Submitted:
24 November 2023
Posted:
27 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
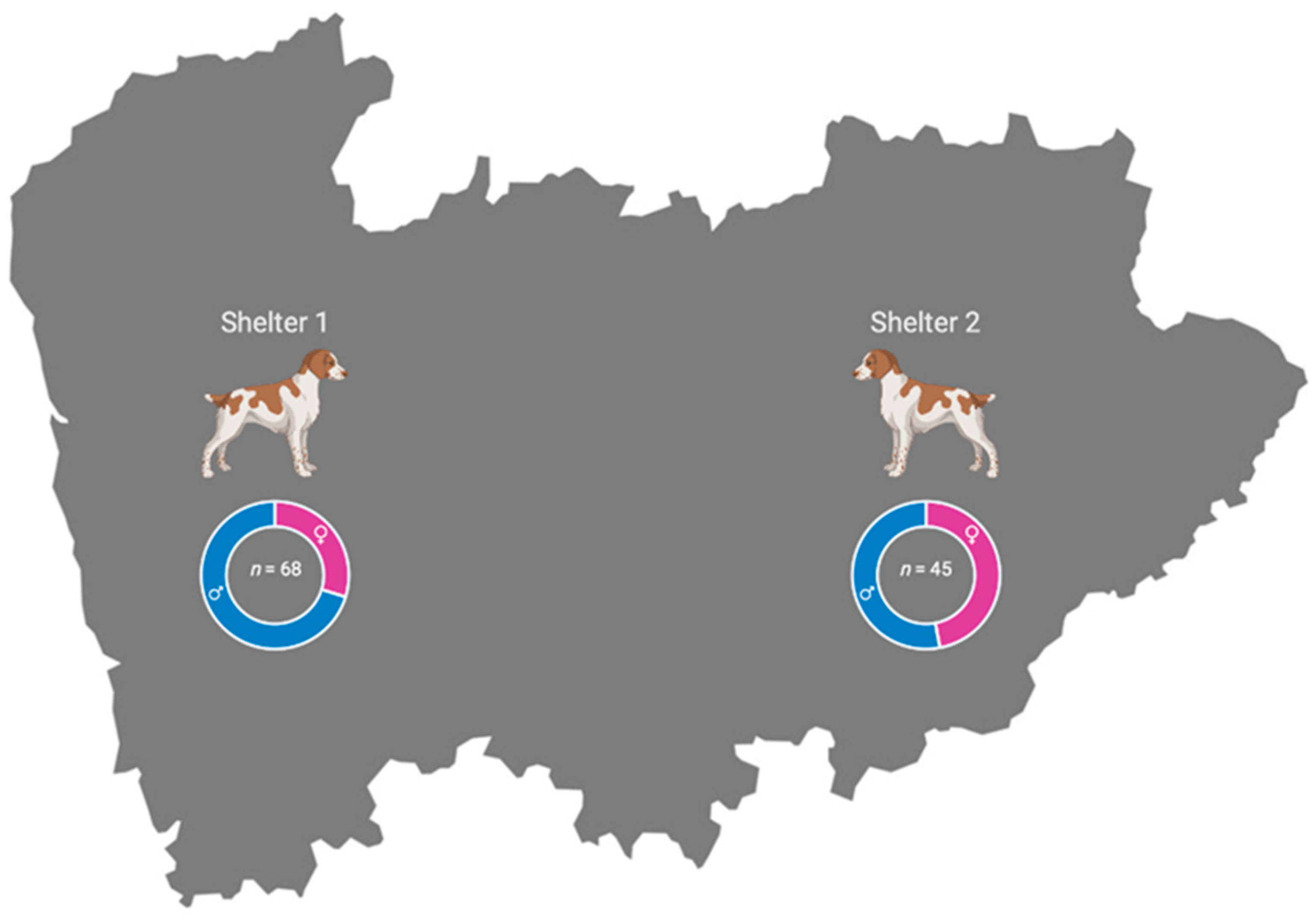
2.1. Study Area
2.2. Animals and Samples
2.3. Data Analysis
3. Results
3.1. Seropositivity to Ehrlichia canis
3.2. Seropositivity to Rickettsia conorii
3.3. Seropositivity to Co-Infections (E. canis + R. conorii)
3.4. Risk Factors for Rickettsia conorii and the Co-Infection (E. canis + R. conorii) in Sheltered Dogs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beugnet, F.; Marié, J.-L. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol 2009, 163, 298–305. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol 2009, 25, 157–163. [Google Scholar] [CrossRef]
- Stich, R.W.; Schaefer, J.J.; Bremer, W.G.; Needham, G.R.; Jittapalapong, S. Host surveys, ixodid tick biology and transmission scenarios as related to the tick-borne pathogen. Ehrlichia canis. Vet Parasitol 2008, 158, 256–273. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Allen, K.E.; McQuiston, J.H.; Breitschwerdt, E.B.; Little, S.E. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 2010, 26, 205–212. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Seasonal dynamics of Ixodes ricinus on ground level and higher vegetation in a preserved wooded area in southern Europe. Vet Parasitol 2013, 192, 253–258. [Google Scholar] [CrossRef]
- Procajło, A.; Skupień, E.M.; Bladowski, M.; Lew, S. Monocytic ehrlichiosis in dogs. Pol J Vet Sci 2011, 14, 515–520. [Google Scholar] [CrossRef]
- Shaw, S.E.; Day, M.J.; Birtles, R.J.; Breitschwerdt, E.B. Tick-borne infectious diseases of dogs. Trends Parasitol 2001, 17, 74–80. [Google Scholar] [CrossRef]
- Harrus, S.; Bark, H.; Waner, T. Canine Monocytic ehrlichiosis: an update. Comp Cont Educ Pract Vet. 1997, 19, 431–444. [Google Scholar]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; Massung, R.F.; Nadelman, R.B.; Nicholson, W.L.; Paddock, C.D.; Pritt, B.S.; Traegerl, M.S. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR. Recommendations and Reports 2016, 65, 1–44. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin Microbiol Rev 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Brouqui, P.; Parola, P.; Fournier, P.E.; Raoult, D. Spotted fever rickettsioses in southern and eastern Europe. FEMS Immunol Med Microbiol 2007, 49, 2–12. [Google Scholar] [CrossRef]
- Rovery, C.; Brouqui, P.; Raoult, D. Questions on Mediterranean spotted fever a century after its discovery. Emerg Infect Dis 2008, 14, 1360–1367. [Google Scholar] [CrossRef]
- Neer, T.M.; Breitschwerdt, E.B.; Greene, R.T.; Lappin, M.R. Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM*. J Vet Intern Med 2002, 16, 309. [Google Scholar] [CrossRef]
- Romaní Vidal, A.; Fernández-Martínez, B.; Herrador, Z.; León Gómez, I.; Gómez Barroso, D. Spatial and temporal trends of Mediterranean spotted fever in Spain, 2005-2015. Ticks Tick Borne Dis 2020, 11, 101353. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Caprì, A.; Pennisi, M.G.; Caldin, M.; Furlanello, T.; Trotta, M. Acute febrile illness is associated with Rickettsia spp. infection in dogs. Parasit Vectors 2015, 8, 216. [Google Scholar] [CrossRef]
- Kovácová, E.; Kazár, J. Rickettsial diseases and their serological diagnosis. Clin Lab 2000, 46, 239–245. [Google Scholar]
- Ericsson, C.D.; Jensenius, M.; Fournier, P.-E.; Raoult, D. Rickettsioses and the international traveler. Clin Infect Dis 2004, 39, 1493–1499. [Google Scholar] [CrossRef]
- Paris, D.H.; Dumler, J.S. State of the art of diagnosis of rickettsial diseases. Curr Opin Infect Dis 2016, 29, 433–439. [Google Scholar] [CrossRef]
- Varela, K.; Goryoka, G.; Suwandono, A.; Mahero, M.; Valeri, L.; Pelican, K.; Salyer, S.J. One Health zoonotic disease prioritization and systems mapping: an integration of two One Health tools. Zoonoses Public Health 2023, 70, 146–159. [Google Scholar] [CrossRef]
- Spindel, M. Strategies for management of infectious diseases in a shelter. In Shelter Medicine for Veterinarians and Staff; Wiley, 2012; pp. 279–286. [Google Scholar]
- European Commission, D.-G. for H. and F.S. TRACES–2022 Annual Report. 2023.
- Sikkema, R.; Schrijver, R.; Dewar, D.; Vries, H.; Bergevoet, R.H.M.; Messori, S.; Barnard, S.; D’Albenzio, S. Study on the welfare of dogs and cats involved in commercial practices. 2015.
- Migliore, S.; Gargano, V.; De Maria, C.; Gambino, D.; Gentile, A.; Vitale Badaco, V.; Schirò, G.; Mira, F.; Galluzzo, P.; Vicari, D.; Di Bella, S. A cross sectional study on serological prevalence of Ehrlichia canis and Rickettsia conorii in different canine population of Sicily (South-Italy) during 2017–2019. Animals 2020, 10, 2444. [Google Scholar] [CrossRef]
- Hazelrig, C.M.; Gettings, J.R.; Cleveland, C.A.; Varela-Stokes, A.; Majewska, A.A.; Hubbard, K.; Burton, K.W.; Yabsley, M.J. Spatial and risk factor analyses of vector-borne pathogens among shelter dogs in the eastern United States. Parasit Vectors 2023, 16, 197. [Google Scholar] [CrossRef]
- Baxarias, M.; Álvarez-Fernández, A.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Ordeix, L.; Rojas, A.; Nachum-Biala, Y.; Baneth, G.; Solano-Gallego, L. Does co-infection with vector-borne pathogens play a role in clinical canine leishmaniosis? Parasit Vectors 2018, 11, 135. [Google Scholar] [CrossRef]
- Patterson, G.; Tanhauser, M.; Schmidt, P.; Spangler, D.; Faulkner, C.; Faulkner, V.; Kish, D.; Gruszynski, K.; Naikare, H.; Coarsey, M.D.; Verma, A. Serosurvey of arthropod-borne diseases among shelter dogs in the Cumberland Gap region of the United States. BMC Vet Res 2020, 16, 221. [Google Scholar] [CrossRef]
- INE Censos 2021. XVI Recenseamento geral da população. VI Recenseamento geral da habitação : Resultados definitivos; Lisboa. 2022.
- Cardoso, L.; Mendão, C.; Madeira de Carvalho, L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal - a national serological study. Parasit Vectors 2012, 5, 62. [Google Scholar] [CrossRef]
- Cardoso, L.; Tuna, J.; Vieira, L.; Yisaschar-Mekuzas, Y.; Baneth, G. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from the north of Portugal. The Veterinary Journal 2010, 183, 232–233. [Google Scholar] [CrossRef]
- Cardoso, L.; Yisaschar-Mekuzas, Y.; Rodrigues, F.T.; Costa, Á.; Machado, J.; Diz-Lopes, D.; Baneth, G. Canine babesiosis in northern Portugal and molecular characterization of vector-borne co-Infections. Parasit Vectors 2010, 3, 27. [Google Scholar] [CrossRef]
- René-Martellet, M.; Lebert, I.; Chêne, J.; Massot, R.; Leon, M.; Leal, A.; Badavelli, S.; Chalvet-Monfray, K.; Ducrot, C.; Abrial, D.; Chabanne, L.; Halos, L. Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in southern Europe. Parasit Vectors 2015, 8, 3. [Google Scholar] [CrossRef]
- Alexandre, N.; Santos, A.S.; Núncio, M.S.; Sousa, R. de; Boinas, F.; Bacellar, F. Detection of Ehrlichia canis by polymerase chain reaction in dogs from Portugal. The Veterinary Journal 2009, 181, 343–344. [Google Scholar] [CrossRef]
- Maia, C.; Almeida, B.; Coimbra, M.; Fernandes, M.; Cristóvão, J.; Ramos, C.; Martins, Â.; Martinho, F.; Silva, P.; Neves, N.; Nunes, M.; Vieira, M.L.; Cardoso, L.; Campino, L. Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasit Vectors 2015, 8, 138. [Google Scholar] [CrossRef]
- Cardoso, L.; Gilad, M.; Cortes, H.; Nachum-Biala, Y.; Lopes, A.; Vila-Viçosa, M.; Simões, M.; Rodrigues, P.A.; Baneth, G. First Report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasit Vectors 2015, 8, 144. [Google Scholar] [CrossRef]
- Pantchev, N.; Schaper, R.; Limousin, S.; Norden, N.; Weise, M.; Lorentzen, L. Occurrence of Dirofilaria immitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in domestic dogs in France: results of a countrywide serologic survey. Parasitol Res 2009, 105, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Pérez Vera, C.; Kapiainen, S.; Junnikkala, S.; Aaltonen, K.; Spillmann, T.; Vapalahti, O. Survey of selected tick-borne diseases in dogs in Finland. Parasit Vectors 2014, 7, 285. [Google Scholar] [CrossRef]
- Amusategui, I.; Tesouro, M.A.; Kakoma, I.; Sainz, Á. Serological reactivity to Ehrlichia canis, Anaplasma phagocytophilum, Neorickettsia risticii, Borrelia burgdorferi and Rickettsia conorii in dogs from northwestern Spain. Vector-Borne and Zoonotic Dis 2008, 8, 797–804. [CrossRef] [PubMed]
- Pennisi, M.-G.; Caprì, A.; Solano-Gallego, L.; Lombardo, G.; Torina, A.; Masucci, M. Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis, and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area (Italy). Ticks Tick Borne Dis 2012, 3, 315–318. [Google Scholar] [CrossRef]
- Geromichalou, A.; Faixová, Z. Haematopathological changes in dogs affected with Ehrlichia canis in Lesvos. Folia Vet 2017, 61, 44–49. [Google Scholar] [CrossRef]
- Batmaz, H.; Nevo, E.; Waner, T.; Sentürk, S.; Yilmaz, Z.; Harrus, S. Seroprevalence of Ehrlichia canis antibodies among dogs in Turkey. Vet Rec 2001, 148, 665–666. [Google Scholar] [CrossRef]
- Schüle, C.; Rehbein, S.; Shukullari, E.; Rapti, D.; Reese, S.; Silaghi, C. Police dogs from Albania as indicators of exposure risk to Toxoplasma gondii, Neospora caninum and vector-borne pathogens of zoonotic and veterinary concern. Vet Parasitol Reg Stud Reports 2015, 1–2, 35–46. [Google Scholar] [CrossRef]
- Mircean, V.; Dumitrache, M.O.; Györke, A.; Pantchev, N.; Jodies, R.; Mihalca, A.D.; Cozma, V. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector-Borne and Zoonotic Dis 2012, 12, 595–604. [Google Scholar] [CrossRef]
- Hamel, D.; Röhrig, E.; Pfister, K. Canine vector-borne disease in travelled dogs in Germany—a retrospective evaluation of laboratory data from the years 2004–2008. Vet Parasitol 2011, 181, 31–36. [Google Scholar] [CrossRef]
- Bogićević, N.; Radovanović, M.E.; Vasić, A.; Manić, M.; Marić, J.; Vojinović, D.; Rogožarski, D.; Gligić, A.; Valčić, M. Seroprevalence of Ehrlichia canis infection in stray dogs from Serbia. Maced Vet Rev 2017, 40, 37–42. [Google Scholar] [CrossRef]
- Laušević, D.; Ilić, T.; Nenadović, K.; Bacić, D.; Obrenović, S. Seroprevalences of Rickettsia conorii, Ehrlichia canis and Coxiella burnetii in dogs from Montenegro. Acta Parasitol 2019, 64, 769–778. [Google Scholar] [CrossRef]
- Alho, A.M.; Lima, C.; Latrofa, M.S.; Colella, V.; Ravagnan, S.; Capelli, G.; Madeira de Carvalho, L.; Cardoso, L.; Otranto, D. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Parasit Vectors 2017, 10, 298. [Google Scholar] [CrossRef]
- Cardoso, L.; Oliveira, A.C.; Granada, S.; Nachum-Biala, Y.; Gilad, M.; Lopes, A.P.; Sousa, S.R.; Vilhena, H.; Baneth, G. Molecular investigation of tick-borne pathogens in dogs from Luanda, Angola. Parasit Vectors 2016, 9, 252. [Google Scholar] [CrossRef]
- Barradas, P.F.; Vilhena, H.; Oliveira, A.C.; Granada, S.; Amorim, I.; Ferreira, P.; Cardoso, L.; Gärtner, F.; de Sousa, R. Serological and molecular detection of spotted fever group Rickettsia in a group of pet dogs from Luanda, Angola. Parasit Vectors 2017, 10, 271. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Llull, J.; Osso, M.; Hegarty, B.; Breitschwerdt, E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet Res 2006, 37, 231–244. [Google Scholar] [CrossRef]
- Torina, A.; Caracappa, S. Dog tick-borne diseases in Sicily. Parassitologia 2006, 48, 145–147. [Google Scholar]
- Schüle, C.; Rehbein, S.; Shukullari, E.; Rapti, D.; Reese, S.; Silaghi, C. Police dogs from Albania as indicators of exposure risk to Toxoplasma gondii, Neospora caninum and vector-borne pathogens of zoonotic and veterinary concern. Vet Parasitol Reg Stud Reports 2015, 1–2, 35–46. [Google Scholar] [CrossRef]
- Punda-Polic, V.; Leko-Grbic, J.; Radulovic, S. Prevalence of antibodies to Rickettsiae in the north-western part of Bosnia and Herzegovina. Eur J Epidemiol 1995, 11, 697–699. [Google Scholar] [CrossRef]
- Spasojevic-Kosic, L.; Savic, S.; Potkonjak, A.; Vracar, V. Retrospective analysis of clinical and laboratory findings in hunting dogs with serologic reactions to tick-borne pathogens (Anaplasma phagocytophilum, Borrelia burgdorferi, Babesia canis, Ehrlichia canis, Ricketsia conorii). Vet Glas 2015, 69, 219–232. [Google Scholar] [CrossRef]
- Selim, A.; Alanazi, A.D.; Sazmand, A.; Otranto, D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasit Vectors 2021, 14, 175. [Google Scholar] [CrossRef]
- Gaunt, S.; Beall, M.; Stillman, B.; Lorentzen, L.; Diniz, P.; Chandrashekar, R.; Breitschwerdt, E. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors 2010, 3, 33. [Google Scholar] [CrossRef]
- Beall, M.J.; Chandrashekar, R.; Eberts, M.D.; Cyr, K.E.; Diniz, P.P.V.P.; Mainville, C.; Hegarty, B.C.; Crawford, J.M.; Breitschwerdt, E.B. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector-Borne and Zoonotic Diseases 2008, 8, 455–464. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, A.S.; Otranto, D.; Dantas-Torres, F.; Capelli, G.; Breitschwerdt, E.B.; de Caprariis, D. Are vector-borne pathogen co-infections complicating the clinical presentation in dogs? Parasit Vectors 2013, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Kass, P.H.; Klement, E.; Waner, T. Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the Disease. Veterinary Record 1997, 141, 360–363. [Google Scholar] [CrossRef]
- Kottadamane, M.R.; Dhaliwal, P.S.; Singla, L. Das; Bansal, B.K.; Uppal, S.K. Clinical and hematobiochemical response in canine monocytic ehrlichiosis seropositive dogs of Punjab. Vet World 2017, 10, 255–261. [Google Scholar] [CrossRef]
- Donnett, U.; Hubbard, K.; Woodruff, K.; Varela-Stokes, A. Prevalence of canine heartworm infection in Mississippi animal shelters. Vet Parasitol 2018, 259, 68–73. [Google Scholar] [CrossRef]
- Mansueto, S.; Vitale, G.; Bentivegna, M.; Tringali, G.; Di Leo, R. Persistence of antibodies to Rickettsia conorii after an acute attack of boutonneuse fever. Journal of Infectious Diseases 1985, 151, 377–377. [Google Scholar] [CrossRef]
- Seaman, R.L.; Kania, S.A.; Hegarty, B.C.; Legendre, A.M.; Breitschwerdt, E.B. Comparison of results for serologic testing and a polymerase chain reaction assay to determine the prevalence of stray dogs in eastern Tennessee seropositive to Ehrlichia canis. Am J Vet Res 2004, 65, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J. Ehrlichiosis. In Canine and feline infectious diseases; Elsevier Saunders: Philadelphia, 2013; pp. 278–289. [Google Scholar]
- Aziz, M.U.; Hussain, S.; Song, B.; Ghauri, H.N.; Zeb, J.; Sparagano, O.A. Ehrlichiosis in dogs: a comprehensive review about the pathogen and its vectors with emphasis on south and east asian countries. Vet Sci 2022, 10, 21. [Google Scholar] [CrossRef]
- Johnson, E.M.; Ewing, S.A.; Barker, R.W.; Fox, J.C.; Crow, D.W.; Kocan, K.M. Experimental transmission of Ehrlichia canis (Rickettsiales: Ehrlichieae) by Dermacentor variabilis (Acari: Ixodidae). Vet Parasitol 1998, 74, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, S.; Portillo, A.; Santibáñez, P.; Palomar, A.M.; Oteo, J.A. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm Infecc Microbiol Clin 2013, 31, 283–288. [Google Scholar] [CrossRef]
- Znazen, A.; Sellami, H.; Elleuch, E.; Hattab, Z.; Ben Sassi, L.; Khrouf, F.; Dammak, H.; Letaief, A.; Ben Jemaa, M.; Hammami, A. Comparison of two quantitative real time PCR assays for Rickettsia detection in patients from Tunisia. PLoS Negl Trop Dis 2015, 9, e0003487. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Caprì, A.; Pennisi, M.G.; Caldin, M.; Furlanello, T.; Trotta, M. Acute febrile illness is associated with Rickettsia spp. infection in dogs. Parasit Vectors 2015, 8, 216. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Trotta, M.; Caldin, M.; Furlanello, T. Molecular survey of Rickettsia spp. in sick dogs in Italy. Zoonoses Public Health 2008, 55, 521–525. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Levy, M.G.; Davidson, M.G.; Walker, D.H.; Burgdorfer, W.; Curtis, B.C.; Babineau, C.A. Kinetics of IgM and IgG responses to experimental and naturally acquired Rickettsia rickettsii infection in dogs. Am J Vet Res 1990, 51, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.L.; Killmaster, L.F.; Zemtsova, G.E. Domestic dogs (Canis familiaris) as reservoir hosts for Rickettsia conorii. Vector-Borne and Zoonotic Dis 2012, 12, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.L.; Zemtsova, G.E.; Montgomery, M.; Killmaster, L.F. Effects of homologous and heterologous immunization on the reservoir competence of domestic dogs for Rickettsia conorii (israelensis). Ticks Tick Borne Dis 2014, 5, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Matthewman, L.A.; Mason, P.R.; Courtney, S.; Katsande, C.; Rukwava, J. Experimental infection of dogs with a zimbabwean strain of Rickettsia conorii. J Trop Med Hyg 1992, 95, 322–326. [Google Scholar] [PubMed]
- Santos-Silva, M.M.; Beati, L.; Santos, A.S.; De Sousa, R.; Núncio, M.S.; Melo, P.; Santos-Reis, M.; Fonseca, C.; Formosinho, P.; Vilela, C.; Bacellar, F. The hard-tick fauna of mainland Portugal (Acari: Ixodidae): an update on geographical distribution and known associations with hosts and pathogens. Exp Appl Acarol 2011, 55, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, H.; Grillini, M.; Simonato, G.; Mondin, A.; Dotto, G.; Frangipane di Regalbono, A.; Kumsa, B.; Cassini, R.; Menandro, M.L. Epidemiological survey on tick-borne pathogens with zoonotic potential in dog populations of southern Ethiopia. Trop Med Infect Dis 2023, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Tzipory, N.; Crawford, P.C.; Levy, J.K. Prevalence of Dirofilaria immitis, Ehrlichia canis, and Borrelia burgdorferi in pet dogs, racing greyhounds, and shelter dogs in Florida. Vet Parasitol 2010, 171, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.K.; Ingwersen, W.; Gray, J.L.; Wintz, D.J. Characterization of animals with microchips entering animal shelters. J Am Vet Med Assoc 2009, 235, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hodo, C.L.; Rodriguez, J.Y.; Curtis-Robles, R.; Zecca, I.B.; Snowden, K.F.; Cummings, K.J.; Hamer, S.A. Repeated cross-sectional study of Trypanosoma cruzi in shelter dogs in Texas, in the context of Dirofilaria immitis and tick-borne pathogen prevalence. J Vet Intern Med 2019, 33, 158–166. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | No. of infected Dogs | Inconclusive results | Prevalence (%) | 95% CI * |
|---|---|---|---|---|
| Ehrlichia canis | 1 | 4 | 0.9 | 0.02–4.8 |
| Rickettsia conorii | 11 | 12 | 9.7 | 4.9–16.8 |
| Co-infection | 12 | 16 | 10.6 | 5.6–17.8 |
| Variables/Categories |
E. canis, Positive/ Total (%) |
95% CI |
R. conorii, Positive/Total (%) |
95% CI | Coinfection (E. canis + R. conorii), Positive/Total (%) |
95% CI |
|---|---|---|---|---|---|---|
| Sex | p = 0.173 | p = 0.003* | p = 0.001* | |||
| Male | 0/68 (0.0%) | 0.0–5.3% | 2/68 (2.9%) | 0.36–10.2% | 2/68 (2.9%) | 0.36–10.2% |
| Female | 1/45 (2.2%) | 0.06–11.8% | 9/45 (20.0%) | 9.6–34.6% | 10/45 (22.2%) | 11.2–37.1% |
| Age | p = 0.235 | p = 0.106 | p = 0.057 | |||
| ≤ 12 months | 0/57 (0.0%) | 0.0–6.3% | 3/57 (5.3%) | 1.1–14.6% | 3/57 (5.3%) | 1.1–14.6% |
| > 12 months | 1/56 (1.8%) | 0.04–9.6% | 8/56 (14.3%) | 6.4–26.2% | 9/56 (16.1%) | 7.6–28.3% |
| Origin | p = 0.312 | p ≤ 0.000* | p ≤0.000* | |||
| Shelter 1 | 1/68 (1.5%) | 0.04–7.9% | 0/68 (0.0%) | 0.0–5.3% | 1/68 (1.5%) | (0.04–7.9%) |
| Shelter 2 | 0/45 (0.0%) | 0.0–7.9% | 11/45 (24.4%) | 12.9–39.5% | 11/45 (24.4%) | 12.9–39.5% |
| Dependent Variable/ /Risk Factor |
p Value | OR | 95% CI |
|---|---|---|---|
| Positivity to R. conorii | |||
| Sex | p = 0.003 | ||
| Male | 1 | ||
| Female | 2.32 | 1.58–3.39 | |
| Origin | p ≤ 0.000 | ||
| Shelter 1 | 1 | ||
| Shelter 2 | 3.0 | 2.28–3.95 | |
| Positivity to mixed infection | |||
| Sex | p = 0.001 | ||
| Male | 1 | ||
| Female | 2.4 | 1.66–3.47 | |
| Origin | p ≤ 0.000 | ||
| Shelter 1 | 1 | ||
| Shelter 2 | 2.72 | 1.97–3.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





