Submitted:
23 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Insects[29]
2.2. Yeast β-galactosidase Assay
2.3. Bioassay
2.4. RNA Extraction, Primers, and Quantitative Real Time Polymerase Chain Reaction (qPCR) Analysis
2.5. Data Analysis
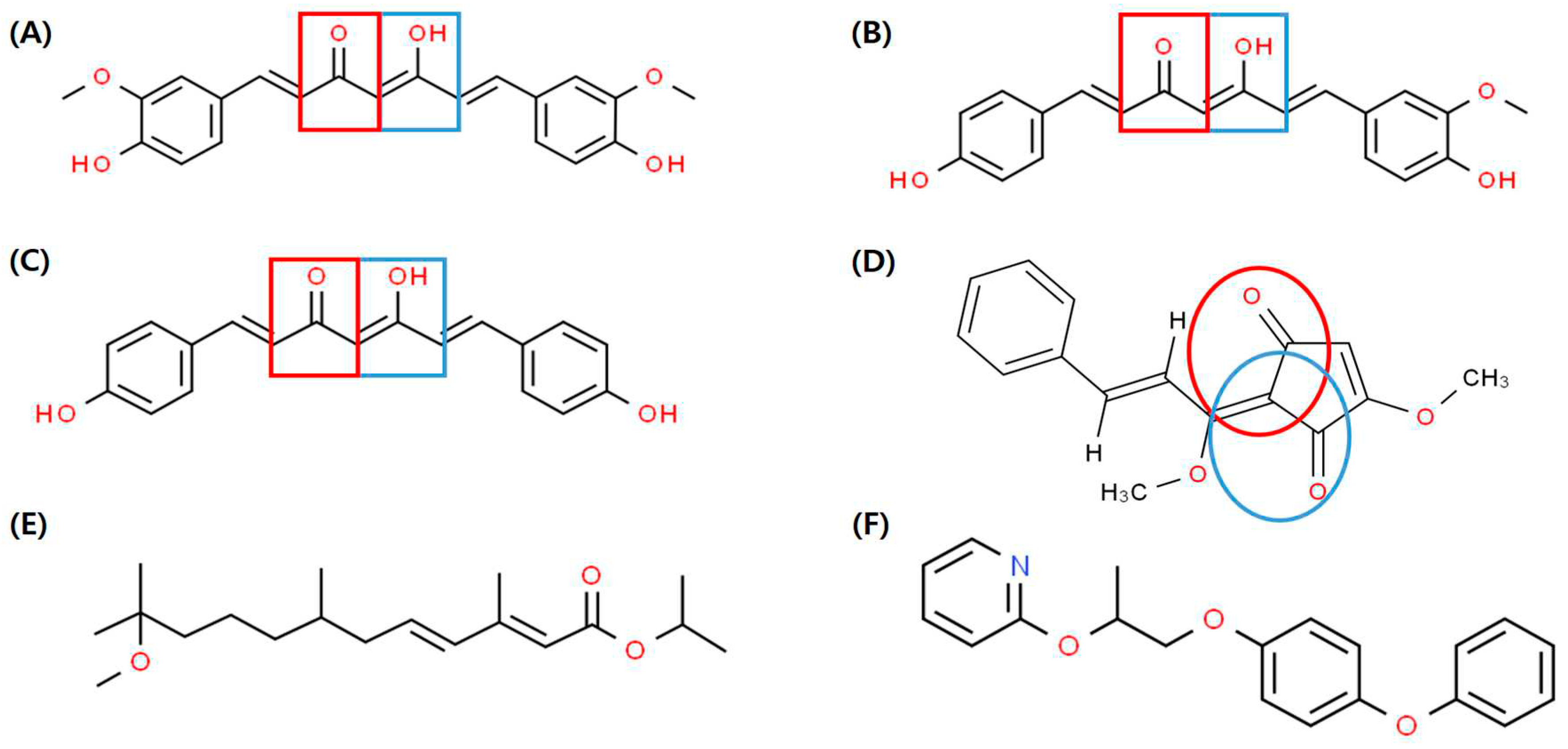
3. Results
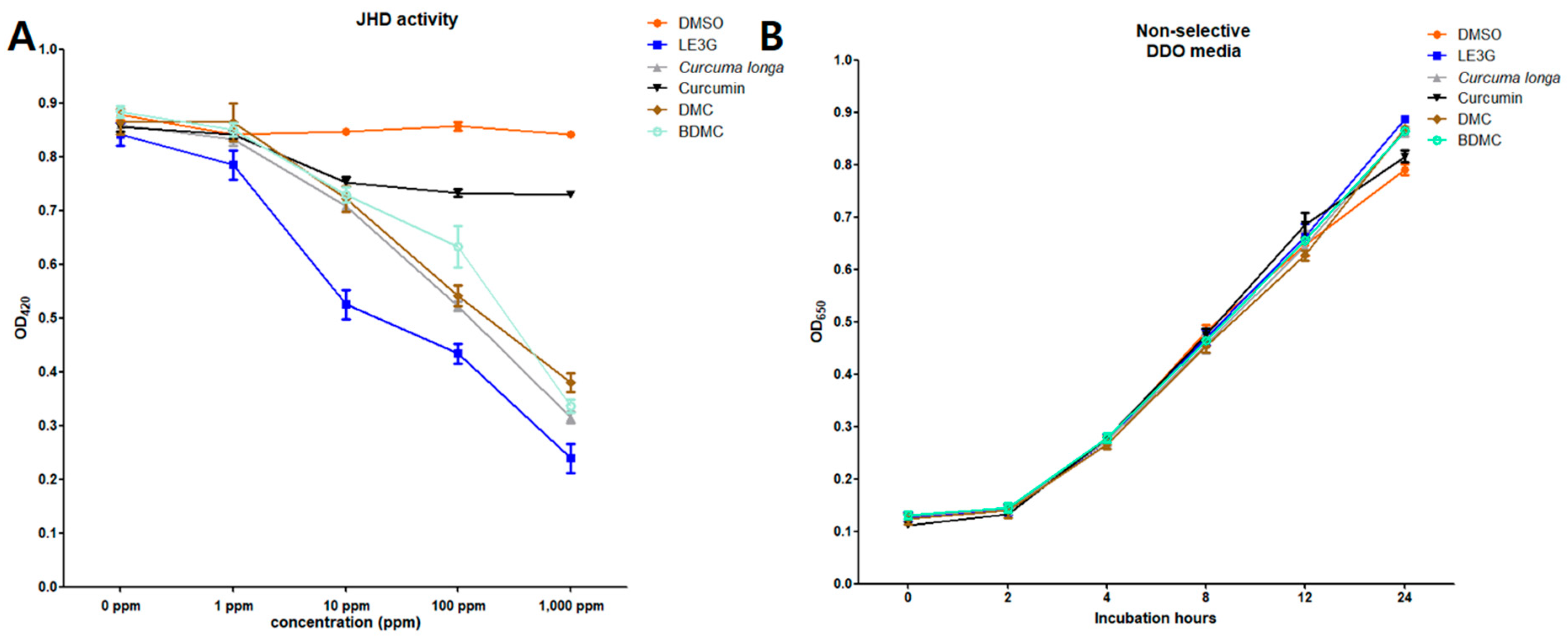
3.1. JHD Activity of Plant Extracts
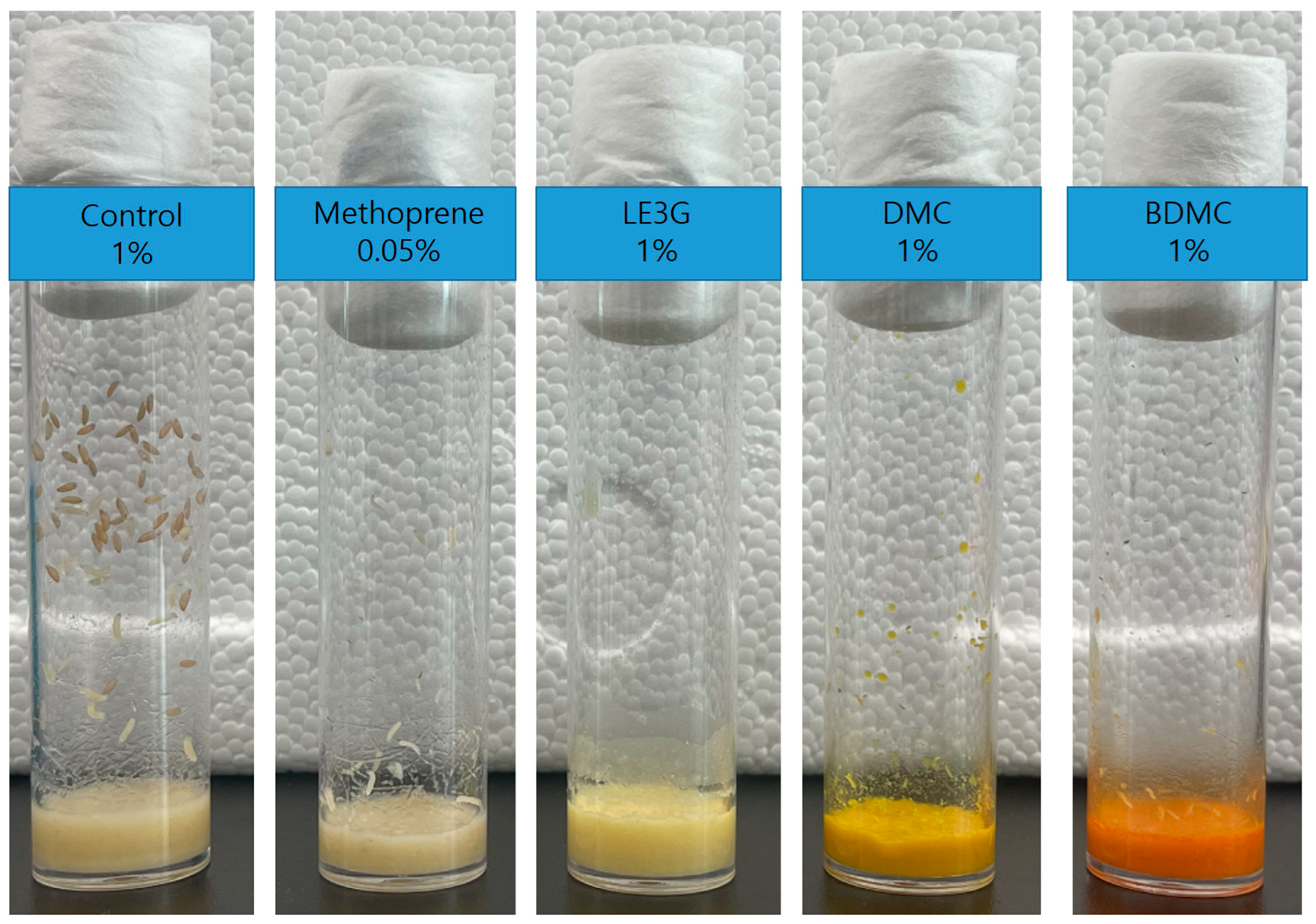
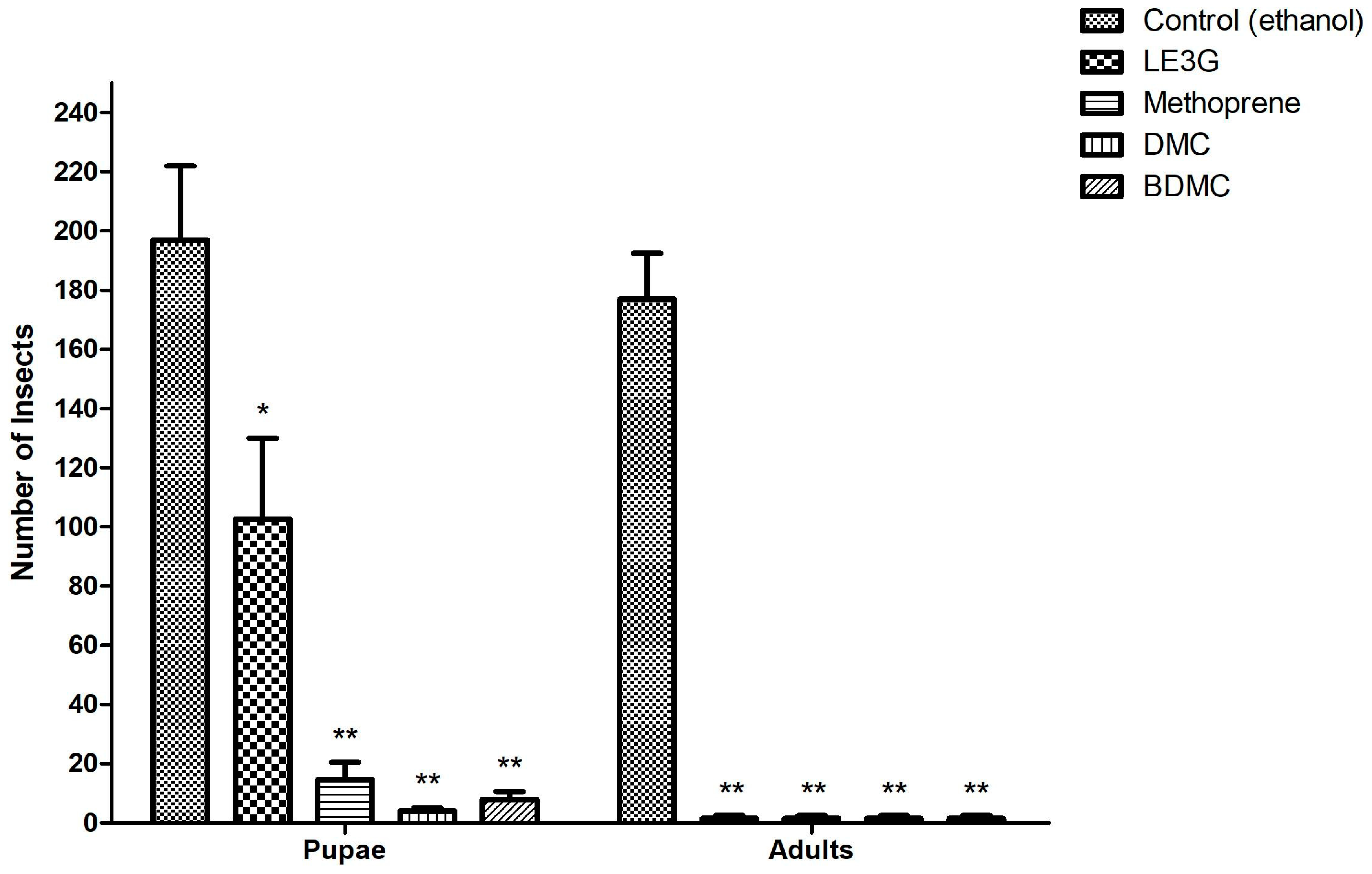
3.2. Changes in the Emergence Rates of D. melanogaster Larvae According to Feeding
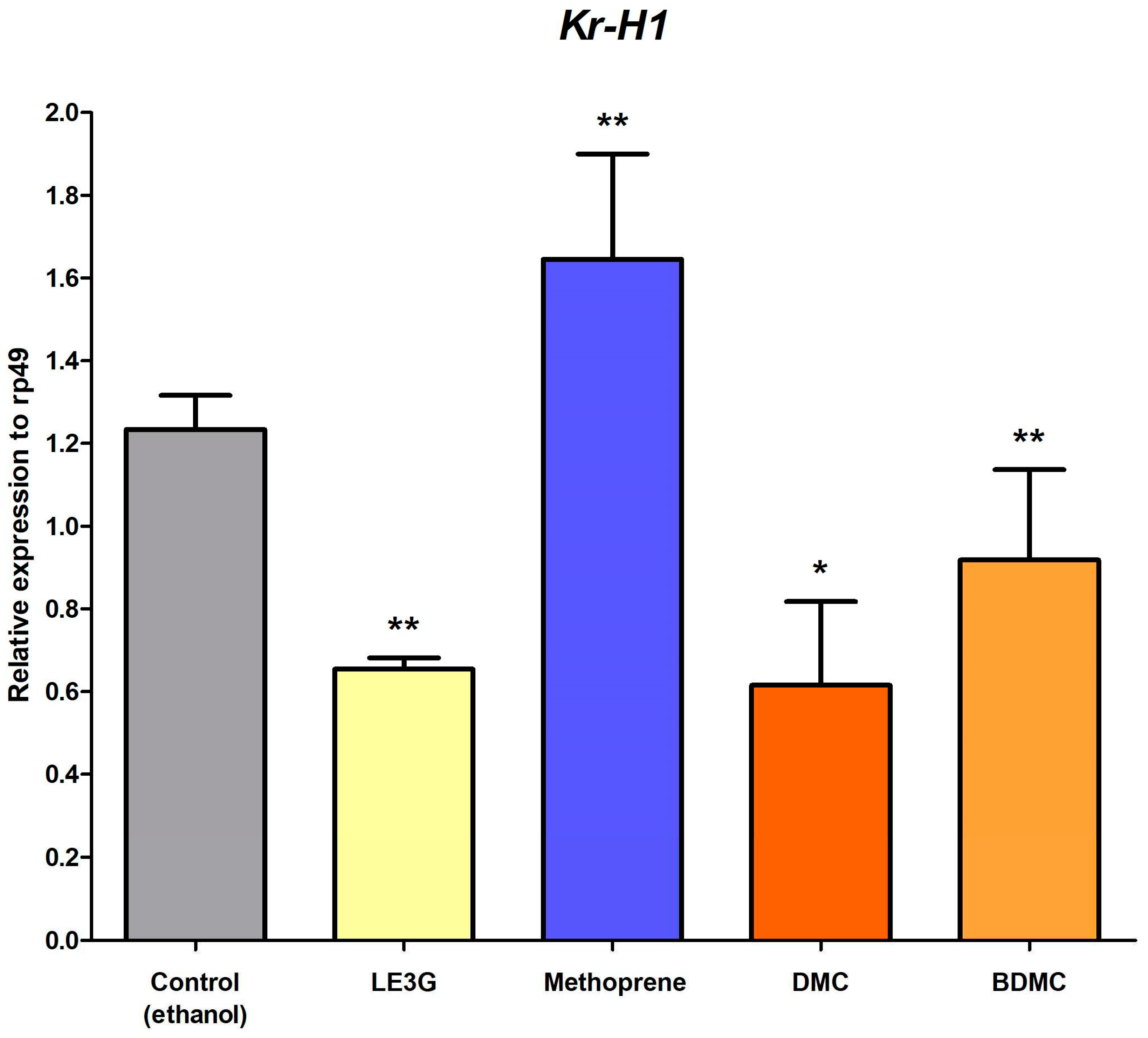
3.3. DMC and BDMC Effects on JH-dependent gene expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lacey, L.A.; Lacey, C.M. The medical importance of riceland mosquitoes and their control using alternatives to chemical insecticides. Journal of the American Mosquito Control Association. Supplement 1990, 2, 1–93. [Google Scholar] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop protection 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Amalraj, A.; Kuttappan, S.; Matharu, A. Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Elsevier, 2022. [Google Scholar]
- Zhang, H.A.; Kitts, D.D. Turmeric and its bioactive constituents trigger cell signaling mechanisms that protect against diabetes and cardiovascular diseases. Molecular and cellular biochemistry 2021, 476, 3785–3814. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: miniperspective. Journal of medicinal chemistry 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnology advances 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—from molecule to biological function. Angewandte Chemie International Edition 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radical Biology and Medicine 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Labban, L. Medicinal and pharmacological properties of Turmeric (Curcuma longa): A review. Int J Pharm Biomed Sci 2014, 5, 17–23. [Google Scholar]
- Durgaprasad, S.; Pai, C.G.; Alvres, J.F. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian Journal of Medical Research 2005, 122, 315. [Google Scholar] [PubMed]
- Ravindran, P.; Babu, K.N.; Sivaraman, K. Turmeric: the genus Curcuma; CRC press, 2007. [Google Scholar] [CrossRef]
- Basak, S.; Sarma, G.C.; Rangan, L. Ethnomedical uses of Zingiberaceous plants of Northeast India. Journal of ethnopharmacology 2010, 132, 286–296. [Google Scholar] [CrossRef]
- Solsoloy, A.; Cacayorin, N.; Cano, L. Insecticidal and fungicidal action of some indigenous plant extracts against cotton pests. Cotton Research Journal (Philippines) 1991. [Google Scholar]
- Aurade, R.M.; Jayalakshmi, S.K.; Sreeramulu, K. Modulatory effects of natural curcuminoids on P-glycoprotein ATPase of insecticide-resistant pest Helicoverpa armigera (Lepidopetera: Noctüidae). The Journal of Membrane Biology 2010, 236, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.N.; Chandra, A.; Nair, M.G. Novel bioactivities of Curcuma longa constituents. Journal of natural products 1998, 61, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Matiadis, D.; Liggri, P.G.; Kritsi, E.; Tzioumaki, N.; Zoumpoulakis, P.; Papachristos, D.P.; Balatsos, G.; Sagnou, M.; Michaelakis, A. Curcumin derivatives as potential mosquito larvicidal agents against two mosquito vectors, Culex pipiens and Aedes albopictus. International Journal of Molecular Sciences 2021, 22, 8915. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Goswami, D.; Rawal, R.M. Revealing the molecular interplay of curcumin as Culex pipiens Acetylcholine esterase 1 (AChE1) inhibitor. Scientific Reports 2021, 11, 17474. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Silva, A.S.; Varshney, R.; Chávez-González, M.L.; Singh, P. Curcuma-based botanicals as crop protectors: From knowledge to application in food crops. Current Research in Biotechnology 2021, 3, 235–248. [Google Scholar] [CrossRef]
- Truman, J.W.; Riddiford, L.M. The morphostatic actions of juvenile hormone. Insect biochemistry and molecular biology 2007, 37, 761–770. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Li, M.; Mead, E.A.; Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci U S A 2011, 108, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Oda, M.; Makita, S.; Chinzei, Y. Characterization of the Drosophila Methoprene -tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J 2005, 272, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci U S A 2011, 108, 21128–21133. [Google Scholar] [CrossRef] [PubMed]
- Kayukawa, T.; Minakuchi, C.; Namiki, T.; Togawa, T.; Yoshiyama, M.; Kamimura, M.; Mita, K.; Imanishi, S.; Kiuchi, M.; Ishikawa, Y.; et al. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci U S A 2012, 109, 11729–11734. [Google Scholar] [CrossRef]
- Kewley, R.J.; Whitelaw, M.L.; Chapman-Smith, A. The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. The international journal of biochemistry & cell biology 2004, 36, 189–204. [Google Scholar] [CrossRef]
- Tumova, S.; Milacek, M.; Šnajdr, I.; Muthu, M.; Tuma, R.; Reha, D.; Jedlicka, P.; Bittova, L.; Novotna, A.; Majer, P. Unique peptidic agonists of a juvenile hormone receptor with species-specific effects on insect development and reproduction. Proceedings of the National Academy of Sciences 2022, 119, e2215541119. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, H.W.; Fang, Y.; An, S.B.; Park, D.S.; Song, H.H.; Oh, S.R.; Kim, S.Y.; Kim, S.; Kim, N.; et al. Identification of plant compounds that disrupt the insect juvenile hormone receptor complex. Proc Natl Acad Sci U S A 2015, 112, 1733–1738. [Google Scholar] [CrossRef]
- Oh, H.W.; Yun, C.S.; Jeon, J.H.; Kim, J.A.; Park, D.S.; Ryu, H.W.; Oh, S.R.; Song, H.H.; Shin, Y.; Jung, C.S.; et al. Conifer Diterpene Resin Acids Disrupt Juvenile Hormone-Mediated Endocrine Regulation in the Indian Meal Moth Plodia interpunctella. J Chem Ecol 2017, 43, 703–711. [Google Scholar] [CrossRef]
- Shin, S.W.; Jeon, J.H.; Jeong, S.A.; Kim, J.A.; Park, D.S.; Shin, Y.; Oh, H.W. A plant diterpene counteracts juvenile hormone-mediated gene regulation during Drosophila melanogaster larval development. PLoS One 2018, 13, e0200706. [Google Scholar] [CrossRef]
- Shin, S.W.; Jeon, J.H.; Yun, C.S.; Jeong, S.A.; Kim, J.A.; Park, D.S.; Shin, Y.; Oh, H.W. Species-Specific Interactions between Plant Metabolites and Insect Juvenile Hormone Receptors. J Chem Ecol 2018, 44, 1022–1029. [Google Scholar] [CrossRef]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction? Front Physiol 2018, 9, 364. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. Journal of Plant Biology 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Science of the Total Environment 2021, 777, 146204. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. International journal of molecular sciences 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Dubey, N. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest biology and Technology 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. Journal of stored products Research 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Damalas, C.A. Potential uses of turmeric (‘Curcuma longa’) products as alternative means of pest management in crop production. Plant omics 2011, 4, 136–141. [Google Scholar]
- Sagnou, M.; Mitsopoulou, K.; Koliopoulos, G.; Pelecanou, M.; Couladouros, E.; Michaelakis, A. Evaluation of naturally occurring curcuminoids and related compounds against mosquito larvae. Acta tropica 2012, 123, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, H.; Walia, S.; Saxena, V.S. Isolation, characterization and insect growth inhibitory activity of major turmeric constituents and their derivatives against Schistocerca gregaria (Forsk) and Dysdercus koenigii (Walk). Pest Management Science: formerly Pesticide Science 2000, 56, 1086–1092. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Industrial crops and products 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. Journal of Herbal Medicine 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Sláma, K. The history and current status of juvenoids. In Proceedings of the Proceedings of 3rd International Conference on Urban Pests, 1999; pp. 9–25.
- Senthil Kumar, K.; Wang, S.-Y. Pharmacological Applications of Lucidone: A Naturally Occurring Cyclopentenedione. Medicinal Plants-Recent Advances in Research and Development 2016, 273–295. [Google Scholar] [CrossRef]
- Shin, S.-W.; Jeon, J.-H.; Kim, J.-A.; Park, D.-S.; Shin, Y.-J.; Oh, H.-W. Inducible Expression of Several Drosophila melanogaster Genes Encoding Juvenile Hormone Binding Proteins by a Plant Diterpene Secondary Metabolite, Methyl Lucidone. Insects 2022, 13, 420. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer |
|---|---|
| Krüppel homolog 1 | Forward 5′-TCACACATCAAGAAGCCAACT-3′ Reverse 5′-GCTGGTTGGCGGAATAGTAA-3′ |
| Rp49 | Forward 5′-ATGCTAAGCTGTCGCACAAATG-3′ Reverse 5′-GTTCGATCCGTAACCGATGT-3′ |
| Species | Plant part | Family | JHD activity |
|---|---|---|---|
| Curcuma longa L. | Root | Zingiberaceae | 1.217175 |
| Pulsatilla koreana Nakai | Root | Ranunculaceae | 1.101688 |
| Machilus thunbergii Siebold & Zucc. | Trunk-bark | Lauraceae | 1.056931 |
| Echinosophora koreensis Nakai | Root | Fabaceae | 0.960778 |
| Pinus densiflora Siebold & Zucc. | Trunk-bark | Pinaceae | 0.936298 |
| Alpinia officinarum | Root | Zingiberaceae | 0.904591 |
| Smilax sieboldii Miq. | Leaf | Liliaceae | 0.886733 |
| Scutellaria baicalensis | Flower | Labiatae | 0.825496 |
| Portulaca oleracea L. | Whole | Portulacaceae | 0.806894 |
| Magnolia kobus DC | Leaf | Magnoliaceae | 0.785417 |
| Broussonetia papyrifera (L.) L’Hér. ex Vent. | Leaf | Moraceae | 0.768187 |
| Syringa patula (Palib.) Nakai | Leaf | Oleaceae | 0.759335 |
| Agrimonia pilosa Ledeb. | Whole | Rosaceae | 0.750968 |
| Cudrania tricuspidata (Carr.) Bureau ex Lavallée | Fruit | Moraceae | 0.745534 |
| Psoralea corylifolia (Babchi) | Seed | Fabaceae | 0.658419 |
| Phlomis umbrosa Turcz. | Whole | Labiatae | 0.65772 |
| Cudrania tricuspidata (Carr.) Bureau ex Lavallée | Trunk | Moraceae | 0.613586 |
| Zingiber officinale | Root | Zingiberaceae | 0.546682 |
| Myristica fragrans | Seed | Myristicaceae | 0.535624 |
| Saururus chinensis (Lour.) Baill. | Whole | Saururaceae | 0.493735 |
| Glycyrrhiza uralensis | Root | Fabaceae | 0.469728 |
| Picrasma quassioides (D. Don) Benn. | Trunk | Simaroubaceae | 0.433324 |
| Sophora flavescens | Root | Fabaceae | 0.391224 |
| Morus alba L. | Root | Moraceae | 0.390836 |
| Actinostemma lobatum Maxim. | Whole | Cucurbitaceae | 0.381283 |
| Salvia miltiorrhiza Bunge | Whole | Labiatae | 0.363577 |
| Cudrania tricuspidata (Carr.) Bureau ex Lavallée | Root | Moraceae | 0.257315 |
| Eclipta prostrata | Whole | Compositae | 0.237714 |
| Magnolia obovata Thunb | Trunk-bark | Lauraceae | 0.176234 |
| Angelica keiskei | Leaf | Apiaceae | 0.134399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





