Submitted:
22 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial strains, identification and culture conditions
2.1. Determination of antimicrobial profiles
2.2. Synthesis and characterization of silver nanoparticles
2.3. Characterization of essential oils
2.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of AgNPs and EOs
2.5. Statistical Analysis
3. Results
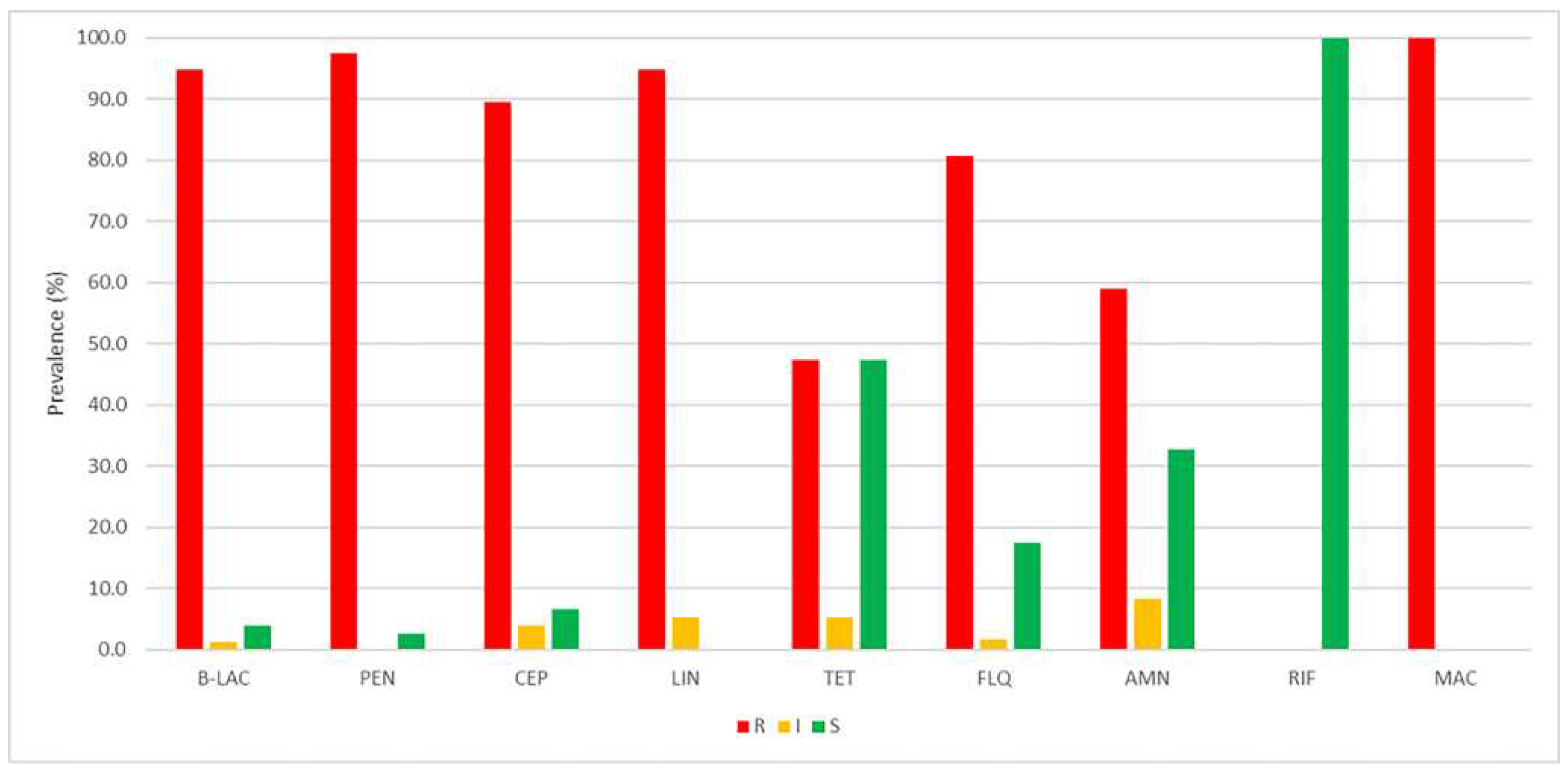
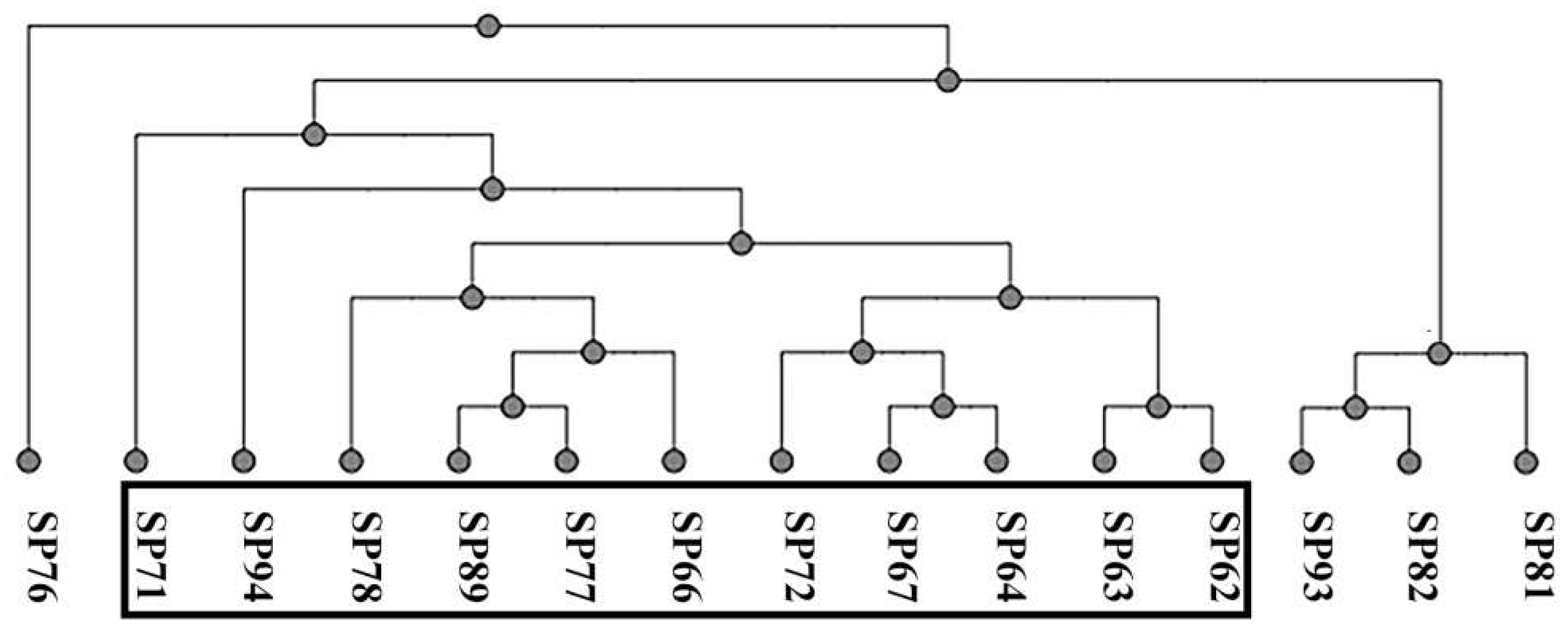
3.1. Phenotypic and molecular profiling of antibiotic resistance
3.2. Characterization of antimicrobial molecules
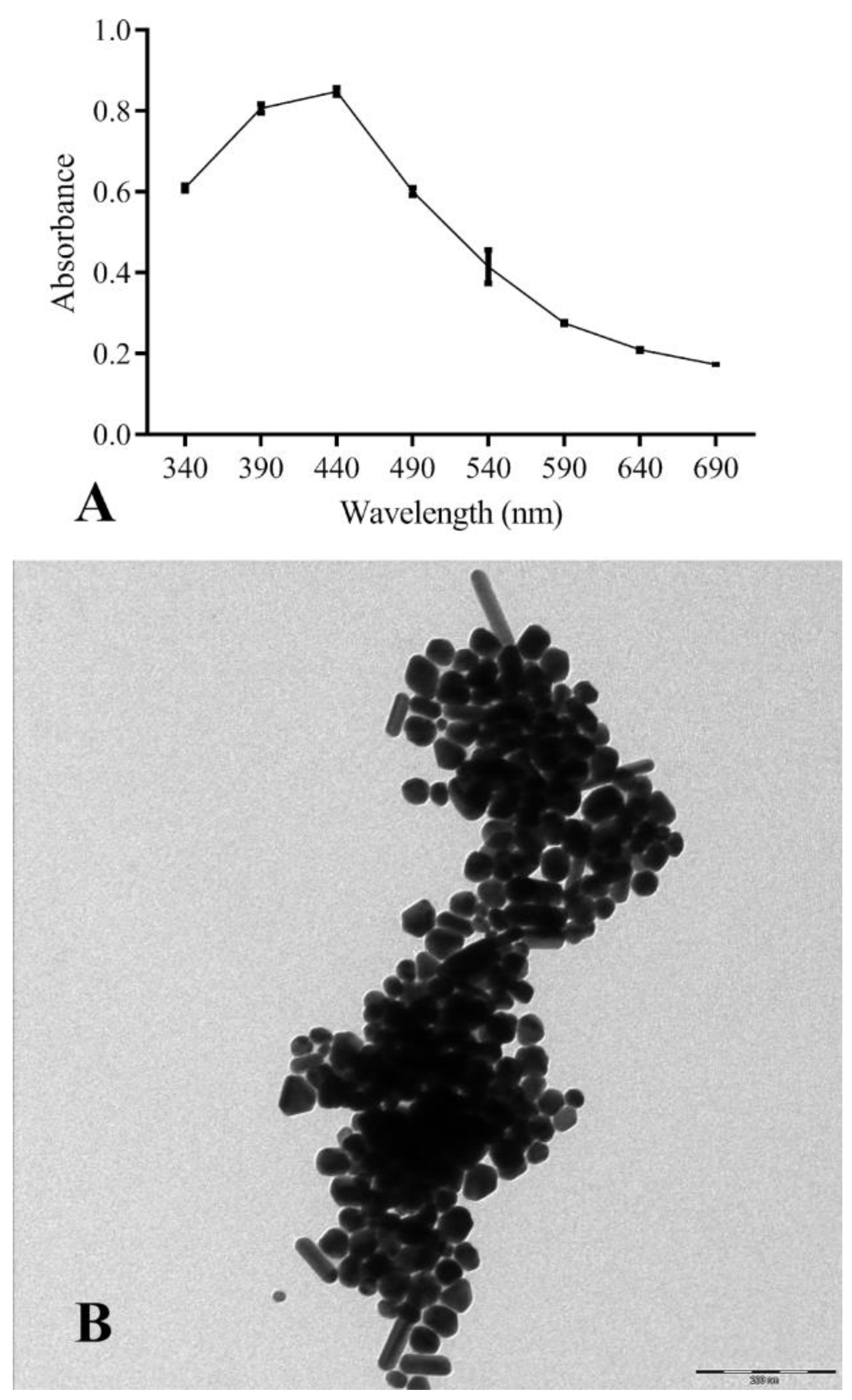
3.2.1. AgNPs
3.2.2. EOs
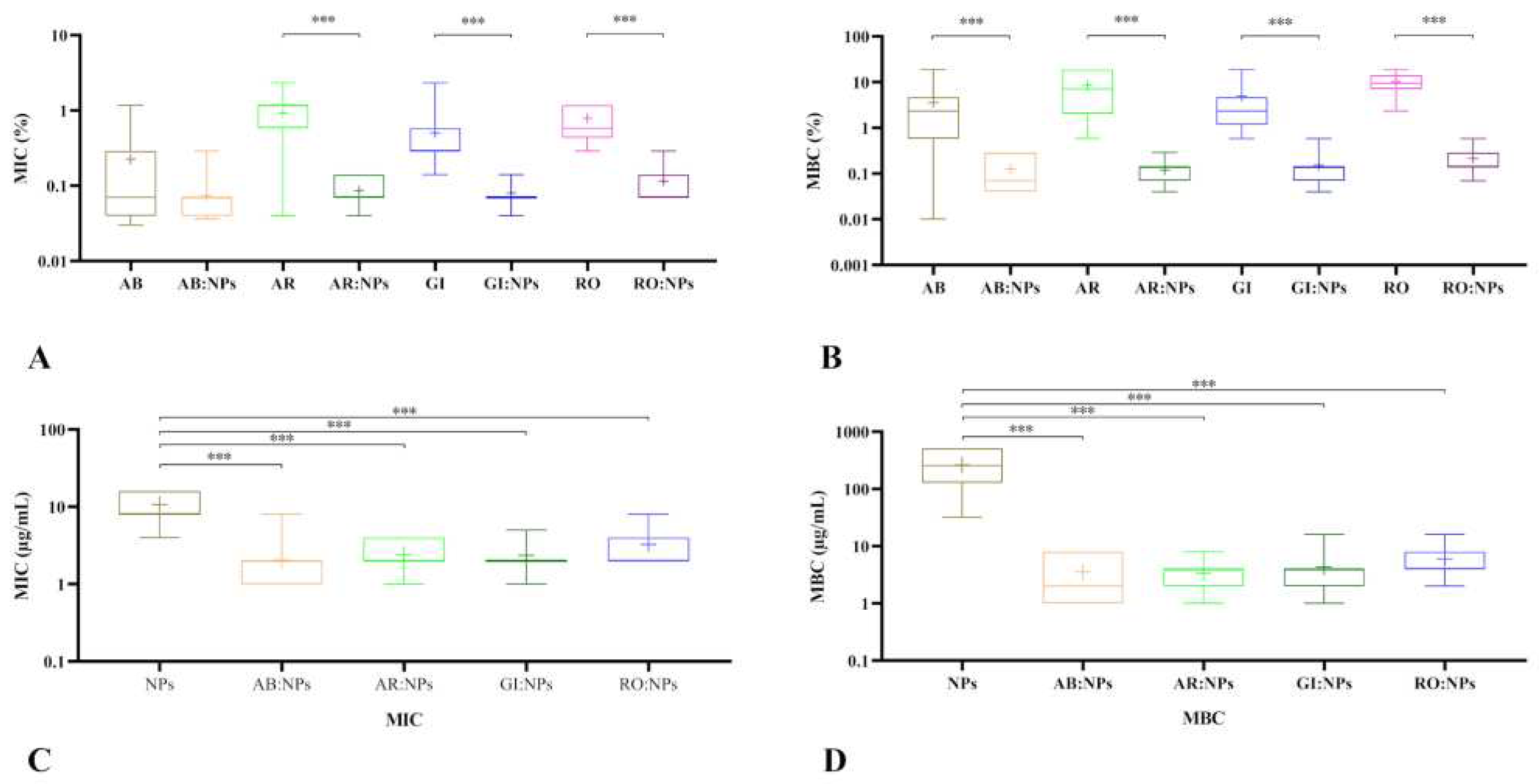
3.3. Antibacterial activity of EOs and NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Machalaba, C.; Raufman, J.; Anyamba, A.; Berrian, A.M.; Berthe, F.C.J.; Gray, G.C.; Jonas, O.; Karesh, W.B.; Larsen, M.H.; Laxminarayan, R.; et al. Applying a One Health Approach in Global Health and Medicine: Enhancing Involvement of Medical Schools and Global Health Centers. Ann. Glob. Health 87, 30. [CrossRef]
- Lerner, H.; Berg, C. A Comparison of Three Holistic Approaches to Health: One Health, EcoHealth, and Planetary Health. Front. Vet. Sci. 2017, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Wernli, D.; Jørgensen, P.S.; Morel, C.M.; Carroll, S.; Harbarth, S.; Levrat, N.; Pittet, D. Mapping Global Policy Discourse on Antimicrobial Resistance. BMJ Glob. Health 2017, 2, e000378. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Joji, R.M.; Shahid, M. Evolution and Implementation of One Health to Control the Dissemination of Antibiotic-Resistant Bacteria and Resistance Genes: A Review. Front. Cell. Infect. Microbiol. 2022, 12, 1065796. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.R. Importance of a One Health Approach in Advancing Global Health Security and the Sustainable Development Goals. Rev. Sci. Tech. Int. Off. Epizoot. 2019, 38, 145–154. [Google Scholar] [CrossRef]
- Meroni, G.; Filipe, J.F.S.; Drago, L.; Martino, P.A. Investigation on Antibiotic-Resistance, Biofilm Formation and Virulence Factors in Multi Drug Resistant and Non Multi Drug Resistant Staphylococcus Pseudintermedius. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Filipe, J.F.S.; Martino, P.A. In Vitro Antibacterial Activity of Biological-Derived Silver Nanoparticles : Preliminary Data. Vet. Sci. 2020, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, R.; Priyantha, M.A.R.; Rubin, J.E.; Church, D. Human Infections Due to Staphylococcus Pseudintermedius, an Emerging Zoonosis of Canine Origin: Report of 24 Cases. Diagn. Microbiol. Infect. Dis. 2016, 85, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.E.; Chirino-Trejo, M. Prevalence, Sites of Colonization, and Antimicrobial Resistance Among Staphylococcus Pseudintermedius Isolated from Healthy Dogs in Saskatoon, Canada. J. Vet. Diagn. Invest. 2011, 23, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Small, C.; Beatty, N.; Helou, G.E.; Small, C.; Beatty, N.; Helou, G.E. Staphylococcus Pseudintermedius Bacteremia in a Lung Transplant Recipient Exposed to Domestic Pets. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Asleh, M.; Feinstein, Y.; Lazar, I.; Rokney, A.; Baum, M.; Sagi, O.; Leibovitz, E.; Danino, D. Severe Pneumonia Caused by Methicillin-Resistant Staphylococcus Pseudintermedius in an Oncology Patient: Case Report and Literature Review. Microb. Drug Resist. Larchmt. N 2022, 28, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, L.D.; Rubin, J.E.; Deneer, H.; Kanthan, R.; Sanche, S.; Beshard, N.; Mpofu, C.; Blondeau, J.M. Bacteremia with Staphylococcus Pseudintermedius in a 4 Month Old Pediatric Oncology Patient. J. Chemother. Florence Italy 2020, 32, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-Y.; Yang, Y.-L.; Hsueh, P.-R.; Lee, P.-I. Catheter-Related Bacteremia Caused by Staphylococcus Pseudintermedius Refractory to Antibiotic-Lock Therapy in a Hemophilic Child with Dog Exposure. J. Clin. Microbiol. 2010, 48, 1497–1498. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 90, 153626. [Google Scholar] [CrossRef]
- Meroni, G.; Cardin, E.; Rendina, C.; Herrera Millar, V.R.; Soares Filipe, J.F.; Martino, P.A. In Vitro Efficacy of Essential Oils from Melaleuca Alternifolia and Rosmarinus Officinalis, Manuka Honey-Based Gel, and Propolis as Antibacterial Agents Against Canine Staphylococcus Pseudintermedius Strains. Antibiotics 2020, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Mol. Basel Switz. 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR Method for Species Identification of Coagulase-Positive Staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J.R. Edinburgh Research Explorer Molecular Diagnostic Identification of Staphylococcus Pseudintermedius Molecular Diagnostic Identification of Staphylococcus Pseudintermedius ᰔ. 2009. [CrossRef]
- CLSI; Dolinsky, A. L.; Ohiro, R.K.; Fan, W.; Xiao, C.; Wu, F. National Committee for Clinical Laboratory Standards. 2000. Performance Standard for Antimicrobial Susceptibility Testing. Document M100–S10. J. Int. Med. Res. 2017, 46, 18. [Google Scholar] [CrossRef]
- Yerragopu, P.S.; Hiregoudar, S.; Nidoni, U.; Ramappa, K.T.; Sreenivas, A.; Doddagoudar, S. Chemical Synthesis of Silver Nanoparticles Using Tri-Sodium Citrate, Stability Study and Their Characterization. Int. Res. J. Pure Appl. Chem. 2020, 21, 37–50. [Google Scholar] [CrossRef]
- Taglienti, A.; Donati, L.; Ferretti, L.; Tomassoli, L.; Sapienza, F.; Sabatino, M.; Di Massimo, G.; Fiorentino, S.; Vecchiarelli, V.; Nota, P.; et al. In Vivo Antiphytoviral Activity of Essential Oils and Hydrosols From Origanum Vulgare, Thymus Vulgaris, and Rosmarinus Officinalis to Control Zucchini Yellow Mosaic Virus and Tomato Leaf Curl New Delhi Virus in Cucurbita Pepo L. Front. Microbiol. 2022, 13, 840893. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; García-Fonticoba, R.; Pérez, D.; Viñes, J.; Fàbregas, N.; Madroñero, S.; Meroni, G.; Martino, P.A.; Martínez, S.; Maté, M.L.; et al. Whole Genome Sequencing and de Novo Assembly of Staphylococcus Pseudintermedius: A Pangenome Approach to Unravelling Pathogenesis of Canine Pyoderma. Vet. Dermatol. 2021, 32, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Riegel, P.; Jesel-Morel, L.; Laventie, B.; Boisset, S.; Vandenesch, F.; Prévost, G. Coagulase-Positive Staphylococcus Pseudintermedius from Animals Causing Human Endocarditis. Int. J. Med. Microbiol. IJMM 2011, 301. [Google Scholar] [CrossRef] [PubMed]
- Starlander, G.; Börjesson, S.; Grönlund-Andersson, U.; Tellgren-Roth, C. ; Melhus, \AAsa Cluster of Infections Caused by Methicillin-Resistant Staphylococcus Pseudintermedius in Humans in a Tertiary Hospital. 2014. [CrossRef]
- Börjesson, S.; Gómez-Sanz, E.; Ekström, K.; Torres, C.; Grönlund, U. Staphylococcus Pseudintermedius Can Be Misdiagnosed as Staphylococcus Aureus in Humans with Dog Bite Wounds. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Darlow, C.; Paidakakos, N.; Sikander, M.; Atkins, B. A Spinal Infection with Staphylococcus Pseudintermedius. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Nocera, F.P.; Meroni, G.; Fiorito, F.; De Martino, L.; Martino, P.A. Occurrence and Antimicrobial Susceptibility Patterns of Canine Staphylococcus Pseudintermedius Strains Isolated from Two Different Italian University Veterinary Hospitals. Vet. Ital. 2020, 56, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Focus: Plant-Based Medicine and Pharmacology: Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291. [Google Scholar]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial Activity of Essential Oils from Different Parts of Plants against Salmonella and Listeria Spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia Coli and Staphylococcus Aureus. Mol. Basel Switz. 2020, 25, 3955. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The Role of Essential Oils against Pathogenic Escherichia Coli in Food Products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.-H.E.; Lai, K.-S. Disruption of KPC-Producing Klebsiella Pneumoniae Membrane via Induction of Oxidative Stress by Cinnamon Bark (Cinnamomum Verum J. Presl) Essential Oil. PloS One 2019, 14, e0214326. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, O.; Sharifi, A.; Ahmadi, A.; Nayeri Fasaei, B. Antibacterial and Anti-PmrA Activity of Plant Essential Oils against Fluoroquinolone-Resistant Streptococcus Pneumoniae Clinical Isolates. Lett. Appl. Microbiol. 2018, 67, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Zouhir, A.; Jridi, T.; Nefzi, A.; Ben Hamida, J.; Sebei, K. Inhibition of Methicillin-Resistant Staphylococcus Aureus (MRSA) by Antimicrobial Peptides (AMPs) and Plant Essential Oils. Pharm. Biol. 2016, 54, 3136–3150. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.O.; Barreto, H.M.; Lima, E. de O.; Souza, E.L. de; Siqueira Júnior, J.P. de Antimicrobial Effect of the Essential Oil from Rosmarinus Officinalis L. against Staphylococcus Pseudintermedius Isolated from Dogs. Rev. Bras. Biociências 2013, 11, 280–283. [Google Scholar]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical Composition and Antimicrobial Activity of Essential Oils of Thymus Algeriensis, Eucalyptus Globulus and Rosmarinus Officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In Vitro Antimicrobial Activity of Essential Oil of Thymus Schimperi, Matricaria Chamomilla, Eucalyptus Globulus, and Rosmarinus Officinalis. Int. J. Microbiol. 2016, 2016, 9545693. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Flamini, G.; Cioni, P.L.; Morelli, I. Composition and Antimicrobial Properties of Essential Oils of Four Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial Activity of Clove and Rosemary Essential Oils Alone and in Combination. Phytother. Res. PTR 2007, 21, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; De Filippis, A.; Fiorito, F.; De Martino, L.; Fratini, F. Antimicrobial Activity of Some Essential Oils against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus Pseudintermedius-Associated Pyoderma in Dogs. Anim. Open Access J. MDPI 2020, 10, 1782. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Singh, V.R.; Verma, S.K.; Singh, N.; Kurmi, A.; Darokar, M.P.; Saikia, D. P-Menthenols Chemotype of Cymbopogon Distans from India: Composition, Antibacterial and Antifungal Activity of the Essential Oil against Pathogens. J. Essent. Oil Res. 2018, 30, 40–46. [Google Scholar] [CrossRef]
- El-Massry, K.F.; El-Ghorab, A.H.; Shaaban, H.A.; Shibamoto, T. Chemical Compositions and Antioxidant/Antimicrobial Activities of Various Samples Prepared from Schinus Terebinthifolius Leaves Cultivated in Egypt. J. Agric. Food Chem. 2009, 57, 5265–5270. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Pinto, J.E.B.P.; Bertolucci, S.K.V.; Alvarenga, A.A.; Alves, M.N.; Duarte, M.C.T.; Sartoratto, A. Chemical Composition and Antimicrobial Activity of the Essential Oil from the Leaves and Flowers of Aloysia Gratissima. Rev. Bras. Plantas Med. 2013, 15, 583–588. [Google Scholar] [CrossRef]
- Martins, R.L.; Simões, R.C.; Rabelo, É. de M.; Farias, A.L.F.; Rodrigues, A.B.L.; Ramos, R. da S.; Fernandes, J.B.; Santos, L. da S.; Almeida, S.S.M. da S. de Chemical Composition, an Antioxidant, Cytotoxic and Microbiological Activity of the Essential Oil from the Leaves of Aeollanthus Suaveolens Mart. Ex Spreng. PLOS ONE 2016, 11, e0166684. [Google Scholar] [CrossRef]
- Demir H; Kalaycı S Chemical Composition and Antimicrobial Activity of Essential Oils of Ocimum Basilicum Var. Album (L.) Benth, Lavandula Angustifolia Subsp. Angustifolia, Melissa Officinalis Belonging to Lamiaceae Family. J. Food Sci. Eng. 2017, 7. [CrossRef]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldaña, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the Selective Antibacterial Activity and Chemical Composition of Citrus Essential Oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [PubMed]
| # | Component1 | LRI2 | AR3 | AB4 | GI5 | RO6 |
| % TIC | ||||||
| 1 | α-Sinensal | 855 | 1.91±0.06 | tr | - | - |
| 2 | Ethanol, 2,2'-oxybis- | 967 | 11.0±5.01 | 5.08±4.67 | 5.19±0.35 | 8.25±0.17 |
| 3 | Methyl DL Leucate | 968 | 3.01±1.14 | 2.63±1.38 | 2.14±1.09 | 2.73±0.84 |
| 4 | Acetic acid, 2-ethylbutyl ester | 976 | 0.28±0.05 | tr | tr | tr |
| 5 | Pseudolimonene | 994 | 0.17±0.04 | 2.92±5.43 | 4.10±5.59 | 6.54±4.69 |
| 6 | Nonane, 3-methyl- | 995 | 0.11±0.02 | - | tr | tr |
| 7 | α-Phellandrene | 996 | 0.22±0.04 | tr | 4.24±5.53 | 0.37±0.29 |
| 8 | 3-Butenoic acid, 3-methyl-, trimethylsilyl ester | 1004 | 2.35±0.55 | 0.18±0.33 | 0.34±0.17 | 0.26±0.27 |
| 9 | α-Terpinene | 1008 | tr | 8.16±7.78 | 0.82±2.09 | 5.21±4.19 |
| 10 | 4-Carene | 1009 | tr | 5.87±5.13 | 1.50±1.99 | 2.76±3.69 |
| 11 | 2-Carene | 1009 | tr | 3.57±4.19 | 1.03±1.43 | 2.67±0.20 |
| 12 | α-Tesabinenene | 1014 | tr | 8.16±7.78 | 0.82±2.09 | 5.21±4.19 |
| 13 | β-cis-Ocimene | 1022 | 0.63±0.24 | 2.94±5.14 | 0.30±0.80 | 5.54±7.41 |
| 14 | 3-Carene | 1029 | tr | tr | tr | 0.58±0.67 |
| 15 | β-Terpinene | 1036 | tr | 3.19±4.80 | 0.27±0.72 | 5.81±7.58 |
| 16 | Crithmene | 1054 | 0.74±0.30 | tr | 2.55±3.69 | 0.30±0.44 |
| 17 | Benzene, butyl- | 1056 | tr | 0.69±0.60 | 0.95±1.32 | 0.19±0.26 |
| 18 | 4-Thujanol | 1065 | 0.47±0.02 | - | 0.15±0.22 | - |
| 19 | Terpinolene | 1080 | tr | 0.10±0.09 | 5.90±8.21 | 0.13±0.18 |
| 20 | 1,6-Octadien-3-ol, 3,7-dimethyl- | 1094 | - | 0.32±0.30 | 6.12±8.51 | 2.00±2.61 |
| 21 | Benzene, 1-ethyl-2,3-dimethyl- | 1096 | tr | 0.14±0.14 | 0.35±0.87 | tr |
| 22 | Benzene, 1,2,4,5-tetramethyl- | 1101 | tr | 0.15±0.14 | 0.40±1.01 | tr |
| 23 | 5,6-dehydrocamphor | 1104 | 0.22±0.09 | 0.15±0.27 | 3.19±8.42 | 0.10±0.14 |
| 24 | p-Mentha-1,3,8-triene | 1106 | tr | tr | 0.37±1.03 | tr |
| 25 | Allo-Ocimene | 1113 | tr | 0.24±0.44 | 0.15±0.21 | 0.37±0.57 |
| 26 | Disulfide, methyl (methylthio)methyl | 1119 | 4.12±0.49 | tr | tr | - |
| 27 | (Z)-p-Menthen-2-en-1-ol | 1125 | 36.14±0.43 | 0.15±0.25 | 0.41±1.06 | 7.22±5.89 |
| 28 | Terpineol, cis-β- | 1133 | 0.98±0.01 | 0.25±0.23 | 0.11±0.14 | 8.10±6.36 |
| 29 | (E)-p-Menthen-2-en-1-ol | 1137 | tr | 6.10±7.95 | 5.96±8.29 | 3.67±0.62 |
| 30 | Benzene, 1-ethenyl-4-methoxy- | 1158 | tr | tr | 0.20±0.44 | 0.36±0.44 |
| 31 | Ethanone, 1-(2-methylphenyl)- | 1176 | tr | 0.33±0.64 | 0.59±1.59 | 5.47±8.30 |
| 32 | γ-Terpineol | 1182 | tr | tr | tr | 0.18±0.23 |
| 33 | Isopentyloxyethyl acetate | 1184 | tr | 0.26±0.49 | tr | tr |
| 34 | α-Terpineol | 1191 | tr | - | 0.19±0.46 | 0.49±0.43 |
| 35 | (L)-α-Terpineol | 1191 | tr | - | 1.66±3.94 | 0.40±0.26 |
| 36 | Ethanone, 1-(3-methylphenyl)- | 1192 | tr | 0.31±0.60 | 0.73±1.58 | tr |
| 37 | Phenol, 2,4,6-trimethyl- | 1202 | tr | - | 1.61±4.47 | 0.20±0.31 |
| 38 | (1S,4S)-Dihydrocarvone | 1202 | tr | 0.24±0.46 | tr | - |
| 39 | Undecanal | 1290 | tr | - | 0.11±0.29 | - |
| 40 | Citronellyl Acetate | 1336 | tr | 0.10±0.09 | tr | tr |
| 41 | 2,3-Dimethyldodecane | 1346 | tr | tr | 0.10±0.19 | 0.20±0.32 |
| 42 | (E,Z)-jasmone | 1349 | tr | tr | 0.29±0.62 | tr |
| 43 | Tridecane, 7-methyl- | 1353 | tr | tr | 0.19±0.19 | 0.23±0.34 |
| 44 | α-Longipinene | 1360 | 0.21±0.05 | tr | 0.44±1.20 | tr |
| 45 | Pentanoic acid, heptyl ester | 1364 | tr | tr | 0.14±0.40 | tr |
| 46 | Cyclosativene | 1367 | 0.13±0.00 | 0.83±1.60 | 0.49±1.36 | tr |
| 47 | 2-Octenal, 2-butyl | 1367 | 1.60±0.05 | 0.20±0.35 | 0.15±0.23 | 0.11±0.15 |
| 48 | Copaene | 1367 | tr | 0.17±0.24 | 0.58±1.59 | tr |
| 49 | α-Cubebene | 1374 | tr | 0.90±1.71 | 0.48±1.29 | tr |
| 50 | Di-epi-α-cedrene | 1384 | tr | 0.88±1.56 | 0.18±0.49 | tr |
| 51 | α-Cedrene | 1384 | tr | tr | 0.19±0.53 | 0.47±0.76 |
| 52 | β-Cubebene | 1394 | tr | tr | 0.22±0.59 | 0.56±0.74 |
| 53 | Acetic acid, decyl ester | 1397 | 0.42±0.02 | 1.93±3.45 | tr | 0.40±0.61 |
| 54 | 2H-Pyran-2-one, 6-pentyl- | 1408 | tr | 2.96±5.53 | tr | 2.74±4.38 |
| 55 | β-Patchoulene | 1408 | tr | 0.14±0.17 | 0.20±0.50 | tr |
| 56 | Terpinyl propionate | 1418 | tr | 2.67±5.10 | tr | - |
| 57 | Lynalyl butyrate | 1429 | tr | tr | tr | 0.84±1.28 |
| 58 | β-Gurjunene | 1429 | tr | tr | 0.44±1.20 | tr |
| 59 | β-Santalene | 1432 | tr | 0.32±0.39 | tr | tr |
| 60 | β-Selinene | 1432 | tr | 0.37±0.64 | tr | - |
| 61 | α-Caryophyllene | 1443 | tr | 0.24±0.22 | tr | 0.13±0.17 |
| 62 | Spathulenol | 1444 | tr | tr | 0.46±1.26 | tr |
| 63 | α Himachalene | 1450 | tr | 0.50±0.66 | - | tr |
| 64 | Acetophenone, 4'-hydroxy- | 1453 | tr | 0.41±0.72 | - | tr |
| 65 | β-Humulene | 1458 | tr | 0.52±0.71 | tr | tr |
| 66 | α-Santalene | 1459 | 0.42±0.1 | 3.71±5.86 | 0.16±0.16 | 0.97±1.47 |
| 67 | 11-Dodecenol | 1461 | tr | 0.56±0.74 | tr | 0.13±0.21 |
| 68 | γ-Gurjunene | 1470 | tr | 0.39±0.73 | tr | 0.12±0.12 |
| 69 | Butanoic acid, 3-methyl-, 1-ethenyl-1,5-dimethyl-4-hexenyl ester | 1471 | 20.78±0.66 | 3.74±6.58 | 0.25±0.40 | 1.59±1.32 |
| 70 | (E)-Isoeugenol | 1474 | tr | 0.15±0.19 | 0.12±0.29 | 0.12±0.18 |
| 71 | β-Chamigrene | 1475 | tr | 0.20±0.29 | 0.10±0.27 | 1.42±2.23 |
| 72 | 10-Dodecenol | 1479 | tr | 1.02±1.74 | tr | 0.24±0.34 |
| 73 | β-Guaiene | 1484 | tr | 0.25±0.43 | 0.14±0.25 | 1.52±1.96 |
| 74 | γ-Cadinene | 1488 | tr | tr | 0.14±0.24 | 0.66±0.99 |
| 75 | α-Bisabolene | 1494 | tr | 0.76±1.00 | tr | 0.64±1.01 |
| 76 | Valencene (isomer R) | 1495 | tr | 0.33±0.38 | tr | 0.56±0.84 |
| 77 | Valencene (isomer S) | 1497 | tr | 0.77±0.81 | tr | tr |
| 78 | β-Bisabolene | 1500 | tr | 0.64±1.18 | tr | tr |
| 79 | 2,4-Dodecadienal, (E,E)- | 1502 | 0.34±0.00 | 0.26±0.37 | tr | tr |
| 80 | (Z,E)-α-Farnesene | 1505 | tr | 0.52±0.49 | 0.41±1.08 | tr |
| 81 | α-Muurolene | 1508 | tr | tr | 2.09±5.77 | tr |
| 82 | δ-Guaiene | 1508 | tr | 0.14±0.19 | tr | tr |
| 83 | Epizonarene | 1522 | tr | tr | 2.21±6.14 | tr |
| 84 | Sesquiphellandrene | 1522 | 0.21±0.04 | 0.80±1.34 | 0.12±0.32 | tr |
| 85 | (+)-Sativene | 1523 | tr | 1.63±2.51 | - | 5.71±9.07 |
| 86 | Hedycaryol | 1528 | tr | tr | tr | 0.56±0.88 |
| 87 | Isocadiene | 1534 | tr | tr | 2.72±7.53 | tr |
| 88 | Butanoic acid, 3,7-dimethyl-6-octenyl ester | 1536 | tr | 1.08±1.07 | tr | 0.14±0.08 |
| 89 | Eudesma-3,7(11)-diene | 1538 | tr | tr | 2.83±7.41 | tr |
| 90 | β-Himachalene | 1561 | tr | 0.12±0.19 | 0.58±0.79 | 0.25±0.38 |
| 91 | Nerolidol | 1567 | tr | 0.28±0.51 | tr | 2.00±3.16 |
| 92 | Caryophyllene oxide | 1576 | tr | 0.36±0.58 | 0.16±0.20 | tr |
| 93 | β-Elemenone | 1578 | tr | 0.51±0.91 | 0.22±0.48 | tr |
| 94 | Carotol | 1583 | - | 0.11±0.11 | 1.13±3.16 | tr |
| 95 | Boronia butenal | 1586 | tr | 2.63±4.99 | tr | tr |
| 96 | Germacrene B | 1589 | tr | 0.28±0.25 | 0.20±0.31 | tr |
| 97 | Dodecan-1-yl acetate | 1590 | 0.39±0.01 | 0.11±0.10 | 0.20±0.35 | tr |
| 98 | Guaiol | 1597 | tr | 0.11±0.10 | 3.98±9.16 | 0.10±0.07 |
| 99 | Cedrenol | 1607 | 1.17±0.55 | 1.19±1.76 | 0.27±0.72 | tr |
| 100 | α-Eudesmol | 1607 | tr | 0.14±0.14 | 0.28±0.76 | tr |
| 101 | Cubenol | 1611 | 0.20±0.01 | 0.48±0.29 | 0.20±0.54 | 0.24±0.34 |
| 102 | Hinesol | 1620 | tr | 0.37±0.58 | 3.55±9.82 | 0.53±0.83 |
| 103 | Ledol | 1620 | 0.30±0.01 | 0.45±0.42 | 0.52±1.44 | tr |
| 104 | γ-Eudesmol | 1625 | 0.10±0.00 | tr | 3.88±5.41 | tr |
| 105 | β-Homocyclocitral | 1627 | - | 0.31±0.47 | - | - |
| 106 | τ-Cadinol | 1642 | tr | 0.32±0.60 | 3.67±9.02 | tr |
| 107 | Geranyl valerate | 1648 | 2.49±0.09 | tr | tr | tr |
| 108 | Blumenol C | 1673 | tr | 1.60±3.05 | tr | tr |
| 109 | 2(1H)-Quinolinone, 1-methyl- | 1673 | tr | tr | 3.53±9.77 | 0.19±0.30 |
| 110 | δ-Cadinol | 1678 | tr | tr | 0.54±0.87 | - |
| 111 | Phenol, 3-methyl-5-(1-methylethyl)-, methylcarbamate | 1693 | tr | 0.72±1.17 | tr | 0.17±0.27 |
| 112 | Cedren-13-ol, 8- | 1692 | 0.22±0.01 | 0.22±0.40 | tr | - |
| 113 | (Z,E) Farnesyl acetate | 1699 | 1.04±0.23 | 0.51±0.50 | tr | tr |
| 114 | 1-Heptadecene | 1711 | tr | 0.18±0.22 | tr | tr |
| 115 | (E,E) Farnesal | 1718 | tr | 0.32±0.59 | - | - |
| 116 | Solavetivone | 1817 | tr | 0.18±0.34 | - | tr |
| 117 | (E,E) Farnesyl acetate | 1833 | tr | 1.11±2.09 | - | tr |
| 118 | Rimuene | 1905 | tr | tr | 0.35±0.93 | - |
| 119 | Kaur-16-ene, (8-β,13-β)- | 2015 | tr | 0.15±0.19 | tr | tr |
| 120 | Epimanool | 2019 | 2.25±0.07 | tr | 0.10±0.27 | tr |
| 121 | (E,E) Farnesyl lactone | 1920 | 0.21±0.08 | - | tr | - |
| 122 | Farnesylacetone | 1926 | 0.29±0.07 | 1.70±3.26 | - | tr |
| 123 | Geranyllinalool | 2025 | tr | 0.38±0.44 | tr | tr |
| 124 | Kaur-16-ene | 2046 | 1.56±0.34 | 1.03±1.79 | 0.10±0.27 | tr |
| SUM | 96.68 | 98.09 | 91.75 | 98.65 | ||
| Monoterpenoids | 40.55 | 39.68 | 39.56 | 60.17 | ||
| Monoterpene hydrocarbons | 1.76 | 26.92 | 21.66 | 29.91 | ||
| Oxygenated monoterpenes | 38.79 | 12.76 | 17.90 | 30.26 | ||
| Diterpenoids | 3.78 | 1.56 | 0.55 | - | ||
| Diterpene hydrocarbons | 1.53 | 1.18 | 0.45 | - | ||
| Oxygenated diterpenes | 2.25 | 0.38 | 0.10 | - | ||
| Sesquiterpenoids | 6.96 | 23.54 | 30.64 | 17.73 | ||
| Sesquiterpene hydrocarbons | 0.34 | 10.97 | 8.66 | 12.41 | ||
| Oxygenated sesquiterpenes | 6.62 | 12.57 | 21.98 | 5.32 | ||
| Others | 45.39 | 33.31 | 21.00 | 20.75 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





