Submitted:
22 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
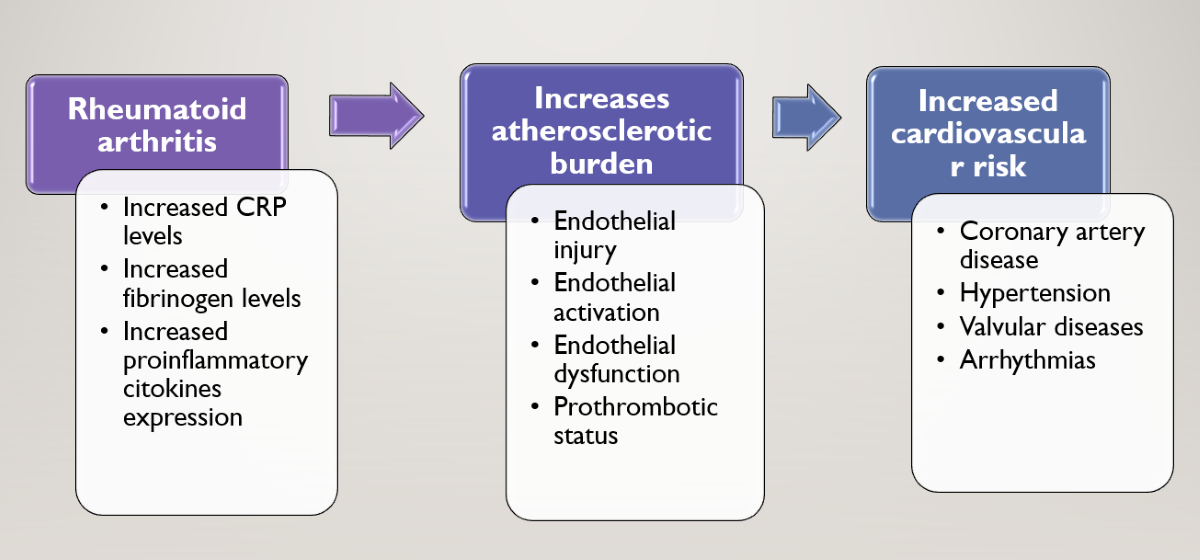
1. Introduction
2. Materials and methods
2.1. Study design and study approval
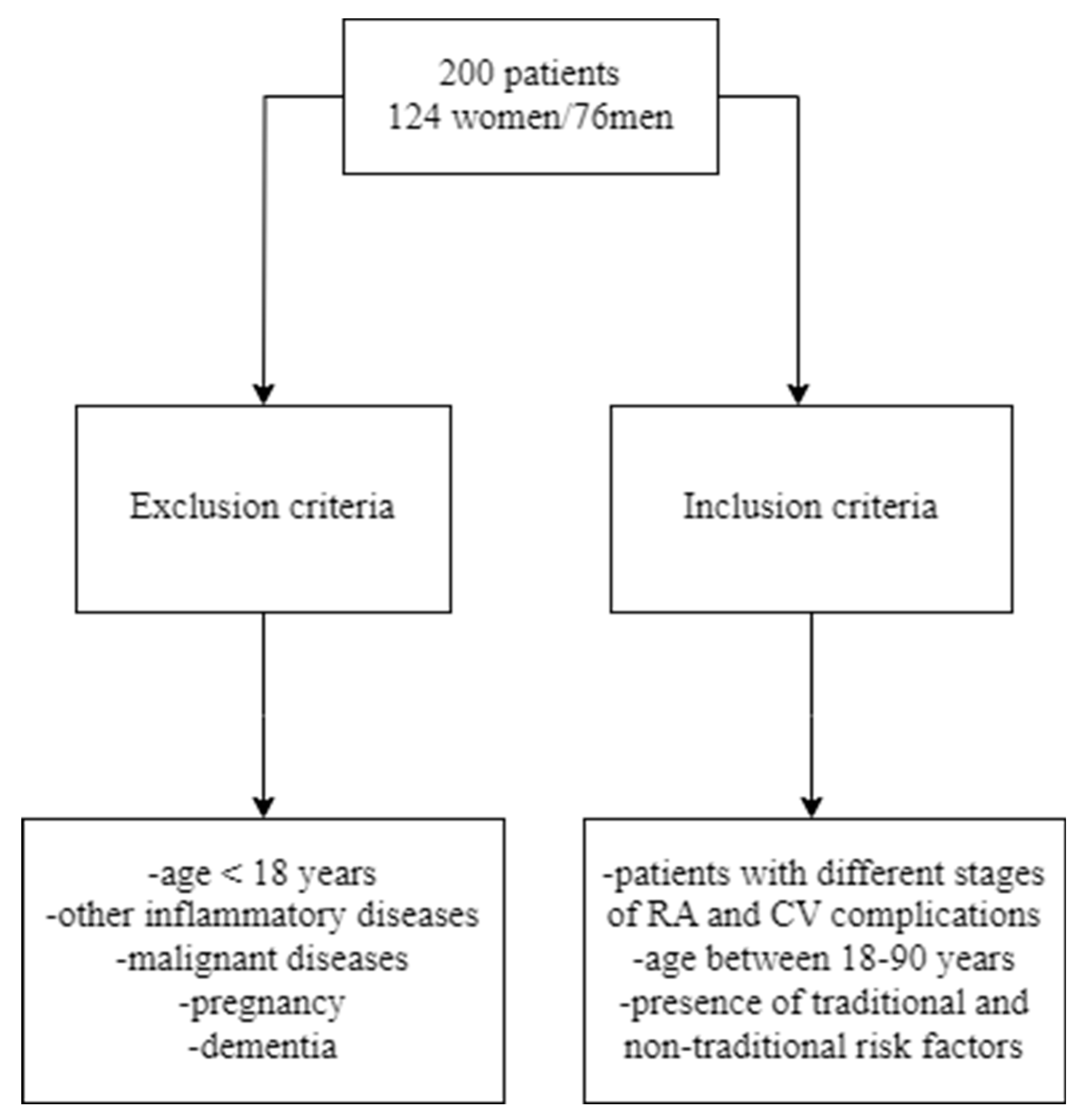
2.2. Inclusion and exclusion criteria
2.3. Data collection
| Clinical Disease Activity Index(CDAI) | |
|---|---|
| 0.0-2.8 | Remission |
| 2.9-10.0 | Low activity |
| 10.1-22.0 | Moderate activity |
| 22.1-76.0 | High activity |
- medical history of joint symptoms (pain, tenderness, stiffness, swelling);
- joint symptoms have lasted more than six weeks;
- elevated levels of CRP or ESR;
- positive biomarker test like rheumatoid factor (RF);
- radiographic changes of bones and joints.
2.4. Statistical Analyses
3. Results
3.1. Patient characteristics and outcomes
- -
- A decrease of total cholesterol (pre: median = 172.5, IQR = 155-229 vs. post: median = 150, IQR = 145-200) (p<0.001) the observed difference being significant (23.78 ± 18.9, median = 20 (IQR = 10-30.75));
- -
- A decrease of triglycerides (pre: median = 120, IQR = 95-160 vs. post: median = 100, IQR = 90-150) (p<0.001) the observed difference being significant (14.32 ± 22.95, median = 10 (IQR = 5-25));
- -
- A decrease of LDL-cholesterol (pre: median = 110, IQR = 92.25-153 vs. post: median = 90.5, IQR = 82.25-129 (p<0.001) the observed difference being significant (22.34 ± 17, median = 18 (IQR = 11-30.75));
- -
- An increase of HDL cholesterol (pre: median = 40, IQR = 40-44 vs. post: median = 42, IQR = 41-45) (p<0.001) the observed difference being significant (1.13 ± 2.5, median = 1 (IQR = 0-2)).
3.2. Correlation of patients‘ parameters
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatology International 2020, 41, 863–877. [Google Scholar] [CrossRef]
- Logstrup, B.B.; Ellingsen, T.; Pedersen, A.B.; Darvalics, B.; Olesen, K.K.W.; Botker, H.E.; Maeng, M. Cardiovascular risk and mortality in rheumatoid arthritis compared with diabetes mellitus and the general population. Rheumatology 2021, 60, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthr. Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Meune, C.; Touze, E.; Trinquart, L.; Allanore, Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years:A systematic review and meta-analysis of cohort studies. Rheumatology 2009, 48, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Crowson, C.S.; Rollefstad, S.; Ikdahl, E.; Kitas, G.D.; van Riel, P.; Gabriel, S.E.; Matteson, E.L.; Kvien, T.K.; Douglas, K.; Sandoo, A.; et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 48–54. [Google Scholar] [CrossRef]
- Boyer, J.-F.; Gourraud, P.-A.; Cantagrel, A.; Davignon, J.-L.; Constantin, A. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Jt. Bone Spine 2011, 78, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Trupin, L.; Shiboski, S.; van der Laan, M.; Graf, J.; Imboden, J.; Yazdany, J.; Schmajuk, G. Smoking Is Associated with Higher Disease Activity in Rheumatoid Arthritis: A Longitudinal Study Controlling for Time-varying Covariates. J. Rheumatol. 2019, 46, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Abbot, S.E.; Whish, W.J.; Jennison, C.; Blake, D.R.; Stevens, C.R. Tumour necrosis factor alpha stimulated rheumatoid synovial microvascular endothelial cells exhibit increased shear rate dependent leucocyte adhesion in vitro. Ann Rheum Dis. 1999; 58, 573–581. [Google Scholar]
- Vaudo G, Marchesi S, Gerli R, Allegrucci R, Giordano A, Siepi D, Pirro M, Shoenfeld Y, Schillaci G, Mannarino E. Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis. 2004, 63, 31–35.
- Hurlimann, D.; Forster, A.; Noll, G.; Enseleit, F.; Chenevard, R.; Distler, O.; Bechir, M.; Spieker, L.E.; Neidhart, M.; Michel, B.A.; et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002, 106, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.A.; van der Heijde, D.M.F.M.; van Rijswijk, M.H.; et al. Interrelationship of outcome measures and process variables in early rheumatoid arthritis: a comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol 1994, 21, 425–429. [Google Scholar] [PubMed]
- Naranjo, A.; Sokka, T.; Descalzo, M.A.; Calvo-Alén, J.; Hørslev-Petersen, K.; Luukkainen, R.K.; Combe, B.; Burmester, G.R.; Devlin, J.; Ferraccioli, G.; Morelli, A.; Hoekstra, M.; Majdan, M.; Sadkiewicz, S.; Belmonte, M.; et al. Cardiovascular disease in patients with rheumatoid arthritis: Results from the quest-ra study. Arthritis Research; Therapy. 2008; 10. [Google Scholar]
- Roman, M.J.; Salmon, J.E. Cardiovascular manifestations of rheumatologic diseases. Circulation 2007, 116, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, L.R.; Woodman, R.J.; Shanahan, E.M.; Mangoni, A.A. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117952. [Google Scholar] [CrossRef] [PubMed]
- Abdulqader, Y.; Al-Ani, M.; Parperis, K. Rheumatoid vasculitis: early presentation of rheumatoid arthritis. BMJ Case Rep. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, M.; De Angelis, V.; Riboldi, P.; Meroni, P.L. Rheumatoid arthritis: a female challenge. Womens Health 2008, 4, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juanatey, C.; Testa, A.; Garcia-Castelo, A.; Garcia-Porrua, C.; Llorca, J.; Ollier, W.E.R.; Gonzalez-Gay, M.A. Echocardiographic and Doppler findings in long-term treated rheumatoid arthritis patients without clinically evident cardiovascular disease. Semin. Arthritis Rheum. 2004, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lassere, M.N.; Rappo, J.; Portek, I.J.; Sturgess, A.; Edmonds, J.P. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern. Med. J. 2013, 43, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, D.; Nishimura, K.; Tamaki, K.; Tsuji, G.; Nakazawa, T.; Morinobu, A.; Kumagai, S. Impact of smoking as a risk factor for developing rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2010, 69, 70–81. [Google Scholar] [CrossRef]
- Gianfrancesco, M.A.; Trupin, L.; Shiboski, S.; van der Laan, M.; Graf, J.; Imboden, J.; Yazdany, J.; Schmajuk, G. Smoking Is Associated with Higher Disease Activity in Rheumatoid Arthritis: A Longitudinal Study Controlling for Time-varying Covariates. J. Rheumatol. 2019, 46, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.-F.; Gourraud, P.-A.; Cantagrel, A.; Davignon, J.-L.; Constantin, A. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Jt. Bone Spine. 2011, 78, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Hallajzadeh, J.; Safiri, S.; Mansournia, M.A.; Khoramdad, M.; Izadi, N.; Almasi-Hashiani, A.; Pakzad, R.; Ayubi, E.; Sullman, M.J.; Karamzad, N. Metabolic syndrome and its components among rheumatoid arthritis patients: A comprehensive updated systematic review and meta-analysis. PLoS ONE. 2017, 12, e0170361. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, J.; Li, B.; et al. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: A systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun Rev. 2018, 17, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Soulaidopoulos, S.; Nikiphorou, E.; Dimitroulas, T.; Kitas, G.D. The Role of Statins in Disease Modification and Cardiovascular Risk in Rheumatoid Arthritis. Front Med 2018, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- de Jong, H.J.I.; Cohen Tervaert, J.W.; Lalmohamed, A.; et al. Pattern of risks of rheumatoid arthritis among patients using statins: A cohort study with the clinical practice research datalink. PLoS ONE 2018, 13, e0193297. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.; Sokka, T.; Descalzo, M.A.; Calvo-Alén, J.; Hørslev-Petersen, K.; Luukkainen, R.K.; Combe, B.; Burmester, G.R.; Devlin, J.; Ferraccioli, G.; Morelli, A.; Hoekstra, M.; Majdan, M.; Sadkiewicz, S.; Belmonte, M.; et al. Cardiovascular disease in patients with rheumatoid arthritis: Results from the quest-ra study. Arthritis Research; Therapy. 2008, 10. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [PubMed]
- Minder, C.M.; Blumenthal, R.S.; Blaha, M.J. Statins for primary prevention of cardiovascular disease. CurrOpinCardiol 2013, 28, 554–60. [Google Scholar] [CrossRef] [PubMed]
- Shenavar-Masooleh, I.; Zayeni, H.; Haji-Abbasi, A.; Azarpira, M.; Hadian, A.; Hassankhani, A.; et al. Cardiac involvement in rheumatoid arthritis: a crosssectional study in Iran. Indian Heart J 2016, 68, 332–335. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, W.J., Jr.; Crawford, M.H.; Klippel, J.H.; Zvaifler, N.J.; O’Rourke, R.A. Echocardiographic assessment of cardiac structure and function in patients with rheumatoid arthritis. Am J Med. 1977, 63, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Toumanidis, S.T.; Papamichael, C.M.; Antoniades, L.G.; Pantelia, M.I.; Saridakis, N.S.; Mavrikakis, M.E.; Sideris, D.A.; Moulopoulos, S.D. Cardiac involvement in collagen diseases. Eur Heart 1995, 16, 257262. [Google Scholar]
- Semb, A.G.; Ikdahl, E.; Wibetoe, G.; Crowson, C.; Rollefstad, S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol. 2020, 6, 361–379. [Google Scholar] [CrossRef]
- Castañeda, S.; Nurmohamed, M.T.; González-Gay, MA. Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016, 30, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Jagpal, A.; Navarro-Millán, I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. 2018, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Discacciati, A.; Orsini, N.; Wolk, A. Cigarette smoking and risk of rheumatoid arthritis: A dose-response meta-analysis. Arthritis Res. 2014, 16, R61. [Google Scholar] [CrossRef] [PubMed]
- Otterness, I.G. The value of C-reactive protein measurement in rheumatoid arthritis. Semin Arthritis Rheum. 1994, 24, 91–104. [Google Scholar] [CrossRef]
- Chaurasia, N.; Singh, A.; Singh, I.L.; Singh, T.; Tiwari, T. Cognitive dysfunction in patients of rheumatoid arthritis. J. Fam. Med. Prim. Care 2020, 9, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Moura, M.D.G.; Lopes, L.C.; Silva, M.T.; Barberato-Filho, S.; Motta, R.H.L.; Bergamaschi, C.C. Use of steroid and nonsteroidal anti-inflammatories in the treatment of rheumatoid arthritis: Systematic review protocol. Medicine 2018, 97, e12658. [Google Scholar] [CrossRef] [PubMed]
- Abbot, S.E.; Whish, W.J.; Jennison, C.; Blake, D.R.; Stevens, C.R. Tumour necrosis factor alpha stimulated rheumatoid synovial microvascular endothelial cells exhibit increased shear rate dependent leucocyte adhesion in vitro. Ann Rheum Dis. 1999, 58, 573–581. [Google Scholar] [CrossRef]
- Hurlimann, D.; Forster, A.; Noll, G.; Enseleit, F.; Chenevard, R.; Distler, O.; Bechir, M.; Spieker, L.E.; Neidhart, M.; Michel, B.A.; et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002, 106, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.A.; van der Heijde, D.M.F.M.; van Rijswijk, M.H.; et al. Interrelationship of outcome measures and process variables in early rheumatoid arthritis: a comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol 1994, 21, 425–429. [Google Scholar] [PubMed]
- Jensen, I.M.H.; Asmussen Andreasen, R.; van Bui Hansen, M.N.; Emamifar, A. The reliability of disease activity score in 28 joints-C-reactive protein might be overestimated in a subgroup of rheumatoid arthritis patients, when the score is solely based on subjective parameters: A cross-sectional, exploratory study. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases, U.S. National Library of Medicine. 2017. [Google Scholar]
- Dissanayake, K.; Jayasinghe, C.; Wanigasekara, P.; Dissanayake, J.; Sominanda, A. Validity of clinical disease activity index (CDAI) to evaluate the disease activity of rheumatoid arthritis patients in Sri Lanka: A prospective follow up study based on newly diagnosed patients. PLoS ONE 2022, 17. [Google Scholar] [CrossRef] [PubMed]
| Therapy | Number of cases | Percentage(%) |
|---|---|---|
| DMARDs | 200 | 100 |
| DMARDs +NSAIDs | 40 | 20 |
| DMARDs in monotherapy | 93 | 46.5 |
| DMARDs in dual therapy | 57 | 28.5 |
| MTX+HCQ+SSZ | 50 | 25 |
| Corticosteroids | 86 | 43 |
| Parameter | Total | Men (N=76) | Women (N=124) | p |
|---|---|---|---|---|
| Age group (Nr., %) | ||||
| 40-49 years | 25 (12.5%) | 11 (14.5%) | 14 (11.3%) | 0.291* |
| 50-69 years | 112 (56%) | 46 (60.5%) | 66 (53.2%) | |
| ≥ 70 years | 63 (31.5%) | 19 (25%) | 44 (35.5%) | |
| Stage of RA (Nr., %) | ||||
| Stage I | 26 (13%) | 13 (17.1%) | 13 (10.5%) | 0.004* |
| Stage II | 38 (19%) | 17 (22.4%) | 21 (16.9%) | |
| Stage III | 85 (42.5%) | 37 (48.7%) | 48 (38.7%) | |
| Stage IV† | 51 (25.5%) | 9 (11.8%) | 42 (33.9%) | |
| Smoking (Nr., %) | 70 (35%) | 50 (65.8%) | 20 (16.1%) | <0.001* |
| Consumption of alcohol (Nr., %) | 47 (23.5%) | 32 (42.1%) | 15 (12.1%) | <0.001* |
| Hypertension (Nr., %) | 106 (53%) | 68 (89.5%) | 38 (30.6%) | <0.001* |
| Hypercholesterolemia (Nr., %) | 91 (45.5%) | 59 (77.6%) | 32 (25.8%) | <0.001* |
| Diabetes mellitus (Nr., %) | 28 (14%) | 15 (19.7%) | 13 (10.5%) | 0.054* |
| CRP (mg/L) (Mean ± SD, Median (IQR)) | 89.04 ± 67.6, 110 (3-142) |
94.68 ± 73.7, 123 (2-153.75) |
79.83 ± 55.5, 105.5 (3-116.5) |
0.006** |
| Fibrinogen (g/L) (Mean ± SD, Median (IQR)) | 4.77 ± 0.32, 4.7 (4.5-5.1) |
5.14 ± 0.15, 5.2 (5.1-5.2) |
4.54 ± 0.14, 4.6 (4.5-4.6) |
<0.001** |
| Rheumatoid factor (UI) (Mean ± SD, Median (IQR)) | 177.52 ± 45.86, 203.5 (125-213) |
212.22 ± 10.6, 210 (205-216.75) |
120.91 ± 12.61, 119 (111-132) |
<0.001** |
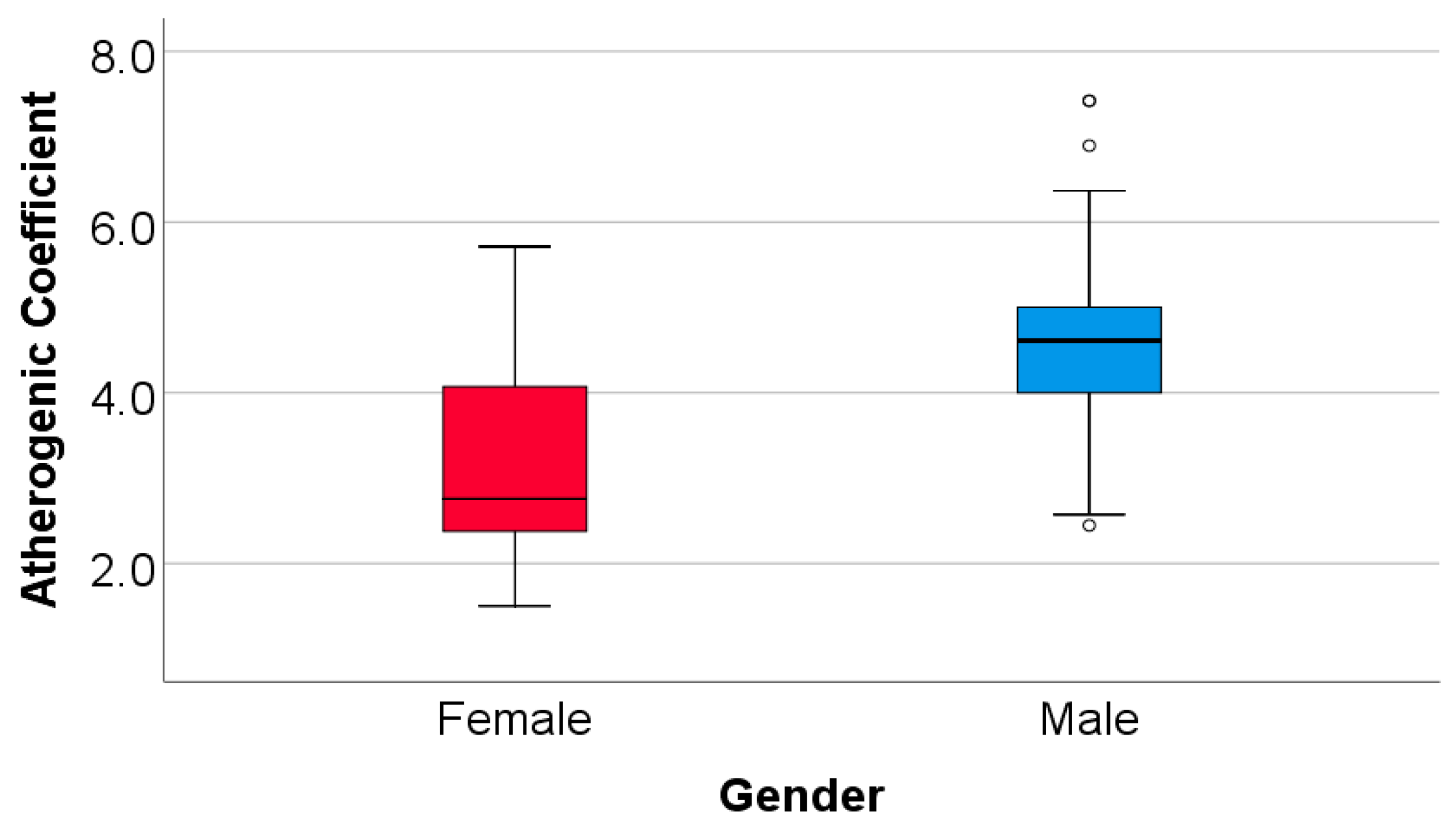
| Atherogenic coefficient (Mean ± SD, Median (IQR)) | 3.655 ± 1.251, 3.25 (2.571-4.731) |
4.518 ± 1.08, 4.61 (4-5) |
3.127 ± 1.04 2.75 (2.37-4.1) |
<0.001** |
| ESR (mm/h) (Mean ± SD, Median (IQR)) | 76.98 ± 44.94, 98 (15-106.75) |
75.21 ± 43.46, 97.5 (15-105) |
78.06 ± 45.97, 98.5 (15-107) |
0.552** |
| Dyslipidemia – Total cholesterol (Nr., %) | 91 (45.5%) | 59 (77.6%) | 32 (25.8%) | <0.001* |
| Dyslipidemia – Tryglicerides (Nr., %) | 94 (47%) | 62 (81.6%) | 32 (25.8%) | <0.001* |
| Dyslipidemia – HDL-cholesterol (Nr., %) | 29 (14.5%) | 19 (25%) | 10 (8.1%) | 0.002* |
| Dyslipidemia – LDL-cholesterol (Nr., %) | 83 (41.5%) | 55 (72.4%) | 28 (22.6%) | <0.001* |
| Coronary artery disease (Nr., %) | ||||
| Absent | 97 (48.5%) | 20 (26.3%) | 77 (62.1%) | <0.001* |
| Monovascular coronary a. disease | 62 (31%) | 32 (42.1%) | 30 (24.2%) | |
| Bivascular coronary a. disease | 27 (13.5%) | 15 (19.7%) | 12 (9.7%) | |
| Trivascular coronary a. disease | 14 (7%) | 9 (11.8%) | 5 (4%) | |
| Sinus tachycardia (Nr., %) | 40 (20%) | 15 (19.7%) | 25 (20.2%) | 1.000* |
| Left ventricular hypertrophy (Nr., %) | 73 (36.5%) | 39 (51.3%) | 34 (27.4%) | 0.001* |
| Secondary ST-T changes (Nr., %) | 63 (31.5%) | 30 (39.5%) | 33 (26.6%) | 0.062* |
| Atrial fibrillation (Nr., %) | 53 (26.5%) | 13 (17.1%) | 40 (32.3%) | 0.021* |
| Supraventricular extrasystole (Nr., %) | 42 (21%) | 20 (26.3%) | 22 (17.7%) | 0.210* |
| Left anterior fascicular block (Nr., %) | 32 (16%) | 14 (18.4%) | 18 (14.5%) | 0.552* |
| Premature ventricular contraction (Nr., %) | 26 (13%) | 12 (15.8%) | 14 (11.3%) | 0.391* |
| Left bundle branch block (Nr., %) | 24 (12%) | 10 (13.2%) | 14 (11.3%) | 0.823* |
| Pericarditis (Nr., %) | 34 (17%) | 9 (11.8%) | 25 (20.2%) | 0.174* |
| Mitral valve prolapse grade I-II (Nr., %) | 46 (23%) | 12 (15.8%) | 34 (27.4%) | 0.083* |
| Mitral regurgitation grade I-II (Nr., %) | 87 (43.5%) | 32 (44.1%) | 55 (44.4%) | 0.771* |
| Pulmonary arterial hypertension (Nr., %) | 39 (19.5%) | 11 (14.5%) | 28 (22.6%) | 0.199* |
| Tricuspid regurgitation grade I-II (Nr., %) | 139 (69.5%) | 52 (68.4%) | 87 (70.2%) | 0.874* |
| Pulmonary regurgitation grade I-II (Nr., %) | 128 (64%) | 48 (63.2%) | 80 (64.5%) | 0.880* |
| Etiology – CHF – High blood pressure (Nr., %) | 89 (44.5%) | 38 (50%) | 51 (41.1%) | 0.243* |
| Etiology – CHF – Coronary artery disease (Nr., %) | 35 (17.5%) | 15 (19.7%) | 20 (16.1%) | 0.567* |
| Etiology – CHF – Valvular heart disease (Nr., %) | 28 (14%) | 12 (15.8%) | 16 (12.9%) | 0.675* |
|
Etiology – CHF – Arrhythmias and conduction disorders (Nr., %) |
15 (7.5%) | 5 (6.6%) | 10 (8.1%) | 0.788* |
| Parameter/Measurement | Pre-Atorvastatin | Post-Atorvastatin | p* | |
|---|---|---|---|---|
| Total cholesterol | Average ± SD | 191 ± 43.33 | 167.22 ± 32.83 | <0.001 |
| Median (IQR) | 172.5 (155-229) | 150 (145-200) | ||
| Triglycerides | Average ± SD | 128.68 ± 39.02 | 114.35 ± 31.45 | <0.001 |
| Median (IQR) | 120 (95-160) | 100 (90-150) | ||
| HDL-cholesterol | Average ± SD | 41.54 ± 2.61 | 42.67 ± 2.81 | <0.001 |
| Median (IQR) | 40 (40-44) | 42 (41-45) | ||
| LDL-cholesterol | Average ± SD | 123.71 ± 38.2 | 101.37 ± 28.61 | <0.001 |
| Median (IQR) | 110 (92.25-153) | 90.5 (82.25-129) | ||
| Joint involvement in RA | Women | Men |
|---|---|---|
| Wrists | 64(51.6%) | 33(43.4%) |
| MCP | 67(54%) | 32(42.1%) |
| PIP | 54(43.5%) | 15(19.7%) |
| Spares DIP | 44(35.4%) | 22(28.9%) |
| First CMC | 30(24.1%) | 12(15.7%) |
| DAS28 | >5.1 | 5.1-3.2 | 3.2-2.6 | <2.6 |
|---|---|---|---|---|
| Men | 15(19.73%) | 20(26.31%) | 24(31.57%) | 17(22.36%) |
| Women | 28(22.58%) | 44(35.48%) | 27(21.77%) | 25(20.16%) |
| CDAI score | 0.0-2.8 | 2.9-10.0 | 10.1-22.0 | 22.1-76.0 |
| Men | 17(22.36%) | 23(30.26%) | 21(27.63%) | 15(19.73%) |
| Women | 26(20.96%) | 28(22.58%) | 46(37.09%) | 24(19.35%) |
| Correlation | p* |
|---|---|
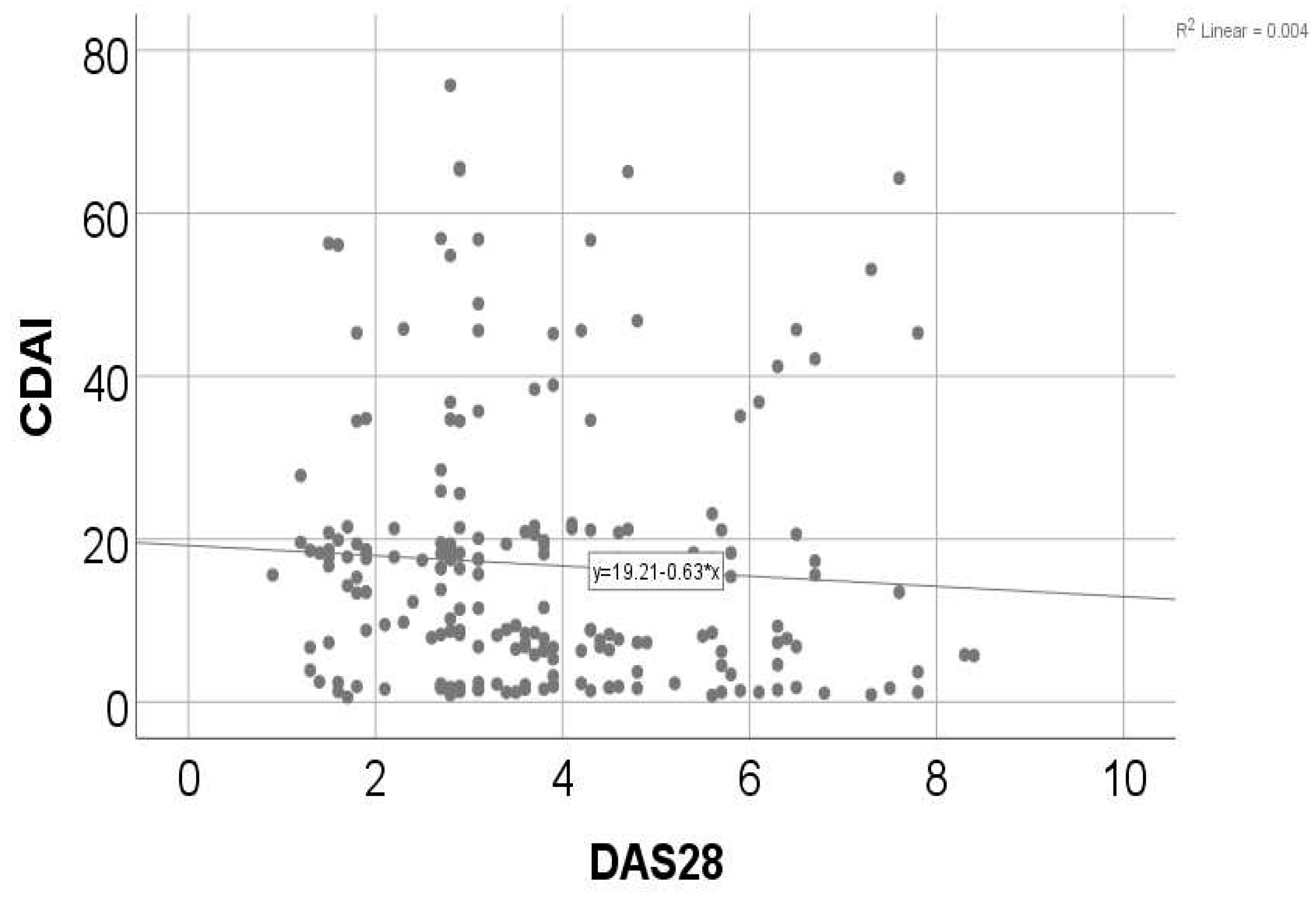
| CDAI (p<0.001**)x DAS28 (p<0.001**) | 0.022, R= -0.162 |
| Inflammation biomarkers | Number of cases | Percentage(%) |
|---|---|---|
| ESR | 140 | 70 |
| CRP | 131 | 65.5 |
| Pericarditis | ||||
|---|---|---|---|---|
| Parameter | Univariable | Multivariable | ||
| OR (95% C.I.) | p* | OR (95% C.I.) | p* | |
| HDL-cholesterol | 1.138 (0.996-1.301) | 0.057 | - | - |
| Atrial fibrillation | 2.279 (1.054-4.931) | 0.036 | - | - |
| Mitral valve prolapse | ||||
| LVH | 0.401 (0.186-0.867) | 0.020 | - | - |
| Mitral regurgitation | ||||
| CRP | 1.005 (1.001-1.009) | 0.019 | 1.005 (1.001-1.009) | 0.025 |
| Dyslipidemia – Triglycerides | 1.793 (1.019-3.154) | 0.043 | 1.760 (0.985-3.145) | 0.056 |
| HDL-cholesterol | 0.895 (0.800-1.002) | 0.055 | - | - |
| Secondary ST-T changes | 0.486 (0.259-0.910) | 0.024 | 0.522 (0.275-0.989) | 0.046 |
| Pulmonary arterial hypertension | ||||
| Rheumatoid factor | 1.008 (1.000-1.016) | 0.063 | - | - |
| HDL-cholesterol | 1.113 (0.979-1.264) | 0.102 | - | - |
| Pulmonary regurgitation | ||||
| Left bundle branch block | 7.264 (1.656-31.869) | 0.009 | - | - |
| Parameter / Group | Atrial fibrillation | p | |
|---|---|---|---|
| Absent (N=147) | Present (N=53) | ||
| Gender (Male) (Nr., %) | 63 (42.9%) | 13 (24.5%) | 0.021* |
| Age (Median (IQR)) | 63 (55-73) | 61 (55-70.5) | 0.642** |
| Stages of RA disease (Nr., %) | 0.890* | ||
| Stage I | 20 (13.6%) | 6 (11.3%) | |
| Stage II | 28 (19%) | 10 (18.9%) | |
| Stage III | 60 (40.8%) | 25 (47.2%) | |
| Stage IV | 39 (26.5%) | 12 (22.6%) | |
| Smoking (Nr., %) | 59 (40.1%) | 11 (20.8%) | 0.012* |
| Alcohol consumption (Nr., %) | 37 (25.2%) | 10 (18.9%) | 0.450* |
| Hypertension (Nr., %) | 85 (57.8%) | 21 (39.6%) | 0.025* |
| Hypercholesterolemia (Nr., %) | 72 (49%) | 19 (35.8%) | 0.110* |
| Diabetes mellitus (Nr., %) | 24 (16.3%) | 4 (7.5%) | 0.165* |
| CRP (Median (IQR)) | 110 (2-135) | 114 (3-147) | 0.505** |
| Fibrinogen (Median (IQR)) | 4.7 (4.5-5.1) | 4.6 (4.5-4.85) | 0.022** |
| ESR (Median (IQR)) | 98 (14-106) | 98 (15.5-110.5) | 0.535** |
| Rheumatoid factor (Median (IQR)) | 201 (121-213) | 206 (168.5-213.5) | 0.047** |
| Atherogenic coefficient (Median (IQR)) | 3.375 (2.75-4.75) | 2.90 (2.29-4.67) | 0.016** |
| Total cholesterol (Median (IQR)) | 180 (157-230) | 160 (145-222.5) | 0.003** |
| Dyslipidemia – Total cholesterol (Nr., %) | 72 (49%) | 19 (35.8%) | 0.110* |
| Triglycerides (Median (IQR)) | 150 (100-170) | 110 (85-157.5) | 0.028** |
| Dyslipidemia – Triglycerides (Nr., %) | 74 (50.3%) | 20 (37.7%) | 0.148* |
| HDL-cholesterol (Median (IQR)) | 40 (40-44) | 40 (40-44.5) | 0.460** |
| Dyslipidemia – HDL-cholesterol (Nr., %) | 24 (16.3%) | 5 (9.4%) | 0.262* |
| LDL-cholesterol (Median (IQR)) | 116 (95-154) | 100 (83.5-149) | 0.008** |
| Dyslipidemia – LDL-cholesterol (Nr., %) | 65 (44.2%) | 18 (34%) | 0.255* |
| Coronary artery disease (Nr., %) | 0.030**** | ||
| Absent | 64 (43.5%) | 33 (62.3%) | |
| Monovascular artery disease | 46 (31.3%) | 16 (30.2%) | |
| Bivascular artery disease | 24 (16.3%) | 3 (5.7%) | |
| Trivascular artery disease | 13 (8.8%) | 1 (1.9%) | |
| Pericarditis (Nr., %) | 20 (13.6%) | 14 (26.4%) | 0.033*** |
| Mitral valve prolapse (Nr., %) | 33 (22.4%) | 13 (24.5%) | 0.849* |
| Mitral regurgitation (Nr., %) | 63 (42.9%) | 24 (45.3%) | 0.872* |
| Pulmonary arterial hypertension (Nr., %) | 24 (16.3%) | 15 (28.3%) | 0.070* |
| Tricuspid regurgitation (Nr., %) | 102 (69.4%) | 37 (69.8%) | 1.000* |
| Pulmonary regurgitation (Nr., %) | 90 (61.2%) | 38 (71.7%) | 0.186* |
| CHF etiology | |||
| Hypertension (Nr., %) | 69 (46.9%) | 20 (37.7%) | 0.264* |
| Coronary artery disease (Nr., %) | 30 (20.4%) | 5 (9.4%) | 0.091* |
| Valvular heart disease (Nr., %) | 18 (12.2%) | 10 (18.9%) | 0.252* |
| Arrhythmias (Nr., %) | 1 (0.7%) | 14 (26.4%) | <0.001* |
| Parameter | Univariable | Multivariable* | ||
|---|---|---|---|---|
| OR (95% C.I.) | p* | OR (95% C.I.) | p* | |
| Gender (Female) | 2.308 (1.139-4.674) | 0.020 | - | - |
| Smoking | 0.391 (0.186-0.820) | 0.013 | - | - |
| Hypertension | 0.479 (0.252-0.908) | 0.024 | - | - |
| Fibrinogen | 0.301 (0.107-0.845) | 0.023 | - | - |
| Rheumatoid factor | 1.009 (1.001-1.017) | 0.020 | - | - |
| Atherogenic coefficient | 0.720 (0.547-0.947) | 0.019 | - | - |
| Total cholesterol | 0.990 (0.982-0.998) | 0.011 | 0.991 (0.983-0.999) | 0.034 |
| Triglycerides | 0.991 (0.983-0.999) | 0.029 | - | - |
| LDL-cholesterol | 0.988 (0.979-0.997) | 0.012 | - | - |
| Coronary artery disease | 0.467 (0.245-0.890) | 0.021 | 0.507 (0.259-0.992) | 0.047 |
| Pericarditis | 2.279 (1.054-4.931) | 0.036 | 2.401 (1.078-5.350) | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





