 0.937 (5p), 1.0 (4d), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Yb II (11 orbitals filled), =39009 | |
| Configurations: | (1), (2), (3), (4), (5), (6), (7), |
| (8) | |
| 1.30 (1s), 1.45 (2s), 1.20 (2p), 1.10 (3s), 1.07 (3p), 1.0 (3d), 1.0 (4s), 1.05 (4p), | |
| 0.918 (5s), 0.90 (5p), 1.025 (4d), 1.0 (4f), 1.1007 (6s),0.97 (6p), 0.97 (5d) | |
| Yb III (11 orbitals filled), = 925575 | |
| Configurations: | (1), (2), (3), (4), , , , , |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.15 (3d), 1.05 (4s), 0.819 (4p), | |
| 1.24 (5s), 0.887 (5p), 0.98 (4d), 1.02 (4f), 1.05 (6s),0.95 (6p), 1.0 (5d) | |
| Yb IV (10 orbitals filled), = 400325 | |
| Configurations: | (1), (2), (3), (4), (5), (6) |
| 1.3 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), | |
| 1.0 (5s), 0.995 (5p), 1.0 (4d), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 (5d) | |
| Yb V (10 orbitals filled), = 208128 | |
| Configurations: | (1), (2), (3), (4), (5), (6) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.0 (5s), | |
| 1.0 (4d), 1.03 (5p), 1.0 (4f), 1.0 (6s),1.15 (6p), 1.2 (5d) | |
| Yb VI (10 orbitals filled), = 486262 | |
| Configurations: | (1), (2), (3), (4) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.1 (5s), | |
| 1.0 (4d), 1.0 (5p), 1.0 (4f), 1.0 (6s),1.0 (6p), 1.0 5d | |
| Lu-I (12 orbitals filled), = 13936 | |
| Configurations: | (1), (2), (3), (4), (5), (6), (7), (8), (9) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), | |
| 0.95 (5s), 1.135 (4d), 0.94 (5p), 1.0 (4f), 1.0 (6s), 0.98 (6p), 1.0 5d | |
| Lu-II (11 orbitals filled), = 109566 | |
| Configurations: | (1), (2), (3), (4), (5), (6), (7), |
| (8), (9), (10) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 0.95 (5s), | |
| 0.937 (5p), 1.0 (4d), 1.0 (4f), 1.0 (6s), 1.0 (6p), 0.99 5d | |
| Lu-III (11 orbitals filled), = 8564 | |
| Configurations: | (1), (2), (3), (4), (5), (6), (7), |
| (8) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 1.05 (4p), 1.00 (5s), | |
| 0.95 (5p), 0.98 (4d), 1.0 (4f), 1.03 (6s), 0.97 (6p), 0.97 5d | |
| Lu-IV (11 orbitals filled), = 926436 | |
| Configurations: | (1), (2), (3), (4), (5), (6), (7), |
| (8), (9) | |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.00 (3d), 1.0 (4s), 0.80 (4p), 1.40 (5s), | |
| 0.90 (5p), 0.98 (4d), 1.02 (4f), 1.05 (6s), 0.92 (6p), 0.97 5d | |
| Lu-V (11 orbitals filled), = 850668 | |
| Configurations: | (1), (2), (3), (4), (5),(6) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.0 (5s), | |
| 0.999 (5p), 0.993 (4d), 1.01 (4f), 0.98 (6s), 1.02 (6p), 1.0 5d | |
| Lu-VI (10 orbitals filled), = 317817 | |
| Configurations: | (1), (2), (3), (4), (5), (6) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 0.80 (3d), 1.0 (4s), 1.00 (4p), 1.40 (5s), | |
| 0.98 (4d), 1.0 (5p), 1.0 (4f), 0.95(6s), 1.3 (6p), 1.3 5d | |
| Lu-VII (10 orbitals filled), = 304178 | |
| Configurations: | (1), (2), (3), (4), (5), (6) |
| 1.30 (1s), 1.25 (2s), 1.12 (2p), 1.07 (3s), 1.05 (3p), 1.0 (3d), 1.0 (4s), 1.0 (4p), 1.0 (5s), | |
| 0.98 (4d), 0.97 (5p), 1.0 (4f), 1.0 (6s), 1.0 (6p), 1.12 5d |
| Number of fine structure levels = 1629 | |||
| ie | SL(cf#) | 2J+1 | E(Ry) |
| 1 | 4Io( 1) | 15 | 0.00000E+00 |
| 2 | 4Io( 1) | 13 | 3.60033E-02 |
| 3 | 4Io( 1) | 11 | 5.85101E-02 |
| 4 | 4Io( 1) | 9 | 7.43454E-02 |
| 5 | 4Me( 2) | 15 | 9.79376E-02 |
| 6 | 6Le( 2) | 13 | 1.01223E-01 |
| 7 | 4Fo( 1) | 9 | 1.09269E-01 |
| 8 | 6Le( 2) | 11 | 1.22576E-01 |
| 9 | 2Ho( 1) | 11 | 1.28064E-01 |
| 10 | 4So( 1) | 3 | 1.35965E-01 |
| Ni- Nj | SLp Ci-SLp Cj | gi-gj | wl(A) | fij | aji(s-1) |
|---|---|---|---|---|---|
| 29- 3 | 4Ie 2- 4Io 1 | 12- 10 | 6454.84 | 4.24E-06 | 8.15E+02 |
| 29- 3 | 4Ie 2- 4Io 1 | 12- 10 | 6454.84 | 4.24E-06 | 8.15E+02 |
| 29- 4 | 4Ie 2- 4Io 1 | 10- 10 | 7270.34 | 4.48E-06 | 5.65E+02 |
| 29- 4 | 4Ie 2- 4Io 1 | 10- 10 | 7270.34 | 4.48E-06 | 5.65E+02 |
| 42- 2 | 4Ke 2- 4Io 1 | 14- 12 | 4678.36 | 9.76E-06 | 3.47E+03 |
| 42- 2 | 4Ke 2- 4Io 1 | 14- 12 | 4678.36 | 9.76E-06 | 3.47E+03 |
| 42- 3 | 4Ke 2- 4Io 1 | 12- 12 | 5289.56 | 1.11E-05 | 2.65E+03 |
| Config | SL | J | E(SS,Ry) | E(NIST,[12],Ry) | |
|---|---|---|---|---|---|
| Ho I, =1629, = 210522 | |||||
| 1 | 4 | 15/2 | 0.0 | 0.0 | |
| 2 | 4 | 13/2 | 0.03603 | 0.0493879 | |
| 3 | 4 | 11/2 | 0.05851 | 0.0784160 | |
| 4 | 4 | 9/2 | 0.07434 | 0.0974668 | |
| 5 | 4 | 15/2 | 0.09794 | 0.0763542 | |
| 6 | 6 | 13/2 | 0.10122 | 0.0767935 | |
| 7 | 6 | 11/2 | 0.12257 | 0.0833543 | |
| 8 | 6 | 13/2 | 0.10122 | 0.0887711 | |
| 9 | 4 | 17/2 | 0.13881 | 0.1031765 | |
| 10 | 6 | 9/2 | 0.14215 | 0.1050742 | |
| Ho II, =924, = 81623 | |||||
| 1 | 5 | 8 | 0.0 | 0.0 | |
| 2 | 5 | 7 | 0.00724 | 0.005808 | |
| 3 | 3 | 7 | 0.05528 | 0.051186 | |
| 4 | 5 | 6 | 0.05793 | 0.053306 | |
| 5 | 5 | 5 | 0.08928 | 0.080652 | |
| 6 | 3 | 6 | 0.09113 | 0.082029 | |
| 7 | 5 | 6 | 0.11003 | 0.098771 | |
| 8 | 5 | 4 | 0.11108 | 0.102111 | |
| 9 | 3 | 9 | 0.11702 | 0.148388 | |
| 10 | 5 | 7 | 0.11832 | 0.152628 | |
| Ho III, =1837, = 258124 | |||||
| 1 | 4 | 15/2 | 0.0 | 0.0 | |
| 2 | 4 | 13/2 | 0.03114 | 0.04956 | |
| 3 | 4 | 11/2 | 0.04969 | 0.07877 | |
| 4 | 4 | 9/2 | 0.06203 | 0.09815 | |
| 6 | 4 | 9/2 | 0.08576 | 0.12147 | |
| 7 | 4 | 7/2 | 0.1149 | 0.16282 | |
| 8 | 4 | 5/2 | 0.1245 | 0.17656 | |
| 9 | 4 | 3/2 | 0.1261 | ||
| 10 | 2 | 11/2 | 0.10275 | 0.15392 | |
| Er I. =993, = 88827 | |||||
| 1 | 3 | 6 | 0.0 | 0.0 | |
| 2 | 3 | 5 | 0.06787 | 0.063408 | |
| 3 | 3 | 4 | 0.06158 | 0.097970 | |
| 4 | 3 | 4 | 0.11386 | 0.045884 | |
| 5 | 3 | 3 | 0.13971 | 0.112792 | |
| 6 | 3 | 2 | 0.15311 | 0.119357 | |
| 7 | 5 | 6 | 0.089544 | 0.065397 | |
| 8 | 5 | 7 | 0.097936 | 0.070140 | |
| 9 | 5 | 9 | 0.104784 | 0.078556 | |
| 10 | 5 | 8 | 0.114163 | 0.085204 | |
| Er II, =1476, = 189738 | |||||
| 1 | 4 | 13/2 | 0.0 | 0.0 | |
| 2 | 2 | 11/2 | 0.00112 | 0.004013 | |
| Config | SL | 2J | E(SS,Ry) | E(NIST,[12],Ry) | |
| 3 | 2 | 9/2 | 0.06211 | 0.046772 | |
| 4 | 4 | 7/2 | 0.06260 | 0.049242 | |
| 5 | 6 | 13/2 | 0.06788 | ||
| 6 | 4 | 11/2 | 0.06837 | 0.0661522 | |
| 7 | 4 | 9/2 | 0.06855 | 0.065567 | |
| 8 | 6 | 15/2 | 0.07743 | 0.121552 | |
| 9 | 6 | 11/2 | 0.08817 | 0.15444 | |
| 10 | 6 | 17/2 | 0.08825 | 0.09720 | |
| Er III, =1000, = 82286 | |||||
| 1 | 4 | 16 | 0.0 | 0.0 | |
| 2 | 4 | 14 | 0.0360 | 0.0493879 | |
| 4 | 3 | 2 | 0.09552 | ||
| 5 | 3 | 3 | 0.08026 | ||
| 6 | 3 | 4 | 0.07184 | 0.04631 | |
| 7 | 5 | 8 | 0.17621 | 0.17602 | |
| 8 | 3 | 7 | 0.21743 | 0.18431 | |
| 9 | 3 | 8 | 0.21743 | 0.18431 | |
| 10 | 3 | 8 | 0.23221 | 0.19617 | |
| Er IV, =1837, = 257713 | |||||
| 1 | 4 | 15/2 | 0.0 | 0.0 | |
| 2 | 4 | 13/2 | 0.0598 | 0.0591 | |
| 3 | 4 | 11/2 | 0.0970 | 0.0921 | |
| 4 | 4 | 9/2 | 0.1230 | 0.1125 | |
| 5 | 4 | 9/2 | 0.1770 | 0.1383 | |
| 6 | 4 | 7/2 | 0.2333 | 0.1863 | |
| 7 | 4 | 5/2 | 0.2525 | 0.2011 | |
| 8 | 4 | 3/2 | 0.2555 | 0.2042 | |
| 9 | 2 | 11/2 | 0.2103 | ||
| 10 | 4 | 3/2 | 0.2193 | 0.1667 | |
| Tm I, =470, = 23804 | |||||
| 1 | 2 | 7/2 | 0.0 | 0.0 | |
| 2 | 2 | 5/2 | 0.0891 | 0.0799 | |
| 3 | 4 | 11/2 | 0.0933 | 0.1195 | |
| 4 | 2 | 13/2 | 0.1017 | 0.1392 | |
| 5 | 4 | 9/2 | 0.1343 | 0.1420 | |
| 6 | 4 | 7/2 | 0.1621 | 0.1591 | |
| 7 | 4 | 9/2 | 0.1639 | 0.1500 | |
| 8 | 4 | 11/2 | 0.1774 | 0.1545 | |
| 9 | 4 | 9/2 | 0.1785 | 0.1703 | |
| 10 | 2 | 15/2 | 0.2030 | 0.1716 | |
| Tm II, =1129, = 2467 | |||||
| 1 | 3 | 4 | 0.0 | 0.0 | |
| 2 | 1 | 3 | 0.000309 | 0.00216 | |
| 3 | 3 | 2 | 0.1050 | 0.0799 | |
| 4 | 3 | 3 | 0.1053 | 0.0816 | |
| 5 | 3 | 6 | 0.1449 | 0.1135 | |
| 6 | 3 | 5 | 0.2272 | 0.1879 | |
| 7 | 3 | 4 | 0.1812 | 0.2272 | |
| 8 | 5 | 5 | 0.1362 | 0.1510 | |
| 9 | 3 | 7 | 0.14733 | 0.17878 | |
| 10 | 3 | 2 | 0.15058 | 0.1606 | |
| Tm III, =1437, = 181768 | |||||
| 1 | 2 | 7/2 | 0.0 | 0.0 | |
| 2 | 2 | 5/2 | 0.06669 | 0.07995 | |
| 3 | 4 | 9/2 | 0.24034 | 0.20866 | |
| 4 | 4 | 15/2 | 0.27574 | 0.235825 | |
| Config | SL | 2J | E(SS,Ry) | E(NIST,[12],Ry) | |
| Tm III, =1437, = 181768 | |||||
| 5 | 4 | 11/2 | 0.27975 | 0.239230 | |
| 6 | 4 | 13/2 | 0.30738 | 0.261724 | |
| 7 | 2 | 13/2 | 0.21097 | 0.230575 | |
| 8 | 2 | 11/2 | 0.21716 | 0.236207 | |
| 9 | 4 | 7/2 | 0.27029 | 0.251029 | |
| 10 | 4 | 17/2 | 0.28719 | 0.251127 | |
| Tm IV, =1606, = 160013 | |||||
| 1 | 3 | 6 | 0.0 | 0.0 | |
| 2 | 3 | 5 | 0.08038 | 0.0737 | |
| 3 | 3 | 4 | 0.06591 | 0.114 ! | |
| 4 | 3 | 4 | 0.12865 | 0.0514! | |
| 5 | 3 | 3 | 0.15760 | 0.1308 | |
| 6 | 3 | 2 | 0.17087 | 0.1353 | |
| 7 | 1 | 4 | 0.21265 | 0.1943 | |
| 8 | 1 | 2 | 0.33334 | ||
| 9 | 1 | 6 | 0.40132 | ||
| 10 | 3 | 0 | 0.43598 | ||
| Tm V, =1837, = 259539 | |||||
| 1 | 4 | 15/2 | 0.0 | 0.0 | |
| 2 | 4 | 13/2 | 0.04067 | ||
| 3 | 4 | 11/2 | 0.06393 | ||
| 4 | 4 | 9/2 | 0.07922 | ||
| 5 | 4 | 9/2 | 0.10784 | ||
| 6 | 2 | 11/2 | 0.12898 | ||
| 7 | 4 | 3/2 | 0.13396 | ||
| 8 | 4 | 7/2 | 0.14556 | ||
| 9 | 4 | 5/2 | 0.15756 | ||
| 10 | 4 | 3/2 | 0.15875 | ||
| Yb I, =455, = 22002 | |||||
| 1 | 1 | 0 | 0.0 | 0.0 | |
| 2 | 3 | 0 | 0.11722 | 0.157544 | |
| 3 | 3 | 1 | 0.12353 | 0.163955 | |
| 4 | 3 | 2 | 0.13864 | 0.179614 | |
| 5 | 3 | 2 | 0.17604 | 0.211309 | |
| 6 | 3 | 5 | 0.21229 | 0.235651 | |
| 7 | 3 | 3 | 0.23746 | 0.250103 | |
| 8 | 3 | 4 | 0.25282 | 0.256836 | |
| 9 | 3 | 1 | 0.37221 | 0.223161 | |
| 10 | 1 | 1 | 0.23039 | 0.228438 | |
| Yb II, =264, = 8033 | |||||
| 1 | 2 | 1/2 | 0.0 | 0.0 | |
| 2 | 2 | 7/2 | 0.12512 | 0.195182 | |
| 3 | 2 | 5/2 | 0.22718 | 0.287669 | |
| 4 | 2 | 3/2 | 0.13701 | 0.209234 | |
| 5 | 2 | 5/2 | 0.15567 | 0.221736 | |
| 6 | 4 | 5/2 | 0.20627 | 0.243846 | |
| 7 | 4 | 3/2 | 0.23529 | 0.262062 | |
| 8 | 4 | 1/2 | 0.22353 | 0.306676 | |
| 9 | 2 | 1/2 | 0.25680 | 0.246605 | |
| 10 | 2 | 3/2 | 0.27636 | 0.276954 | |
| Yb III, =1485, = 203904 | |||||
| 1 | 1 | 0 | 0.0 | 0.0 | |
| 2 | 3 | 2 | 0.31312 | 0.304234 | |
| 3 | 3 | 5 | 0.35368 | 0.337353 | |
| Config | SL | 2J | E(SS,Ry) | E(NIST,[12],Ry) | |
| Yb III, =1485, = 203904 | |||||
| 4 | 1 | 3 | 0.381433 | 0.356681 | |
| 5 | 3 | 4 | 0.397868 | 0.365965 | |
| 6 | 3 | 4 | 0.313955 | 0.315810 | |
| 7 | 3 | 3 | 0.314706 | 0.318858 | |
| 8 | 3 | 12 | 0.387298 | 0.365965 | |
| 9 | 3 | 2 | 0.393126 | 0.361962 | |
| 10 | 3 | 4 | 0.406379 | 0.367132 | |
| Yb IV, =963, = 40767 | |||||
| 1 | 2 | 7/2 | 0.0 | 0.0 | |
| 2 | 2 | 5/2 | 0.10849 | 0.093077 | |
| 3 | 4 | 9/2 | 0.68093 | 0.715611 | |
| 4 | 2 | 15/2 | 0.71781 | 0.748930 | |
| 5 | 4 | 11/2 | 0.72798 | 0.753373 | |
| 6 | 4 | 13/2 | 0.75809 | 0.779549 | |
| 7 | 4 | 7/2 | 0.74697 | 0.768627 | |
| 8 | 4 | 17/2 | 0.75311 | 0.775715 | |
| 9 | 4 | 9/2 | 0.78667 | 0.802991 | |
| 10 | 4 | 11/2 | 0.79105 | 0.803512 | |
| Yb V, =873, = 59027 | |||||
| 1 | 3 | 6 | 0.0 | 0.0 | |
| 2 | 3 | 4 | 0.031148 | ||
| 3 | 3 | 5 | 0.056967 | ||
| 4 | 3 | 4 | 0.085510 | ||
| 5 | 3 | 3 | 0.10029 | ||
| 6 | 3 | 2 | 0.10611 | ||
| 7 | 1 | 4 | 0.13819 | ||
| 8 | 1 | 2 | 0.21423 | ||
| 9 | 1 | 6 | 0.26570 | ||
| 10 | 3 | 0 | 0.28940 | ||
| Yb VI, = 1407, = 13807 | |||||
| 1 | 4 | 15/2 | 0.0 | 0.0 | |
| 2 | 4 | 11/2 | 0.020645 | ||
| 3 | 4 | 13/2 | 0.021470 | ||
| 4 | 2 | 9/2 | 0.024775 | ||
| 5 | 2 | 11/2 | 0.036494 | ||
| 6 | 2 | 5/2 | 0.072901 | ||
| 7 | 2 | 11/2 | 0.074037 | ||
| 8 | 4 | 11/2 | 0.082992 | ||
| 9 | 4 | 7/2 | 0.093051 | ||
| 10 | 2 | 9/2 | 0.103729 | ||
| Lu I, = 148, = 3220 | |||||
| 1 | 2 | 3/2 | 0.0 | 0.0 | |
| 2 | 2 | 5/2 | 0.020658 | 0.018170 | |
| 3 | 2 | 1/2 | 0.083090 | 0.037691 | |
| 4 | 2 | 3/2 | 0.097783 | 0.068130 | |
| 5 | 4 | 3/2 | 0.116829 | 0.158809 | |
| 6 | 4 | 5/2 | 0.123975 | 0.168626 | |
| 7 | 4 | 7/2 | 0.137319 | 0.186195 | |
| 8 | 4 | 9/2 | 0.152215 | 0.206033 | |
| 9 | 4 | 1/2 | 0.121224 | 0.189202 | |
| 10 | 4 | 3/2 | 0.125580 | 0.193146 | |
| Config | SL | 2J | E(SS,Ry) | E(NIST,[12],Ry) | |
| Lu II, =455, = 22154 | |||||
| 1 | 1 | 0 | 0.0 | 0.0 | |
| 2 | 3 | 1 | 0.088774 | 0.107495 | |
| 3 | 3 | 2 | 0.098492 | 0.113319 | |
| 4 | 3 | 3 | 0.132393 | 0.129392 | |
| 5 | 1 | 2 | 0.162822 | 0.157946 | |
| 6 | 3 | 0 | 0.235055 | 0.248452 | |
| 7 | 3 | 1 | 0.248583 | 0.259740 | |
| 8 | 3 | 2 | 0.288652 | 0.295736 | |
| 9 | 3 | 2 | 0.286572 | 0.267974 | |
| 10 | 3 | 3 | 0.318543 | 0.281482 | |
| Lu III, = 145, = 159 | |||||
| 1 | 2 | 1/2 | 0.0 | 0.0 | |
| 2 | 2 | 3/2 | 0.0510604 | 0.052011 | |
| 3 | 2 | 5/2 | 0.0956229 | 0.078805 | |
| 4 | 2 | 1/2 | 0.342017 | 0.349932 | |
| 5 | 2 | 3/2 | 0.379465 | 0.407384 | |
| 6 | 4 | 5/2 | 0.741428 | ||
| 7 | 4 | 7/2 | 0.743474 | ||
| 8 | 4 | 3/2 | 0.745181 | ||
| 9 | 4 | 11/2 | 0.758697 | ||
| 10 | 4 | 7/2 | 0.766942 | ||
| Lu IV, =1485, = 204567 | |||||
| 1 | 1 | 0 | 0.0 | 0.0 | |
| 2 | 3 | 2 | 0.860650 | 0.824085 | |
| 3 | 3 | 5 | 0.894914 | 0.863590 | |
| 4 | 3 | 3 | 0.919319 | 0.886969 | |
| 5 | 3 | 4 | 0.934682 | 0.898131 | |
| 6 | 3 | 6 | 0.927311 | 0.895222 | |
| 7 | 3 | 1 | 0.939756 | 0.897644 | |
| 8 | 3 | 2 | 0.943373 | 0.908169 | |
| 9 | 3 | 4 | 0.971074 | 0.931466 | |
| 10 | 3 | 3 | 0.971264 | 0.939247 | |
| Lu V, =1437, = 182086 | |||||
| 1 | 2 | 7/2 | 0.0 | 0.0 | |
| 2 | 2 | 5/2 | 0.127518 | 0.107464 | |
| 3 | 4 | 9/2 | 1.392281 | 1.373872 | |
| 4 | 2 | 15/2 | 1.417875 | 1.412341 | |
| 5 | 4 | 11/2 | 1.428745 | 1.429373 | |
| 6 | 4 | 17/2 | 1.448544 | 1.448762 | |
| 7 | 4 | 13/2 | 1.451578 | 1.449241 | |
| 8 | 4 | 7/2 | 1.457972 | 1.476007 | |
| 9 | 2 | 11/2 | 1.484829 | 1.481346 | |
| 10 | 4 | 9/2 | 1.485680 | 1.483601 | |
| Lu VI, =873, = 59028 | |||||
| 1 | 3 | 6 | 0.0 | 0.0 | |
| 2 | 3 | 4 | 0.030443 | ||
| 3 | 3 | 5 | 0.055132 | ||
| 4 | 3 | 4 | 0.083617 | ||
| 5 | 3 | 3 | 0.099283 | ||
| 6 | 3 | 2 | 0.106151 | ||
| 7 | 1 | 4 | 0.133692 | ||
| 8 | 1 | 2 | 0.219180 | ||
| Config | SL | 2J | E(SS,Ry) | E(NIST,[12],Ry) | |
| Lu VII, = 777, = 68947 | |||||
| 9 | 1 | 6 | 0.280176 | ||
| 10 | 3 | 0 | 0.305074 | ||
| 1 | 2 | 11/2 | 0.0 | 0.0 | |
| 2 | 2 | 9/2 | 0.0125831 | ||
| 3 | 4 | 3/2 | 0.0421028 | ||
| 4 | 4 | 7/2 | 0.0666561 | ||
| 5 | 4 | 5/2 | 0.104410 | ||
| 6 | 4 | 5/2 | 0.104410 | ||
| 7 | 4 | 3/2 | 0.123075 | ||
| 8 | 4 | 1/2 | 0.127465 | ||
| 9 | 2 | 9/2 | 0.147603 | ||
| 10 | 4 | 7/2 | 0.224123 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





