1. Introduction
The liver is the biggest organ in the vertebrate body and the principal location of xenobiotic absorption. Toxic substances, medicines, and viral infiltration by consumption or infection can all harm the liver. Carbon tetrachloride (CCl4) is a xenobiotic that has been linked to hepatotoxicity [
1]. In animal models, CCl4 has been frequently utilized to explore the effects of chemical poisons on the liver [
2]. The reductive dehalogenation of CCl4 by the P450 enzyme structure to the exceedingly sensitive trichloromethyl radical commences the development of lipid peroxidation, which is regarded to be the most significant mechanism in the pathophysiology of CCl4-induced liver impairment [
3]. Trichloromethyl radical’s canister easily respond through sulfhydryl groups of glutathione (GSH) and protein thiols. CCl4 also affects in antioxidant architecture of the liver, with antioxidant enzymes with catalase, glutathione peroxidase (GPx), glutathione reductase (GR), glutathione transferase (GST) and SOD, [
4].
Sulfated polysaccharides from seaweeds take remained publicized to protect against liver damage in hepatic cells due to their non-toxicity. Furthermore, a polysaccharide (low molecular weight) from
Laminaria japonica (moderate acid hydrolysis) displayed hepatoprotective effects into acute liver damage animal representations caused by carbon tetrachloride (CCl4) [
5]. Chen et al. [
6] developed an agaro-oligosaccharides produced from polysaccharide (red seaweed) and tested them for hepatoprotective activity in rats and were structurally characterized. Guo et al. [
7] studied the protective effect of
Porphyra yezoensis polysaccharide on rat against hepatotoxic impact produced by carbon tetrachloride. Furthermore, the sulfated polysaccharide has piqued the interest of biomedical researchers due to its unique biological happenings, which include antioxidant, antibacterial and anti-tumor properties, as well as immune-boosting properties [
8]. The current study aims to evaluate the preventive impact of
G. edulis sulfated polysaccharide against carbon tetrachloride (CCl4)-induced liver damage in Wister rats, taking into account the numerous biological features of sulfated polysaccharides.
2. Materials and Methods
2.1. Seaweed Collection and Pre-Treatment
The seaweed
G. edulis was collected from the sub-tidal location of Thiruchendur (Lat. 09°44' N; Long. 079°02' E) in Tamil Nadu, on the Southeast coast of India, during the lowest low-tide period. Umamaheswara Rao's preferred systematic key reference was used to identify the alga [
9]. Then the seaweed was washed in seawater and remove the epiphytes, muck, and sand, and further washed with tap water followed by distilled water and shade dried. Then the seaweed was broken down into slight pieces and ground into a powder in a blender crusher.
2.2. Extraction of Polysaccharide
The extraction of polysaccharide was carried out according to method of Seedevi et al. [
10] was followed. 50g of powder (seaweed) was soaked in 1:50 volume of distilled water (2.5 L) for 2 hours before being stirred and refluxed for another 2 hours at 100°C. Then the extract was cooled and filtered through cheesecloth; the supernatant was centrifuged at 6000 rpm for 20 minutes. The remainder was then extracted 3 times in 2 hours above the same. Then the all supernatant were collective and intense using rotary evaporator and dialyzed (molecular weight cut off 12,000 kDa) in cellulose membrane for three days against distilled water. Then the sample was lyophilized (Penguin plus -4 kg, Lark India).
2.3. Deproteinization of Polysaccharide
The polysaccharide was deproteinized following the Sevag method [
11]. The polysaccharide was liquified in distilled water and miscellaneous with Sevag reagent (CHCl3: n-BuOH - 5:1) as described the
2.4. Fractionation of Polysaccharides
2.4.1. Ion-Exchange Chromatography
10mg of polysaccharide was dissolved in 10ml of distilled water and centrifuged at 6000 rpm for 10 minutes, and the supernatant was applied to a Q-Sepharose™ (GE- Heathcare) Fast Flow column (EX 150 mm×50 cm) equilibrated with distilled water. The column was eluted stepwise with distilled water, using a linear gradient of 0–3 mol/ NaCl at a flow rate of 0.55 ml/min, and 15 fractions were collected. The carbohydrate content of the collected fractions was estimated using a phenol-sulfuric acid reaction [
12]. The fractions with the highest yield were combined, dialyzed, and freeze-dried.
2.5. Purification of Fractionated Polysaccharide
2.5.1. Gel Filtration Chromatography
Gel permeation chromatography was used to further purify the fractionated polysaccharide using a High-Resolution Sepharose 4-LB Fast Flow column (EX 100 mm25 cm) equilibrated with distilled water. The column was eluted in steps with distilled water and 0.2 mol/l sodium acetate buffer at a rate of 0.33 ml/min. The carbohydrate content of the collected fractions was estimated using the phenol-sulphuric acid reaction [
12]. The fractions with the maximum yield were combined, dialyzed, and freeze-dried.
2.6. Analysis of Biochemical Composition
2.6.1. Determination Total Carbohydrate and Protein Content
The total carbohydrate content was calculated using the phenol-sulphuric acid method and D-glucose as a standard [
12]. The protein content was determined using the Bradford method [
13], with bovine serum albumin serving as a standard.
2.6.2. Determination of Ash and Moisture Content
The ash content was determined using a gravimetric method [
14]. In a muffle furnace, 0.5 g of sulfated polysaccharide was burned at 600°C for 8 hours in a porcelain crucible. The ash content was represented by the weight of the residue, and the results were given as a percentage. Micro-oven was used to determine the moisture content of sulfated polysaccharide. The results were given as a percentage after drying 0.5 mg of sample at 13°C for 2 hours.
2.6.3. CHN/S Analysis
The carbon, hydrogen, nitrogen, and sulfur content of the sulfated polysaccharide were determined using the CHNS/O analyzer EA1112 (CE Instrument, Italy). TCD was used to elute 1mg of sulfated polysaccharide (Thermal Conductivity Detector, CE Instrument). As reference standards, 2,5-Bis (5′ tert-butyl-2-benzoxazol-2-yl) thiophene (BBOT), and aspartic acid were used.
2.6.4. Polyacrylamide Gel Electrophoresis (PAGE)
The molecular weight of the sulfated polysaccharides was appraised using PAGE analysis using the method of Nikapitiya et al. [
15]. 10µg of sample was applied to a 6% polyacrylamide gel slab (1mm thickness) in 0.02M sodium barbital (pH 8.6) and run at 100 V for 30 minutes. Then the gel was stained with 0.1% toluidine blue in 1% acetic acid for 4 hours before being washed in 1% acetic acid. The dextran sulfate, chondroitin sulfate, and heparan sulfate (Sigma) were used as standard. Total Lab software (Version 1.11 of Total Lab) was used to calculate the molecular weight.
2.7. Spectral Analysis of Sulfated Polysaccharide
2.7.1. Monosaccharide Analysis of Sulfated Polysaccharide
1mg of sulfated polysaccharide was dissolved in 1ml of distilled water and mixed with an equivalent volume of 4.0 M trifluoroacetic acid (TFA), according to Seedevi et al [
10]. The mixer was hydrolyzed for 4 hours at 100
oC and filtered through a 0.45m syringe filter, and the remaining acid was detached three times finished decompressing and distillation through methanol. Then 100µl sample was injected in a Gas Chromatography-Mass Spectrometer (GC-MS) (Perkin-Elmer Clarus 680 SQ8 with autosampler) with a carbohydrate analysis column (RTX-5MS 30M, 0.32mm), and a refractive index (RI) detector. The mono-sugars such as arabinose, glucose, galactose, fucose, mannose, and xylose (Sigma) were used as references.
2.7.2. FT-IR Analysis of Sulfated Polysaccharide
10 mg of sulfated polysaccharide was mixed with 100mg of dried potassium bromide (KBr) and compressed to form a salt disc (10 mm diameter) and analyzed using a Bio-Rad FT-IR (USA). Spectra were collected between wave numbers of 4000 and 500 cm-1.
2.7.3. 1H-NMR Analysis of Sulfated Polysaccharide
The
1H-NMR spectrum of sulfated polysaccharide was obtained using the Luo and Fan method [
16]. In an NMR tube, 30 mg of sample was dissolved in 0.5 ml of D
2O (99.9%). At 27°C, the 1H-NMR spectra were obtained, and the chemical shift was expressed in parts per million (ppm).
2.8. Experimental Animals
The experiments animals in male albino Wister rats (180-220 g) obtained from the Central Animal House, Department of Pharmacology, K.M. Pharmacy College, Madurai, Tamil Nadu, India. The animals were housed in (47 × 34 × 20 cm) polypropylene cages wrinkled with husk, and transformed every 24 h, under a 12-h light: 12-h dark cycle at 22± 20C temperature in A/C condition, and permissible to have free access to consumption water and food ad libitum. The exertion was passed out giving to the rules of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and approved by the Animal Ethical Committee of Periyar University (PU IAEC/2018/M1/16; dated 08.2018).
2.9. Acute Toxicity Study of Sulfated Polysaccharide
Sulfated polysaccharide from
G. edulis was studied for its acute toxicity using Wistar rats, conferring to the procedures set by OECD [
17]. 180 - 220 g of healthy Wistar rats were used for the investigation. Different dosages of sulfated polysaccharide were 0.5, 1.0, 1.5 and 2.0 g/kg body weight (PW) for oral administration (p.o.) were given to I- IV groups with 5 rats in each group. The general behavior and mortality of the treated animals (groups I-IV) were observed for 14 days.
2.10. Hepatoprotective Experimental Design
The animals were divided into five groups (I-V), with six rats (n = 6) in each group.
Group I: Normal control rats: Animals were allowed to have free access to a normal diet for 7 weeks.
Group II: CCl4 control rats: Animals were treated with CCl4 @ 1.0 ml/kg body weight in 50% corn oil solution twice a week by intraperitoneal injection for 7 weeks.
Group-III: Silymarin - treated hepatic injured rats (positive control): The hepatic injured rats were treated with normal diet and silymarin (50 mg/kg/day, p.o.) for 7 weeks.
Group IV: Sulfated polysaccharide- treated hepatic injured rats: The hepatic injured rats were treated with normal diet and sulfated polysaccharide (50 mg/kg/day, p.o.) for 7 weeks.
2.11. Collection of Blood Serum and Organs
After the experiment, all the animals were sacrificed by cervical dislocation. The blood was collected and centrifuged at 3000 × g at 4°C for 10 min and and to collect the serum. And the liver was separated out, washed with PBS saline (two times) and homogenate. Then the homogenate was centrifuged at 4000 × g at 4°C for 10 min to remove cellular debris. Finally, the collected supernatant was analysis for further, and one portion of the liver sample of (each group) animals was expunged for the histopathological investigation.
2.12. Estimation of Serum Marker Enzymes
The serum aspartate aminotransferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), and albumin (ALB) were deliberated using the analytical kits.
2.13. Estimation of SOD, Catalase GPx and GSH
The liver homogenate was assessed for the of SOD, CAT, GPx, and GSH levels. All of these enzymes were determined according to the producer’s instructions.
2.14. Histopathological Studies
The brain, spleen, heart, liver, lung, and kidney of each group of acute toxicity experimental rats, as well as the liver of the hepatoprotective experimental rats, were immediately dissected out. All tissues were washed twice with PBS saline and fixed in 10% formalin. Each tissue was desiccated and fixed in paraffin wax; 3-5 m impenetrable sections were bowdlerized with a rotary microtome, further dry in categorized alcohol, and stained with hemotoxylin and eosin. The samplings were inspected for pathological changes using a light microscope (Motic, India) and pictures were taken.
2.15. Statistical Analysis
The experimental data were exposed to one-way ANOVA, and Dunnett's multiple comparison (GraphPad Software, USA) were used to determine the variance between means at the 0.05 level.
3. Results and Discussion
3.1. Structural Characterization of Sulfated Polysaccharide
3.1.1. Yield and Chemical Composition
The yield of the present sulfated polysaccharide recorded 12.90% based on the dry weight basis. The sulfated polysaccharide form
G. edulis noted the carbohydrate, protein, ash, and moisture content of the sulfated polysaccharide from
G. edulis were found to be 83.07%, 0%, 15.02% and 2.09%; whereas the sulfated polysaccharide of
Gracilaria corticata exposed the yield, carbohydrate, protein, ash and moisture content were 29.08%, 84%, 0%, 19.7% and 29.4% respectively [
14]. The CHN/S analysis such as carbon, hydrogen, nitrogen and sulfur content of the sulfated polysaccharide were estimated to be 25.90%, 4.28%, 2.65% and 3.61% respectively, in the current study. However, the purified sulfated polysaccharide from the seaweed of
G. corticata contained 33.19% of carbon, 5.91% of hydrogen, 7.21% of nitrogen and 3.75% of sulphur [
14]. The yield variations of the polysaccharides, are species-specific, and the chemical composition of seaweeds varies significantly, possibly due to differences in cell wall components and the effect of the extraction process.
3.1.2. Molecular Weight of Sulfated Polysaccharide
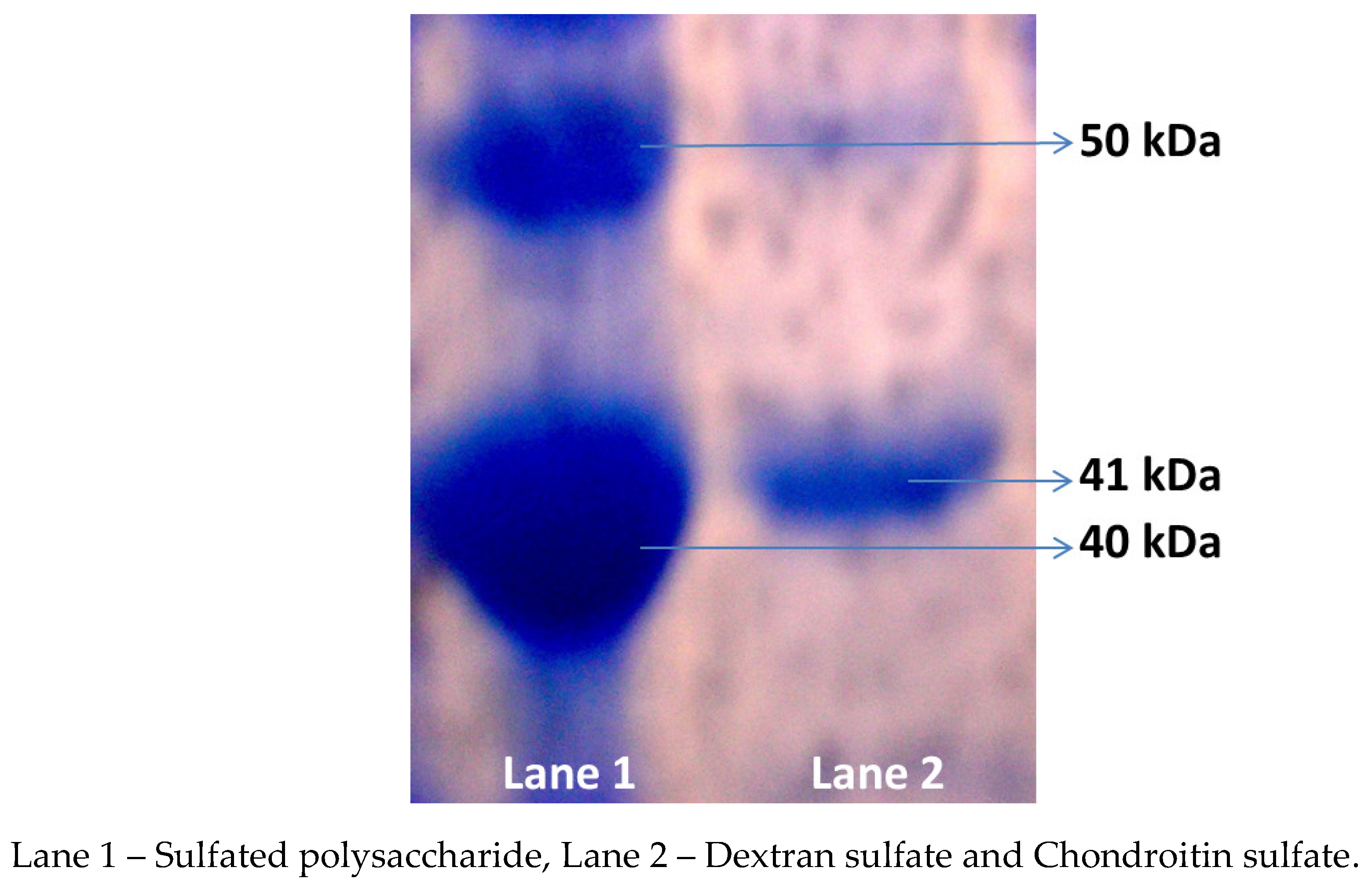
The sulfated polysaccharide from
G. edulis reported the molecular weight of 41 kDa, which was equated to the ideals 50 kDa (Chondrioitin sulfate) and 40 kDa (Dextran sulfate) respectively (
Figure 1). In the present study, the molecular weight of the sulfated polysaccharide from
G. edulis was originate to be higher in the current study when compared to that of brown seaweed
Sargassum fulvellum in which fermented polysaccharide presented 8 kDa and 20 kDa [
18]. In a previous study, the
Sargassum plagiophyllum sulfated polysaccharide fractions (F1, F2, and F3) noted the molecular weight were 30 kDa, 35 kDa, and 20 kDa, respectively [
19]. Sudharsan et al. [
20] determined the molecular weights of the crude, fraction and purified polysaccharide from
Gracilaria. debilis to be 44 kDa, 32 kDa, and 20 kDa, respectively.
3.1.3. FT-IR Spectrum of Sulfated Polysaccharide
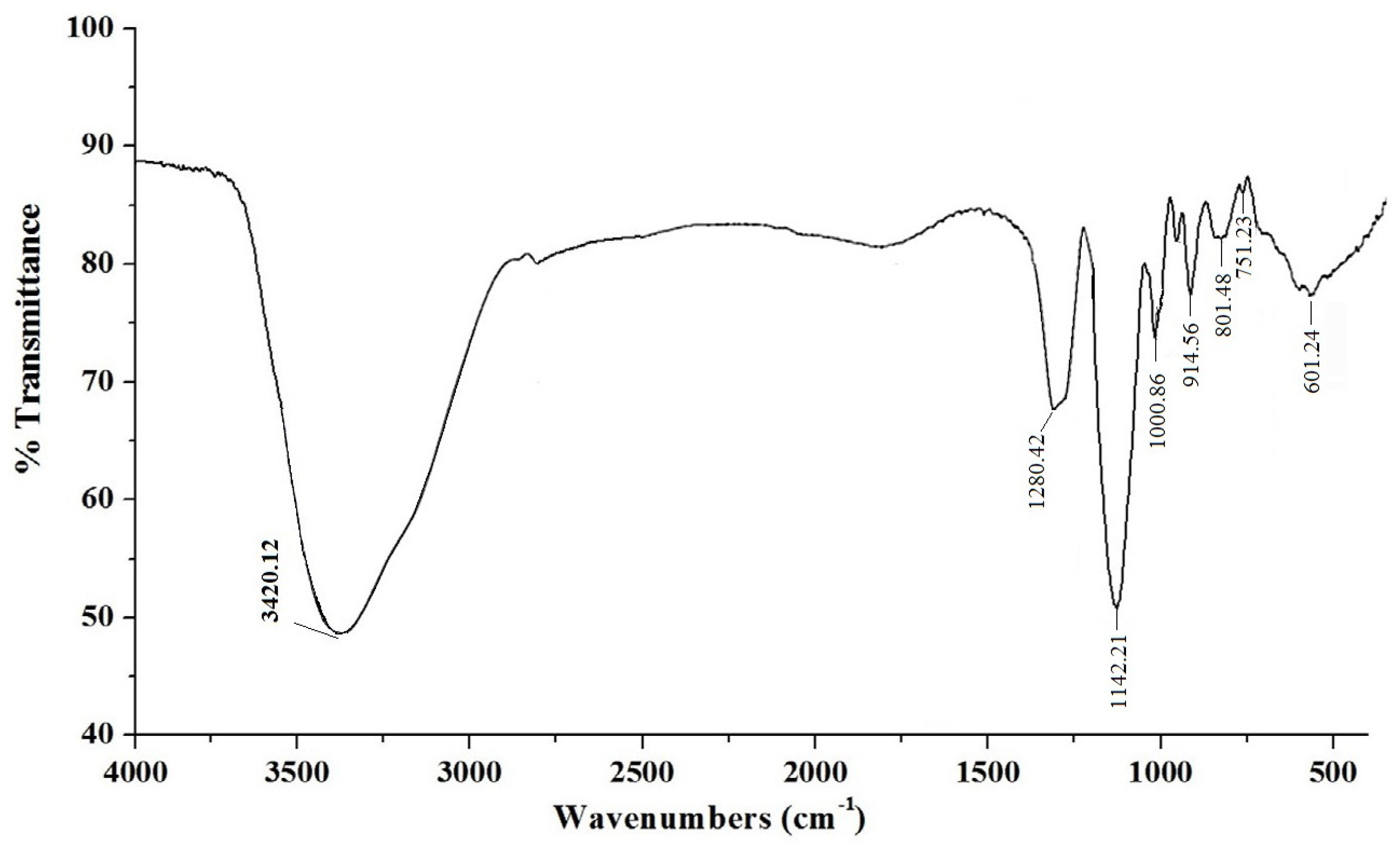
In the FT-IR spectral analysis of the sulfated polysaccharide showed an absorbance band at 3420.12 cm
−1 that characterized the extending vibrations of O-H in the essential sugar residues. The band at 1280.42 cm
−1 was ascribed to O=S=O stretching shuddering of sulfate esters in the polysaccharide. The strong extensive absorption bands at 1142.21 and 1000.86 cm
−1 attributed the presence of galactan residues in the polysaccharide (
Figure 2). The characteristic preoccupation band at 914.24 cm
−1 revealed the presence of sulfate substitution of β-d-galactose. The band at 801.48 cm
−1 showed the existence of sulfate group on 4-linked 3,6-anhydro-α-D-galactose 2-sulfate remains [
21]. The weak engagement band at 601.24 cm
−1 showed the sulfate group in the polysaccharide [
22].
3.1.4. Monosaccharide Composition of Sulfated Polysaccharide
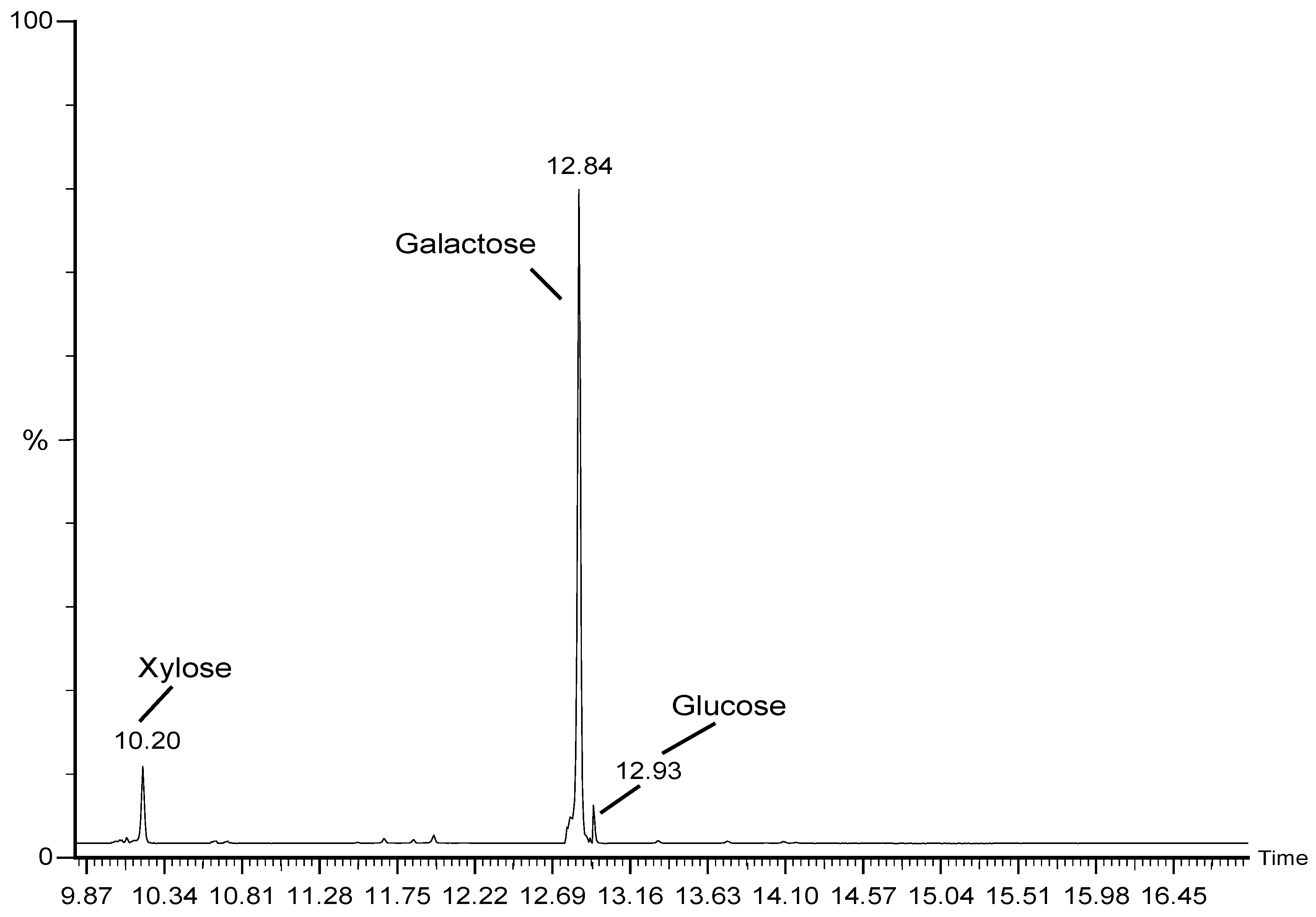
When compared to the monosaccharide configuration of the typical monosugars, the sulfated polysaccharide from
G. edulis had 78.20% galactose, 9.21% xylose, and 4.59% glucose (
Figure 3). Similar to this, the cold-water, hot-water, acid, and alkali extraction of sulfated polysaccharide of the red seaweed from
Mastocarpus stellatus had a difficult concentration of galactose were 87.76%, 95.19%, 93.85% and 70.17% and lower amounts of xylose and glucose [
23]. In contrast, the sulfated polysaccharide fractions (P1, P2, P3, P4 and P5) from
M. oxyspermum had monosaccharide contents that were higher in rhamnose 84.94%, 84.65%, 85.77%, 80.35% and 78.28% and lower in glucose 7.29-10.07%, xylose 4.79-8.60%, mannose 1.43-1.66%, and galactose 1.22-2.87% [
24]. In the present study molecular weight findings showed that the sulfated polysaccharide differed chemically from other species in that it was a sulfated polymer rich in galactose.
3.1.5. 1H-NMR Spectrum of Sulfated Polysaccharide
The NMR spectrum of the sulfated polysaccharide signals started from 2.392 ppm to 5.182ppm (
Figure 4). The signal at 5.072 – 5.182ppm exposed the α-D-galactose conformation and the signal at 4.532 – 4.799 designated β-D-glucopyranosyl outline in the polysaccharide. The signal at 4.012 – 4.472 expended the α-D-galactose configuration and the indication at 3.976 - 3.987 ppm characterized the sulfate group linked with α-galactose in the sulfated polysaccharide. The signals at 5.072 – 5.182 ppm showed α-anomeric residues and the sign at 4.532 – 4.799 ppm disclosed β- anomeric proton. Similarly, Sudharsan et al. [
20] stated the sulfate group linked with α-galactose and mentioned as galactan sulfate and conformed through NMR spectrum. The results indicated that the repeating unit of sulfated polysaccharide consisted of terminal Gal
p, terminal Glc
p, (1→6)α-D-Gal
iv(1→3)β-D-Glc
iii(1→4,6)β-D-Gal
ii(1→2,4)α-D-Glc
i(1→ moieties.
3.2. Acute Toxicity Effect of Sulfated Polysaccharide
In acute toxicity study, the sulfated polysaccharide from
G. edulis was administrated different doses were 0.5, 1, 1.5, and 2g/kg (body weight) in rats, revealed non-toxic and did not affect the brain, spleen, heart, liver, and kidney; when compared to normal (control) rats' brain, spleen, heart, liver, and kidney. And almost in the higher concentration of sulfated polysaccharide at 2g/kg had normal structure with no tissue and cellular impairment. As a result, the sulfated polysaccharide was found to be non-toxic, necessitating further in-vivo biological testing. Furthermore, Seedevi et al [
25] reported non-toxic in rats at the dose of >2g/kg body weight for the oral administration of crude and rhamnose enriched polysaccharide from
Gateloupia lithophilla, and also observed with non-mortality or symptoms.
3.3. Hepatoprotective Activity
3.3.1. Effect of Sulfated Polysaccharide on Serum ALT, AST, ALP, and ALB
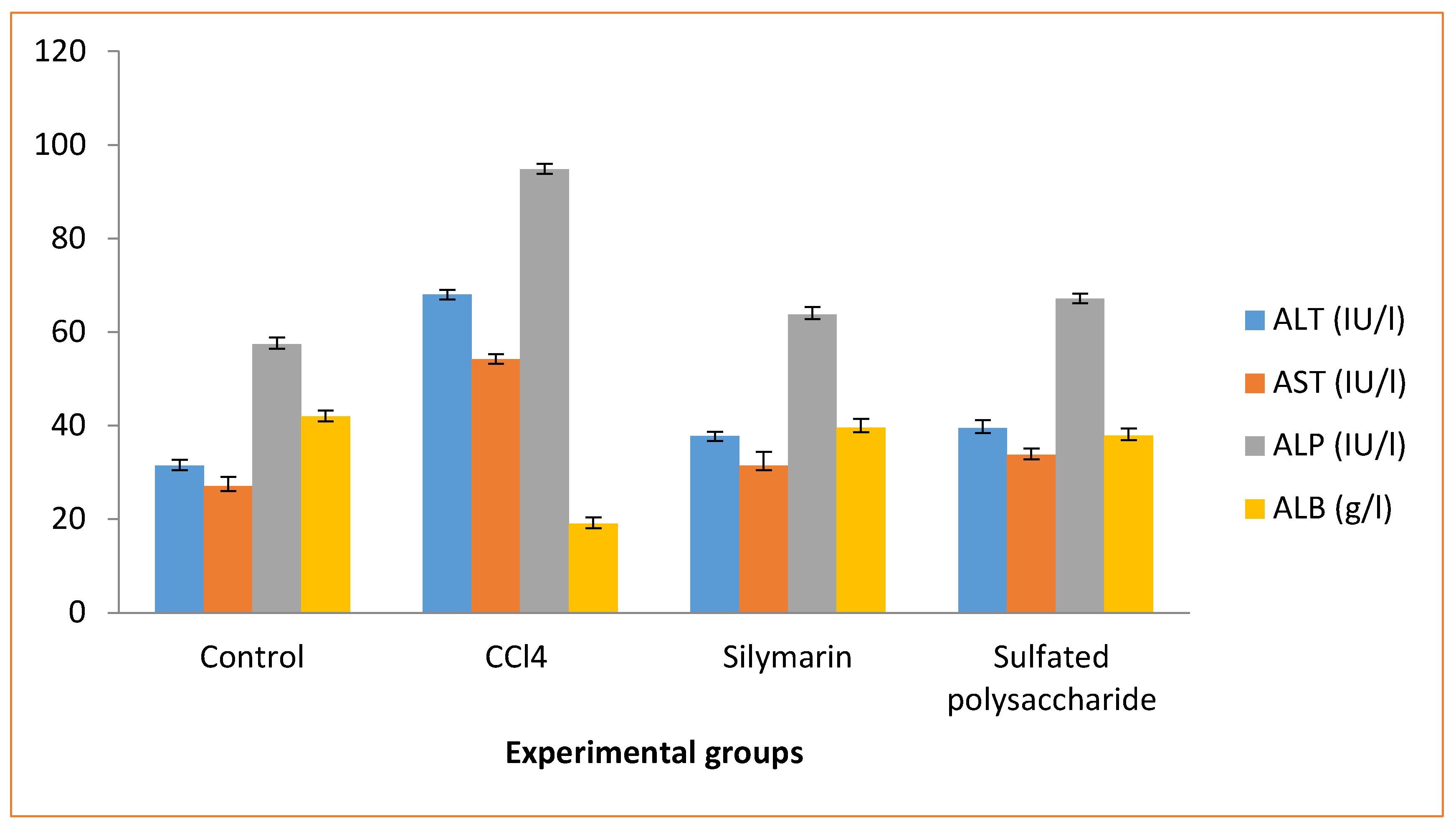
The sulfated polysaccharide pretreatment on CCl4-induced (group III) rats revealed in changes of serum ALT, AST, ALP, and ALB levels in near normal level, when compared to the normal control (group I) rats (
Figure 5). The CCl4 induced (group II) rats caused hepatotoxicity as evidenced by an increase in serum ALT, AST, and ALP levels and a decrease in ALB levels, compared to the control (group I) rats. When compared to the CCl4-induced (group III) rats, the animals pretreated with sulfated polysaccharide (group IV) rats showed a significant decrease in ALT, AST, and ALP levels, as well as an increase in ALB levels, when compared to the normal control (group I) rats. CCl4-induced rats with silymarin administration (group III) rats reversed the changes in ALT, AST, ALP, and ALB levels. Similar results were observed in single dose CCl4 induced rats, which showed an increase in serum ALT, AST, and ALP levels and a decrease in ALB levels; however, total saponins from
Taraphochlamys affinis (TSTA) treated with CCl4 induced rats showed the opposite effect, i.e., decreased ALT, AST, and ALP levels and an increase in ALB level [
26]. The reversed plasma ALT and AST levels have been linked to liver cell damage [
27]. In contrast, the present sulfated polysaccharide pretreatment of CCl4-induced rats results in serum ALT, AST, ALP, and ALB levels that are near normal, which may steady the cell membrane and prevent intracellular enzyme outflow into the blood.
3.3.2. Effect of Sulfated Polysaccharide on Hepatic Antioxidant Enzyme Activities
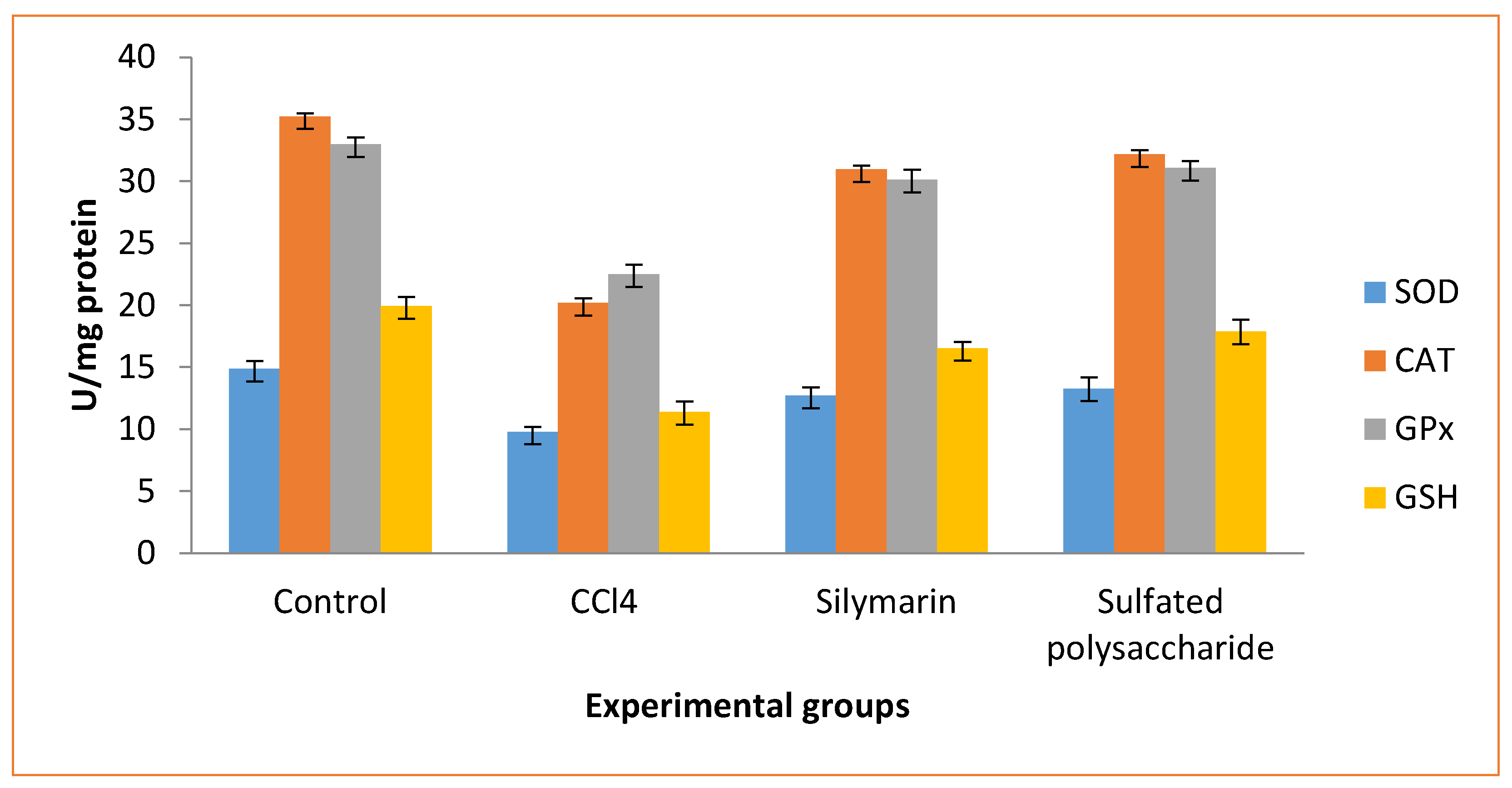
The SOD, catalase, GPx, and GSH levels were significantly lower in CCl4-induced (group II) rats compared to normal control (group I) rats (
Figure 6). But, when compared to the normal control rats (group I) and CCl4 induced (group II) rats, the sulfated polysaccharide treated (group IV) rats improved in SOD, catalase, GPx, and GSH levels. In contrast, silymarin administration (group III) rats increased SOD, catalase, GPx, and GSH levels when compared to normal control (group I) rats and CCl4-induced (group II) rats. These finding is consistent with the activity of SOD, catalase, GPx, and GSH at 200mg/kg (b.wt) concentrations of β-chitosan from the cuttlebone of
Sepioteuthis lessoniana and chitosan from
Sepia kobiensis on CCl
4-induced rat liver tissue [
28,
29]. Thus, the findings support the hepatoprotective effect of sulfated polysaccharide from
G. edulis, which may provide liveliness to prevent the liver tissue impairment.
3.3.3. Histopathological Examination
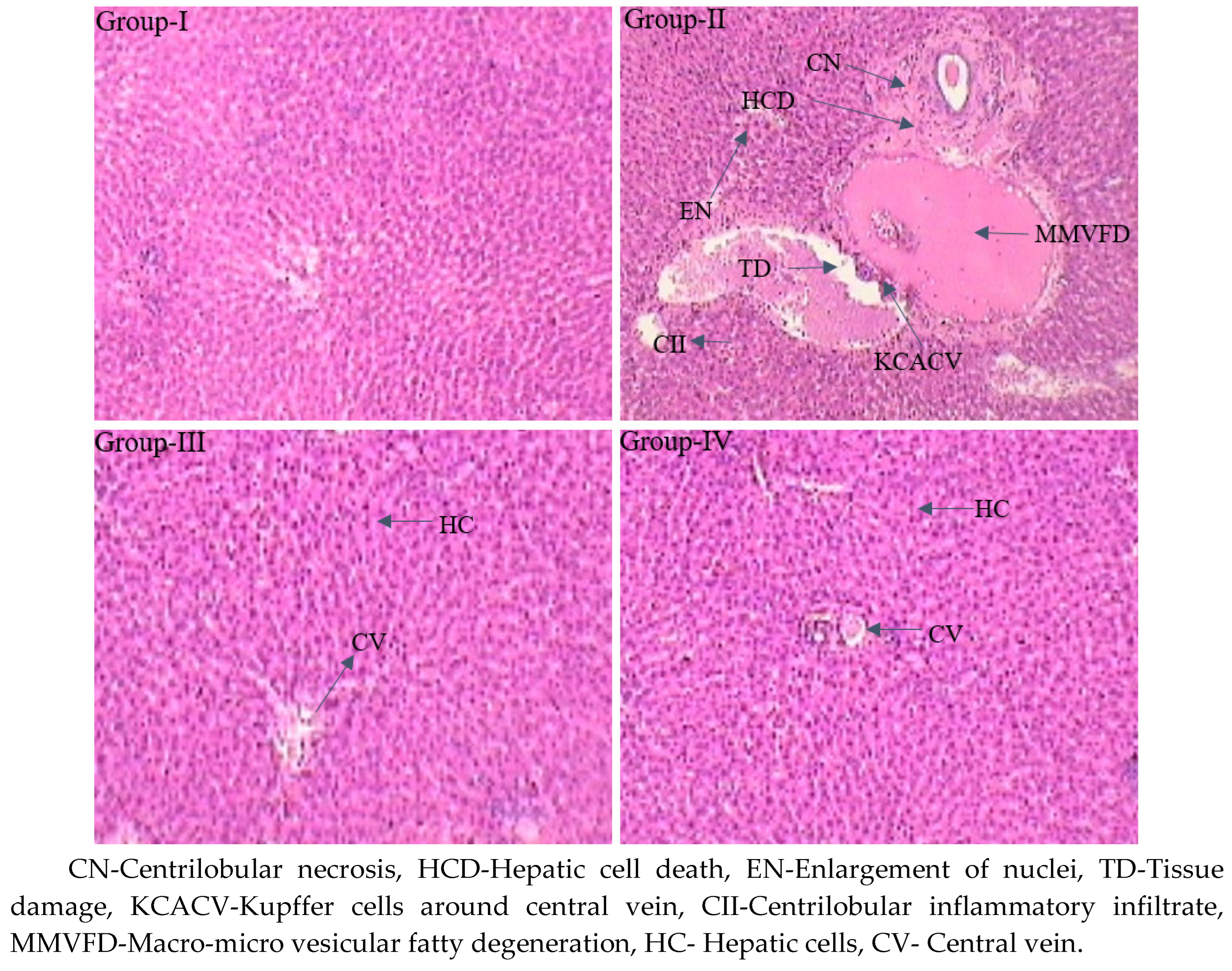
Histopathological examination of the liver tissue of normal control (group I) rats revealed regular cellular construction with well-designed hepatocytes shining from a central vein unglued by blood sinusoids and the hepatocytes covers central pale stained nuclei. CCl4 induced (group II) rats displayed irregular pathological variations reliable with hepatotoxicity, with tissue damage, necrosis in hepatic lobules (centrilobular necrosis), amplification of nuclei, macrovesicular and microvesicular fatty deterioration, centrilobular inflammatory penetrate, hypertrophy, Kupffer cells about the central vein, and fibrosis. The liver tissue of silymarin-treated hepatic injured (group III) rats disclosed significant retrieval in the form of normal hepatocytes and less centrilobular necrosis. Nevertheless, in the sulfated polysaccharide-treated hepatic injured (group IV) rats, well-known recovered the liver tissue in the arrangement of normal hepatocytes, controlling centrilobular necrosis and centrilobular inflammatory infiltrates while inhibiting microvesicular, and macrovesicular fatty degeneration (
Figure 7). In contrast, the polysaccharide from
Porphyra yezoensis disclosed a decrease in cells inflammation at a dose of 50mg/kg b.wt when treated against CCl
4-induced hepatic injured mice [
7]. In the current study, the sulfated polysaccharide demonstrated good recovery of hepatic liver damage at a lower dose of 50mg/kg body weight. The hepato-productive effect of sulfated polysaccharide may the boost up the immune system in influence of molecular weight with higher combination of galactose.
4. Conclusions
In conclusion, the sulfated polysaccharide was effective in preventing CCl4-induced liver damage in rats in the current study. The sulfated polysaccharide pretreatment of CCl4-induced rats results in serum ALT, AST, ALP, and ALB levels that are near normal, which may alleviate the cell membrane and stop intracellular enzyme outflow into the blood. Thus, the findings demonstrate the hepatoprotective effect of sulfated polysaccharide, which may provide liveliness to prevent tissue impairment. As a result, G. edulis sulfated polysaccharide can be considered as an alternative in the treatment of liver injury and/or preventive medicine.
Author Contributions
Palaniappan Seedevi - Conceptualization, methodology, writing-original draft preparation and supervision, Pasiyappazham Ramasamy - Formal analysis, Ramya Mohan - Software; Khalid Mujasam Batoo - language editing, Sajjad Hussain - manuscript review, Shanmugam Vairamani - Data curation, Annaian Shanmugam – Editing and validation.
Funding
The author K M Batoo would like to thank Researchers Supporting Project No. (RSP2023R148), King Saud University, Riyadh, Saudi Arabia for the financial support.
Institutional Review Board Statement
The work was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and approved by the Animal Ethical Committee of Periyar University (PU IAEC/2018/M1/16; dated 08.2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author K M Batoo would like to thank Researchers Supporting Project No. (RSP2023R148), King Saud University, Riyadh, Saudi Arabia for the financial support. Data is contained within the article or supplementary material. The author (P.S. PDF/2017/002512) is thankful to Department of Sciences and Technology, Science and Engineering Research Board (DST-SERB), National Postdoctoral Fellowship (N-PDF), Government of India. The authors would like to express their gratitude to the Director, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), for allowing them to carry out this research successfully.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kodavanti, P.R.S.; Joshi, U.M.; Young, R.A.; Meydrech, E.F.; Mehendale, H.M. Protection of Hepatotoxic and Lethal Effects of CCl by Partial Hepatectomy. Toxicol. Pathol. 1989, 17, 494–505. [Google Scholar] [CrossRef]
- Srivastava, A.; Shivanandappa, T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010, 118, 411–417. [Google Scholar] [CrossRef]
- Demirdag, K.; Bahcecioglu, I.H.; Ozercan, I.H.; Özden, M.; Yilmaz, S.; Kalkan, A. Role of L-carnitine in the prevention of acute liver damage induced by carbon tetrachloride in rats. J. Gastroenterol. Hepatol. 2004, 19, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Abd El-Gabar, M.; Bastawy, M. Carbon tetra chloride changes the activity of cytochrome P450 system in the liver of male rats: role of antioxidants. Toxicology 2001, 169, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, C.H.; Li, Z.J.; Cai, Y.P.; Liu, H.Y.; Qi, H.T. Antioxidant and hepatoprotective activities of low molecular weight sulfated polysaccharide from Laminaria japonica. J. Appl. Phycol. 2004, 16, 111–115. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Zhu, P.; Lin, J. Antioxidant activity and hepatoprotective potential of agaro-oligosaccharides in vitro and in vivo. Nutr. J. 2006, 12, 1–12. [Google Scholar] [CrossRef]
- Guo, T.-T.; Xu, H.-L.; Zhang, L.-X.; Zhang, J.-P.; Guo, Y.-F.; Gu, J.-W.; He, P.-M. In vivo protective effect of Porphyra yezoensis polysaccharide against carbon tetrachloride induced hepatotoxicity in mice. Regul. Toxicol. Pharmacol. 2007, 49, 101–106. [Google Scholar] [CrossRef]
- Smit A J, (2004) Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 16:245-262. [CrossRef]
- Umamaheshwara, R.M. Key for identification of economical important seaweeds. CMFRI Bull. 1987, 41, 1–116. [Google Scholar]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Vasanthkumar, S.; Srinivasan, A.; Vairamani, S.; Shanmugam, A. Structural characterization and bioactivities of sulfated polysaccharide from Monostroma oxyspermum. Int. J. Biol. Macromol. 2015, 72, 1459–1465. [Google Scholar] [CrossRef]
- Staub, A.M. Removal of proteins from polysaccharides. Methods Carbohydr. Chem. 1965, 5, 5–7. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Ann. Chem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Vairamani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulphated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Nikapitiya, C.; De Zoysa DZ, M.; Jeon, Y.J.; Lee, J. Isolation of sulphated anticoagulant compound from fermented red seaweed Grateloupia filicina. J. World Aquac. Soc. 2007, 38, 407–417. [Google Scholar] [CrossRef]
- Luo, A.; Fan, Y. In vitro antioxidant of a water soluble polysaccharide from Dendrobium fimhriatum Hook.var.oculatum Hook. Int. J. Mol. Sci. 2011, 12, 4068–4079. [Google Scholar] [CrossRef]
- OECD/OCDE. Guideline for the testing of chemicals. Revised Draft Guideline 423: Acute Oral Toxicity October 2000.
- Zoysa, M.D.; Nikapitiya, C. Anticoagulant activity of sulfated polysaccharide isolated from fermented brown seaweed Sargassum fulvellum. J. Appl. Phycol. 2008, 20, 67–74. [Google Scholar] [CrossRef]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process. Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Sudharsan, S.; Seedevi, P.; Subhapradha, N.; Vasanth kumar, S.; Vairamani, S.; Madeswaran, P.; Shanmugam, A.; Srinivasan, A. Antioxidant and anticoagulant activity sulphated polysaccharide from Gracilaria debilis (Forsskal) Southeast coast of India, Int. J. Biol. Macromol. 2015, 81, 1031–1038. [Google Scholar] [CrossRef]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The system of sulfated galactans from the red seaweed Gymnogongrus torulosus (Phyllophoraceae, Rhodophyta): Location and structural analysis. Carbohydr. Polym. 2008, 73, 594–605. [Google Scholar] [CrossRef]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A viruses. Carbohydr. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef]
- Gomez-Ordonez, E.; Jimenez-Escrig, A.; Ruperez, P. Bioactivity of sulphated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef]
- Zhang, H.J.; Mao, W.J.; Fang, F.; Li, H.Y.; Sun, H.H.; Chen, Y.; Qi, X.H. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008, 71, 428–434. [Google Scholar] [CrossRef]
- Seedevi, P.; Ramu Ganesan, A.; Moovendhan, M.; Mohan, K.; Sivasankar, P.; Loganathan, S.; Vairamani, S.; Shanmugam, A. Anti-diabetic activity of crude polysaccharide and rhamnose-enriched polysaccharide from G. lithophila on Streptozotocin (STZ)-induced in Wistar rats. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.F.; Zhang, S.; Zheng, L.; He, M.; Huang, R.; Lin, X. Hepatoprotective effects of total saponins isolated from Taraphochlamys affinis against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2012, 50, 713–718. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, N.S.; Abdel-Rahman, M.S. The mechanism of chloroform toxicity in isolated rat hepatocytes. Toxicol. Lett. 1993, 69, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Subhapradha, N.; Saravanan, R.; Ramasamy, P.; Srinivasan, A.; Shanmugam, V.; Shanmugam, A. Hepatoprotective Effect of β-Chitosan from Gladius of Sepioteuthis lessoniana Against Carbon Tetrachloride-Induced Oxidative Stress in Wistar Rats. Appl. Biochem. Biotechnol. 2014, 172, 9–20. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Protective effect of chitosan from Sepia kobiensis (Hoyle 1885) cuttlebone against CCl4 induced hepatic injury. Int. J. Biol. Macromol. 2014, 65, 559–563. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).