Submitted:
25 November 2023
Posted:
27 November 2023
You are already at the latest version
Abstract
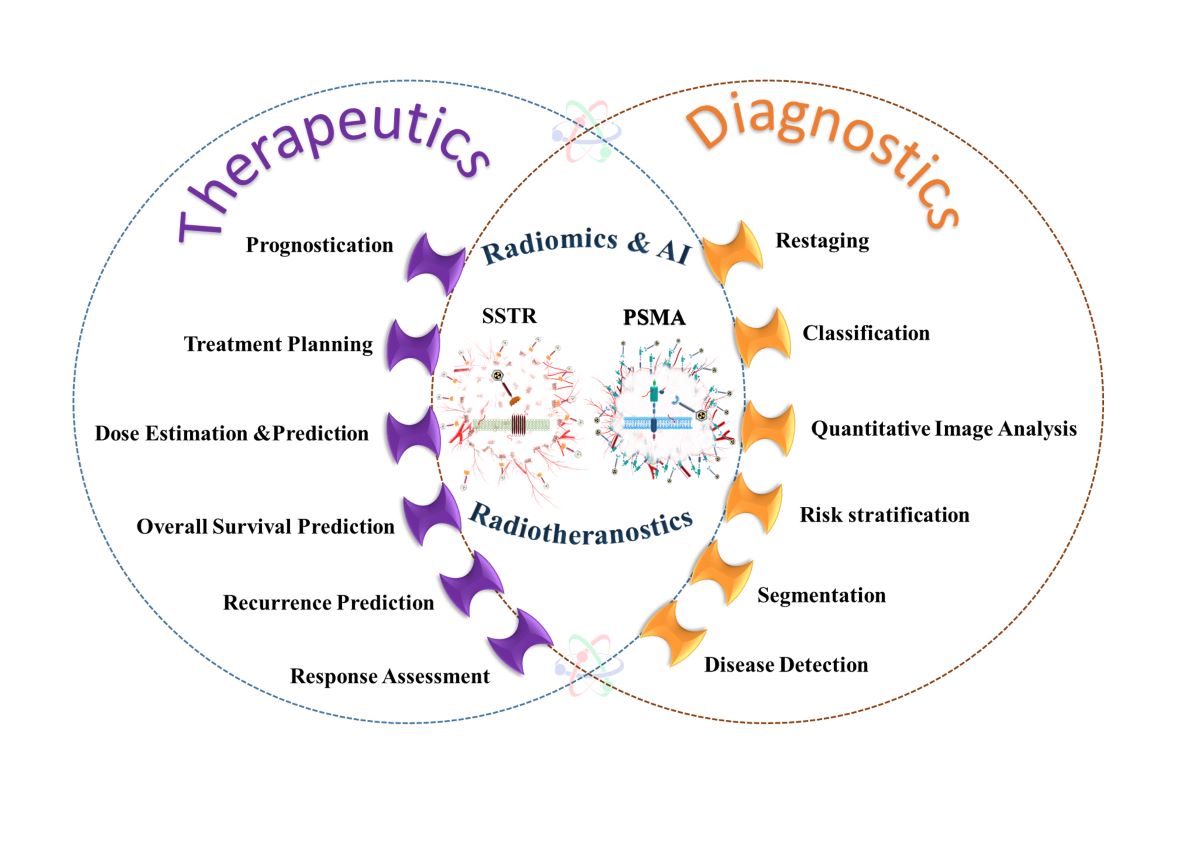
Keywords:
1. Introduction
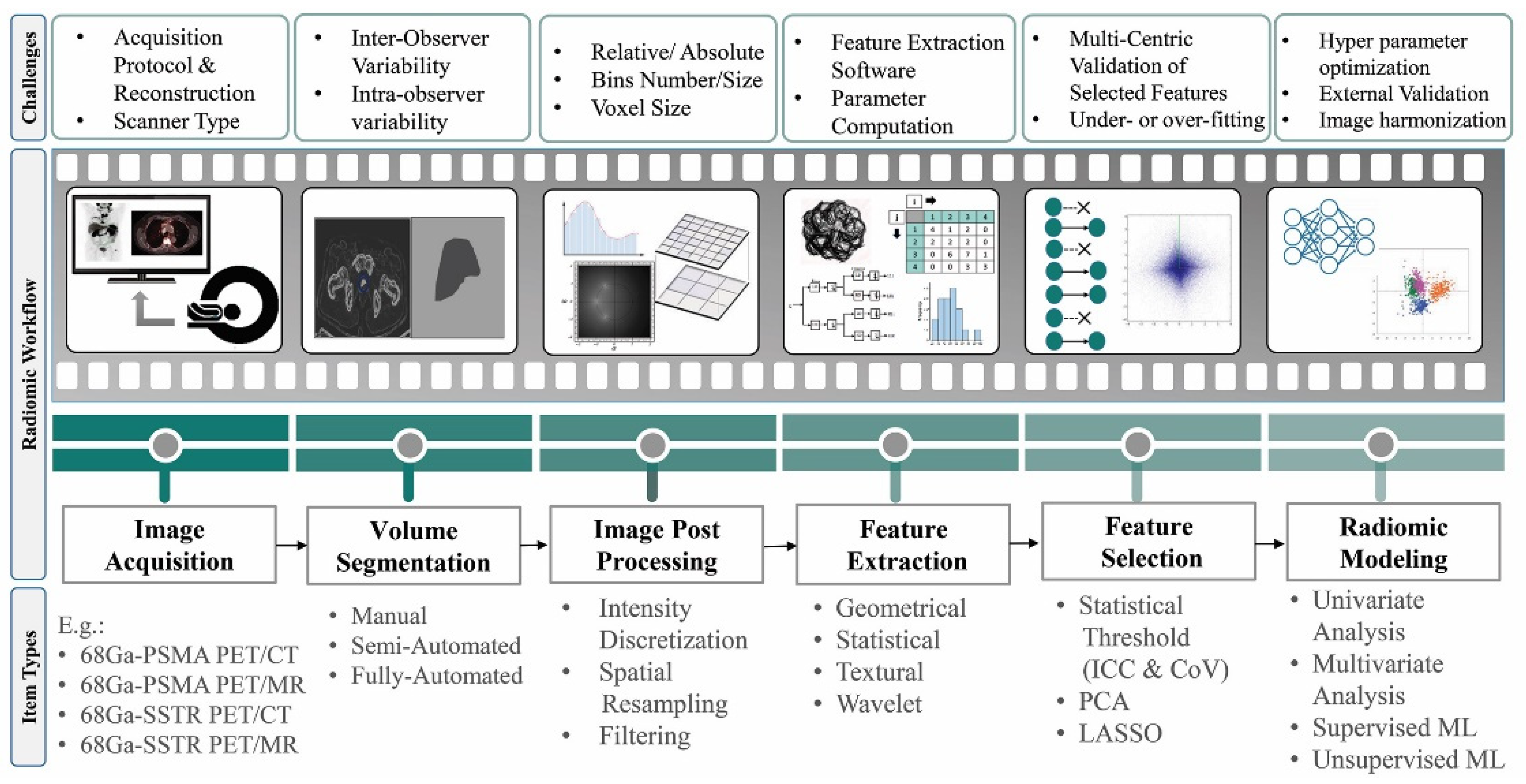
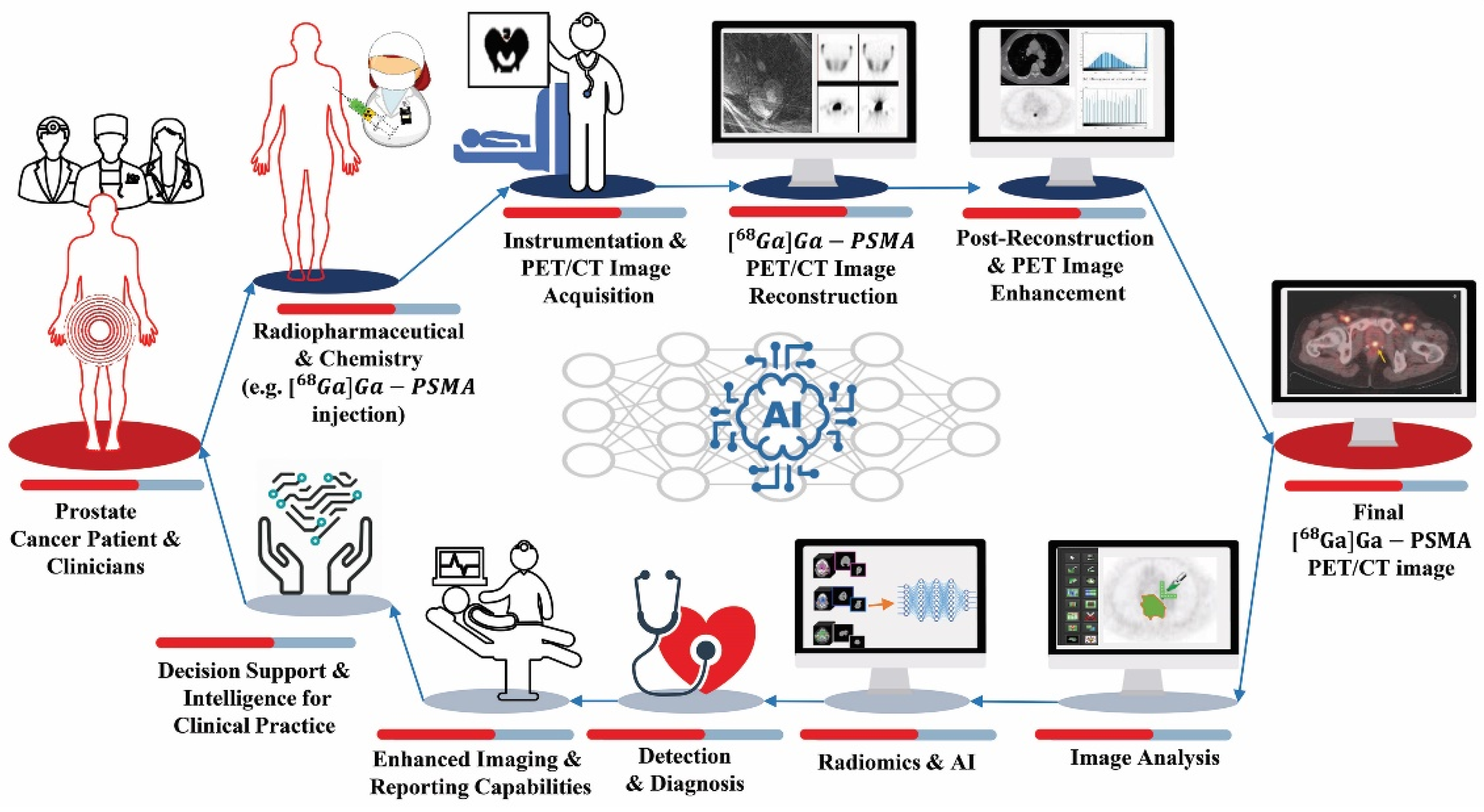
2. Radiomics and AI Workflow
- Geometric or shape features: based on the segmented regions.
- Statistical or intensity features: computed using intensity values in each image region.
- Textural features (TFs): quantification of image intensity and regularity via mathematical functions.
- Wavelet or high-order features: the image transformation process is essential to obtain these features.
3. Application of Radiomics and AI in 68GA SSTR and PSMA Image-Guided RPTS
3.1. RPT Response Assessment
3.1.1. 68. Ga/177Lu-SSTR
| # | First author, Year [Ref] | Radiopharmaceutical, Modality | # Pats |
Site | Utility | Feature Class | Stats, ML/DL Algorithms | Software | Finding RFs | Result | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Grubmüller et al., 2018 [50] | [68Ga]Ga-PSMA-11 PET/CT | 38 | 77 primary prostate & metastatic LNs, bone & visceral metastases | OS prediction | First order (shape & intensity) | Unavailable Cox proportional hazards model, KM, & Cohen's kappa (κ) | Hermes Hybrid3D |
TTV | TTV was significantly associated with OS & its changes were significantly associated with PSA response (p=0.58), contrary to SUVmean changes (p=0.15). | PSMA-TTV is a promising tool for RPT response evaluation. |
| 2 | Khurshid et al., 2018 [58] | [68Ga]Ga-PSMA-11 PET/CT | 70 | 118 primary prostate & metastatic LNs, bone & liver metastases | Therapy response prediction | First order (intensity)/ second order (texture) | Spearman correlation | NM | NGLCM (Entropy & homogeneity) | Entropy (r = -0.327) & homogeneity (r=0.315) TFs of bone lesions correlated ∆PSA. | Better treatment response for more heterogeneous lesions. |
| 3 | Acar et al., 2019 [59] | [68Ga]Ga-PSMA-11 PET/CT | 75 | 257 metastatic bone lesions | Therapy response prediction | First order (shape & intensity)/ second order (texture) | Decision tree, discriminant analysis, SVM, KNN, & ensemble classifier | LIFEx | GLZLM_SZHGE & histogram-based kurtosis | Weighted KNN achieved the best classification performance with AUC = 0.76 (ACU = 73.5%, SE=73.5%, SP=73.7%). | Metastatic or responded sclerotic bone lesions discrimination using CT texture analysis & ML. |
| 4 | Seifert et al., 2020 [71] | [68Ga]Ga-PSMA-11 PET/CT | 110 | 136 metastatic LNs, bone & visceral (liver, lung, & pleura) lesions | OS prediction/ restaging/ Seg | First order (shape & intensity)/ | Univariate & multivariate regression, spearman correlation, & Mann Whitney U tests |
MIWBAS, v.1.0, Siemens | PSMA-TV | Lesion number (HR=1.255), PSMA-TV (HR =1.299), & PSMA-TLQ (HR=1.326) prognosticators of OS. | - Baseline PSMA-PET TV was a significant negative prognosticator of OS in prostate cancer before RPT. - In compression with PSMA-TV, PSMA-TLQ was an independent & superior prognosticator of OS. |
| 5 | Widjaja et al., 2021 [52] | [68Ga]Ga-PSMA-11 PET/CT | 71 | 208 primary prostate & metastatic LNs, bone, liver, & soft tissue lesions |
Biochemical response prediction | first order (shape & intensity) | Kruskal–Wallis, Fisher's exact, & KM | syngo.via; V50B; Siemens | SUVmax | SUVmax was an independent predictor for early PSA response in the treatment course. | Higher PSMA expression was related to a better early biochemical response. |
| 6 | Gafita et al., 2021 [60] | [68Ga]Ga-PSMA-11 PET/CT | 414 | 463 metastatic LNs, bone, & liver lesions | OS & PFS prediction | First order (Intensity) | LASSO, & Wilcoxon Mann-Whitney | qPSMA v.1.0 | SUVmean | PSM SUV: correlated significantly with tumor PSMA expression. | - Higher PSMA expression correlated with longer OS & PSA-PFS. - Patients with metastatic bone disease had shorter OS & PSA-PFS. |
| 7 | Khreish et al., 2021 [53] | [68Ga]Ga-PSMA-11 PET/CT | 51 | 322 primary prostate & metastatic LNs, bone, liver & soft tissue lesions | PFS prediction | First order (intensity) | KM, Cox proportional-hazards modeling, Spearman, & Cohen's κ | NM | TLR |
- ΔTLR & ΔSUV significantly correlated with ΔPSA. Univariate analysis: SUVpeak failed to predict survival. - Multivariable analysis: TLR was independently associated with PFS. |
TLR (normalization of the total lesion PSMA over healthy liver tissue uptake) biomarker can be a predictor of PFS in RPT. |
| 8 | Moazemi et al., 2021 [61] | [68Ga]Ga-PSMA-11 PET/CT | 83 | 2,070 primary prostate & metastatic lesions | Therapy response prediction | First order (intensity)/ second order (texture) | 5 ML classifiers [linear, RBF, & polynomial kernel SVM, ET, & random forest] | InterView Fusion | Task I: PET (Min & Correlation) & CT (Min, Coarseness, & Busyness) | Strong correlations between ML SVM classifier (RBF kernel) on a selection of RFs & clinical parameters with ΔPSA (with AUC=80%, SE=75%, & SP=75%). | RFs were superior to clinical parameters in terms of correlation with ΔPSA. |
| 9 | Moazemi et al., 2021 [62] | [68Ga]Ga-PSMA-11 PET/CT | 100 | 2067 pathological hotspots | Therapy response prediction/ auto Seg | First order (shape & intensity)/ second order (texture) | UNet & 6 ML classifiers (logistic regression, SVM (linear, polynomial RBF kernels), ET, & random forest) | PyRadiomics | 14 features from both PET & CT modalities | Seg. task (0.88 precision, 0.77 recall, & 0.82 Dice). In predicting the response task, logistic regression performed the best (with AUC=0.73, SE=0.81, & SP=0.58). |
In 177Lu-PSMA RPT, the facilitated automated decision support tool has an assistant potential for patient screening. |
| 10 | Moazemi et al., 2021 [63] | [68Ga]Ga-PSMA-11 PET/CT | 83 | 2,070 primary prostate & metastatic lesions | OS prediction/ restaging | First order (shape & intensity)/ second order (texture) | LASSO regression & KM estimator | InterView Fusion | PET kurtosis & SUVmin | The relevant RFs significantly correlated with OS (r=0.2765, p=0.0114). | 68Ga-PSMA-PET/CT scans & patient-specific clinical parameters have the potential for the prediction of OS in advanced PC patients under 177Lu-PSMA RPT. |
| 11 | Roll et al., 2021 [64] | [68Ga]Ga-PSMA-11 PET/MRI | 21 | 49 metastatic lesions in bone, LNs, liver & lung |
Biochemical response & OS prediction |
First order (intensity) |
KM analysis & log-rank test | 3D slicer, v.4.11.2 |
T2-weighted (interquartile range) |
The logistic regression model revealed the highest accuracy (AUC=0.83). | There was a high survival for patients with a biochemical response & higher T2 interquartile range values. |
| 12 | Rosar et al., 2022 [54] | [68Ga]Ga-PSMA-11 PET/CT | 66 | 139 metastatic lesions in bone, LNs, liver, & other soft tissue | OS prediction | First order (shape & intensity) | Spearman's rank correlation & KM | Syngo. Via |
TLP | There was a strong correlation between ∆PSA & ∆TLP (r=0.702). | TLP (summed products of volume × uptake (SUVmean) of all lesions) biomarker independently & strongly predicted OS. |
| 13 | Gafita, et al., 2022 [55] | [68Ga]Ga-PSMA-11 PET/CT | 406 | normal liver, spleen, salivary gland & kidney, & metastatic lesions in bone, LNs & visceral organs | Therapy response prediction/ restaging | First order (shape & intensity) | Spearman CC & Kruskal–Wallis testing | gPSMA | PSMA-VOL | - Salivary glands, kidneys, & liver: a moderate & negative correlation between PSMA-VOL & SUVmean. - Spleen: a weak correlation between PSMA-VOL & SUVmean. |
Decreasing the activity concentration in OARs due to the tumor sequestration affecting the biodistribution of 68Ga-PSM showed the tumor sink effect. |
| 14 | Hartrampf et al., 2022 [56] | [68Ga]Ga-PSMA-11 PET/CT | 65 | 144 primary prostate & metastatic bone, LNs, liver & lung lesion | Therapy response assessment | First order (shape & intensity) | Shapiro–Wilk tests & Spearman's rank CC |
FIJI (ImageJ) | ΔPSMA-TV | ΔPSA was correlated with ΔSUVmaxall (r = 0.51), ΔPSMA-TVall (r ≥ 0.59), ΔPSMA-TV10 (r ≥ 0.57), & ΔPSMA-TV5 (r ≥ 0.53). | The RPT response assessment was possible through PSMA-TV. |
| 15 | Pathmanandav et al., 2022 [57] | [68Ga]Ga-PSMA-11 PET/CT /[18F]FDG PET/CT | 56 | 92 metastatic lesions in bone, LNs, & visceral organs | Therapy Response Prediction | First order (shape & intensity) | KM, Cox proportional-hazards regression, logistic regression, & LASSO | MIM | PSMA_TV & SUVmean | PSMA SUVmean was an independent predictor of treatment response, but SUVmax was not. | A higher SUVmean correlated with treatment response, but a higher PSMA_TV was associated with worse OS. |
| 16 | Gieselet al., 2017 [65] | [18F]FDG PET/CT, [68Ga]Ga-PSMA-11 PET/CT, & [68Ga]Ga-DOTA-TOC PET/CT | 148 (40 PCa) | 254 metastatic LNs | Restaging | first order (shape & intensity) | 2-sided paired-sample t-test, 2-sided Wilcoxon signed-rank testing | In-house | PET (SUVmax) CT (short-axis diameter (SAD) & Histogram) | CT densities correlated with the PET uptake (with a 7.5 HU threshold to discriminate between malignant & benign LNs infiltration) & 20 HU to exclude benign LN. | CT density measurements & PET uptake analysis increased the differentiation between malignant & benign LN. |
| 17 | Moazemi et al., 2020 [66] | [68Ga]Ga-PSMA-11 PET/CT | 72 | 2419 hotspots in normal kidney, bladder & salivary glands, &metastatic lesions | Restaging | First order (shape & intensity)/ second order (texture) | 5 ML classifiers [SVM (linear, RBF, & polynomial kernels), ET & random forest] | InterView FUSION | PET (kurtosis; busyness, & coarseness) | - AUC = 0.98, (SE=0.94 & SP=0.89). - ET & RF showed the best results. |
Using ML & considering features from both the CT & PET images outperformed using either separately. |
| 18 | Erle et al., 2021 [67] | [68Ga]Ga-PSMA-11 PET/CT | 87 |
2452 hotspots in normal liver, kidney, lacrimal & salivary glands, & metastatic lesions |
Restaging | First order (intensity)/ second order (texture) | SVM (linear kernel), ET & random forest | InterView FUSION | 77 RFs | The ET classifier resulted in an (AUC=0.95, SE=.0.95, & SP=0.80). | Combining manual & ML-based diagnosis has the potential to predict hotspot labels with high sensitivity. |
| 19 | Hinzpeter et al., 2021 [68] | [68Ga]Ga-PSMA-11 PET/CT | 67 | 205 bone metastases | Restaging | First order (intensity)/ second order (texture) |
Gradient-boosted tree | 3D Slicer, V.4.11 | 11 most important & independent features2 | Model classification AUC=0.85 (with SE=78%. & SP=93%). | The distinction of healthy bone from metastatic bone accurately using PET/CT texture analysis & ML. |
| 20 | Hammes et al., 2018 [69] | [68Ga]Ga-PSMA-11 PET/CT | 38 | 100 metastatic bone lesions | Staging/ therapy response prediction/ Seg | First order (intensity) | Linear regression & ANOVA | NA | SUVmax & SUVmean | SUVmax, r2=0.97; SUVmean, r2= 0.88; lesion count, r2=0.97. HU threshold: not significant. |
EBONI has the potential to semi-automatically quantify TVs in PSMA PET/CT in a fast (3 min per scan), robust, & reproducible manner. |
| 21 | Zhao et al., 2019 [70] | [68Ga]Ga-PSMA-11 PET/CT | 193 | 1,756 primary prostate & metastatic lesions in bone & LNs | Staging/ restaging/ Seg | NA | 2.5DU-Net | NA | NA | Bone lesion detection (precision=99%, recall=99%, & F1 score=99%) LN lesion detection (precision=94%, recall=89%, & F1 score=92%). |
CNN has the potential to automatically segment disease sites on 68Ga-PSMA PET/CT images to confirm whether a voxel is a lesion or not. |
| 22 | Seifert et al., 2020 [51] | [68Ga]Ga-PSMA-11 PET/CT | 40 | 100 metastatic lesions in the bone, LNs, liver, & lung | Seg/ OS prediction | First order (shape & intensity) | Seg: GAN t-tests, log-rank tests, Cox regression,ICC, Pearson correlation |
MIWBAS, v.1.0 | PET_TV50 | PSMATV50: R2=1.000 & SUVmax: R2=0.988. |
PSMATV50 was a significant predictor of OS. |
| 23 | Xue et al., 2022 [81,82] | [68Ga]Ga-PSMA-11 PET/CT | 23 | WB, kidney, liver, spleen, & salivary | Dose prediction | First order (shape & intensity) | RFR & ANN | NA | SUVmax & TV |
The dose prediction based on the literature population means had a significantly larger MAPE for each organ compared to the optimal ML methods. - Average prediction error for kidneys = 15.76%. |
It is possible to estimate the dose before RPT, which may support the treatment planning role. |
| # | First author, Year [Ref] | Radiopharmaceutical, Modality | # pats |
Site | Utility | Feature Class | Stats, ML/ DL Algorithms | Software |
Finding RFs |
Result | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Werner et al., 2017 [42] | [68Ga]Ga-DOTA-TATE PET/CT | 142 | 872 primary tumors of GEP-NETs (pancreatic, stomach & intestine), lung & metastatic lesions in LNs, bone, liver & lung | OS & PFS prediction | First order (intensity)/ second order (texture) | Cox multi-parametric regression, Youden index, & KM | Interview FUSION | Entropy, Correlation, Short Zone Emphasis & Homogeneity | Eight statistically independent TFs for time-to-progression & time-to-death were identified with Cox analysis, among which entropy was that predicts both PFS & OS. | The prognostic performance of intratumoral TFs analysis outperformed conventional PET parameters. |
| 2 | Werner et al., 2018 [43] | [68Ga]Ga-DOTA-TATE/ DOTA-TOC PET/CT | 31 | 162 metastatic lesions in LNs, bone, liver & lung | OS prediction | First order (intensity)/ second order (texture) |
Youden Index, KM, multivariate Cox hazard analysis, & relative risks |
Interview Fusion |
Entropy | - SUVmean/max was not able to Prognosticate. - Entropy was a significant RF to distinct high- & low-risk groups. |
Differently from conventional PET parameters, higher entropy (a texture feature) values were associated with more prolonged survival. |
| 3 | Önner et al., 2020 [44] | [68Ga]Ga-DOTA-TATE PET/CT | 22 |
326 primary tumors of the pancreas, stomach, intestine & metastatic lesions in LNs, bone, liver & lung |
Treatment response prediction | First order (intensity)/ second order (texture) | Kolmogorov–Smirnov, Mann–Whitney U, & Youden Index | LIFEx | skewness & kurtosis | AUC: for skewness & kurtosis (0.619 & 0.518, resp.). | Skewness & kurtosis predicted PRRT response. |
| 4 | Weber et al., 2020 [45] | [68Ga]Ga-DOTA-TOC PET/MRI | 9 PRRT | 80 metastatic liver lesions | Treatment response prediction | First order (intensity)/ second order (texture) | Mann-Whitney test |
LIFEx | ADC maps (Lesion Vol & Entropy) |
- No PET parameter values predicted PRRT response. - In the treatment responders group: a significant decrease in ADCmaps_lesion volumes & ADCmaps_entropy. . |
No parameters of PET or ADC maps predicted PRRT response. However, the study sample size was small, so further research is suggested. |
| 5 | Ortega et al., 2021 [46] | [68Ga]Ga-DOTA-TATE PET/CT | 91 | 872 primary tumors of GEP-NETs (pancreatic, intestine & stomach), lung & metastatic lesions in LNs, bone, liver & lung | PFS prediction | First order (intensity)/ second order (texture) | 2-sided Wilcoxon rank sum test & cox proportional hazards model |
In-house | Multivariate analysis: mean SUVmax & mean lesion SUVmax/liver SUVmax |
- Significantly higher mean SUVmax in responders than that in non-responders. - A higher mean SUVmax & mean SUVmax tumor-to-liver ratio was associated with therapy response. - Higher kurtosis values were observed in non-responders than in responders (mean 8.6 vs. 5.8). |
SSTR expression & tumor heterogeneity metrics associated with PFS. |
| 6 | Atkinson et al., 2021[47] | [68Ga]Ga-DOTA-TATE PET/CT | 44 | GEP-NETs primary tumors (pancreatic, stomach, intestine), lung, thyroid & phaeochromocytoma/ paraganglioma & metastatic lesions in LNs, bone, liver, lung, peritoneum & brain | OS & PFS prediction | First order (intensity)/ second order (texture) | Univariate KM & multivariate Cox regression | TexRAD, Cambridge, UK | CT-coarse kurtosis, PET_entropy, & PET_skewness | - SUVmax & SUVmean were not significant in outcome prediction - Higher kurtosis, higher entropy, & lower skewness: predict shorter PFS. - CT-TA (coarse kurtosis): independently predicates PFS (HR=2.57 & CI=1.22–5.38). - PET-TA (unfiltered skewness): independently predicates OS (HR=9.05, 95% CI=1.19–68.91). |
Texture analysis yielded prognostic biomarkers that had the potential to assess outcomes in NETs patients with more aggressive diseases. |
| 7 | Liberini et al., 2021 [48] | [68Ga]Ga-DOTA-TATE PET/CT & [18F]FDG PET/CT | 2 | 22 metastatic lesions in LNs, bone & liver | Prognosis prediction | First order (intensity)/ second order (texture) | Mann–Whitney, Pearson correlation matrix, & PCA | LIFEx v.5.10 (IMIV/CEA, Orsay, France) |
TLSREwb-50 & SRETVwb-50 |
- Mann–Whitney test: 28 RFs showed significant differences between the two patients. - Pearson correlation matrix: identified seven second-order RFs, with poor correlation with SUVmax & PET vol. |
Defining inter-patient heterogeneity & therapy response prediction may be possible using RFs. |
| 8 | Laudicella et al., 2022 [49] | [68Ga]Ga-DOTA-TOC PET/CT | 38 | 324 metastatic lesions in LNs, bone, liver & other soft tissue | Treatment response prediction | First order (intensity)/ second order (texture) | t-test, Mann– Whitney U, & Youden index |
LIFEx | HISTO_Skewness & HISTO_Kurtosis |
- HISTO_Skewness & HISTO_Kurtosis: able to predict the response (AUC ROC, SE. & SP. of 0.745, 80.6%, 67.2% & 0.722, 61.2%, 75.9%, resp.). - SUVmax was not able to predict the response (AUC= 0.523). |
The developed theragnomics (THERAGNOstics +radiOMICS) predictive model was superior to conventional quantitative parameters to predict the GEP-NET lesion's response to 177Lu-DOTA-TOC PRRT. |
| 9 | Giesel et al., 2017 [65] | [18F]FDG PET/CT, [68Ga]Ga-PSMA-11 PET/CT, & [68Ga]Ga-DOTA-TOC PET/CT | 148 (35 GEP-NET) | 217 metastatic LNs | Restaging | First order (shape & intensity) | 2-sided paired-sample t-testing, 2-sided Wilcoxon signed-rank testing | In-house | PET (SUVmax) CT (short-axis diameter (SAD) & Histogram) | CT densities correlated with the PET uptake (with a 7.5 HU threshold to discriminate between malignant & benign LNs infiltration & 20 HU to exclude benign LN). | CT density measurements & PET uptake analysis increased the differentiation between malignant & benign LN. |
| 10 | Liberini et al., 2021 [72] | [68Ga]Ga-DOTA-TOC PET/CT | 49 | 60 primary tumors of GEP-NETs (pancreatic, stomach, intestine) & metastatic lesions in LNs, liver & other soft tissue | Prognosis prediction/Seg. /restaging | First order (intensity)/ second order (texture) | Pearson's CCs, DSC, ICC, & coefficient of variance |
LifeX v.4.81 (IMIV/CEA, Orsay, France) | GLZLM (also called GLSZM) features & zones with low gray-level (SZLGE & LZLGE), & SUVmax thresh. of 40% | SAEB Seg. & operators: DSC mean= 0.75 ± 0.11 (0.45–0.92) SAEB Seg. & 4 manual Seg.= 0.78 ± 0.09 (0.36–0.97). |
- Superior RFs stability among operators was provided using SUVmax thresholds of 40% but led to a possible biological information loss. - SAEB performed better than manual segmentation; however, further validation is suggested. |
| 11 | Wehrend et al., 2021 [73] | [68Ga]Ga-DOTA-TATE PET/CT | 125 | 223 liver lesions |
Seg | NA | CNN: 2D U-Net Stats: F1 score |
MIM | NA |
- Highest precision-recall AUC (0.73±0.03): using a noise filter (15-pixel). - Highest mean PPV (0.94±0.01): 20-pixel filter. - Highest mean F1 score (0.79±0.01): 20-pixel filter. - Highest mean SE. (0.74±0.02): 5-pixel filter. |
- DNN can automatically facilitate the detection of hepatic metastases. - For further validation, it suggested the need for more studies with larger sample sizes. |
| 12 | Akhavanallaf et al., 2023 [83] | [68Ga]Ga-DOTA-TATE PET/CT | 25 | 90 NETs: 75 liver, 11 LNs, three Primary Pancreas tumors, & one Chest tumor | Dose Prediction | First order (shape & intensity) | Spearman rank correlation, univariate linear regression model, ElasticNet & Permutation-based RF variable-Importance feature selection | NM | SUVmean, TLSUVmean (SUVmean of total-lesion-burden) & SUVpeak | Tumor dose prediction using an optimal trivariate RF model composed of SUVmean, TLSUVmean, and total liver SUVmean: R2 = 0.64, MAE = 0.73 Gy/GBq, and MRAE = 0.20. |
PET-based metrics combined with ML models can improve dose prediction, which may be useful for stratifying patients and personalizing treatment. |
| 13 | Plachouris et al., 2023 [84] | [68Ga]Ga-DOTA-TOC PET/CT | 20 | 3412 features from 4 OARs (liver, spleen, and left- and right kidneys) |
Dose Prediction | First order (intensity)/ second order (texture) + dosiomic features | Multivariate analysis & nine supervised linear & non-linear-based ML regression algorithms: linear, ridge, extra tree, AdaBoost, gradient boost, random forest, decision tree, SVR,& XGBoost regression algorithms trained for every OAR. | PyRadiomics | Differed for each OAR (Table 3 in [84]) | - Wavelet-based features had highly correlated predictive value. - More precise prediction using non-linear-based ML regression algorithms than linear-based ones. |
The combination of radiomics and dosiomics may be useful for individualized molecular radiotherapy response assessment and OAR dose prediction. |
3.1.2. 68. Ga/177Lu-PSMA
3.2. Restaging
3.3. Segmentation
3.4. Dose Prediction
4. Application of Radiomics and AI in [18F]PSMA PET/CT Image-Guided RPTS
4.1. RPT Response Assessment
4.2. Segmentation
5. Application of Radiomics and AI in 64Cu SSTR and PSMA Image-Guided RPTS
5.1. RPT Response Assessment
5.2. Segmentation
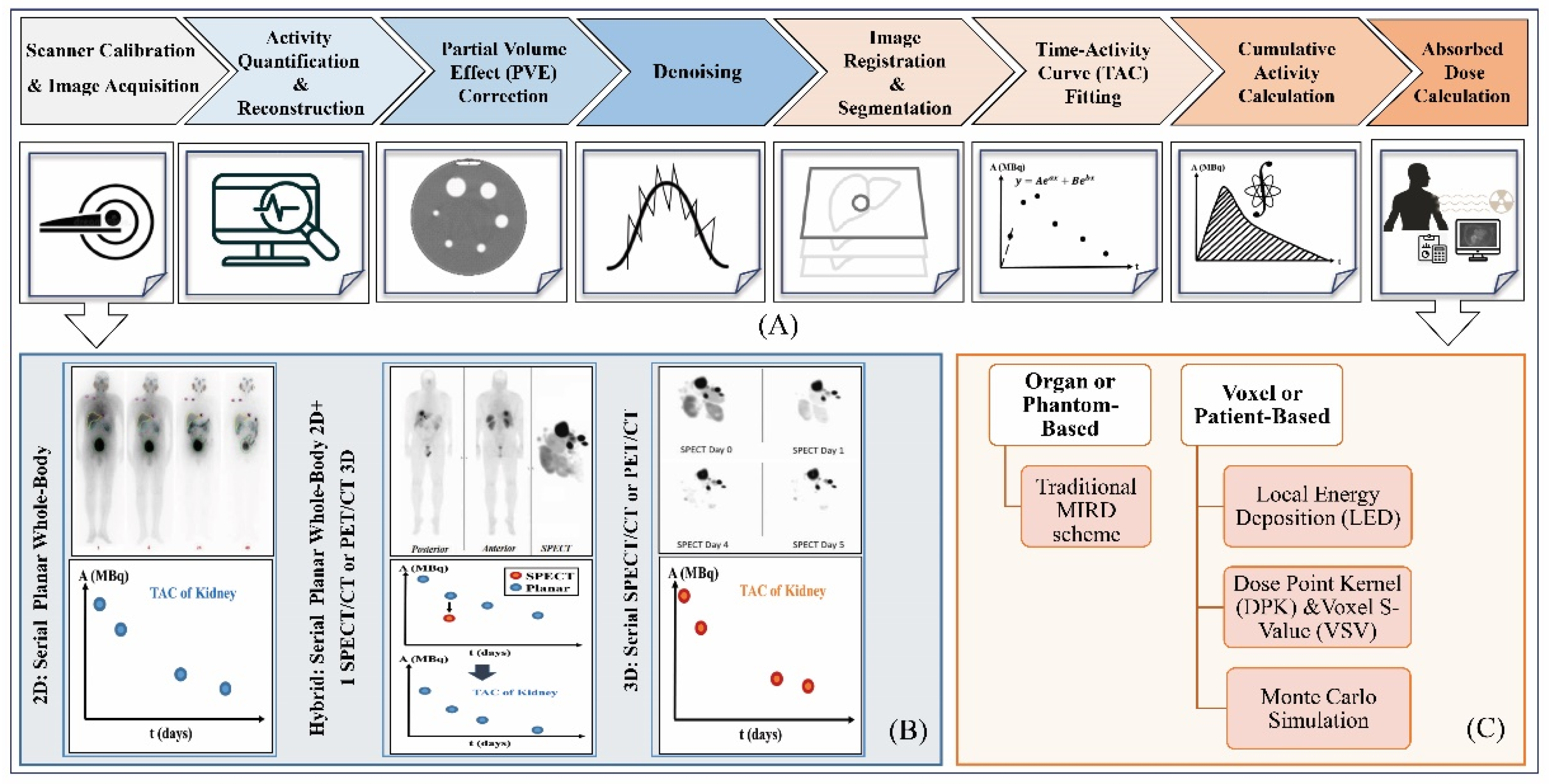
6. Dosimetry Workflow and Treatment Planning
7. Role of AI in Dosimetry Workflow of 177Lu-SSTR and PSMA RPT
7.1. Image Acquisition and Quantification
7.2. Image Segmentation
7.3. Dose Estimation
8. Role of AI in Dosimetry Workflow of 90Y SSTR and PSMA RPT
9. Discussion and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, T.; Ao, E.C.; Lambert, B.; Brans, B.; Vandenberghe, S.; Mok, G.S. Quantitative imaging for targeted radionuclide therapy dosimetry-technical review. Theranostics 2017, 7, 4551. [Google Scholar] [CrossRef] [PubMed]
- Teker, F.; Elboga, U. Is SUVmax a useful marker for progression-free survival 177 in patients with metastatic GEP-NET receiving Lu-DOTATATE therapy? Hellenic Journal of Nuclear Medicine 2021, 24, 122–131. [Google Scholar] [PubMed]
- Huizing, D.; Aalbersberg, E.A.; Versleijen, M.W.; Tesselaar, M.E.; Walraven, I.; Lahaye, M.J.; de Wit–van der Veen, B.; Stokkel, M.P. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.; Reddy, R.J.; Maheswaran, T.; Asokan, G.; Dany, A.; Anand, B. Theranostics: A treasured tailor for tomorrow. Journal of pharmacy & bioallied sciences 2014, 6, S6. [Google Scholar]
- Sjögreen Gleisner, K.; Chouin, N.; Gabina, P.M.; Cicone, F.; Gnesin, S.; Stokke, C.; Konijnenberg, M.; Cremonesi, M.; Verburg, F.A.; Bernhardt, P. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor-and PSMA-targeting ligands. European Journal of Nuclear Medicine and Molecular Imaging 2022, 49, 1778–1809. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Z.; Jarrahi, A.M.; Momenabadi, V.; Ghorat, F.; Adineh, H.; Sohrabivafa, M.; Goodarzi, E. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide stomach cancers and their relationship with the human development index (HDI). World Cancer Res J 2019, 6. [Google Scholar]
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C. Global burden of urologic cancers, 1990–2013. European urology 2017, 71, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.B.; Li, J.; Huang, B.; Weir, H.K. Prostate cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer 2017, 123, 5160–5177. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Moul, J.W.; Carroll, P.R. The changing face of prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2005, 23, 8146–8151. [Google Scholar] [CrossRef]
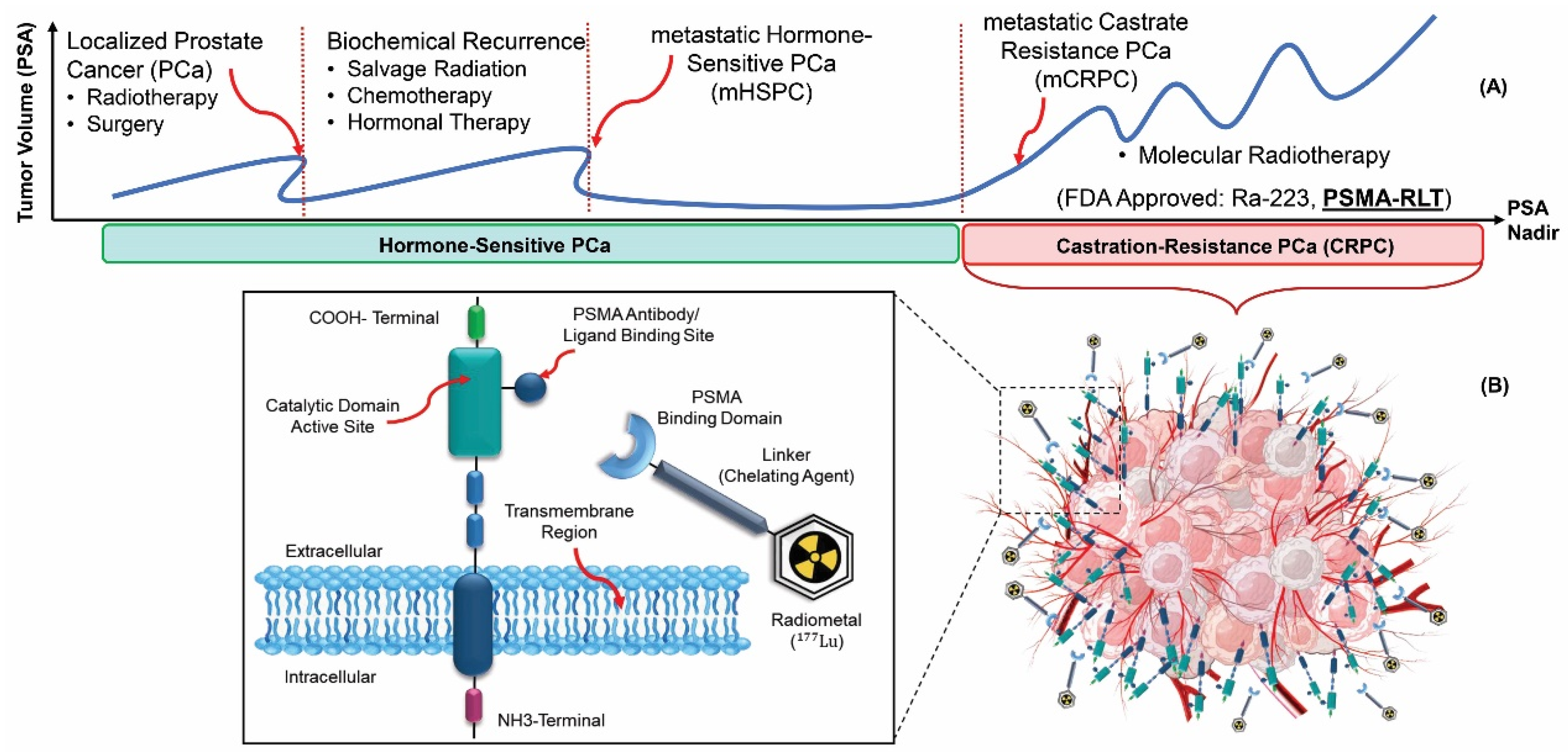
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA theranostics: review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Jadvar, H.; Ahmadzadehfar, H. PSMA theranostics: current status and future directions. Molecular imaging 2018, 17, 1536012118776068. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Aryana, K.; Pirayesh, E.; Farzanehfar, S.; Assadi, M.; Fallahi, B.; Shafiei, B.; Ayati, N.; Amoui, M. The Iranian Society of Nuclear Medicine practical guideline on radioligand therapy in metastatic castration-resistant prostate cancer using 177Lu-PSMA. Iranian Journal of Nuclear Medicine 2018, 26, 2. [Google Scholar]
- Shakeri, S.; Askari, E.; Zarehparvar, S.; Farahmandfar, F.; Norouzbeigi, N.; Salek, R.; Aryana, K. [68Ga] Ga-PSMA-11 PET/CT for staging and patient management of high-risk prostate cancer: A single-center experience from Iran. Iranian Journal of Nuclear Medicine 2022, 30, 33–39. [Google Scholar]
- Liberini, V.; Laudicella, R.; Balma, M.; Nicolotti, D.G.; Buschiazzo, A.; Grimaldi, S.; Lorenzon, L.; Bianchi, A.; Peano, S.; Bartolotta, T.V. Radiomics and artificial intelligence in prostate cancer: new tools for molecular hybrid imaging and theragnostics. European Radiology Experimental 2022, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Food, U.; Administration, D. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. US FDA website 2022. [Google Scholar]
- Fallahi, B.; Khademi, N.; Karamzade-Ziarati, N.; Fard-Esfahani, A.; Emami-Ardekani, A.; Farzanefar, S.; Eftekhari, M.; Beiki, D. 99mTc-PSMA SPECT/CT versus 68Ga-PSMA PET/CT in the evaluation of metastatic prostate cancer. Clinical Nuclear Medicine 2021, 46, e68–e74. [Google Scholar] [CrossRef]
- Harsini, S.; Fallahi, B.; Ziarati, N.K.; Razi, A.; Amini, E.; Emami-Ardekani, A.; Fard-Esfahani, A.; Parizi, M.K.; Farzanehfar, S.; Beiki, D. A Prospective Study on [68Ga]-PSMA PET/CT Imaging in Newly Diagnosed Intermediate-and High-Risk Prostate Cancer. Asia Oceania Journal of Nuclear Medicine and Biology 2021, 9, 101. [Google Scholar]
- Das, S.; Dasari, A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: are there global differences? Current oncology reports 2021, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Taal, B.; Visser, O. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004, 80, 3–7. [Google Scholar] [CrossRef]
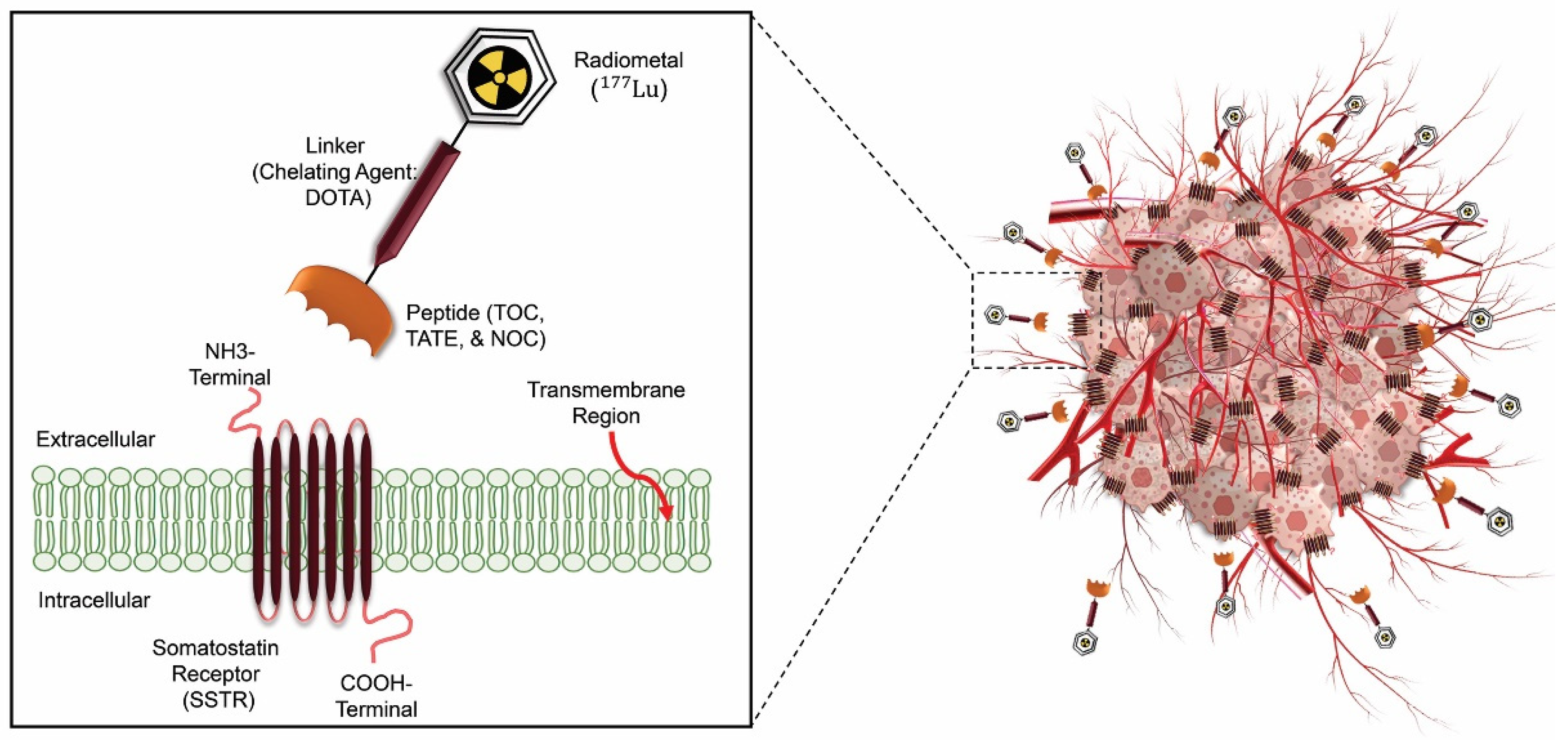
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C. Procedure guidelines for pet/ct tumour imaging with 68Ga-dota-conjugated peptides: 68Ga-dota-toc, 68Ga-dota-noc, 68Ga-dota-tate. European journal of nuclear medicine and molecular imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Food; Administration, D.; approves lutetium Lu, F. 177 dotatate for treatment of GEP-NETS. Accessed January 2018, 26.
- Miller, C.; Rousseau, J.; Ramogida, C.F.; Celler, A.; Rahmim, A.; Uribe, C.F. Implications of physics, chemistry and biology for dosimetry calculations using theranostic pairs. Theranostics 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Yousefirizi, F.; Decasez, P.; Amyar, A.; Ruan, S.; Saboury, B.; Rahmim, A. Artificial Intelligence-Based Detection, Classification and Prediction/Prognosis in PET Imaging: Towards Radiophenomics. arXiv preprint arXiv:2110.10332 2021.
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: images are more than pictures, they are data. Radiology 2016, 278, 563. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to radiomics. Journal of Nuclear Medicine 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.; Deist, T.M.; Peerlings, J.; De Jong, E.E.; Van Timmeren, J.; Sanduleanu, S.; Larue, R.T.; Even, A.J.; Jochems, A. Radiomics: the bridge between medical imaging and personalized medicine. Nature reviews Clinical oncology 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Ray, S. A quick review of machine learning algorithms. In Proceedings of the 2019 International conference on machine learning, big data, cloud and parallel computing (COMITCon), 2019; pp. 35–39.
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. Journal of big Data 2021, 8, 1–74. [Google Scholar]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D. Radiomics: the process and the challenges. Magnetic resonance imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Reuzé, S.; Schernberg, A.; Orlhac, F.; Sun, R.; Chargari, C.; Dercle, L.; Deutsch, E.; Buvat, I.; Robert, C. Radiomics in nuclear medicine applied to radiation therapy: methods, pitfalls, and challenges. International Journal of Radiation Oncology* Biology* Physics 2018, 102, 1117–1142. [Google Scholar] [CrossRef] [PubMed]
- Orlhac, F.; Nioche, C.; Klyuzhin, I.; Rahmim, A.; Buvat, I. Radiomics in PET imaging: a practical guide for newcomers. PET clinics 2021, 16, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.J.; Boellaard, R.; Dutta, J.; Jha, A.K.; Jacobs, P.; Li, Q.; Liu, C.; Sitek, A.; Saboury, B.; Scott, P.J. Nuclear medicine and artificial intelligence: best practices for algorithm development. Journal of Nuclear Medicine 2022, 63, 500–510. [Google Scholar] [CrossRef]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: a roadmap for future development. The Lancet Oncology 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Kręcisz, P.; Czarnecka, K.; Królicki, L.; Mikiciuk-Olasik, E.b.; Szymański, P. Radiolabeled peptides and antibodies in medicine. Bioconjugate chemistry 2020, 32, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Reischl, G. Production of radionuclides: Cyclotrons and reactors. 2021.
- Berry, D.J.; Ma, Y.; Ballinger, J.R.; Tavaré, R.; Koers, A.; Sunassee, K.; Zhou, T.; Nawaz, S.; Mullen, G.E.; Hider, R.C. Efficient bifunctional gallium-68 chelators for positron emission tomography: tris (hydroxypyridinone) ligands. Chemical communications 2011, 47, 7068–7070. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Vorobyeva, A. Radiometals—Chemistry and radiolabeling. Reference Module in Biomedical Sciences [Internet]. Elsevier 2021.
- Arabi, H.; Zaidi, H. Applications of artificial intelligence and deep learning in molecular imaging and radiotherapy. European Journal of Hybrid Imaging 2020, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.; O’Dorisiol, T.; Howe, J.; Cremonesi, M. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. European journal of nuclear medicine and molecular imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Manafi-Farid, R.; Harsini, S.; Saidi, B.; Ahmadzadehfar, H.; Herrmann, K.; Briganti, A.; Walz, J.; Beheshti, M. Factors predicting biochemical response and survival benefits following radioligand therapy with [177Lu] Lu-PSMA in metastatic castrate-resistant prostate cancer: A review. European Journal of Nuclear Medicine and Molecular Imaging 2021, 48, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsótér, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K. Pre-therapy somatostatin receptor-based heterogeneity predicts overall survival in pancreatic neuroendocrine tumor patients undergoing peptide receptor radionuclide therapy. Molecular Imaging and Biology 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Önner, H.; Abdülrezzak, Ü.; Tutuş, A. Could the skewness and kurtosis texture parameters of lesions obtained from pretreatment Ga-68 DOTA-TATE PET/CT images predict receptor radionuclide therapy response in patients with gastroenteropancreatic neuroendocrine tumors? Nuclear Medicine Communications 2020, 41, 1034–1039. [Google Scholar] [CrossRef]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from 68Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC cancer 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Ortega, C.; Wong, R.K.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, A.; Metser, U. Quantitative 68Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-DOTATATE. Journal of Nuclear Medicine 2021, 62, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Ganeshan, B.; Endozo, R.; Wan, S.; Aldridge, M.D.; Groves, A.M.; Bomanji, J.B.; Gaze, M.N. Radiomics-based texture analysis of 68Ga-DOTATATE positron emission tomography and computed tomography images as a prognostic biomarker in adults with neuroendocrine cancers treated with 177Lu-DOTATATE. Frontiers in Oncology 2021, 2942. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Dionisi, B.; Thuillier, P.; Ciuffreda, L.; Piovesan, A.; Fioroni, F. 68Ga-DOTATOC PET/CT-based radiomic analysis and PRRT outcome: A preliminary evaluation based on an exploratory radiomic analysis on two patients. Frontiers in medicine 2021, 7, 601853. [Google Scholar] [CrossRef] [PubMed]
- Laudicella, R.; Comelli, A.; Liberini, V.; Vento, A.; Stefano, A.; Spataro, A.; Crocè, L.; Baldari, S.; Bambaci, M.; Deandreis, D. [68Ga] DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu] DOTATOC PRRT: The “Theragnomics” Concept. Cancers 2022, 14, 984. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, B.; Senn, D.; Kramer, G.; Baltzer, P.; D’Andrea, D.; Grubmüller, K.H.; Mitterhauser, M.; Eidherr, H.; Haug, A.R.; Wadsak, W. Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. European journal of nuclear medicine and molecular imaging 2019, 46, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Herrmann, K.; Kleesiek, J.; Schäfers, M.; Shah, V.; Xu, Z.; Chabin, G.; Grbic, S.; Spottiswoode, B.; Rahbar, K. Semiautomatically quantified tumor volume using 68Ga-PSMA-11 PET as a biomarker for survival in patients with advanced prostate cancer. Journal of Nuclear Medicine 2020, 61, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, L.; Werner, R.A.; Ross, T.L.; Bengel, F.M.; Derlin, T. PSMA expression predicts early biochemical response in patients with metastatic castration-resistant prostate cancer under 177Lu-PSMA-617 radioligand therapy. Cancers 2021, 13, 2938. [Google Scholar] [CrossRef] [PubMed]
- Khreish, F.; Wiessner, M.; Rosar, F.; Ghazal, Z.; Sabet, A.; Maus, S.; Linxweiler, J.; Bartholomä, M.; Ezziddin, S. Response assessment and prediction of progression-free survival by 68Ga-PSMA-11 PET/CT based on tumor-to-liver ratio (TLR) in patients with mCRPC undergoing 177Lu-PSMA-617 radioligand therapy. Biomolecules 2021, 11, 1099. [Google Scholar] [CrossRef]
- Rosar, F.; Wenner, F.; Khreish, F.; Dewes, S.; Wagenpfeil, G.; Hoffmann, M.A.; Schreckenberger, M.; Bartholomä, M.; Ezziddin, S. Early molecular imaging response assessment based on determination of total viable tumor burden in [68Ga] Ga-PSMA-11 PET/CT independently predicts overall survival in [177Lu] Lu-PSMA-617 radioligand therapy. European Journal of Nuclear Medicine and Molecular Imaging 2022, 49, 1584–1594. [Google Scholar] [CrossRef]
- Gafita, A.; Wang, H.; Robertson, A.; Armstrong, W.R.; Zaum, R.; Weber, M.; Yagubbayli, F.; Kratochwil, C.; Grogan, T.R.; Nguyen, K. Tumor sink effect in 68Ga-PSMA-11 PET: Myth or Reality? Journal of Nuclear Medicine 2022, 63, 226–232. [Google Scholar] [CrossRef]
- Hartrampf, P.E.; Krebs, M.; Peter, L.; Heinrich, M.; Ruffing, J.; Kalogirou, C.; Weinke, M.; Brumberg, J.; Kübler, H.; Buck, A.K. Reduced Segmentation of Lesions Is Comparable to Whole-Body Segmentation for Response Assessment by PSMA PET/CT: Initial Experience with the Keyhole Approach. Biology 2022, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Pathmanandavel, S.; Crumbaker, M.; Yam, A.O.; Nguyen, A.; Rofe, C.; Hovey, E.; Gedye, C.; Kwan, E.M.; Hauser, C.; Azad, A.A. 177Lu-PSMA-617 and Idronoxil in Men with End-Stage Metastatic Castration-Resistant Prostate Cancer (LuPIN): Patient Outcomes and Predictors of Treatment Response in a Phase I/II Trial. Journal of Nuclear Medicine 2022, 63, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Ahmadzadehfar, H.; Gaertner, F.C.; Papp, L.; Zsóter, N.; Essler, M.; Bundschuh, R.A. Role of textural heterogeneity parameters in patient selection for 177Lu-PSMA therapy via response prediction. Oncotarget 2018, 9, 33312. [Google Scholar] [CrossRef] [PubMed]
- Acar, E.; Leblebici, A.; Ellidokuz, B.E.; Başbınar, Y.; Kaya, G.Ç. Machine learning for differentiating metastatic and completely responded sclerotic bone lesion in prostate cancer: a retrospective radiomics study. The British journal of radiology 2019, 92, 20190286. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. The Lancet Oncology 2021, 22, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Moazemi, S.; Erle, A.; Khurshid, Z.; Lütje, S.; Muders, M.; Essler, M.; Schultz, T.; Bundschuh, R.A. Decision-support for treatment with 177Lu-PSMA: machine learning predicts response with high accuracy based on PSMA-PET/CT and clinical parameters. Annals of translational medicine 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Moazemi, S.; Essler, M.; Schultz, T.; Bundschuh, R.A. Predicting treatment response in prostate cancer patients based on multimodal PET/CT for clinical decision support. In Proceedings of the International Workshop on Multimodal Learning for Clinical Decision Support, 2021; pp. 22–35.
- Moazemi, S.; Erle, A.; Lütje, S.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Estimating the potential of radiomics features and radiomics signature from pretherapeutic PSMA-PET-CT scans and clinical data for prediction of overall survival when treated with 177Lu-PSMA. Diagnostics 2021, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Roll, W.; Schindler, P.; Masthoff, M.; Seifert, R.; Schlack, K.; Bögemann, M.; Stegger, L.; Weckesser, M.; Rahbar, K. Evaluation of 68Ga-PSMA-11 PET-MRI in Patients with Advanced Prostate Cancer Receiving 177Lu-PSMA-617 Therapy: A Radiomics Analysis. Cancers 2021, 13, 3849. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Schneider, F.; Kratochwil, C.; Rath, D.; Moltz, J.; Holland-Letz, T.; Kauczor, H.-U.; Schwartz, L.H.; Haberkorn, U.; Flechsig, P. Correlation between SUVmax and CT radiomic analysis using lymph node density in PET/CT-based lymph node staging. Journal of Nuclear Medicine 2017, 58, 282–287. [Google Scholar] [CrossRef]
- Moazemi, S.; Khurshid, Z.; Erle, A.; Lütje, S.; Essler, M.; Schultz, T.; Bundschuh, R.A. Machine learning facilitates hotspot classification in PSMA-PET/CT with nuclear medicine specialist accuracy. Diagnostics 2020, 10, 622. [Google Scholar] [CrossRef]
- Erle, A.; Moazemi, S.; Lütje, S.; Essler, M.; Schultz, T.; Bundschuh, R.A. Evaluating a machine learning tool for the classification of pathological uptake in whole-body PSMA-PET-CT scans. Tomography 2021, 7, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hinzpeter, R.; Baumann, L.; Guggenberger, R.; Huellner, M.; Alkadhi, H.; Baessler, B. Radiomics for detecting prostate cancer bone metastases invisible in CT: a proof-of-concept study. European radiology 2022, 32, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Hammes, J.; Täger, P.; Drzezga, A. EBONI: A tool for automated quantification of bone metastasis load in PSMA PET/CT. Journal of Nuclear Medicine 2018, 59, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gafita, A.; Vollnberg, B.; Tetteh, G.; Haupt, F.; Afshar-Oromieh, A.; Menze, B.; Eiber, M.; Rominger, A.; Shi, K. Deep neural network for automatic characterization of lesions on 68Ga-PSMA-11 PET/CT. European journal of nuclear medicine and molecular imaging 2020, 47, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [177Lu] Lu-PSMA-617 radioligand therapy in a bicentric analysis. European Journal of Nuclear Medicine and Molecular Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; De Santi, B.; Rampado, O.; Gallio, E.; Dionisi, B.; Ceci, F.; Polverari, G.; Thuillier, P.; Molinari, F.; Deandreis, D. Impact of segmentation and discretization on radiomic features in 68Ga-DOTA-TOC PET/CT images of neuroendocrine tumor. EJNMMI physics 2021, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wehrend, J.; Silosky, M.; Xing, F.; Chin, B.B. Automated liver lesion detection in 68Ga DOTATATE PET/CT using a deep fully convolutional neural network. EJNMMI research 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F. SUV of [68Ga] DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Molecular Imaging and Biology 2015, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Ezziddin, S.; Lohmar, J.; Yong-Hing, C.J.; Sabet, A.; Ahmadzadehfar, H.; Kukuk, G.; Biersack, H.-J.; Guhlke, S.; Reichmann, K. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clinical nuclear medicine 2012, 37, e141–e147. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. Journal of nuclear medicine 2019, 60, 517–523. [Google Scholar] [CrossRef]
- Bruvoll, R.; Blakkisrud, J.; Mikalsen, L.; Connelly, J.; Stokke, C. Correlations between [68Ga] Ga-DOTA-TOC uptake and absorbed dose from [177Lu] Lu-DOTA-TATE. 2022.
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W. The 68Ga/177Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: correlation of SUVmax values and absorbed dose estimates. European journal of nuclear medicine and molecular imaging 2017, 44, 788–800. [Google Scholar] [CrossRef]
- Stenvall, A.; Gustafsson, J.; Larsson, E.; Roth, D.; Sundlöv, A.; Jönsson, L.; Hindorf, C.; Ohlsson, T.; Sjögreen Gleisner, K. Relationships between uptake of [68Ga] Ga-DOTA-TATE and absorbed dose in [177Lu] Lu-DOTA-TATE therapy. EJNMMI research 2022, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zang, J.; Wang, H.; Liu, Q.; Li, F.; Lin, Y.; Huo, L.; Jacobson, O.; Niu, G.; Fan, X. Pretherapeutic 68Ga-PSMA-617 PET may indicate the dosimetry of 177Lu-PSMA-617 and 177Lu-EB-PSMA-617 in main organs and tumor lesions. Clinical nuclear medicine 2019, 44, 431. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Gafita, A.; Dong, C.; Zhao, Y.; Tetteh, G.; Menze, B.H.; Ziegler, S.; Weber, W.; Afshar-Oromieh, A.; Rominger, A. Application of machine learning to pretherapeutically estimate dosimetry in men with advanced prostate cancer treated with 177Lu-PSMA I&T therapy. European journal of nuclear medicine and molecular imaging 2022, 1–9. [Google Scholar]
- Xue, S.; Gafita, A.; Dong, C.; Zhao, Y.; Tetteh, G.; Menze, B.H.; Ziegler, S.; Weber, W.; Afshar-Oromieh, A.; Rominger, A. Proof-of-concept Study to Estimate Individual Post-Therapy Dosimetry in Men with Advanced Prostate Cancer Treated with 177Lu-PSMA I&T Therapy. 2022.
- Akhavanallaf, A.; Peterson, A.B.; Fitzpatrick, K.; Roseland, M.; Wong, K.K.; El-Naqa, I.; Zaidi, H.; Dewaraja, Y.K. The predictive value of pretherapy [68Ga] Ga-DOTA-TATE PET and biomarkers in [177Lu] Lu-PRRT tumor dosimetry. European Journal of Nuclear Medicine and Molecular Imaging 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Plachouris, D.; Eleftheriadis, V.; Nanos, T.; Papathanasiou, N.; Sarrut, D.; Papadimitroulas, P.; Savvidis, G.; Vergnaud, L.; Salvadori, J.; Imperiale, A. A radiomic-and dosiomic-based machine learning regression model for pretreatment planning in 177Lu-DOTATATE therapy. Medical Physics 2023. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Derlin, T.; Lapa, C.; Sheikbahaei, S.; Higuchi, T.; Giesel, F.L.; Behr, S.; Drzezga, A.; Kimura, H.; Buck, A.K. 18F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M.; Babich, J.W.; Kelly, J.M. Advances in PSMA theranostics. Translational Oncology 2022, 22, 101450. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. European journal of nuclear medicine and molecular imaging 2017, 44, 678–688. [Google Scholar] [CrossRef]
- Szabo, Z.; Mena, E.; Rowe, S.P.; Plyku, D.; Nidal, R.; Eisenberger, M.A.; Antonarakis, E.S.; Fan, H.; Dannals, R.F.; Chen, Y. Initial evaluation of [18F] DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Molecular imaging and biology 2015, 17, 565–574. [Google Scholar] [CrossRef]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. Journal of Nuclear Medicine 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Cardinale, J.; Schäfer, M.; Neels, O.; Benešová, M.; Mier, W.; Haberkorn, U.; Kopka, K.; Kratochwil, C. 18F-labelled PSMA-1007 shows similarity in structure, biodistribution and tumour uptake to the theragnostic compound PSMA-617. European Journal of Nuclear Medicine and Molecular Imaging 2016, 43, 1929–1930. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Song, I.H.; Kim, S.Y.; Ko, H.Y.; Kil, H.S.; Chi, D.Y.; Giesel, F.L.; Kopka, K.; Hoepping, A.; Chun, J.-H. Preclinical Evaluation of a Companion Diagnostic Radiopharmaceutical,[18F] PSMA-1007, in a Subcutaneous Prostate Cancer Xenograft Mouse Model. Molecular Pharmaceutics 2022. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.A.; Debowski, M.; Gulhane, B.; Arnfield, E.G.; Pelecanos, A.M.; Garcia, P.L.; Latter, M.J.; Lin, C.Y.; Roberts, M.J.; Ramsay, S.C. Prospective intra-individual blinded comparison of [18F] PSMA-1007 and [68 Ga] Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging 2022, 49, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Saule, L.; Radzina, M.; Liepa, M.; Roznere, L.; Lioznovs, A.; Ratniece, M.; Mamis, E.; Vjaters, E. Recurrent Prostate Cancer Diagnostics with 18F-PSMA-1007 PET/CT: A Systematic Review of the Current State. Diagnostics 2022, 12, 3176. [Google Scholar] [CrossRef] [PubMed]
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. European Journal of Nuclear Medicine and Molecular Imaging 2021, 48, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Cordes, M.; Schmidt, D.; Bäuerle, T.; Goetz, T.I.; Beck, M.; Prante, O.; Cavallaro, A.; Uder, M.; Wullich, B. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. European journal of nuclear medicine and molecular imaging 2018, 45, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, L.M.; Beyer, L.; Zacherl, M.J.; Gildehaus, F.J.; Todica, A.; Kunte, S.C.; Holzgreve, A.; Sheikh, G.T.; Herlemann, A.; Casuscelli, J. Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T. Biomedicines 2022, 10, 946. [Google Scholar]
- Draulans, C.; De Roover, R.; van der Heide, U.A.; Kerkmeijer, L.; Smeenk, R.J.; Pos, F.; Vogel, W.V.; Nagarajah, J.; Janssen, M.; Isebaert, S. Optimal 68Ga-PSMA and 18F-PSMA PET window levelling for gross tumour volume delineation in primary prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging 2021, 48, 1211–1218. [Google Scholar] [CrossRef]
- Spohn, S.K.; Kramer, M.; Kiefer, S.; Bronsert, P.; Sigle, A.; Schultze-Seemann, W.; Jilg, C.A.; Sprave, T.; Ceci, L.; Fassbender, T.F. Comparison of manual and semi-automatic [18F] PSMA-1007 PET based contouring techniques for intraprostatic tumor delineation in patients with primary prostate cancer and validation with histopathology as standard of reference. Frontiers in oncology 2020, 10, 600690. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Brendel, M.; Beyer, L.; Albert, N.L.; Todica, A.; Zacherl, M.J.; Wenter, V.; Herlemann, A.; Kretschmer, A.; Ledderose, S.T. Feasibility of Different Tumor Delineation 18F-PSMA-1007 Approaches PET/CT Imaging for in Prostate Cancer Patients. Exploring the Potential of PSMA-PET Imaging on Personalized Prostate Cancer Treatment 2022.
- Lau, Y.C.; Chen, S.; Ho, C.L.; Cai, J. Reliability of gradient-based segmentation for measuring metabolic parameters influenced by uptake time on 18F-PSMA-1007 PET/CT for prostate cancer. Frontiers in Oncology 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Trägårdh, E.; Enqvist, O.; Ulén, J.; Jögi, J.; Bitzén, U.; Hedeer, F.; Valind, K.; Garpered, S.; Hvittfeldt, E.; Borrelli, P. Freely available, fully automated ai-based analysis of primary tumour and metastases of prostate cancer in whole-body [18f]-psma-1007 pet-ct. Diagnostics 2022, 12, 2101. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. Journal of Nuclear Medicine 2017, 58, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Revheim, M.-E.; Raynor, W.; Zehetner, W.; Knoll, P.; Zandieh, S.; Alavi, A. 64Cu-DOTATOC PET-CT in patients with neuroendocrine tumors. Oncology and therapy 2020, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Ranganathan, D.; Wagh, N.; Shafie, A.; Gaber, A.; Abbasi, A.; Kjaer, A.; Tworowska, I.; Núñez, R. 64Cu-DOTATATE PET/CT for imaging patients with known or suspected somatostatin receptor–positive neuroendocrine tumors: Results of the first US prospective, reader-masked clinical trial. Journal of Nuclear Medicine 2020, 61, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Anderson, C.J. Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals. Journal of labelled compounds and radiopharmaceuticals 2014, 57, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-D.; Liu, C.; Liu, F.; Xie, Q.-H.; Liu, T.-L.; Guo, X.-Y.; Xu, X.-X.; Yang, X.; Zhu, H.; Yang, Z. 64Cu-PSMA-617: A novel PSMA-targeted radio-tracer for PET imaging in gastric adenocarcinoma xenografted mice model. Oncotarget 2017, 8, 74159. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C. 64Cu-PSMA-617 PET/CT imaging of prostate adenocarcinoma: first in-human studies. Cancer Biotherapy and Radiopharmaceuticals 2016, 31, 277–286. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Johnbeck, C.B.; Binderup, T.; Loft, M.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W. 64Cu-DOTATATE PET/CT and prediction of overall and progression-free survival in patients with neuroendocrine neoplasms. Journal of Nuclear Medicine 2020, 61, 1491–1497. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Johnbeck, C.B.; Loft, M.; Pfeifer, A.; Oturai, P.; Langer, S.W.; Knigge, U.; Ladefoged, C.N.; Kjaer, A. Semiautomatic tumor delineation for evaluation of 64Cu-DOTATATE PET/CT in patients with neuroendocrine neoplasms: prognostication based on lowest lesion uptake and total tumor volume. Journal of Nuclear Medicine 2021, 62, 1564–1570. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Lindholm, K.; Hindsholm, A.; Gæde, M.; Ladefoged, C.N.; Loft, M.; Johnbeck, C.B.; Langer, S.W.; Oturai, P.; Knigge, U. A convolutional neural network for total tumor segmentation in [64Cu] Cu-DOTATATE PET/CT of patients with neuroendocrine neoplasms. EJNMMI research 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Danieli, R.; Milano, A.; Gallo, S.; Veronese, I.; Lascialfari, A.; Indovina, L.; Botta, F.; Ferrari, M.; Cicchetti, A.; Raspanti, D. Personalized Dosimetry in Targeted Radiation Therapy: A Look to Methods, Tools and Critical Aspects. Journal of Personalized Medicine 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- James, S.S.; Bednarz, B.; Benedict, S.; Buchsbaum, J.C.; Dewaraja, Y.; Frey, E.; Hobbs, R.; Grudzinski, J.; Roncali, E.; Sgouros, G. Current status of radiopharmaceutical therapy. International Journal of Radiation Oncology* Biology* Physics 2021, 109, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nature Reviews Drug Discovery 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Singh, S.B.; Thapaliya, S.; Shrestha, S.; Deo, S.; Khanal, K. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Rathke, H.; Flechsig, P.; Mier, W.; Bronzel, M.; Mavriopoulou, E.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Kratochwil, C. Dosimetry estimate and initial clinical experience with 90Y-PSMA-617. Journal of Nuclear Medicine 2019, 60, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Boschi, A.; Cittanti, C.; Martini, P.; Panareo, S.; Tonini, E.; Nieri, A.; Urso, L.; Caracciolo, M.; Lodi, L. 90Y/177Lu-DOTATOC: from preclinical studies to application in humans. Pharmaceutics 2021, 13, 1463. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Thomas, S.R.; Stubbs, J.B.; Stabin, M.G.; Hays, M.T.; Koral, K.F.; Robertson, J.S.; Howell, R.W.; Wessels, B.W.; Fisher, D.R. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. Journal of Nuclear Medicine 1999, 40, 37S–61S. [Google Scholar] [PubMed]
- Dewaraja, Y.K.; Frey, E.C.; Sgouros, G.; Brill, A.B.; Roberson, P.; Zanzonico, P.B.; Ljungberg, M. MIRD pamphlet no. 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. Journal of Nuclear Medicine 2012, 53, 1310–1325. [Google Scholar] [CrossRef]
- Ljungberg, M.; Celler, A.; Konijnenberg, M.W.; Eckerman, K.F.; Dewaraja, Y.K.; Sjögreen-Gleisner, K. MIRD pamphlet no. 26: joint EANM/MIRD guidelines for quantitative 177Lu SPECT applied for dosimetry of radiopharmaceutical therapy. Journal of nuclear medicine 2016, 57, 151–162. [Google Scholar] [CrossRef]
- Bolch, W.E.; Bouchet, L.G.; Robertson, J.S.; Wessels, B.W.; Siegel, J.A.; Howell, R.W.; Erdi, A.K.; Aydogan, B.; Costes, S.; Watson, E.E. MIRD pamphlet no. 17: the dosimetry of nonuniform activity distributions—radionuclide S values at the voxel level. Journal of Nuclear Medicine 1999, 40, 11S–36S. [Google Scholar] [PubMed]
- Bolch, W.E.; Eckerman, K.F.; Sgouros, G.; Thomas, S.R. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. Journal of Nuclear Medicine 2009, 50, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Furhang, E.E.; Chui, C.S.; Sgouros, G. A Monte Carlo approach to patient-specific dosimetry. Medical physics 1996, 23, 1523–1529. [Google Scholar] [CrossRef]
- Brosch-Lenz, J.; Yousefirizi, F.; Zukotynski, K.; Beauregard, J.-M.; Gaudet, V.; Saboury, B.; Rahmim, A.; Uribe, C. Role of AI in Theranostics: Towards Routine Personalized Radiopharmaceutical Therapies. arXiv preprint arXiv:2107.13913 2021.
- Arabi, H.; AkhavanAllaf, A.; Sanaat, A.; Shiri, I.; Zaidi, H. The promise of artificial intelligence and deep learning in PET and SPECT imaging. Physica Medica 2021, 83, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Santoro, L.; Mora-Ramirez, E.; Trauchessec, D.; Chouaf, S.; Eustache, P.; Pouget, J.-P.; Kotzki, P.-O.; Bardiès, M.; Deshayes, E. Implementation of patient dosimetry in the clinical practice after targeted radiotherapy using [177Lu-[DOTA0, Tyr3]-octreotate. EJNMMI research 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hippeläinen, E.; Tenhunen, M.; Mäenpää, H.; Sohlberg, A. Quantitative accuracy of 177 Lu SPECT reconstruction using different compensation methods: phantom and patient studies. EJNMMI research 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rydén, T.; Van Essen, M.; Marin, I.; Svensson, J.; Bernhardt, P. Deep-learning generation of synthetic intermediate projections improves 177Lu SPECT images reconstructed with sparsely acquired projections. Journal of Nuclear Medicine 2021, 62, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Odland, A.; Server, A.; Saxhaug, C.; Breivik, B.; Groote, R.; Vardal, J.; Larsson, C.; Bjørnerud, A. Volumetric glioma quantification: comparison of manual and semi-automatic tumor segmentation for the quantification of tumor growth. Acta Radiologica 2015, 56, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Starmans, M.P.; van der Voort, S.R.; Tovar, J.M.C.; Veenland, J.F.; Klein, S.; Niessen, W.J. Radiomics: data mining using quantitative medical image features. In Handbook of medical image computing and computer assisted intervention; Elsevier: 2020; pp. 429–456.
- van Heeswijk, M.M.; Lambregts, D.M.; van Griethuysen, J.J.; Oei, S.; Rao, S.-X.; de Graaff, C.A.; Vliegen, R.F.; Beets, G.L.; Papanikolaou, N.; Beets-Tan, R.G. Automated and semiautomated segmentation of rectal tumor volumes on diffusion-weighted MRI: can it replace manual volumetry? International Journal of Radiation Oncology* Biology* Physics 2016, 94, 824–831. [Google Scholar] [CrossRef]
- Gudi, S.; Ghosh-Laskar, S.; Agarwal, J.P.; Chaudhari, S.; Rangarajan, V.; Paul, S.N.; Upreti, R.; Murthy, V.; Budrukkar, A.; Gupta, T. Interobserver variability in the delineation of gross tumour volume and specified organs-at-risk during IMRT for head and neck cancers and the impact of FDG-PET/CT on such variability at the primary site. Journal of medical imaging and radiation sciences 2017, 48, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Peterson, A.; Van, B.; Fedrigo, R.; Carlson, J.; Sunderland, J.; Frey, E.; Dewaraja, Y.K. An international study of factors affecting variability of dosimetry calculations, part 1: design and early results of the SNMMI dosimetry challenge. Journal of Nuclear Medicine 2021, 62, 36S–47S. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Hardcastle, N.; Dawe, N.; Kron, T.; Hofman, M.S.; Hicks, R.J. Deep learning renal segmentation for fully automated radiation dose estimation in unsealed source therapy. Frontiers in oncology 2018, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Ryden, T.; van Essen, M.; Svensson, J.; Bernhardt, P. Deep learning-based SPECT/CT quantification of 177Lu uptake in the kidneys. 2020.
- Nazari, M.; Jiménez-Franco, L.D.; Schroeder, M.; Kluge, A.; Bronzel, M.; Kimiaei, S. Automated and robust organ segmentation for 3D-based internal dose calculation. EJNMMI research 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tsougos, I.; Loudos, G.; Georgoulias, P.; Theodorou, K.; Kappas, C. Patient-specific internal radionuclide dosimetry. Nuclear medicine communications 2010, 31, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Hwang, D.; Kim, J.H.; Lee, J.S. Deep-dose: a voxel dose estimation method using deep convolutional neural network for personalized internal dosimetry. Scientific reports 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Götz, T.I.; Schmidkonz, C.; Chen, S.; Al-Baddai, S.; Kuwert, T.; Lang, E.W. A deep learning approach to radiation dose estimation. Physics in Medicine & Biology 2020, 65, 035007. [Google Scholar]
- Melodia, L. Deep Learning Estimation of Absorbed Dose for Nuclear Medicine Diagnostics. arXiv preprint arXiv:1805.09108 2018.
- Götz, T.I.; Lang, E.W.; Schmidkonz, C.; Kuwert, T.; Ludwig, B. Dose voxel kernel prediction with neural networks for radiation dose estimation. Zeitschrift für medizinische Physik 2021, 31, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Akhavanallaf, A.; Shiri, I.; Arabi, H.; Zaidi, H. Whole-body voxel-based internal dosimetry using deep learning. European journal of nuclear medicine and molecular imaging 2021, 48, 670–682. [Google Scholar] [CrossRef]
- Li, Z.; Fessler, J.A.; Mikell, J.K.; Wilderman, S.J.; Dewaraja, Y.K. DblurDoseNet: A deep residual learning network for voxel radionuclide dosimetry compensating for single-photon emission computerized tomography imaging resolution. Medical Physics 2022, 49, 1216–1230. [Google Scholar] [CrossRef]
- Vinjamuri, S.; Gilbert, T.; Banks, M.; McKane, G.; Maltby, P.; Poston, G.; Weissman, H.; Palmer, D.; Vora, J.; Pritchard, D. Peptide receptor radionuclide therapy with 90Y-DOTATATE/90Y-DOTATOC in patients with progressive metastatic neuroendocrine tumours: assessment of response, survival and toxicity. British journal of cancer 2013, 108, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Zhang, J.; Tweedle, M.F.; Knopp, M.V.; Hall, N.C. Theranostic imaging of Yttrium-90. BioMed research international 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Elschot, M.; Lam, M.G.; van den Bosch, M.A.; Viergever, M.A.; de Jong, H.W. Quantitative monte carlo–based 90y spect reconstruction. Journal of Nuclear Medicine 2013, 54, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Dewaraja, Y.K.; Chun, S.Y.; Srinivasa, R.N.; Kaza, R.K.; Cuneo, K.C.; Majdalany, B.S.; Novelli, P.M.; Ljungberg, M.; Fessler, J.A. Improved quantitative 90Y bremsstrahlung SPECT/CT reconstruction with Monte Carlo scatter modeling. Medical physics 2017, 44, 6364–6376. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Lim, H.; Fessler, J.A.; Dewaraja, Y.K. A deep neural network for fast and accurate scatter estimation in quantitative SPECT/CT under challenging scatter conditions. European journal of nuclear medicine and molecular imaging 2020, 47, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.C.; Ho, T.T.; Le, T.A.; Nguyen, D.T.; Nguyen, D.K.; Nguyen, H.H. A convolutional neural network for Y90 SPECT/CT scatter estimation. Nuclear Science and Technology 2021, 11, 9–13. [Google Scholar] [CrossRef]
- Lim, H.; Chun, I.Y.; Dewaraja, Y.K.; Fessler, J.A. Improved low-count quantitative PET reconstruction with an iterative neural network. IEEE transactions on medical imaging 2020, 39, 3512–3522. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Rowe, S.P.; Du, Y. Artificial intelligence in single photon emission computed tomography (SPECT) imaging: a narrative review. Annals of Translational Medicine 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Bradshaw, T.J.; Buvat, I.; Hatt, M.; Prabhat, K.; Liu, C.; Obuchowski, N.F.; Saboury, B.; Slomka, P.J.; Sunderland, J.J. Nuclear medicine and artificial intelligence: best practices for evaluation (the RELAINCE guidelines). Journal of Nuclear Medicine 2022, 63, 1288–1299. [Google Scholar] [CrossRef]
- Babak, S. Artificial Intelligence in Nuclear Medicine: Opportunities, Challenges, and Responsibilities Toward a Trustworthy Ecosystem. Journal of Nuclear Medicine 2023. [CrossRef]
- Afshar, P.; Mohammadi, A.; Plataniotis, K.N.; Oikonomou, A.; Benali, H. From handcrafted to deep-learning-based cancer radiomics: challenges and opportunities. IEEE Signal Processing Magazine 2019, 36, 132–160. [Google Scholar] [CrossRef]
- Dewaraja, Y.K.; Mirando, D.M.; Peterson, A.; Niedbala, J.; Millet, J.D.; Mikell, J.K.; Frey, K.; Wong, K.K.; Wilderman, S.; Nelson, A.S. A pipeline for automated voxel dosimetry: application in patients with multi-SPECT/CT imaging following 177Lu peptide receptor radionuclide therapy. Journal of Nuclear Medicine 2022. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S. Digital twins to personalize medicine. Genome medicine 2020, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Boussard, T.; Macklin, P.; Greenspan, E.J.; Gryshuk, A.L.; Stahlberg, E.; Syeda-Mahmood, T.; Shmulevich, I. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nature medicine 2021, 27, 2065–2066. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Brosch-Lenz, J.; Fele-Paranj, A.; Yousefirizi, F.; Soltani, M.; Uribe, C.; Saboury, B. Theranostic digital twins for personalized radiopharmaceutical therapies: Reimagining theranostics via computational nuclear oncology. Frontiers in Oncology 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Bradshaw, T.J.; Buvat, I.; Dutta, J.; Jha, A.K.; Kinahan, P.E.; Li, Q.; Liu, C.; McCradden, M.D.; Saboury, B. Issues and Challenges in Applications of Artificial Intelligence to Nuclear Medicine--The Bethesda Report (AI Summit 2022). arXiv preprint arXiv:2211.03783 2022.
- Abdollahi, H.; Rahmim, A. Digital Twins for Personalized Healthcare: Application to Radiopharmaceutical Therapies. Frontiers in Biomedical Technologies 2022. [Google Scholar] [CrossRef]
- Brosch-Lenz, J.; Uribe, C.; Rahmim, A.; Saboury, B. Theranostic Digital Twins: An indispensable prerequisite for personalized cancer care. J Nucl Med 2022. [Google Scholar] [CrossRef]
| Therapeutic Radioisotopes |
Diagnostic Radioisotopes- Pharmaceuticals | |
|---|---|---|
| SSTRs Target/ NET | PSMA target/ mCRPC | |
| 177Lu | [68Ga]Ga-DOTA-TATE PET | [68Ga]Ga-PSMA-617 PET |
| [68Ga]Ga -DOTA-TOC PET | [68Ga]Ga-PSMA-I&T PET | |
| [68Ga]Ga-PSMA-11 PET | ||
| [64CuCu]-DOTA-TATE PET | [64Cu]Cu-PSMA-617 PET | |
| [64Cu]Cu-DOTA-TOC PET | ||
| [18F]PSMA-617 PET | ||
| [44Sc]Sc-PSMA-617 PET | ||
| 225Ac | [177Lu]Lu-DOTA-TATE SPECT | [177Lu]Lu-PSMA-617 SPECT |
| [177Lu]Lu-DOTA-TOC SPECT | ||
| 90Y | [177Lu]Lu-DOTA-TATE SPECT | [177Lu]Lu-PSMA-617 SPECT |
| [177Lu]Lu-DOTA-TOC SPECT | [177Lu]Lu-J591 SPECT | |
| [111In]In-DOTA-TATE SPECT | [111In]In-J591 SPECT | |
| [111In]In-DOTA-TOC SPECT | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





