Submitted:
25 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- A Nikon LV100POL polarizing microscope with Marzhauser X, Y, and Z-axis motorization, Prior Lumen200 fiber optic fluorescence system, and Nikon NIS Elements image analysis software were used for the analyses (Figure 2A).
- Adsorption measurements.
- Densimetric research
3. Results
3.1. Microscopic Analyses
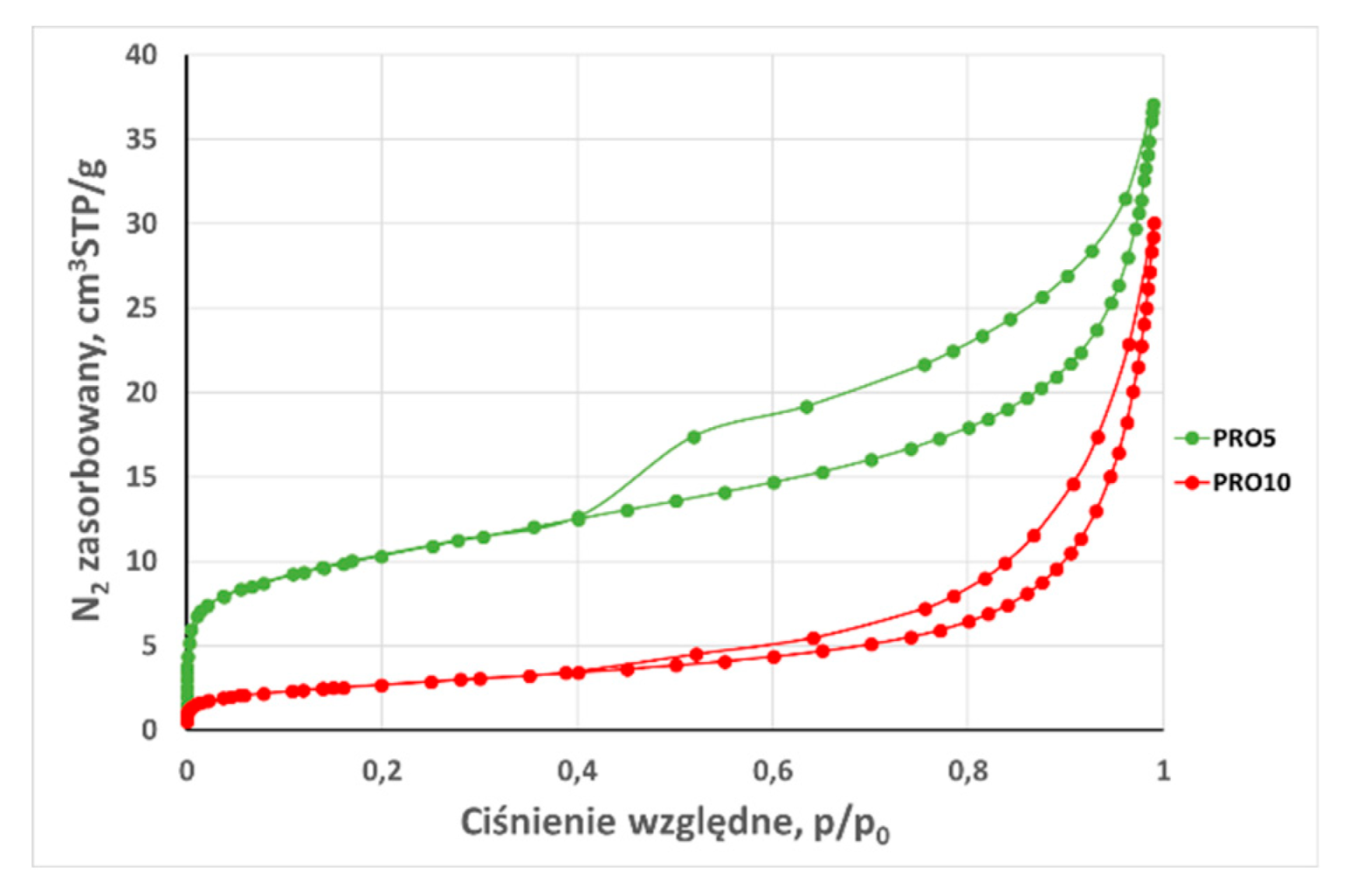
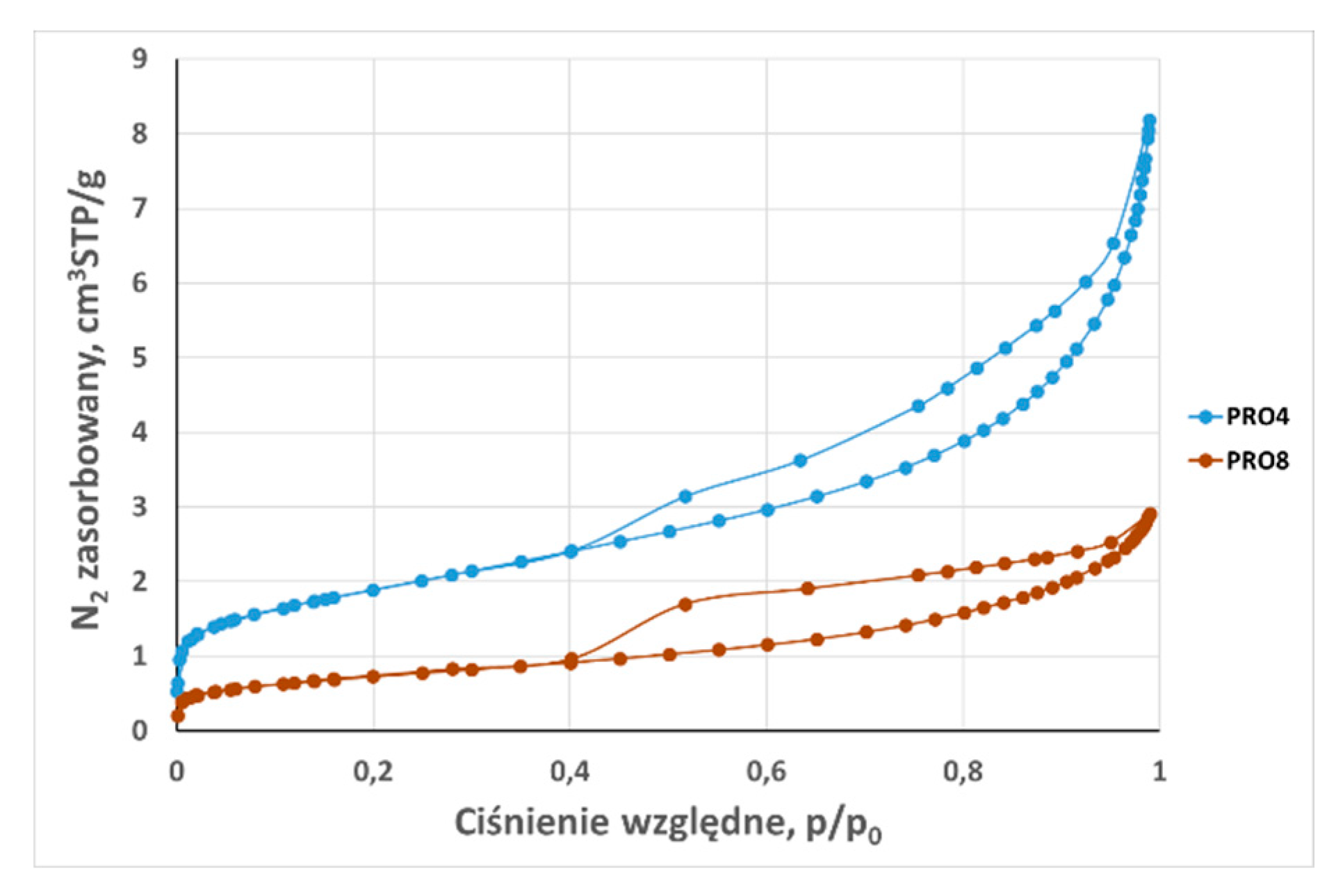
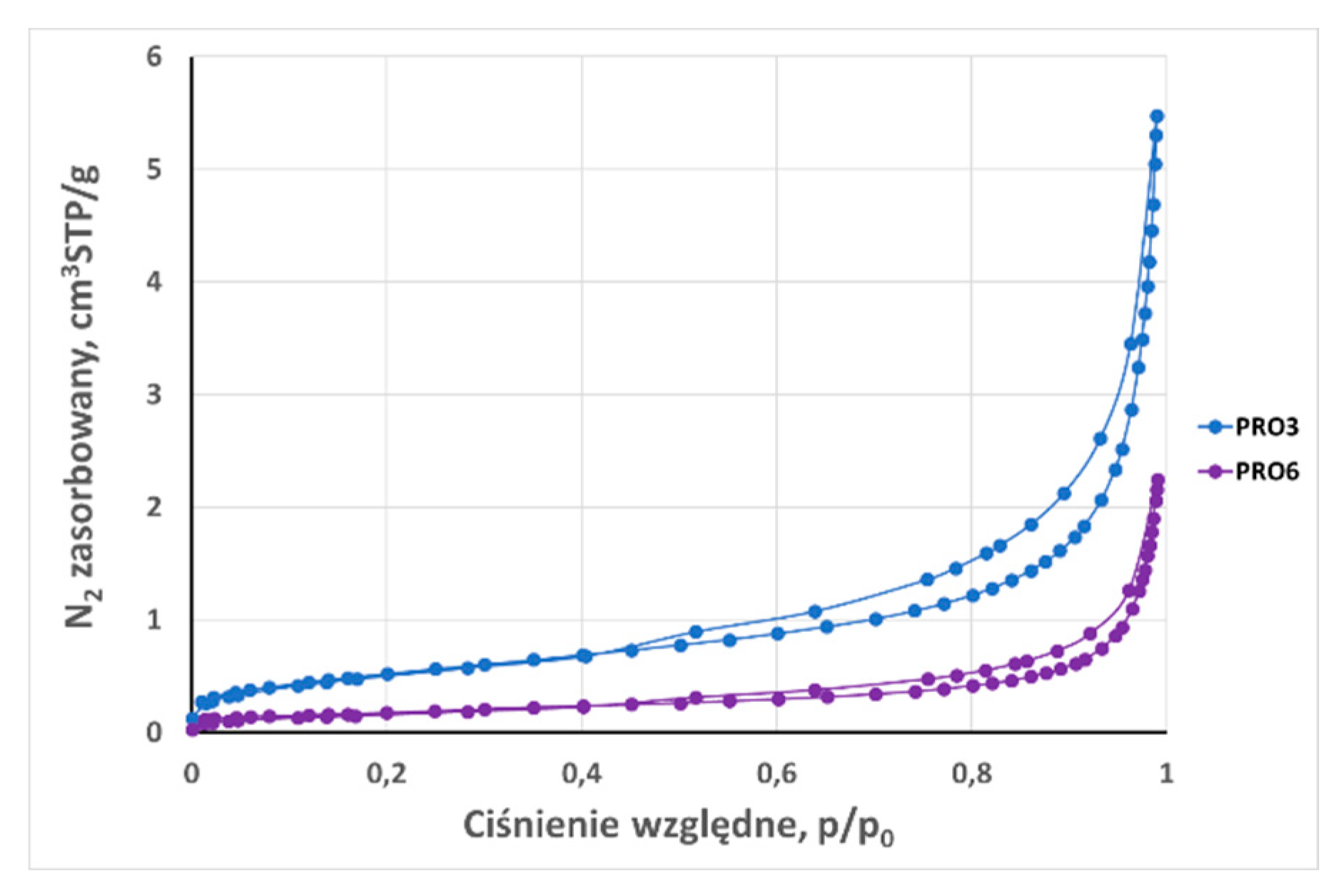
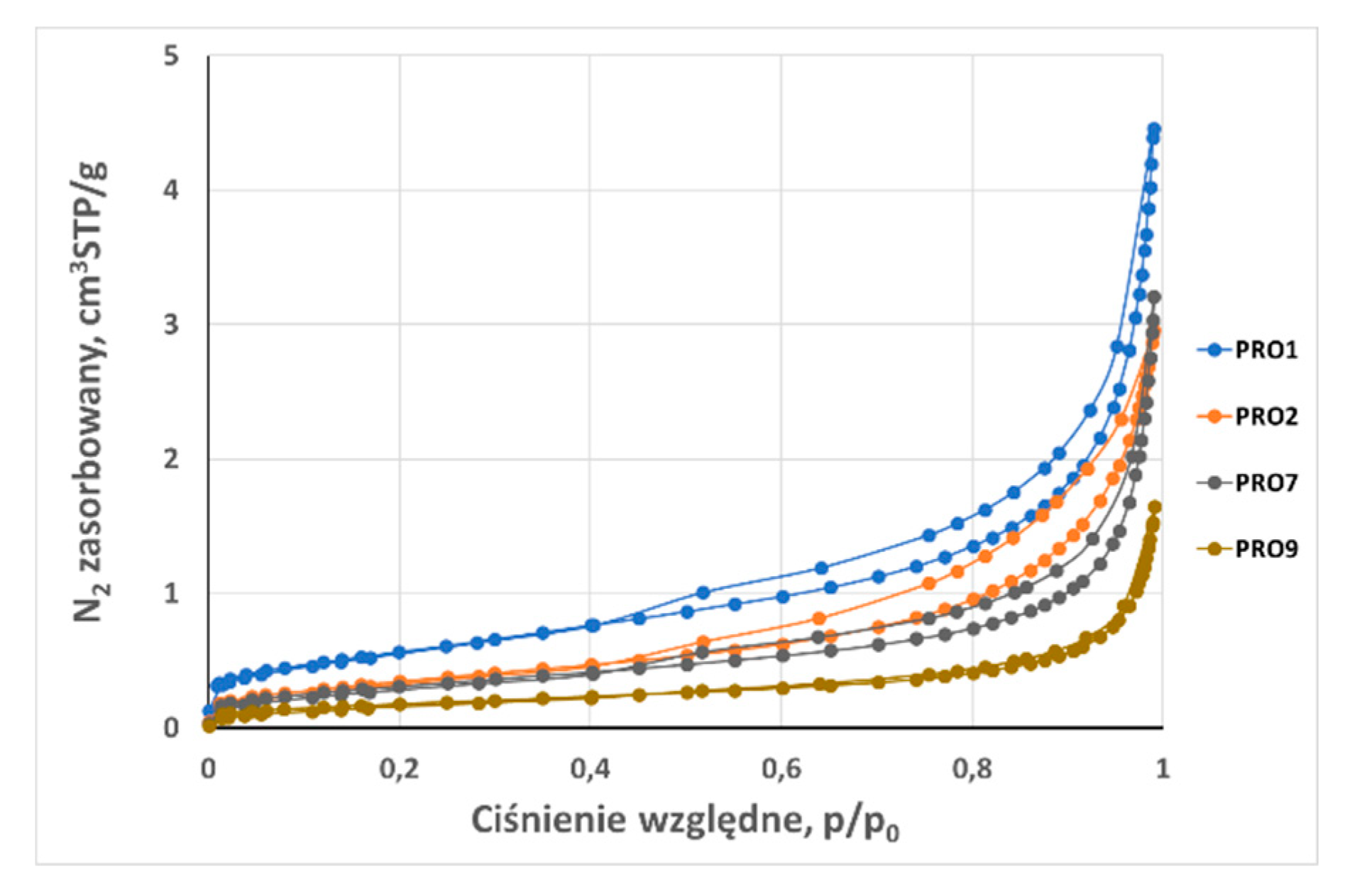
3.2. Analysis of low-pressure isotherms
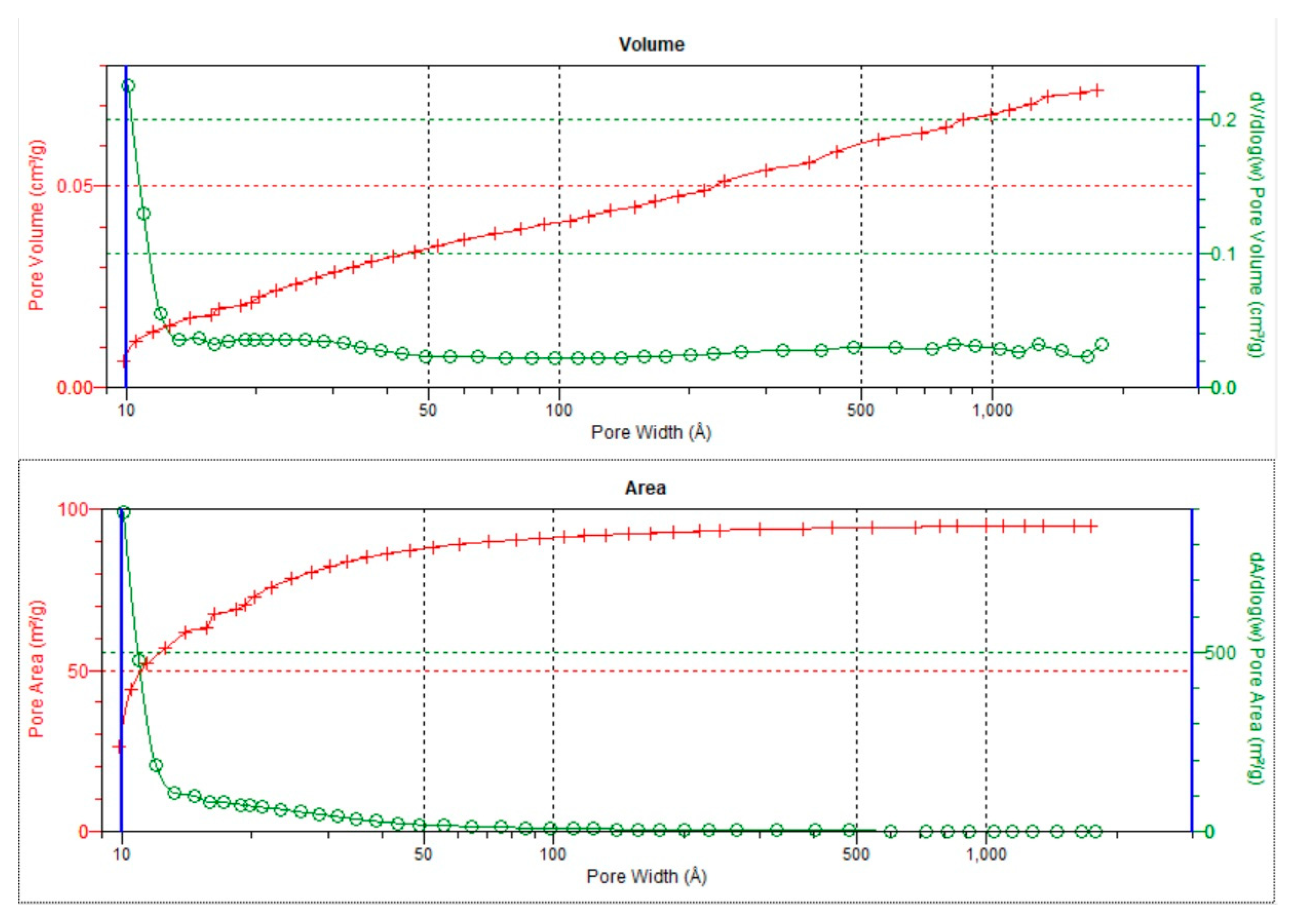
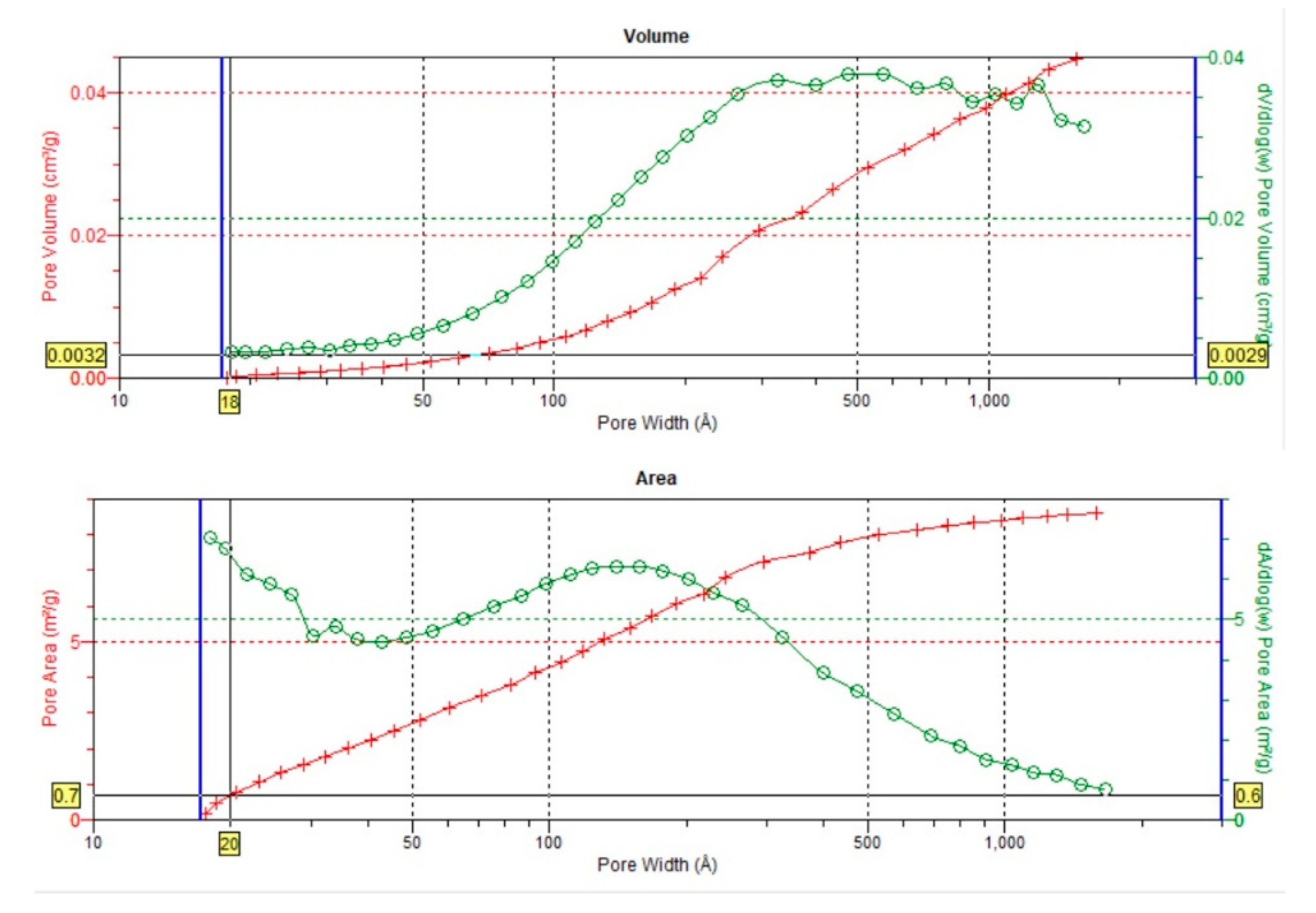
3.3. Textural parameters
| N2, -196°C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRO1 | PRO2 | PRO3 | PRO4 | PRO5 | PRO6 | PRO6 | PRO7 | PRO8 | PRO9 | PRO10 | ||
| Total adsorption capacity (aN2) | cm3STP /g | 4,44 | 2,95 | 5,48 | 8,18 | 37,06 | 2,96 | 2,245 | 3,21 | 2,908 | 1,6498 | 30,04 |
| BET surface area (SBET) 0,05 < p/p0 ≤ 0,35 | m2 /g | 2,080 | 1,320 | 1,920 | 6,650 | 35,800 | 1,670 | 0,642 | 1,161 | 2,560 | 0,650 | 9,490 |
| t-plot micropore area | 0,000 | 0,000 | 0,000 | 0,515 | 12,070 | 0,000 | 0,249 | 0,000 | 0,101 | 0,000 | 0,694 | |
| t-plot external surface area | 2,080 | 1,290 | 1,922 | 6,133 | 23,014 | 1,654 | 0,476 | 1,170 | 2,449 | 0,619 | 8,791 | |
| BJH cumulative surface area of pores 1.7-300 nm | 2,080 | 1,320 | 1,996 | 5,900 | 35,800 | 1,670 | 0,630 | 1,237 | 2,550 | 0,670 | 8,700 | |
| BJH total pore volume (Vt) | cm3 /g | 0,0069 | 0,0046 | 0,0085 | 0,0127 | 0,0750 | 0,0046 | 0,0035 | 0,0048 | 0,0045 | 0,0026 | 0,0465 |
| t-plot micropore volume | 0 | 0 | 0 | 0,0003 | 0,0205 | 0 | 0,0001 | 0 | 0,0001 | 0 | 0,0032 | |
| BJH cumulative volume of pores 1.7-300 nm | 0,0068 | 0,0045 | 0,0082 | 0,0129 | 0,0545 | 0,0044 | 0,0034 | 0,0047 | 0,0046 | 0,0024 | 0,0446 | |
| BJH average pore diameter | nm | 12 | 12 | 16,3 | 7,39 | 7,05 | 9,2 | 19,2 | 15,1 | 6,7 | 13,6 | 18 |
3.4. Densiometric studies results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Tiab D., Donaldson E.C Petrophysics : theory and practice of measuring reservoir rock and fluid transport properties Edition: Third Elsevier/Gulf Professional Publishing, Amsterdam, 2012.
- Mastalerz, M., Schimmelmann, A., Drobniak, A., & Chen, Y. (2013). Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bulletin, 97(10), 1621–1643. [CrossRef]
- Zou C., Qiu Z., Zhang J., Li Z., Wei H., Liu B.,... & Li Y. (2022). Unconventional petroleum sedimentology: A key to understanding unconventional hydrocarbon accumulation. Engineering. [CrossRef]
- Hatherly, P. 2013. Overview on the application of geophysics in coal mining. International Journal of Coal Geology, 114, 74–84. [CrossRef]
- Safaei-Farouji M, Misch D., Sachsenhofer R F., A review of influencing factors and study methods of carbon capture and storage (CCS) otential in coals, International Journal of Coal Geology, Volume 277, 2023, 104351, ISSN 0166-5162. [CrossRef]
- Pajdak, A. , Kudasik M., Skoczylas N., Wierzbicki M.,Teixeira Palla Braga L., Studies on the competitive sorption of CO2 and CH4 on hard coal, International Journal of Greenhouse Gas Control, Volume 90, 2019, 102789. [CrossRef]
- Godyń, K.; Dutka, B.; Chuchro, M.; Młynarczuk, M. Synergy of Parameters Determining the Optimal Properties of Coal as a Natural Sorbent. Energies 2020, 13, 1967. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B. Sorption and Micro-Scale Strength Properties of Coals Susceptible to Outburst Caused by Changes in Degree of Coalification. Materials 2021, 14, 5807. [Google Scholar] [CrossRef] [PubMed]
- Dutka B., Godyń K., Coalification as a process determining the methane adsorption ability of Coal seams Arch. Min. Sci. 66 (2021), 2, 181-195. https://doi.org/10.24425/ams.2021.137455. [CrossRef]
- Lerner, D.N. , Burston M.W., Bishop P.K., Hydrogeology of the Coventry region (UK): an urbanized, multilayer, dual porosity aquifer system, Journal of Hydrology, Volume 149, Issues 1–4, 1993, Pages 111-135. [CrossRef]
- Jarzyna, J. A. , Puskarczyk, E., Motyka, J. (2019). Estimating porosity and hydraulic conductivity for hydrogeology on the basis of reservoir and elastic petrophysical parameters. Journal of Applied Geophysics. [CrossRef]
- Németh, G. , Mlinárik, L., & Török, Á. (2016). Adsorption and chemical precipitation of lead and zinc from contaminated solutions in porous rocks: Possible application in environmental protection. Journal of African Earth Sciences, 122, 98–106. [CrossRef]
- Chen, J. , Liu S., Shen W., Shao J., Vu M-N, Enhancing the elastoplastic damage constitutive model for clayey rocks: Incorporating anisotropy, saturation, time-dependent, and debonding effects, Journal of Rock Mechanics and Geotechnical Engineering, Volume 15, Issue 9, 2023, Pages 2291-2312. [CrossRef]
- Farquhar, R. A. , Somerville, J. M., & Smart, B. G. D. (1994). Porosity as a Geomechanical Indicator: An Application of Core and Log Data and Rock Mechanics. European Petroleum Conference. [CrossRef]
- Choren, J. A. , Heinrich, S. M., & Silver-Thorn, M. B. (2013). Young’s modulus and volume porosity relationships for additive manufacturing applications. Journal of Materials Science, 48(15), 5103–5112. [CrossRef]
- Peng S., Zhang J., Engineering Geology for Underground Rocks. Springer-Verlag Berlin Heidelberg 2007.
- Stalin, V.K. , Muttharam M., (red.) Geotechnical Characterisation and Geoenvironmental Engineering IGC 2016 Volume 1, Springer Nature Singapore Pte Ltd. 2019. [Google Scholar] [CrossRef]
- Bhawani Singh., Goel R. K., Engineering Rock Mass Classification Tunneling, Foundations, and Landslides. Butterworth-Heinemann is an imprint of Elsevier 225 Wyman Street, Waltham, MA 02451, USA The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK. 2011 Elsevier.
- Zinszner B., Pellerin F-M. A Geoscientist's Guide to Petrophysics (2007) Editions TECHNIP, 2007 – 384).
- Gradziński R. Sedymentologia, 1976 Wydawnictwo Geologiczne Warszawa.
- Manecki, A. , Muszyński, M., 2008. Przewodnik do petrografii. Akademia Górniczo-Hutnicza, Uczelniane Wydawnictwa Naukowo – Dydaktyczne, Kraków.
- Milliken, K. L, Curtis M.E., Imaging pores in sedimentary rocks: Foundation of porosity prediction,Marine and Petroleum Geology, Volume 73, 2016, Pages 590-608, ISSN 0264-8172. [CrossRef]
- Zubrzycki, A. , 2011. Podstawy geologii naftowej. Zakład Poligraficzny S.C.M.R. Wioska, Kraków.
- Lucia, F.J., 2007. Carbonate reservoir characterization, second edi. ed. Springer - Verlag, New York.
- Pajdak, A. , Godyń, K., Kudasik, M. et al. The use of selected research methods to describe the pore space of dolomite from copper ore mine, Poland. Environ Earth Sci 76, 389 (2017). [CrossRef]
- Amyx, J.W., Bass, D.M., and Whiting, R.L., Petroleum Reservoir Engineering, McGraw Hill Book Company, New York, 1960.
- Pappalardo, G., Punturo, R., Mineo, S., & Contrafatto, L. (2017). The role of porosity on the engineering geological properties of 1669 lavas from Mount Etna. Engineering Geology, 221, 16–28. https://doi.org/10.1016/j.enggeo.2017.02.020. [CrossRef]
- Tanyıldızı, M. , Gökalp İ., Utilization of pumice as aggregate in the concrete: A state of art, Construction and Building Materials, Volume 377, 2023, 131102, ISSN 0950-0618. [CrossRef]
- Plewa, M. , Plewa, S., 1992. Petrofizyka. Wydawnictwa Geologiczne, Warszawa.
- Ryncarz, T., 1993. Zarys fizyki górotworu. Śląskie Wydawnictwo Techniczne, Katowice.
- Twardowski, K., Traple, J., 2006. Charakterystyka ilościowa porowatości ośrodków gruntowo-skalnych a zjawiska molekularno-powierzchniowe. Wiertnictwo, Naft. Gaz 23, 487–495.
- IUPAC, Recommendations for the characterization of porous solids, Pure Appl. Chem., 66 (1994) 1739-1758.
- IUPAC Reporting Physisorption Data, Pure Appl. Chem., 57 (1985) 603J.
- Skoczylas, N., & Godyń, K. (2014). Evaluating selected lithological features using photographs taken with an introscopic camera in boreholes. International Journal of Rock Mechanics and Mining Sciences, 72, 319–324. https://doi.org/10.1016/j.ijrmms.2014.09.017. [CrossRef]
- Karbownik, M.; Krawczyk, J.; Godyń, K.; Schlieter, T.; Ščučka, J. Analysis of the Influence of Coal Petrography on the Proper Application of the Unipore and Bidisperse Models of Methane Diffusion. Energies 2021, 14, 8495. [Google Scholar] [CrossRef]
- Dandekar, A. , 2006. Petroleum Reservoir Rock and Fluid Properties. CRC, Wiley.
- Peters, E.J. , 2012. Advanced petrophysics - volumes 1 and 2. Greenleaf Book Group, Austin.
- Semyrka, R. , Semyrka, G., Zych, I., 2008. Zmienność parametrów petrofizycznych subfacji dolomitu głównego zachodniej strefy półwyspu Grotowa w świetle badań porozymetrycznych. Geologia 34, 445 – 468.
- Such, P. , Leśniak, G., 2012. Porowatość, przepuszczalność i parametry przestrzeni porowej, w: Rzeczpospolita łupkowa: Studium wiedzy o gazie z formacji łupkowych. Prace Instytutu Nafty i Gazu 183, Kraków, ss. 159–170.
- Zalewska, J. (red.)., 2010. Rentgenowska mikrotomografia komputerowa w badaniu skał węglanowych : praca zbiorowa. Pr. Nauk. Inst. Naft. i Gazu 171, 263.
- Zalewska, J. , Dohnalik, M., 2012. Rentgenowska mikrotomografia komputerowa. Zobaczyć niewidzialne. Rynek Pol. Naft. i gazu 90–103.
- Vieira, L.D. , Moreira A.C., Mantovani I.F., Honorato A.R., Prado O.F., Becker M., Fernandes C.S., Waichel B.L, The influence of secondary processes on the porosity of volcanic rocks: A multiscale analysis using 3D X-ray microtomography, Applied Radiation and Isotopes, Volume 172, 2021, 109657. [CrossRef]
- Choma, J. Kloske, M. & Jaroniec, M., An improved methodology for adsorption characterization of unmodified and modified silica gels. Journal of Colloid and Interface Science. 266, pp. 168-174, 2003.
- Godyń, K.; Dutka, B. Preliminary Studies of Slag and Ash from Incinerated Municipal Waste for Prospective Applications. Energies 2023, 16, 117. [Google Scholar] [CrossRef]
- https://stat.gov.pl/ listopad 2022.
- Wielgosiński, G. , Wasiak, D., & Zawadzka, A., 2014. The Use of Sequential Extraction for Assessing Environmental Risks of Waste Incineration Bottom Ash. Ecological Chemistry and Engineering S, 21(3). [CrossRef]
- Wielgosinski, G. Termiczne przekształcanie odpadów. Wydawnictwo Nowa Energia 2020.
- Rosik-Dulewska, C. , Podstawy gospodarki odpadami. Środowisko. Wydawnictwo Naukowe PWN, 2023.
- Białowiec, A. , Pulka, J., Stępień, P., Manczarski, P., & Gołaszewski, J. (2017). The RDF/SRF torrefaction: An effect of temperature on characterization of the product – Carbonized Refuse Derived Fuel. Waste Management, 70, 91–100. [CrossRef]
- Mądrawski, J. , Kostrzewski W., Smoczkiewicz-Wojciechowska A., Problematyka wykorzystania odpadów typu żużle ze spalarni śmieci komunalnych do produkcji betonów Przegląd Budowlany, 2017 tom R. 88, nr 9 105—107.
- Gisele de Lorena Diniz Chaves, Renato Ribeiro Siman, Glaydston Mattos Ribeiro, Ni-Bin Chang, Synergizing environmental, social, and economic sustainability factors for refuse derived fuel use in cement industry: A case study in Espirito Santo, Brazil, Journal of Environmental Management, Volume 288, 2021, 112401. [CrossRef]
- Rozporządzenie Ministra Klimatu z dnia 2 stycznia 2020 r. w sprawie katalogu odpadów.
- Szewski, A. , Budziszewski A., Ewidencja odpadów 2023 Poradnik BDO - Ewidencja odpadów oraz sprawozdawczość odpadowa. www.kartaewidencji.pl.
- Ambaye, T.G. , Djellabi R., Vaccari M., Prasad S., Aminabhavi T.M, Rtimi S., Emerging technologies and sustainable strategies for municipal solid waste valorization: Challenges of circular economy implementation, Journal of Cleaner Production, Volume 423, 2023, 138708. [CrossRef]
- https://portalprzemyslowy.pl/ listopad 2022.
- Ryś, J. , 1995. Stereology of materials. Fotobit Design, Kraków.
- Faass, G.S. : Correlation of Gas Adsorption, Mercury Intrusion, and Electron Microscopy Pore Property Data for Porous Glasses. Georgia Institute of Technology (1981), p. 260.
- Pettijohn F., J. , Potter P. E., 1972: Sand and Sandstone. Springer Verlag. Berlin.
- Dunham, R.J. (1962) Classification of Carbonate Rocks According to Depositional Texture. In: Ham, W.E., Ed., Classification of Carbonate Rocks, AAPG, Tulsa, 108-121.
- Ryka, W. , Maliszewska A.: Słownik petrograficzny. Warszawa 1991. Wydawnictwo Geologiczne.
- Olszewska, K. , Magnes C., Ziółkowski J., Kuhl J., Atlas petrograficzny górnośląskich kamiennych węgli humusowych. Główny Instytut Górnictwa. Wydawnictwo Śląsk, Katowice 1965.
- Gao, H.; Lan, Y.; Guo, N. Pore Structural Features of Granite under Different Temperatures. Materials 2021, 14, 6470. [Google Scholar] [CrossRef] [PubMed]
- Godyń, K.; Dutka, B. Slags and ashes generated in the process of incineration of municipal waste as one of the cogs of the circular economy. In Proceedings of SEEP 2022; Brunel University: London, UK, 2022; pp. 12–15. [Google Scholar]


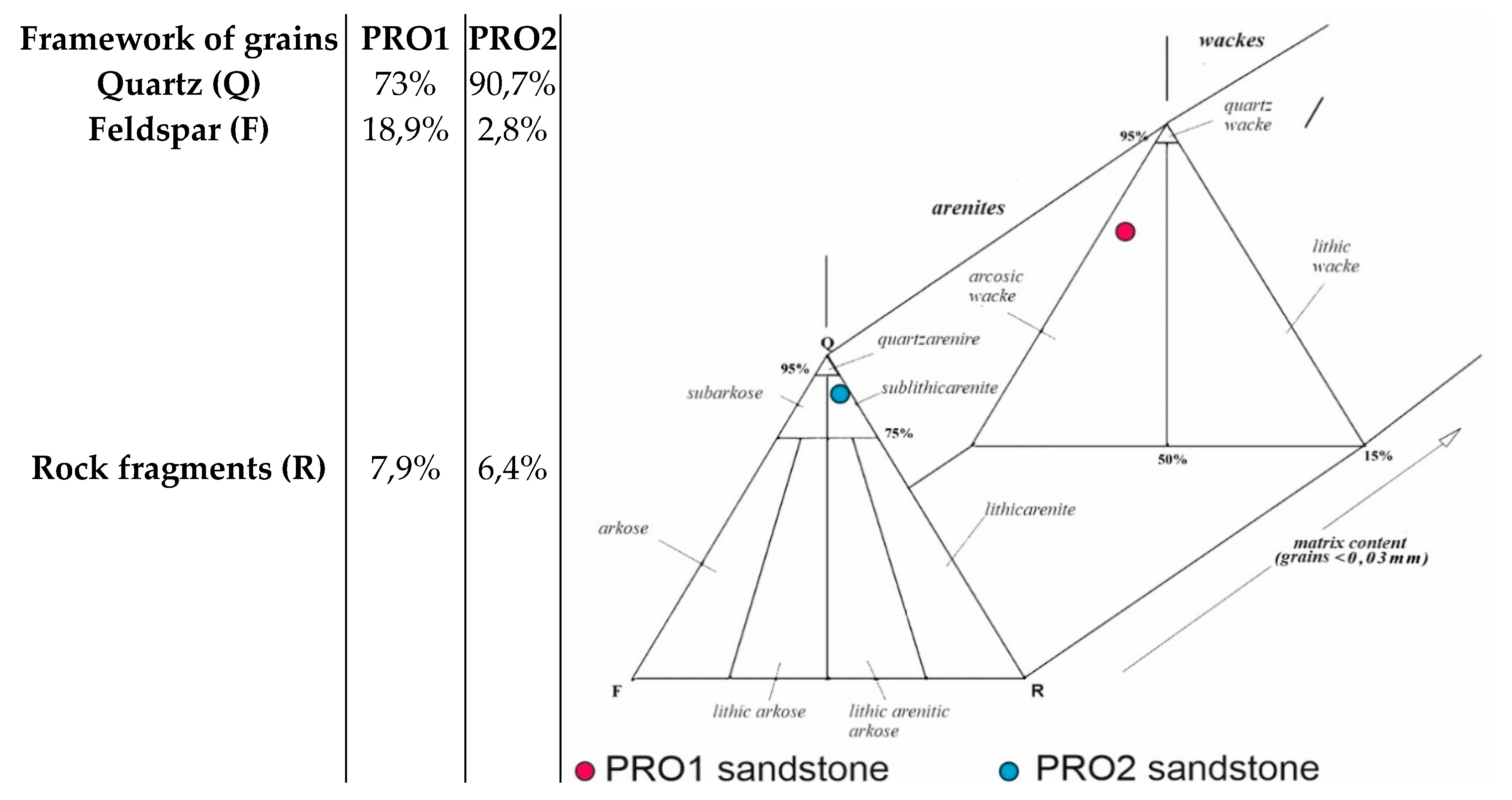
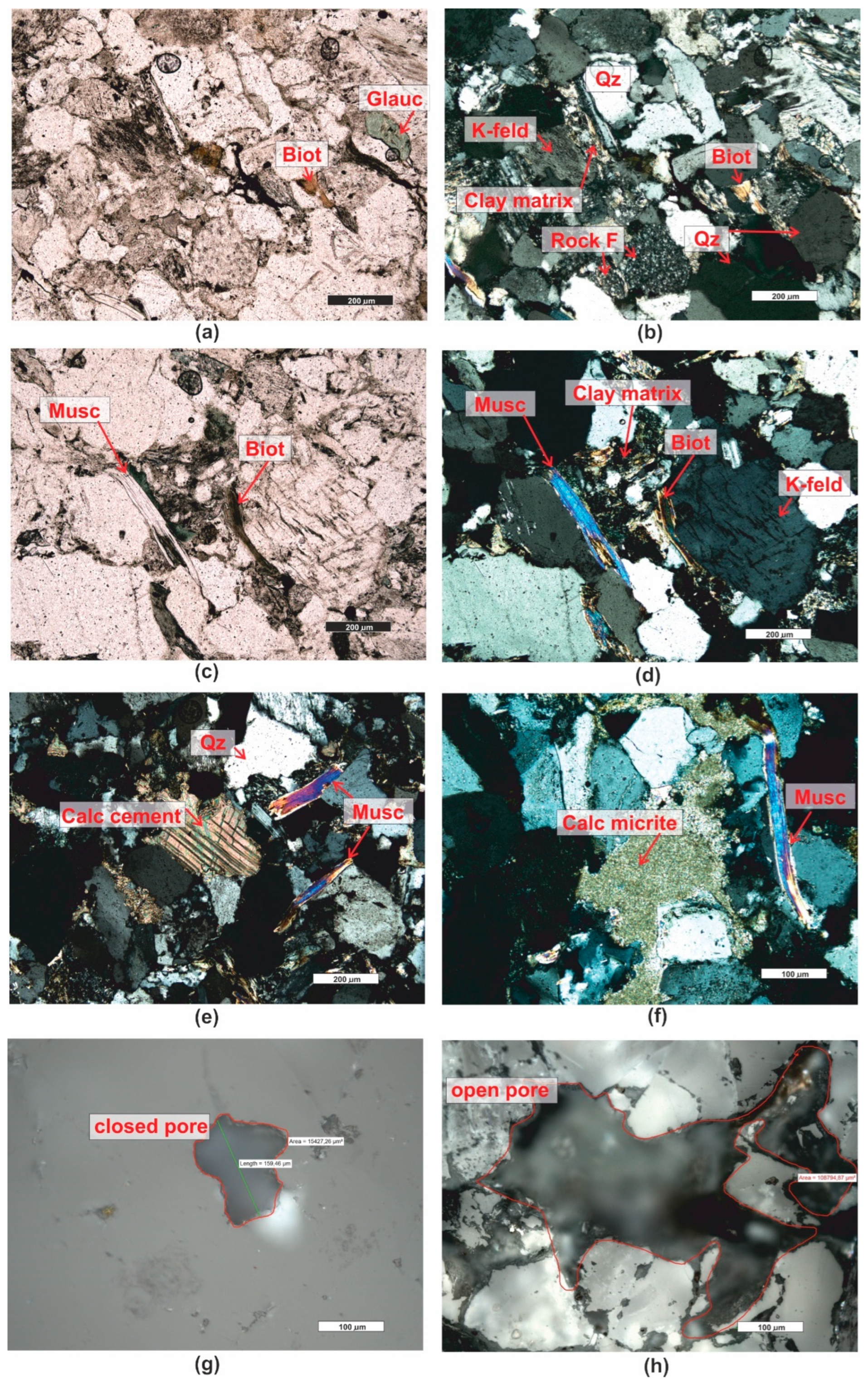

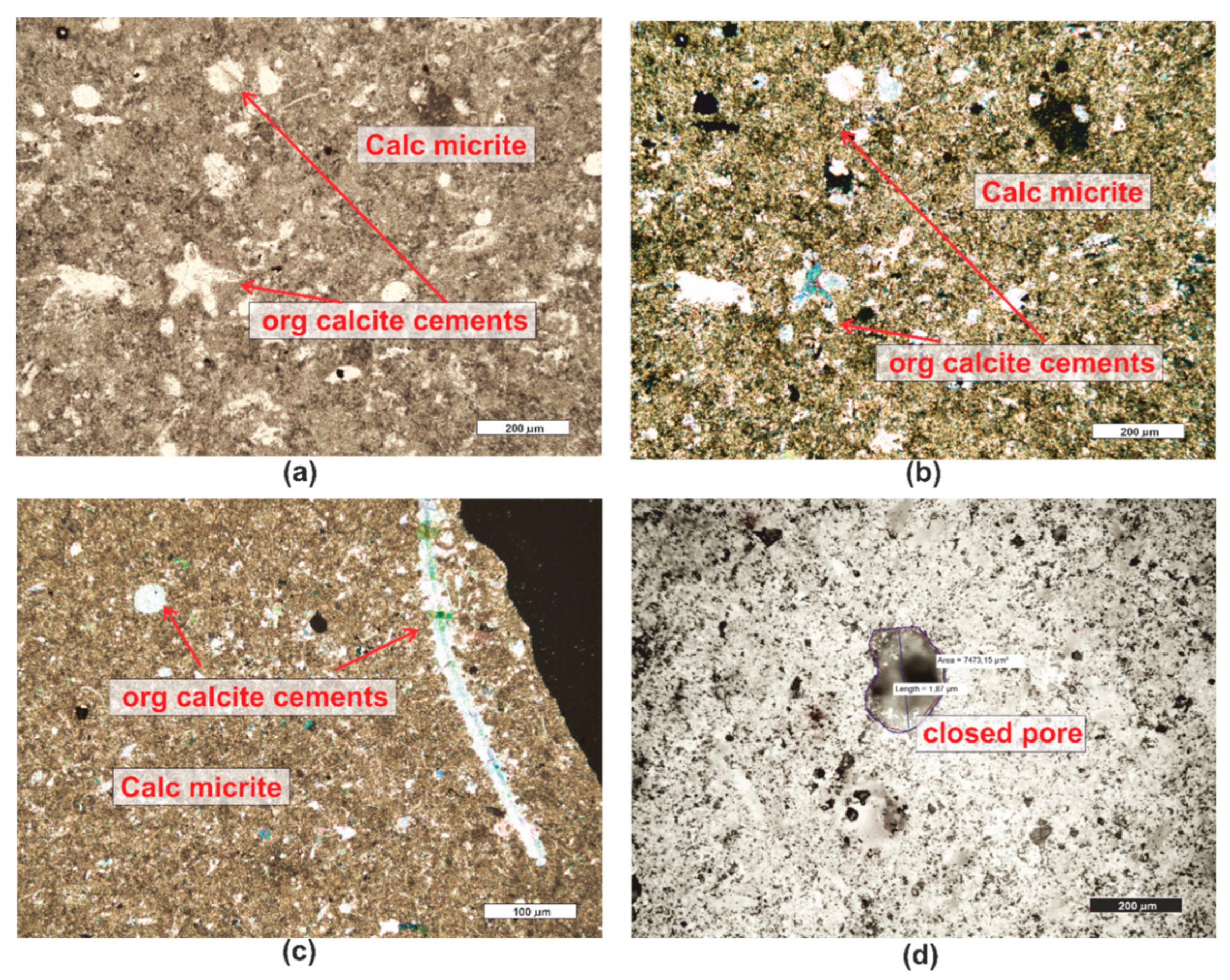
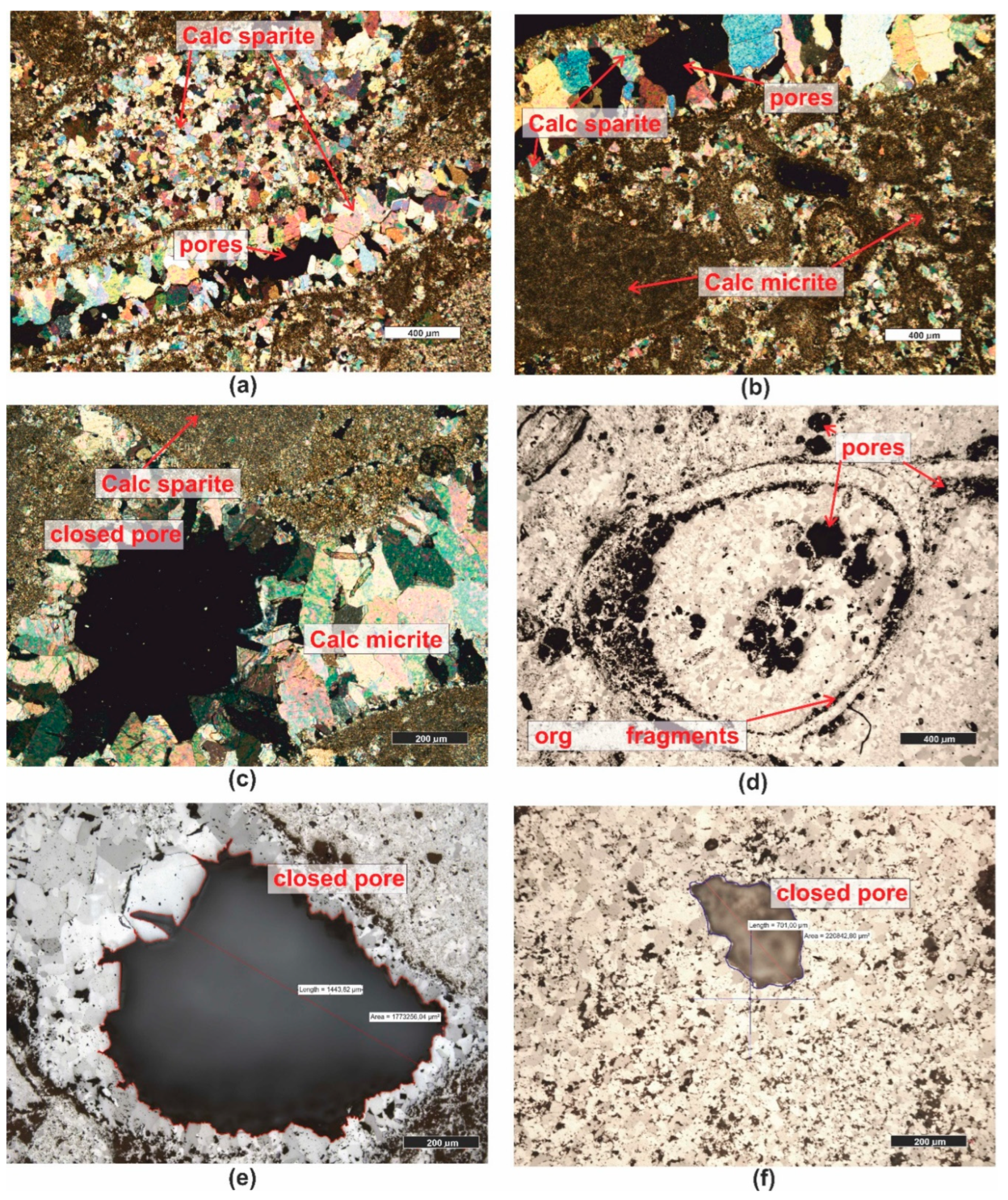
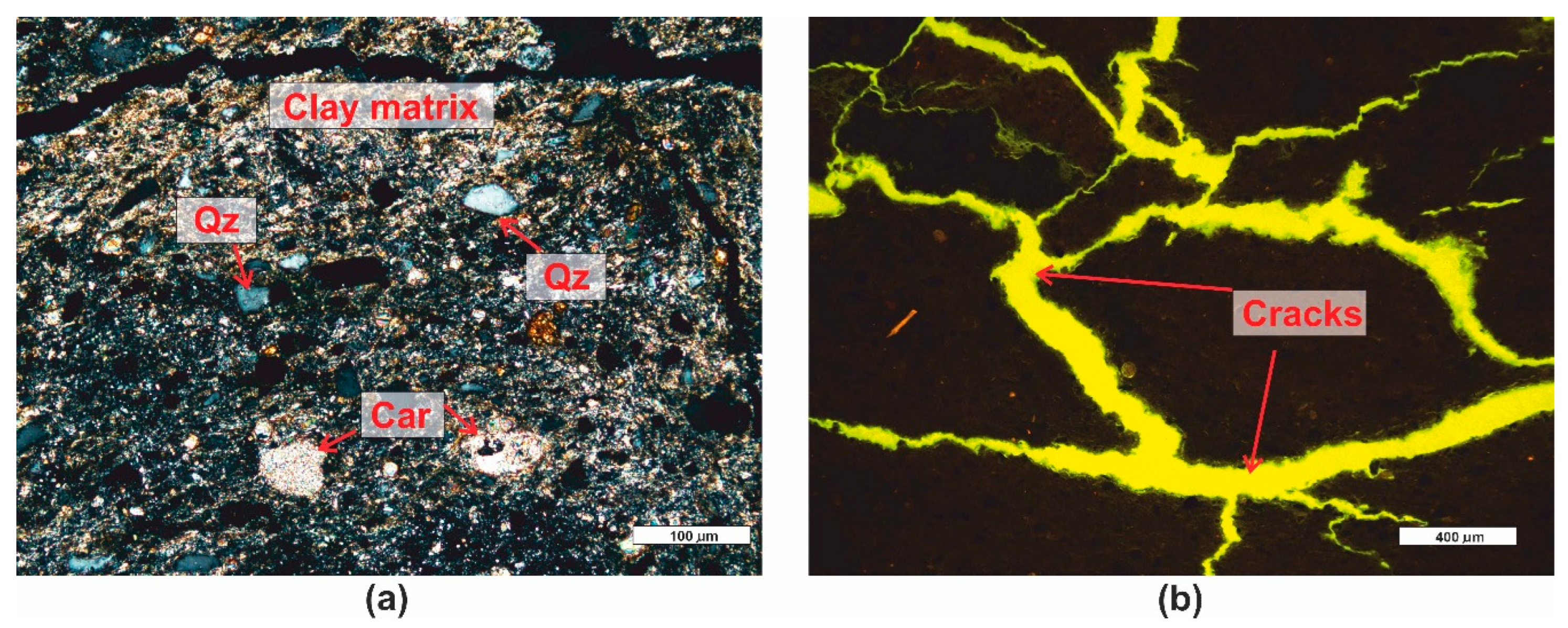
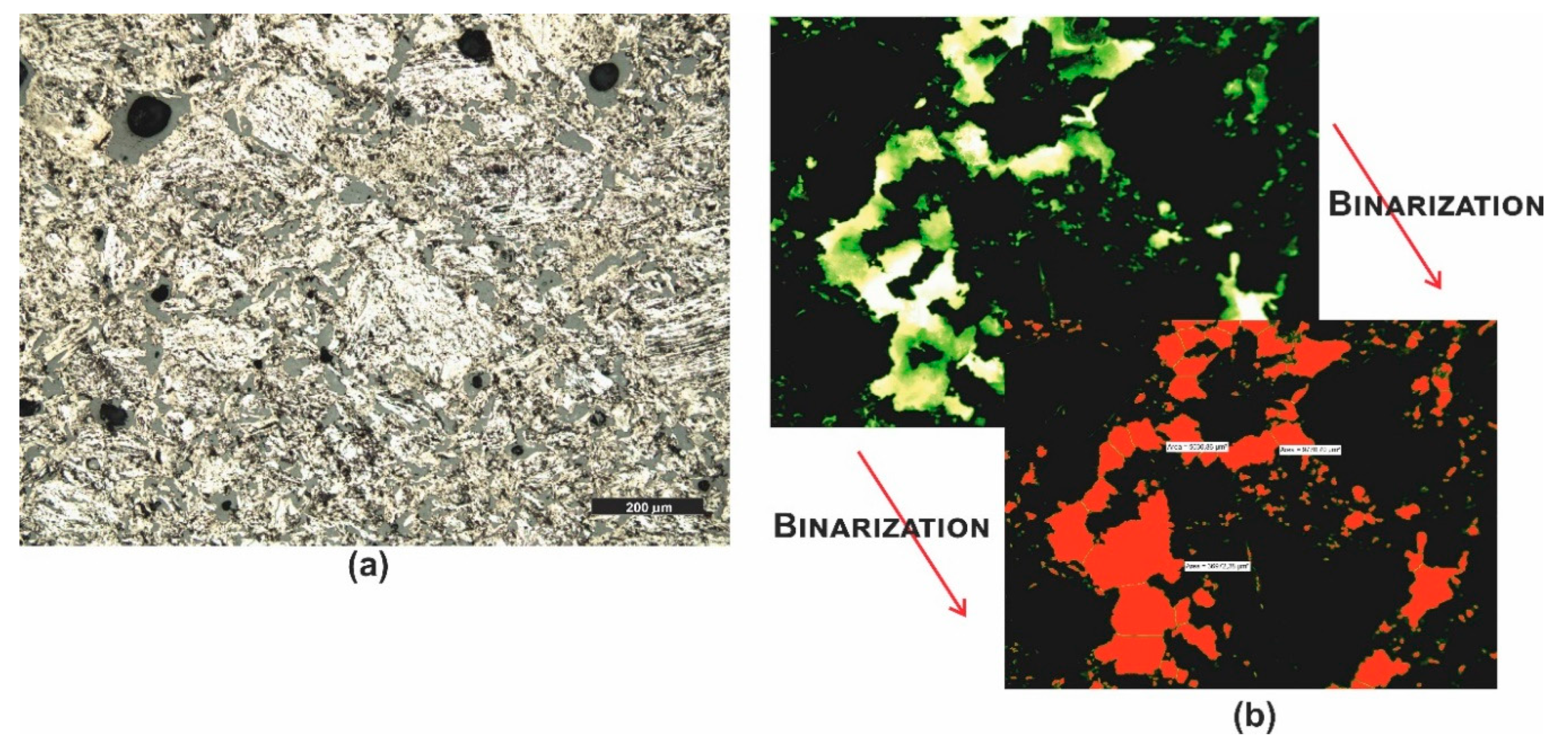

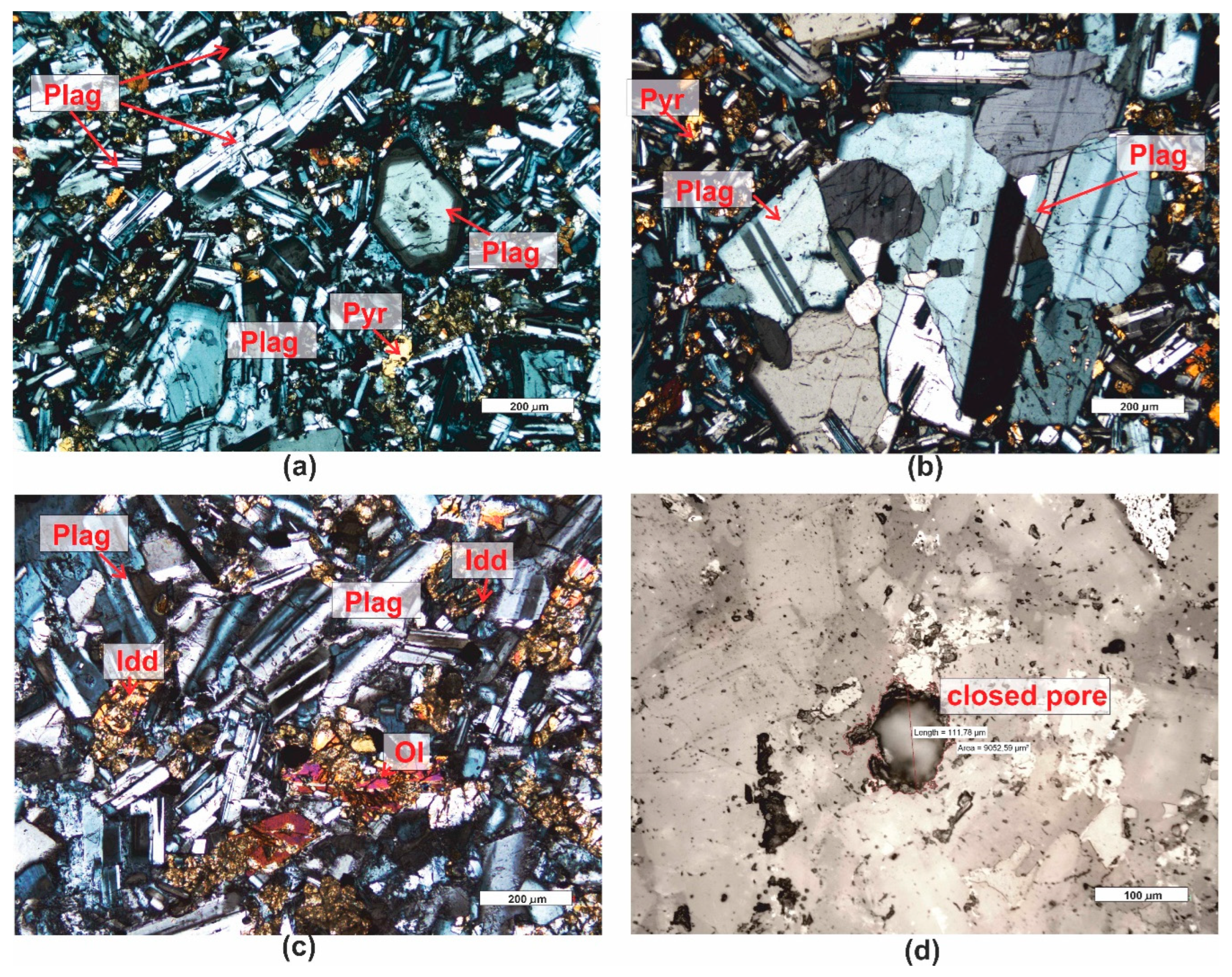
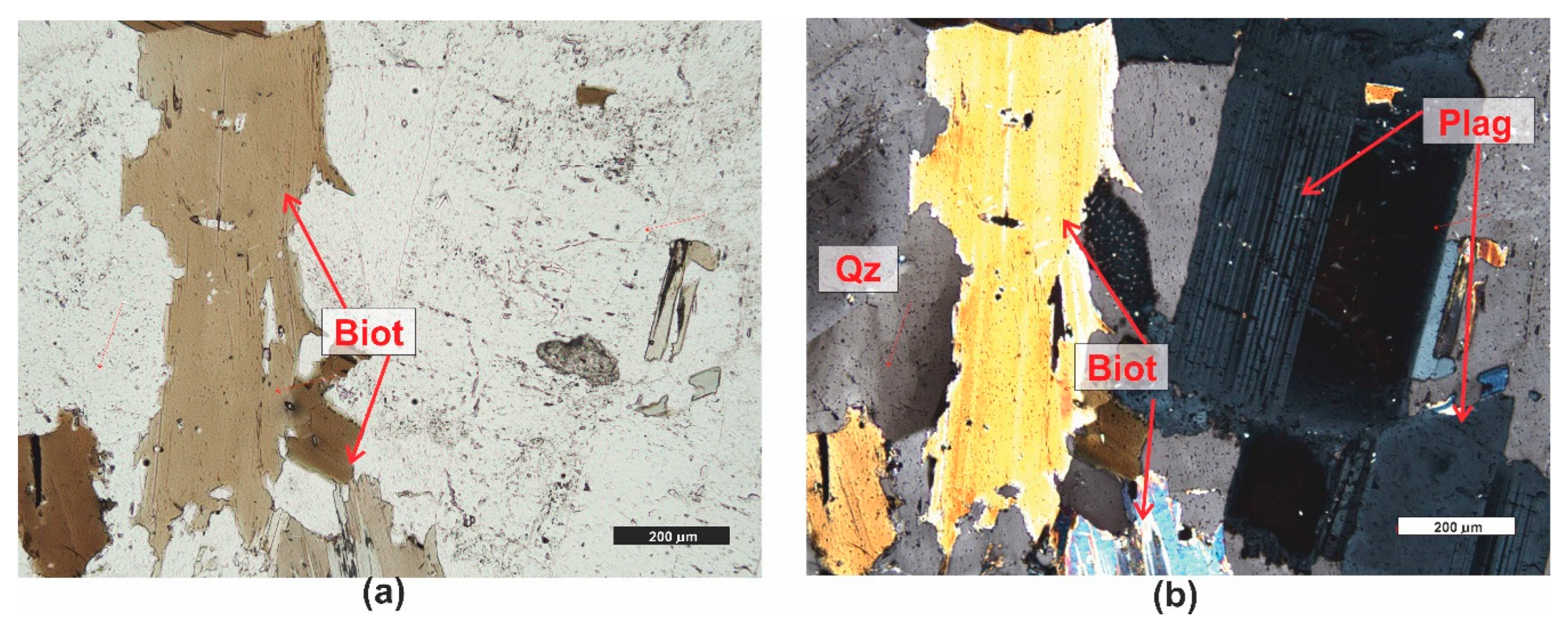

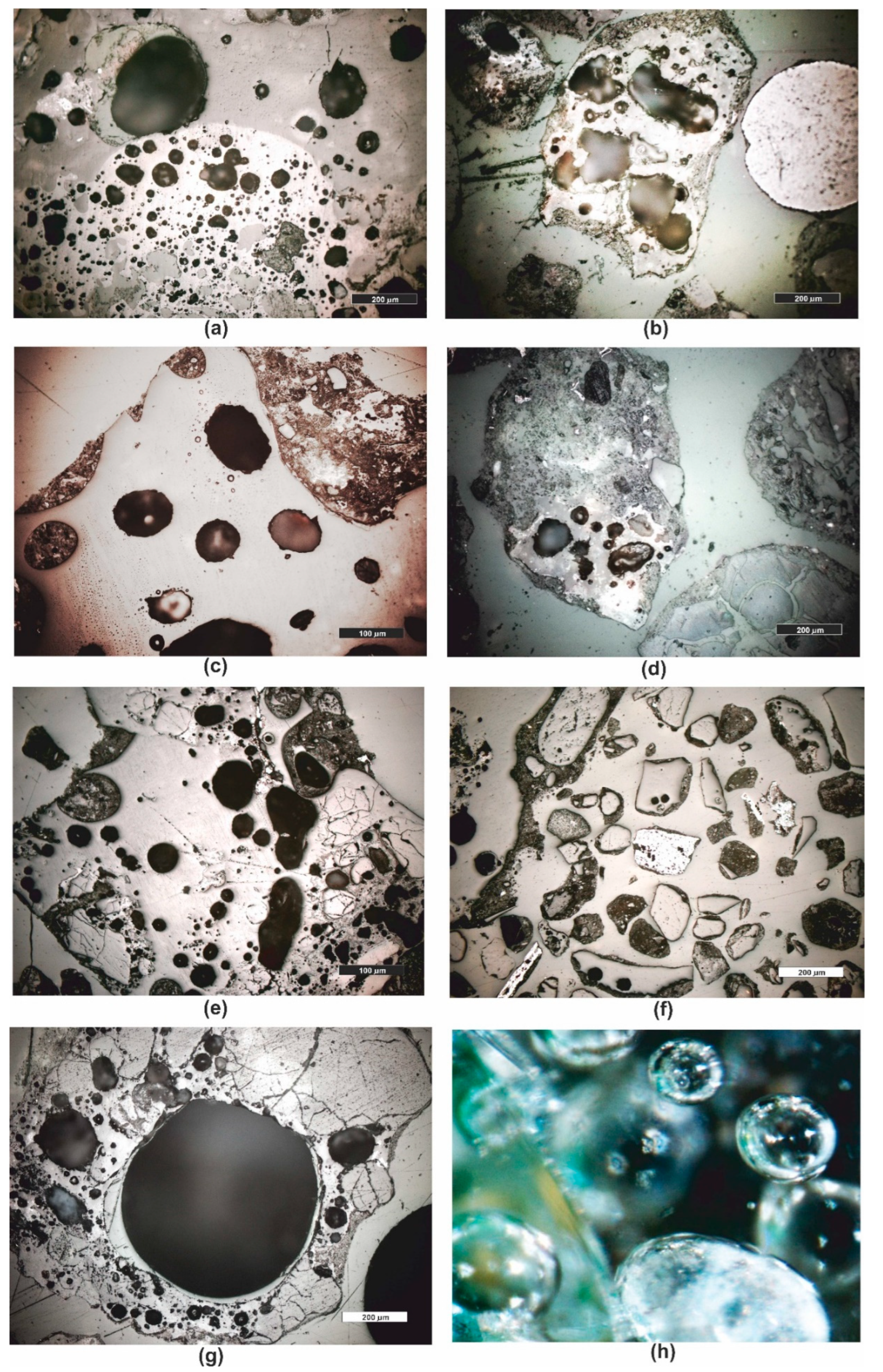
| Grain fraction | 4-2,5 | 2,5-1,6 | 1,6-1 | 1-0,63 | 0,63-0,4 | 0,4-0,25 |
| Porosity [% ] | 19,81 | 9,97 | 11,55 | 11,32 | 5,87 | 4,15 |
| Sample | Real density [g/cm3] | Aparent density [g/cm3] | Porosity [%] | Pore volume [cm3/g] |
|---|---|---|---|---|
| PRO1 | 2,6926 | 2,3592 | 12,4 | 0,0525 |
| PRO2 | 2,6707 | 2,3000 | 13,9 | 0,0603 |
| PRO3 | 2,7247 | 2,4384 | 10,5 | 0,0431 |
| PRO4 | 2,7108 | 2,4906 | 8,1 | 0,0326 |
| PRO5 | 2,5252 | 2,2260 | 11,8 | 0,0532 |
| PRO6 | 1,8681 | 1,4303 | 23,4 | 0,1639 |
| PRO7 | 2,7073 | 1,8554 | 31,5 | 0,1696 |
| PRO8 | 2,7550 | 2,6453 | 4,0 | 0,0151 |
| PRO9 | 2,7895 | 2,5907 | 7,1 | 0,0275 |
| PRO10 | 2,5850 | 1,9570 | 24,3 | 0,1241 |
| Sample | Mikroscopic [% porosity] |
Densimetric [% porosity] |
Adsorption [% porostiy] |
|---|---|---|---|
| > 1000 nm (makroporosity) | < 25000 nm (micro, meso, macro) | < 300 nm (micro and meso) | |
| PRO1 | 17,5 | 12,4 | 0 |
| PRO2 | 11,9 | 13,9 | 0 |
| PRO3 | 11,6 | 10,5 | 0 |
| PRO4 | 10,4 | 8,1 | 2,7 |
| PRO5 | 0 | 11,8 | 8,5 |
| PRO6 | 25 | 23,4 | 2,6 |
| PRO7 | 26,4 | 31,5 | 0 |
| PRO8 | 1,5 | 4,0 | 2,0 |
| PRO9 | 0 | 7,1 | 0 |
| PRO10 | 4,2 | 24,3 | 6,9 |
| Comparison - features / analysis | Clastic rock of the sandstone type | Slags and process ashes |
|---|---|---|
| Color/pleochroism | yes | yes /maybe |
| Relief | yes | yes |
| Cracks and pores | yes | yes |
| Structure and texture | yes | yes or not |
| Cement, matrix | yes | if present yes |
| Shape and size of ingredients | yes | yes |
| Interference colours | yes | yes or not |
| Crystal twinning | yes | yes or not |
| Qualitative assessment | yes | yes or not |
| Stereological analyzes – quantitative analysis | yes | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





