Introduction
In the competitiveness of thin-sheet rolled products, a special role is given to the radical improvement and stabilization of the quality of rolled products produced. Its practical solution largely depends on the quality of the steel ingot. During the formation of an ingot, all types of macroheterogeneity are greatly developed: physical, structural and chemical. This significantly degrades the quality of cast and rolled metal. This problem is especially acute in the production of sheet metal.
The waste-free production of thin sheet metal is inextricably linked with the quality of the metal produced, i.e. the presence of “hidden” internal defects in the structure of castings, which in subsequent processing can lead to both direct losses of metal in the form of trim (head or end), and to losses of metal at the final stage of end-to-end technology.
Analysis of technical literature [1-10] showed that, despite a large number of studies on the problem of quality of thin-sheet rolled products, the results are ambiguous and contradictory. There is no consensus on the source and causes of the formation of surface defects in rolled sheet metal. This is due to the fact that the morphological characteristics of defects of steelmaking and rolling origin may be similar, since all defects are elongated in the direction of deformation and have similar characteristics and shape in the transverse direction. In addition, the metallographic method for determining the causes of the formation of surface defects is applicable for metal that has experienced one high-temperature heating, while in practice the metal undergoes 2-3 high-temperature heating.
Despite the existence of a proven connection between the structure transformation of macro- and micro-inhomogeneity of the ingot into surface defects of rolled sheets.
Research Methodology
To study the nature and sources of surface defects in rolled sheets, a comparative method of structural-concentration analysis of metal at the end-to-end metallurgical process of ingot - slab - sheet metal has been developed, based on the metallographic method of studying the structure and qualitative analysis of non-metallic inclusions.
To study chemical heterogeneity and contamination with non-metallic inclusions, characteristic ingots were isolated. Oxygen cutters were used to cut axial plates parallel to the wide edge 120-150 mm above the axial plane. In the machine shop, they planed it to the axial plane, sanded it, and then made sulfur prints on photographic paper after etching the surface of the axial template with a sulfuric acid solution.
To study chemical heterogeneity and contamination with non-metallic inclusions, metal samples were taken. The content of elements [C], [Mn], [Si], [S], [P], [AL], [N] was determined by a chemical method. Metal contamination with non-metallic inclusions was determined by electrolytic deposition and the LT metallographic method. To study the macrostructure of the cortical zone of the ingot, a corner template was cut out at 5 levels along the height of the ingot, corresponding to 5, 25, 50, 75 and 95% of the height from the head of the ingot. The cut templates were planed, polished and etched in a sulfuric acid solution to remove sulfur prints.
To study the causes of defective areas, samples were taken to determine non-metallic inclusions in them and the chemical composition of the metal along the boundaries of the defect. From the selected samples, thin sections were made to study the microstructure and contamination of steel with non-metallic inclusions, as well as samples to determine the number of non-metallic inclusions.
The chemical composition of phases and structural components was determined by the local microprobe method on a Samesa microanalyzer.
In general, the scheme for determining the nature and origin of surface defects included:
- -
study of the topography of defects on the surface of rolled sheets;
- -
metallographic examination of the steel microstructure in defective areas;
- -
determination of the composition and nature of non-metallic inclusions, both in the defect zone and in the volume of “healthy” metal;
- -
analysis of technological parameters of smelting, out-of-furnace processing, steel casting and metal rolling at the rolling stage;
- -
study of the structure of the cortical layer of the ingot and identification of structural heterogeneity using macroanalysis methods.
- -
study of the influence of high-temperature heating on the behavior of inclusions and gas bubbles located in the crustal layer of the ingot.
Research results
Metallographic analysis of the microstructure of samples of cold-rolled steel sheets in areas where surface defects appear makes it possible to identify mainly 3 types of characteristic structures:
- 1–
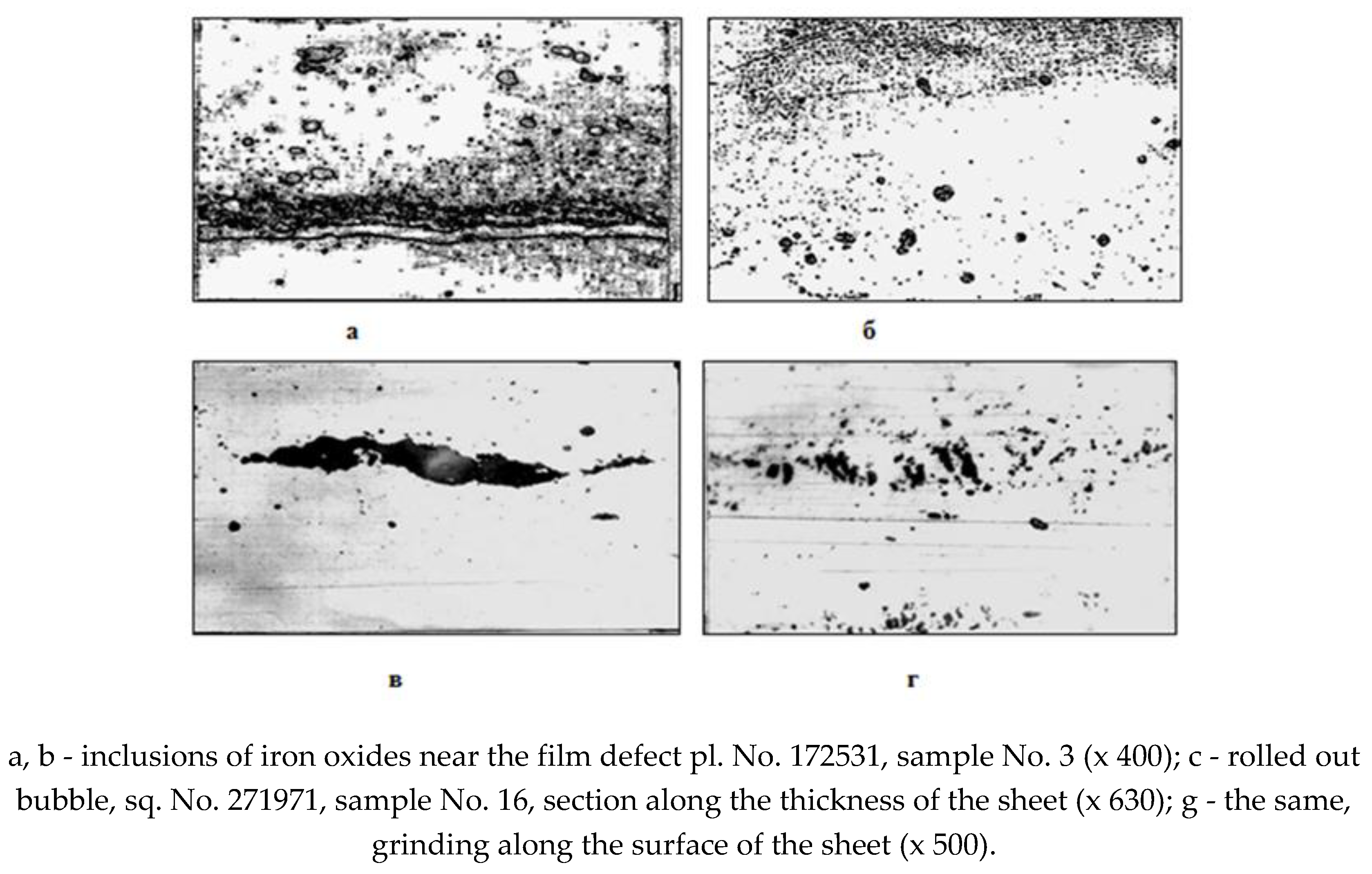
coarse films (Fig. 1, a), affecting a significant area of metal in the subsurface layers with a specific type of non-metallic inclusions in the form of globules of iron oxides (wustite) and pinpoint oxide rash (Fig. 1, b)
- 2–
areas of bubbles filled with iron oxides such as wustite (Fig. 1, c), which often form films on the surface of the sheet during rolling (Fig. 1, d);
- 3–
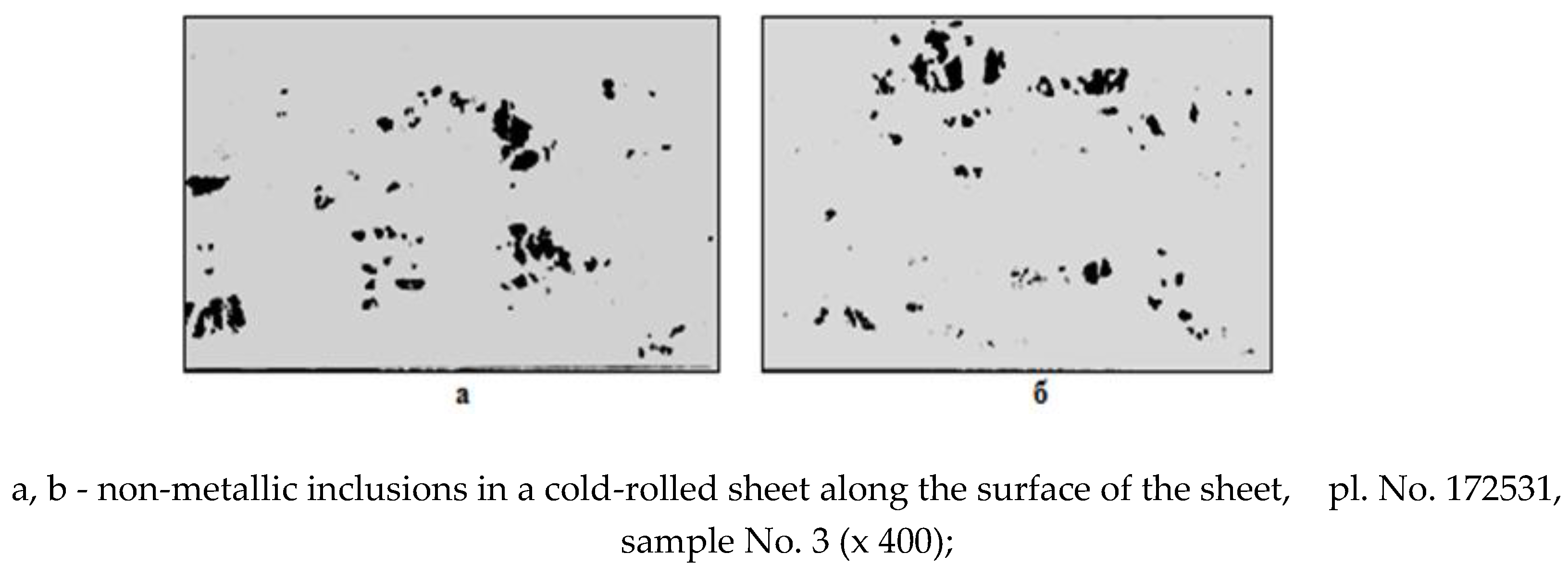
group accumulation of oxide non-metallic inclusions of complex composition (Fig. 2, a, b).
In table
Figure 1 shows a point chemical analysis of non-metallic inclusions from clusters, where it is clear that the inclusions correspond to oxides of the SiO2 – MnO – FeO system.
Non-metallic inclusions identified in places of defects in cold-rolled sheets belong to brittlely destroyed manganese silicates and have the following chemical composition: 12-18% SiO2; up to 58% MnO; up to 10% FeO.
Rice. 1.
Microstructure of cold-rolled steel 08 KP in place manifestations of the defect.
Rice. 1.
Microstructure of cold-rolled steel 08 KP in place manifestations of the defect.
Rice. 2.
Microstructure of steel at the location of the defect.
Rice. 2.
Microstructure of steel at the location of the defect.
Table 1.
Chemical composition of inclusions.
Table 1.
Chemical composition of inclusions.
| Oxides content,% by weight |
| SiO2
|
МпО |
FeO |
Al2O3
|
CaO |
MgO |
| 11,52 |
58,20 |
19,15 |
0,68 |
0,32 |
0,11 |
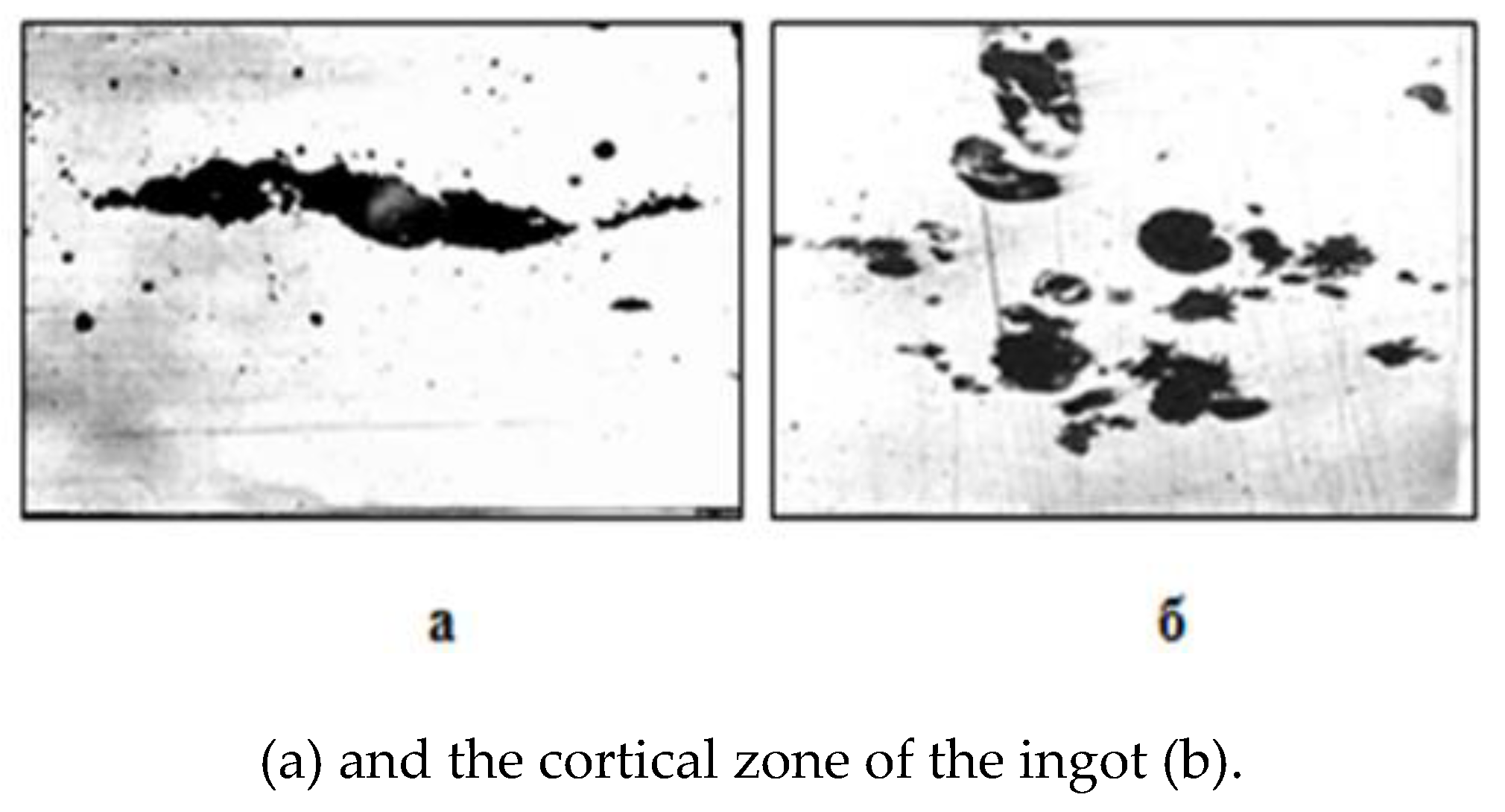
Another type of characteristic steel microstructure in areas of surface defects is revealed in the form of accumulations of iron oxides of the wustite type (Fig. 3 a, b) between the cavities of pores and discontinuities (gray in the microphotograph - wustite inclusions, dark - pores and discontinuities). Microprobe point analysis of areas of non-metallic inclusions confirms the presence of 91.61% FeO and 2.50% MnO in them.
The analysis results show that the following types of surface defects are formed in cold-rolled sheets: 1 - large films; 2- small captivity; 3- rolled out oxidized bubbles; 4- coarse non-metallic inclusions. In almost 1-3 groups of defects, the main non-metallic phase is iron oxides, the content of which is 90% or more.
A comparative structural-concentration analysis of metal in cold-rolled steel at the site of a defect in the base metal and the cortical zone of the ingot before and after high-temperature heating revealed the identity of the morphological signs of structural heterogeneity (Fig. 4). The difference is only in the shape, distribution of inclusions and concentration of FeO in wüstite. An increase in the FeO content from 80-85% to 87-93% indicates the oxidation of subcortical bubbles and micropores during the oxidative heating of ingots in the cells of heating wells.
To determine the moment of formation of these defects, the macro- and microstructure of the near-surface zones of the ingot and slab was studied.
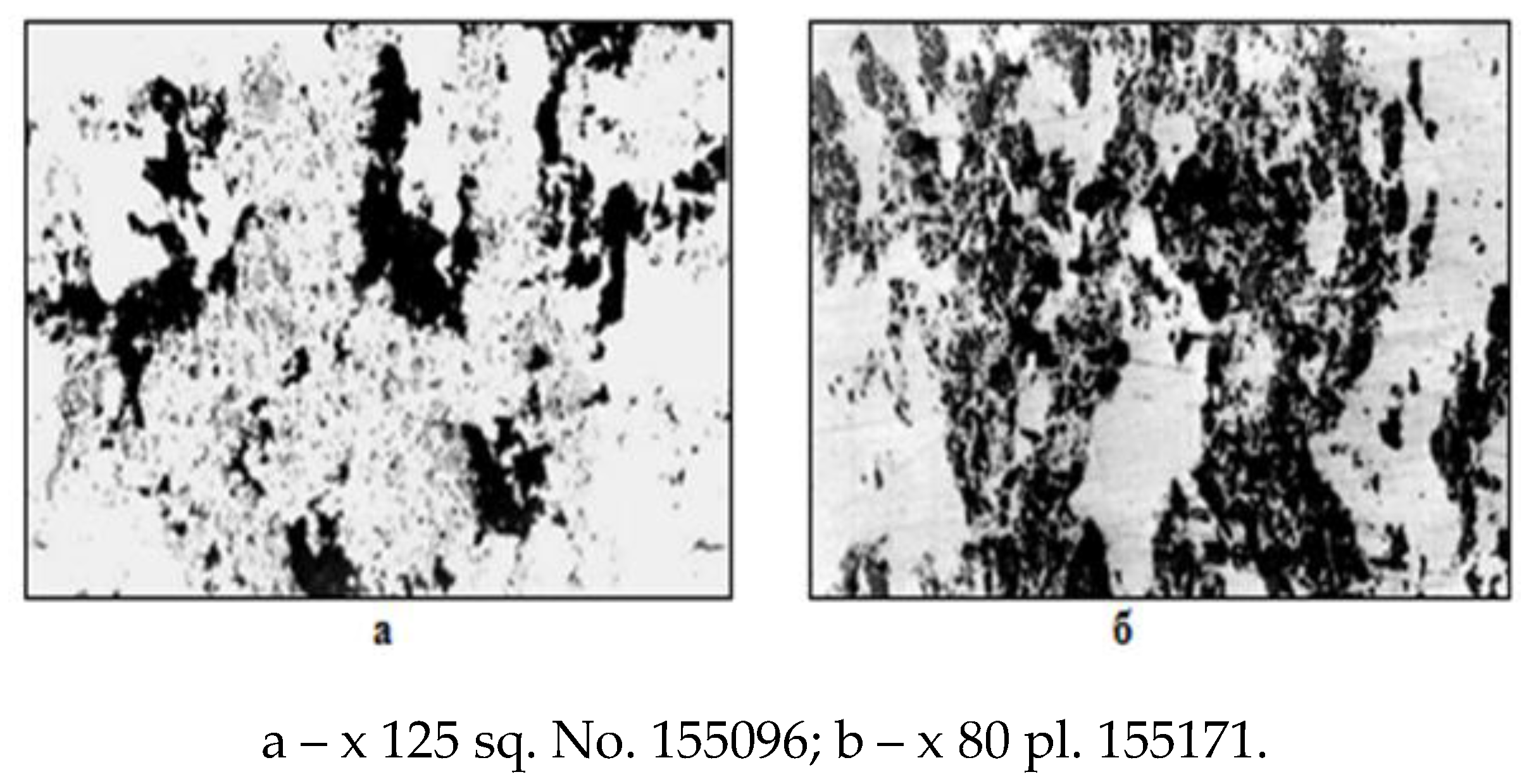
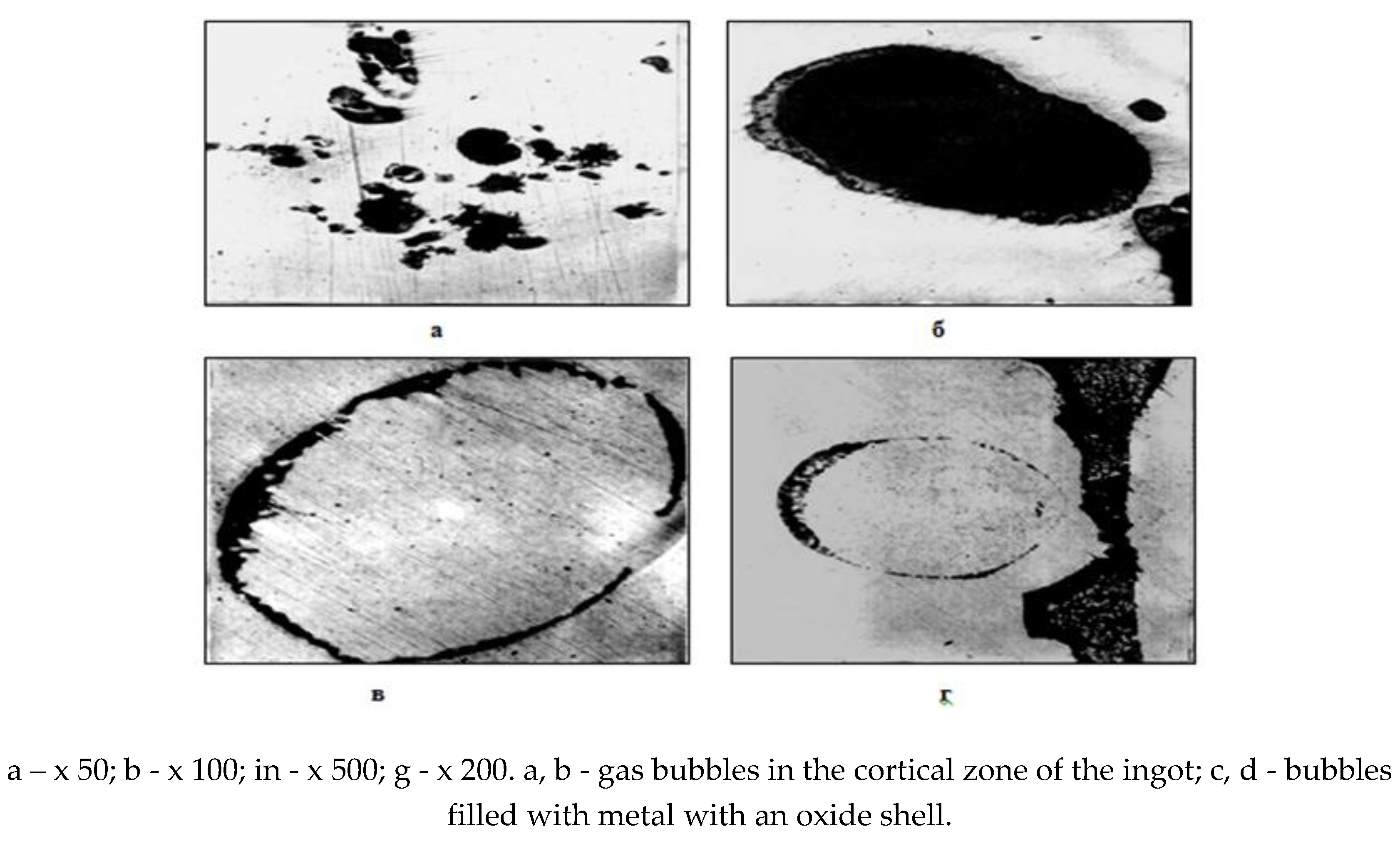
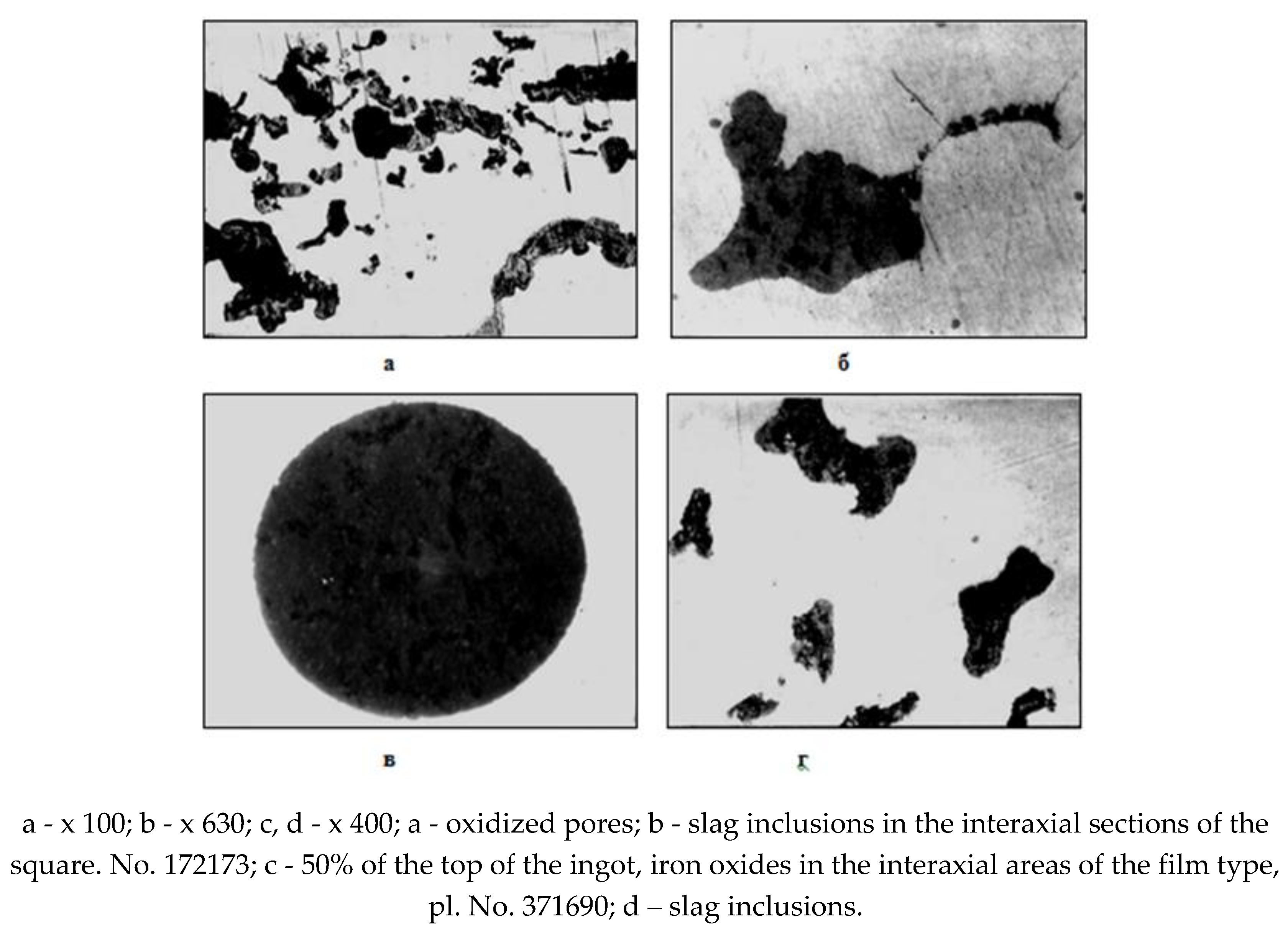
A detailed macro- and microstructural analysis of the structure of the surface zones of the ingots indicates the unsatisfactory condition of the crustal layer of the ingot surface. The steel crust zone has an increased content of gas bubbles (subcortical, pores, shells), often oxidized along the inner walls of the bubble or bubbles filled with metal, but with an oxide shell of composition ~ 85% FeO and up to 7% MnO (Fig. 5). The structure of the metal of the surface crust is loose, porous and contaminated with non-metallic inclusions in the form of iron oxides such as wüstite, products of secondary oxidation during casting, as well as slag particles (Fig. 6).
Rice. 3.
Accumulation of iron oxides at defects on the surface cold-rolled steel sheets grade 08 PS.
Rice. 3.
Accumulation of iron oxides at defects on the surface cold-rolled steel sheets grade 08 PS.
Rice. 4.
Microstructure of a gas bubble in a cold-rolled product.
Rice. 4.
Microstructure of a gas bubble in a cold-rolled product.
Rice. 5.
Microstructure of ingot corner templates.
Rice. 5.
Microstructure of ingot corner templates.
Rice. 6.
Accumulation of non-metallic inclusions in a boiling crust.
Rice. 6.
Accumulation of non-metallic inclusions in a boiling crust.
Discussion of Research Results
A comparative analysis of defects in cold-rolled steel shows that defects of steelmaking origin account for 62 to 84% of all surface defects (
Table 2). The results of metallographic studies show that 90% of surface defects in cold-rolled sheets are represented by a non-metallic oxide phase consisting of iron and manganese oxides. A high proportion of surface defects in the film from oxide non-metallic inclusions (33–38%) and a rolled bubble filled with the oxide phase FeO-MnO indicates their nucleation at the crystallization front in a two-phase zone enriched in manganese, oxygen, carbon, sulfur and phosphorus.
Oxidation of manganese occurs at all horizons of the forming ingot, including in the zone of predominant gas release, and the formation of the oxide phase from FeO and MnO occurs both at the crystallization front in the interdendritic sections of the liquid steel - solid surface phase boundary, and on the forming outer surface of the bubble CO.
The oxide liquid phase of FeO and MnO, which envelops the CO bubble upon separation, is carried away from the crystallization zone to the head part of the ingot, as evidenced by the appearance of slag foam (ingot slag) on the metal mirror in the mold during boiling. As a result of the movement of bubbles, a specific circulation of the melt occurs at the crystallization front, which promotes the drawing in of liquids and their oxidation products after the bubbles and the formation of micro-discontinuity and channels filled with an oxide slag phase, mainly FeO and MnO, or metal, but having an oxide rim along the inner surface of the discontinuity, which was recorded during metallographic analysis. The presence of pores filled with oxides is explained by much lower values of interfacial tension at the oxide phase - metal boundary than at the gas - metal boundary and, therefore, it is easier for an oxide phase nucleus (inclusion of oxides - FeO and MnO) to arise in a liquid metal than a gas nucleus.
The contamination of the cortical zone of the ingot with oxide non-metallic inclusions formed both on the surface of CO bubbles and in the interdendritic space is influenced by the intensity of metal boiling in the mold. With developed boiling, firstly, the thickness of the two-phase zone, enriched in liquates and the products of their interaction with oxygen, decreases and, secondly, intensive leaching of the resulting slag oxide phases into the head part of the ingot occurs.
The intensity of gas formation is determined by the degree of oxidation of the steel by the time it enters the mold, the liquid mobility of the metal, the solidification rate and casting temperature, as well as the chemical composition (oxygen, carbon, manganese content).
The main reason for the deterioration of the bottom part of the ingot is boiling (“swelling”) of the metal due to the formation of numerous small CO bubbles covered with a ferromanganese oxide film. The viscous oxide film prevents the free release of CO from the metal, which leads to foaming of the metal. The foamed slag-metal layer rises upward and, due to the large cooling effect of the cold walls of the mold, settles on its walls to form a frozen “shirt”. The high oxidative potential of the atmosphere in the cavity of the mold, due to the injection of air by a jet of metal, leads to oxidation of the surface of the “splash” frozen on the walls of the mold.
It has been established that the main share of defects is of steelmaking origin and is determined by three factors:
- a-
small thickness, porosity and low gas density of the crust layer of the ingot;
- b-
the formation of internal (hidden) films from splashes (splashes) of metal in the mold during casting;
- c-
contamination of the metal of the cortical zone of the ingot with oxide non-metallic inclusions.
In rolled sheets, this manifests itself in the presence of non-metallic inclusions of iron and manganese oxides or complex ferromanganese silicates in defective areas of the surface and subsurface layers of sheets.
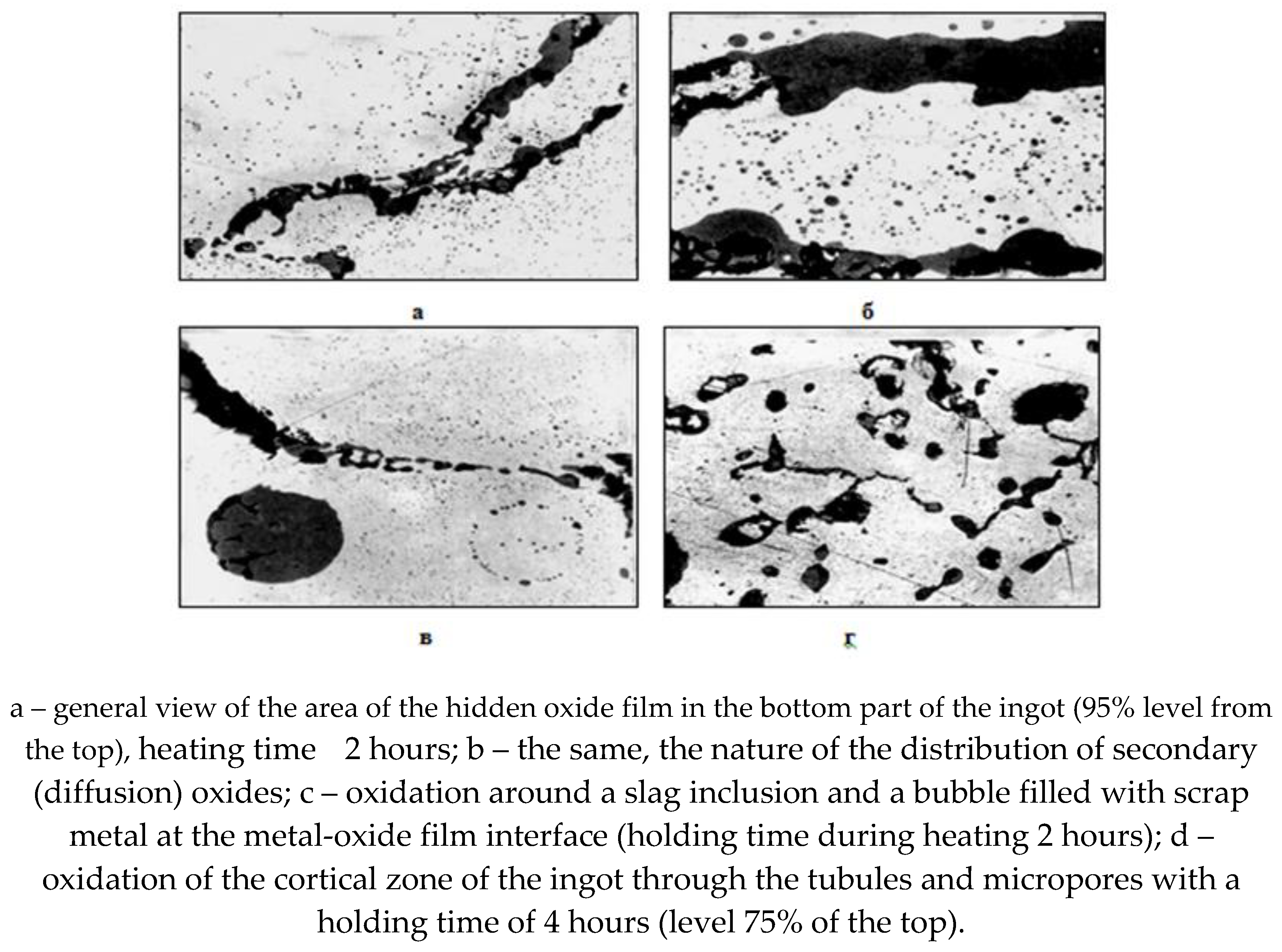
The following mechanism is proposed for the transformation of microdefects in the ingot into continuity defects in rolled sheets. When heating boiling steel ingots with microdefects in the structure of the crustal zone in the cells of the heating wells, further oxidation of the “hidden” film, subcrustal bubbles, and microporosity occurs, which leads to a change in the quantitative composition of oxide inclusions, i.e. to increase the FeO content from 34-85% to 91-94%. In addition, the diffusion nucleation of oxide satellite inclusions in the form of an oxide rash additionally occurs and the area of the defective metal increases (Fig. 7).
Rice. 7.
The nature of the distribution of non-metallic phases after high-temperature heating of steel grade 08 KP, pl. No. 172191.
Rice. 7.
The nature of the distribution of non-metallic phases after high-temperature heating of steel grade 08 KP, pl. No. 172191.
The source of oxygen for the diffusion oxidation of macro- and micro-discontinuities, the nucleation of new satellite oxide phases of the crust zone of the ingot is the oxidizing furnace atmosphere, as well as the oxide phase in the form of a “hidden” film, oxide non-metallic inclusions, as well as scale on the surface of the heating ingot. Point quantitative analysis showed that the content of total oxygen in the “hidden” film is 23-31%, which may well serve as an additional oxidizing agent for internal diffusion oxidation of the metal.
When rolling an ingot, the shape changes and the distribution of microdefects occurs. In the surface zones of the steel ingot, accumulations of inclusions are found in the form of films along the interaxial areas of the dendrites (Fig. 8, a).
Coarse defects of the ingot located close to the surface, already at the first rolling stage, roll out onto the surface of the slab and give a defect in the form of honeycomb torn and film, or after a fire cleaning machine, where a 4-6 mm layer of the metal surface is removed, revealing internal defects of the ingot (Fig. 9 , a, b).
As the layer of healthy metal above the defect thins at subsequent rolling stages, due to the unequal plasticity of the steel matrix and inclusions, they come to the surface of the strip and form defects such as rolled bubbles and films, for example, when heating the slab in methodical furnaces and rolling in a hot mill line rolling (Fig. 9, c, d, e) or during cold-rolled rolling and temper training (Fig. 9, f, g). Large inclusions in the form of oxide films, which are formed mainly in the bottom part of the ingot, form rough defects on the surface of the sheets, usually with peeling or scanlines.
Rice. 8.
Microstructure of steel at the site of surface defects cold rolled sheets.
Rice. 8.
Microstructure of steel at the site of surface defects cold rolled sheets.
Conclusion
Thus, the results of the experiments make it possible to reveal the nature of non-metallic inclusions contaminating the surface zones of the ingot, to clarify the sources and causes of the formation of the main types of defects in rolled sheets:
Rice. 9.
Mechanism of transformation of ingot defects into surface defects sheet metal.
Rice. 9.
Mechanism of transformation of ingot defects into surface defects sheet metal.
- 1
-
The main share of surface defects in thin-sheet cold-rolled steel is of steelmaking origin (62-84%) and is determined by three factors:
- a-
the presence of a thin and loose outer cortical layer of the ingot, damaged by subcortical bubbles, pores, and tubules due to the high oxidation of the steel;
- b-
the presence of an internal (“hidden”) film from boiling of the metal in the mold during casting. In rolled sheets, this manifests itself in the presence of non-metallic inclusions of iron and manganese oxides or complex ferromanganese silicates at places of defects in the surface and subsurface layers of sheets;
- c-
contamination of the cortical zone of the ingot with oxide non-metallic inclusions.
- 2
The identity of the morphological signs of structural heterogeneity in the cortical zone of the ingot and in the place of manifestation of the defect in cold-rolled steel has been established. The difference lies in the shape, distribution of inclusions and concentration of FeO in wüstite.
- 3
An increase in the concentration of FeO in wustite from 80-85% in the cortical zone of the ingot to 87-93% at the site of the defect indicates that a significant part of the defects are formed during the process of oxidative heating and subsequent hot deformation. During the heating process, oxidation of the internal cavities of microdefects occurs, as well as the formation of additional satellite oxide inclusions in the form of dispersed oxide rash and globules of diffusion wustite oxides near areas of large non-metallic inclusions, increasing the area affected by defects.
- 4
Surface defects of cold-rolled sheets are 90% represented by a non-metallic oxide phase consisting of iron and manganese oxides. A high proportion of surface defects in the film from oxide non-metallic inclusions (33–38%) and a rolled bubble filled with the oxide phase FeO-MnO indicates their nucleation at the crystallization front in a two-phase zone enriched in manganese, oxygen, carbon, sulfur and phosphorus.
- 5
The formation of structural heterogeneity in the form of a “hidden” film in the bottom part of the ingot is associated with the boiling of the first portions of steel and its crystallization on the surface of the mold. The boiling of steel is associated with the formation of a large number of small bubbles of CO, covered with a thin slag film of viscous ferromanganese oxides, which prevent rupture of the film and the release of CO from the metal.
- 6
Coarse defects of the ingot located close to the surface are rolled out onto the surface of the slab in the form of honeycomb waste and film already at the first rolling stage. As the layer of healthy metal above the defect thins at subsequent rolling stages, due to the unequal ductility of the steel matrix and inclusions, they come to the surface of the sheets and form defects such as rolled bubbles and film. Large inclusions, in the form of oxide films, which are formed mainly in the bottom part of the ingot, form rough films on the surface of the sheets, usually with peeling or scaliness.
Based on a study of the structural and concentration features of the macro- and microstructure of the metal at the end-to-end metallurgical processing of ingot - thin-sheet rolled products, the following was established:
- –
mechanism and causes of microstructural heterogeneity, increased contamination of the cortical zone of the ingot;
- –
the nature of the relationship between the type of structure of the cortical zone of the ingot and the development of defects in the rolled surface;
- –
mechanism of transformation of structural heterogeneity of the cortical zone of the ingot into defects in the surface of rolled sheets;
- –
significant influence of the metal heating mode on the further development of defects in the ingot and finished rolled products.
References
- Sychkov A.B. Transformation of continuously cast billet defects into rolled surface defects / A.B. Sychkov, M.A. Zhigarev, A.V. Perchatkin // Metallurgist. – 2006. – No. 2. – pp. 60–64. 2. Pravosudovich V.B. et al. Defects in steel ingots and rolled products: Reference. ed. M.: Intermet Engineering, 2006. 384 p.
- Steel defects: Ref. ed. / Ed. S. M. Novokshchenova, M. I. Vinograd. M.: Metallurgy, 1984. 199 p.
- Zaitsev A.I., Rodionova I.G., Khoroshilov A.D., Mezin F.I. et al. Analysis of the nature of the occurrence of surface defects in cold-rolled IF steels // Journal "Electrometallurgy" 2012. No. 7. pp. 36-40.
- L. Zhang, J. Zhi, F. Mei, L. Zhu, X. Jiang, J. Shen, J. Cui, K. Cai and B. G. Thomas. Basic oxygen furnace based steelmaking processes and cleanliness control at Baosteel // Institute of Materials, Minerals and Mining Published by Maney on behalf of the Institute, 2006.
- O.B. Isaev. Improving the technology of steel refining in the tundish of a continuous casting machine in order to improve the quality of continuously cast billets and rolled plates, Azovstal OJSC // Metallurg 2009, No. 11.
- Yang Wen’, Cao Jing’, Wang Xin-hua. Investigation on Non-Metallic Inclusions in LCAK Steel Produced by BOF-LF-FTSC Production Route // Journal of iron and steel research, international. 2011. Vol.18, No.9, P. 06-12, 20.
- Cong Wang, Neerav Verma, Youjong Kwon. Study on the Transient Inclusion Evolution during Reoxidation of a Fe–Al–Ti–O Melt // ISIJ International. 2011.Vol. 51, No. 3, pp. 375–381.
- M. Lind. Mechanism and kinetics of transformation of aluminum inclusions in steel by calcium treatment. Doctoral Thesis. Helsinki University of Technology Publications in Materials Science and Engineering., Helsinki, 2006. 89 p.
- G.Cabai, F.Cabai. Continuous casting of steel. Some principles and practical notes. – STS s.r.l., 2010. – 112 p.
Table 2.
Classification of defects in cold-rolled sheets.
Table 2.
Classification of defects in cold-rolled sheets.
Classification
defects |
pl. No. 380520
08KP |
pl. No. 180521
08 KP |
pl. No. 0180654 08 PS |
Average
values |
| peal bubble, % |
48,3 |
45,8 |
66,0 |
56,6 |
| captivity from n/inclusions, % |
37, 9 |
45,8 |
22,6 |
32,1 |
| ingot film, % |
10,3 |
- |
1,9 |
3,8 |
| other, % |
3,4 |
8,3 |
9,4 |
7,5 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).