1. Introduction
As primary sources of essential nutrients, fruits and vegetables are a vital part of our daily diet [
1,
2]. Phytonutrients they contain, prevent cardiovascular diseases as well as certain cancers [
3]. These valuable constituents (fatty acids, vitamins, minerals and fibers) are intact in freshly harvested fruits. Low consumption of fruits and vegetables may potentially leads to nutritional deficiencies and favor diseases such are malnutrition in children, overweight or obesity in adults [
4,
5]. Consequently, regular intake of fruit and vegetable is an important part of a healthy diet [
6,
7] and nutritionists recommend to ideally consume at least five portions per day (5/d) [
3,
8]. The sale of fruits and vegetables in the streets and open-air markets is a widespread practice in low-and-middle income countries (LMIC) and represent an important part of daily food consumption in urban and peri-urban areas [
9,
10,
11,
12]. This activity constitutes a business opportunity and has a significant socio-economic impact, especially with regard to the value chain of horticultural products. Indeed, in LMIC, sale of fruits and vegetables in batch or even in single of portion of a piece provide a regular source of income for millions of people. In addition, this activity contributes to the economic empowerment of smallholder farmers, local food processors, wholesalers and different retailers [
11,
13].
According to the 2018
Codex alimentarius report, despite many education and training initiatives, and efforts to frame the horticulture sector, many actors involved in food processing still have limited food safety skills [
14]. This raises concerns on the quality, safety [
7,
15], management and proper preservation of fruits and vegetables in LMIC. For example, post-harvest losses of mangoes are estimated at around 60% [
12,
16]. This is one of the main reasons that promote the sale of these food matrices on the streets as fourth-range products [
17].
Fresh vegetables are rich in carbohydrates, low in proteins and have a pH ranging from neutrality to a slight acidity. They constitute a favorable ecosystem for the development of microorganisms that can cause their putrefaction making these products highly perishable. Additionally, when these products are frequently marketed without proper guidelines [
18], they can act as vehicles for infection by bacteria, parasites, viruses, fungi, especially when eaten uncooked [
1,
2,
17,
19]. In LMIC where poor hygiene conditions are frequent, fruits and vegetables can be contaminated with pathogens through fecal transmission or from the environment (irrigation water or contaminated soil) [
2,
15]. These contaminants cause a food safety concern and therefore food poisonings which pose a persistent threat to public health [
2,
15]. Indeed, most raw-eaten foods have been recognized as sources of transmission of infectious diseases [
1,
2,
3].
Although the majority of food poisoning events are due to the consumption of animal source foods [
15], the number of cases associated with fresh fruits and vegetables continues to increase [
20]. Thus, a wide range of contaminated fresh fruits and vegetables have recently caused major outbreaks of microbial infections [
21]. Moreover, several studies showed that unsafe fresh fruits and vegetables can be vectors for various human pathogens [
1,
18,
19,
22].
In Senegal as in many LMIC, food safety remains one of the major concerns with multiple challenges. Street foods have developed strongly over the past thirty years [
11,
15,
23,
24,
25]. This phenomenon is linked to the combined effect of changing eating habits [
19], rural exodus, population growth in cities and also due to the high annual production of horticultural products [
21]. In a context of food transition, the agri-food sector has experienced a considerable change since the beginning of the 2000s. In addition, compliance with health standards in the fruit and vegetable value chain remains a controversial subject given the prevalence of foodborne diseases in resource-limited countries [
26].
This study aimed to assess the microbiological quality of set of fruit and vegetable sold on stalls in the streets and open-air markets in Dakar.
2. Materials and Methods
2.1. Collection and sampling sites
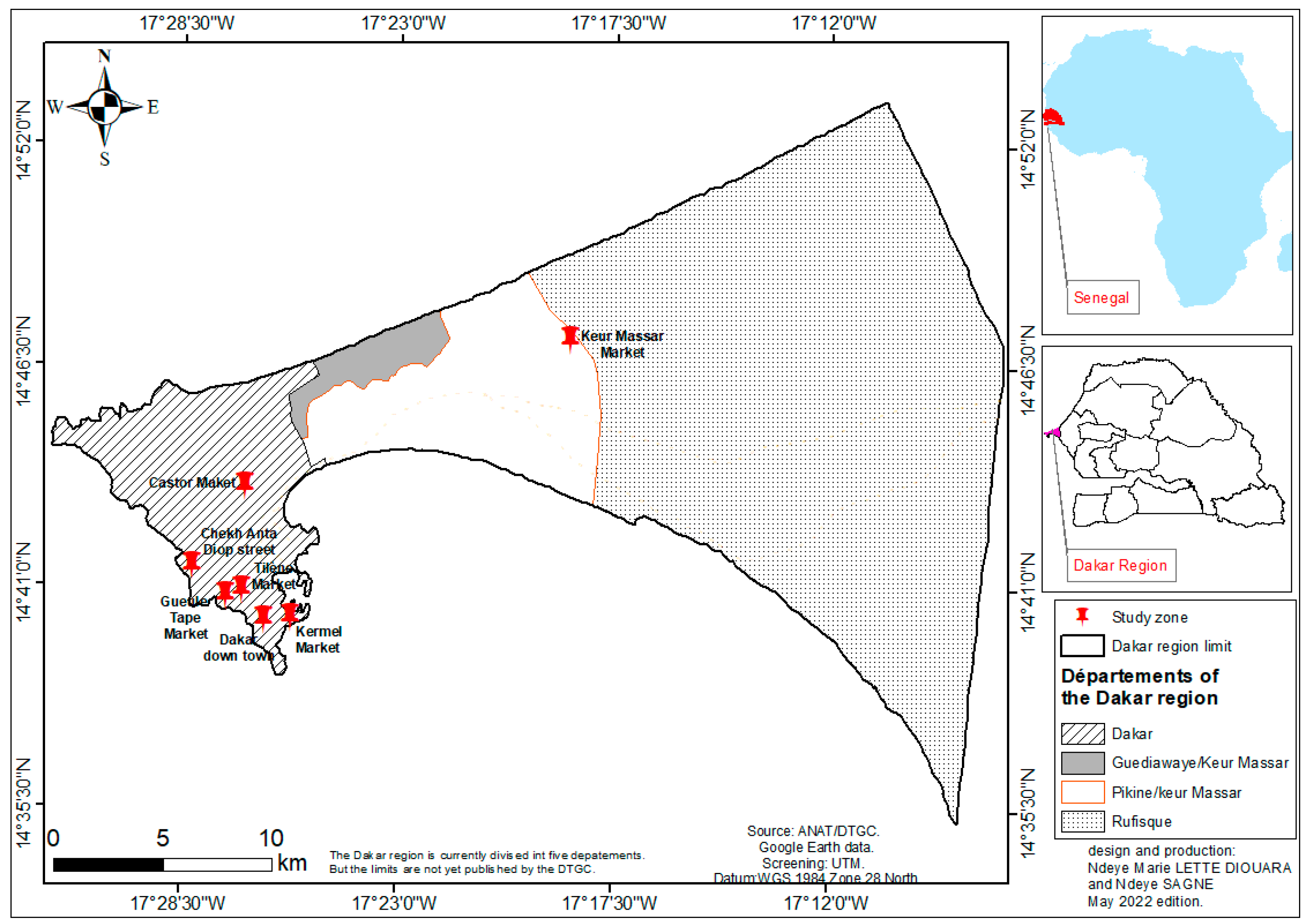
Samples were purchased in seven sites including five in the district of Dakar city (Tilène, Kermel, Castor markets, Dakar – down town and Cheikh Anta Diop Avenue) and two in its suburbs (Gueule Tapée, Parcelles Assainies and Keur Massar markets) (
Figure 1). In order to obtaining representative samples, study sites were chosen according to the high density of the surrounding populations reflecting level of frequentation. For each type of sample and regardless of the collection site, a quantity of 250 g was purchased from different sellers. We have arbitrarily set to 3 and 5 for a minimum and maximum of samples purchased per seller. It is noteworthy that one of the sites i.e “Kermel market” is located in Dakar city center, a neighborhood where high-income individuals reside. Samples collection was carried out over the period from June to December 2021 on all sites.
The collected samples were individually packaged in sterile plastic bags and hermetically sealed. Each sample was clearly identified by a code with a permanent marker. Samples were transported to the laboratory in a cooler box bag containing ice packs maintained at 4°C, within 2 hours following collection. Each sample was split in two with 150 g stored in a biobank at - 80°C for molecular tests and the remaining immediately submitted to rub-shake-rub to dislodge microbial populations from the surface.
2.2. Bacterial culture
For each sample, 25g were mixed with 225mL of Buffered Peptone Water in a sterile filter bag stomacher. This matrix was subjected to rub-shake-rub to dislodge microbial populations from the surface. Part of the mixture obtained was incubated at 37°C during 24 hours for detection of
Salmonella spp. For detection of
E. coli and
Vibrio spp., a serial dilution (up to 10
−4) was carried out from this pre-enrichment solution, all according to the matrix for inoculating the microbial suspension at different concentrations. Then we used specific culture media for
E. coli,
Vibrio spp. and
Salmonella spp. suspicion i.e Tryptone Bile Glucuronate (TBX), Thiosulfate Citrate Sucrose (TCBS) and Rappaport, respectively. For
E. coli investigation, a deep inoculation is carried out by pouring the TBX culture medium into a Petri dish containing 1mL of microbial suspension. Dishes are then incubated at 42°C for 48 hours. The determination of
Vibrio spp. was carried out by seeding on the surface by spreading 100 μL of microbial suspension on the surface of the TCBS culture medium. The dishes are incubated at 37°C for 24 hours. For the suspicion of
Salmonella, 100 µL of the pre-incubated pre-enrichment are inoculated into 10 mL of Rappaport. The tubes are then incubated at 42°C for 24 hours. All microbial analyses were carried out in duplicate. Characteristic blue colonies of
E. coli were counted in TBX, a chromogenic medium. For
Vibrio spp., yellow colonies around 2 mm diameter were counted. Counting is performed in accordance with the ISO 16649 2001 standard. For
Salmonella spp., suspicion would result in a change in the Rappaport medium which becomes colorless whereas initially blue. After counting characteristic colonies of each germ, bacterial contamination was recorded and expressed as the number of colonies forming units per gram of sample (CFU g
-1) according to the following formula ISO 7218 [
27] : N=(∑Colonies)/V(n1+0,1n2)D.
ΣColonies: sum of the numbers of bacterial colonies in dishes considered;
N: CFU number per gram of initial product;
V: Volume (in mL) of the inoculated suspension;
n1 & n2: number of interpretable dishes chosen at the 1st and at the 2nd dilution considered;
D: dilution factor of the 1st dilution considered.
The interpretation of the microbiological results was carried out according to European Commission Regulation (EC) No. 2073/2005 based on two and three class plans for Salmonella spp. and Vibrio spp. on one side and for the E. coli respectively. The presence of Salmonella spp. and Vibrio spp. in fresh fruits and vegetables indicates fecal contamination which declares the product corrupted and therefore not suitable for human consumption. The following parameters were considered: m, M, Sat and Cor.
m: minimal number of CFU of bacteria per gram of sample tolerated in the product
M: maximal number of CFU/g tolerated in the product; M = 10m
Sat: degree of satisfaction with the established standards; Sat = 3m
Cor: degree of corruption of product; this means the number of CFU beyond which the product is declared not suitable for human consumption which is set as follow Cor = 1000m. Below this threshold, the product may be contaminated and does not meet the standards established by Codex Alimentarius but suitable for human consumption. In case of the product is declared unsatisfactory but fit for consumption because inferior to Cor level; this situation would indicate a margin of tolerance for consumption of contaminated product without health risk.
For each product, according to standards established by
Codex Alimentarius [
14], the different values of these parameters were summarized in
Table 1.
2.3. Nucleic Acids isolation and Sequencing
Nucleic acids were extracted using the Quick-DNA/RNA™ Miniprep Plus kit (Zymo Research Quick- DNA/RNA™ Miniprep Plus Catalog D7003; lot 207477; 50 preps) according to manufacturer's instructions. The quantity and integrity of the DNA was examined using Qubit 4 fluorometer (Thermo Scientific) and Qubit ™ 1X dsDNA High Sensitivity (HS) and Broad Range (BR) Assay Kits.
The Rapid Barcoding kit 96 (SQK-RBK110.96) was used for sequencing according to the manufacturer’s recommendations. An amount of 50 ng was used for library preparation. This step included sample barcoding, purification and washes with AMPure XP beads and ethanol 80% respectively, elution with 15µL of Elution Buffer (EB), and addition of Rapid Adapter (RAP-F) to barcoded fragments. The library was loaded into a R9.4.1 (FLO-MIN 106) flow cell according to the kit manufacturer's instructions.
2.4. Data analysis
Statistical analysis for microbiological results was performed using Microsoft excel program. For metagenomic analysis, sequences obtained after 3 hours of run were retrieved and analyzed using the EPI2ME Agent Desktop version 3.6.2 (
https://epi2me.nanoporetech.com) of Oxford Nanopore Technologies (ONT) for real-time sequence analysis. The What’s In My Pot for taxonomic classification workflow was used with microbial quantification from metagenomic samples according to sequences available in NCBI RefSeq database that is linked to EPI2ME [
28,
29]. This pipeline utilizes “centrifuge” kmer-based read identification through FastQ files outpout generated after basecalling. The abundance of species obtained after this analysis was used for the subsequent steps, which consisted in describing the microbial community of each sample using Microsoft Excel program. We labeled « OTHERS » all species that have relative a abundance less than 5%.
For intra sample diversity [
30], we used the following metrics: Observed OTUs (for different taxa observed in a sample at taxonomic level), Chao1 (for the estimation of diversity through abundances), Simpson (based on the probability that two species taken from the sample at random are different) and Shannon index (estimator for both species richness and evenness, but with weight on the richness) [
31]. Theses metrics were calculated with the vegan and phyloseq packages in Rstudio. Wilcox-Test were performed to find out the significance between observed differences. All plots were carried out with ggplot2 package in R.
For the inter samples diversity (beta), non-phylogenetic based metrics were used for the dissimilarity analysis: Bray-Curtis Dissimilarity (examines the abundances of microbes that are shared between two samples, and the number of microbes found in each; ranged from 0 to 1) and Jaccard distances (abundances are not taken into account; just the presence of microbes in one or both samples) [
32,
33]. These parameters were calculated in R with ecodist package. Dissimilarities were plotted with the Principal Coordinates Analysis (PCoA) approach using ggplot2’s R package
3. Results
In this study, we analyzed 240 fruit and vegetable samples collected at different supply sites (streets and open-air markets) in Dakar, Senegal. The samples included lettuce (n = 40), tomatoes (n = 40), mango slices (n = 40), onion slices (n = 40), mint leaves (n = 40), strawberries (n = 20) and grapes (n = 20) (Table 2).
3.1. Fruit and vegetables contamination by Escherichia coli and Vibrio spp
Microbiological analysis revealed the presence of
E. coli and
Vibrio spp. in most of the samples (
Table 3).
E. coli was isolated from all mint samples (100%) and from 97.5% (39/40) of the lettuce. In addition, most of the onion samples (85%, 34/40) were contaminated by
E. coli. Other samples including strawberries (40%, 8/20), mango slices (22.5%, 9/40) and tomatoes (2.5%, 1/40) were less contaminated by
E. coli (
Table 3). Contamination of
Vibrio spp was recorded in most of the mint leaves (92.5%, 37/40), lettuce (97.5%, 39/40) and onion samples (90%, 36/40).
Vibrio spp. was also significantly present in mango slices (47.5%, 19/40), tomatoes (27.5%, 11/40) and strawberries (15%, 3/20) (
Table 3). Interestingly, no sample was positive for
Salmonella. It is important to note that no positive culture was obtained from grape samples.
When analyzing product contamination according to the supply sites (
Figure 1), we found 90.91% (50/55) and 87.27% (48/55) of the samples from Keur Massar market were contaminated by
E. coli and
Vibrio spp respectively (
Table 4). Tilene and Castor markets showed a comparable level of contamination by
E. coli and
Vibrio spp. (
Table 4). Contrary to what was found in the Dakar downtown,
Vibrio spp. was more frequently found than
E. coli in samples from the Kermel market and Cheikh Anta Diop street (
Table 4).
The high level of contamination of samples from Kermel market was somehow unexpected since this market is located within downtown area and is mainly used by high-income households, contrary to the other supply sites that are located in densely populated suburbs with mostly low-income and deprived inhabitants. In this regard, it is interesting to note that none of the samples collected at Gueule-Tapée market contained any of the analyzed bacteria. These results suggest that the location of the supply sites has a little contribution to the contamination of the products.
3.2. Samples contamination and standard criteria for human consumption
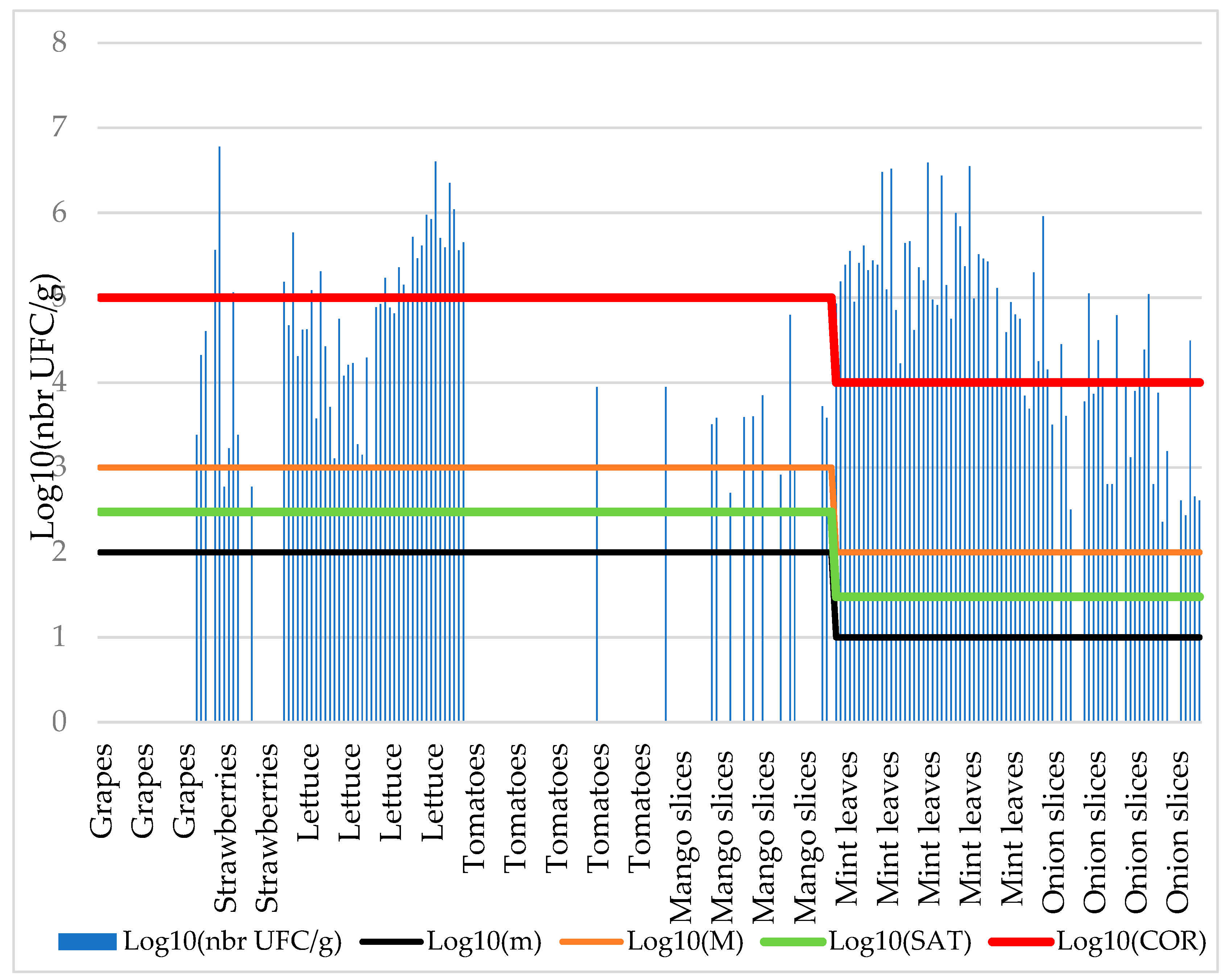
We determined the level of contamination by calculating the bacterial densities in positive fruit and vegetables samples. For each product, the proportions of samples contaminated by
E. coli were shown in
Figure 2. The most contaminated samples are lettuce and mint leaves.
As shown in
Table 5, contamination levels ranged from 1,300 to 6,000,000 CFU g
-1 for
E. coli and thus, most of the samples were significantly above the acceptable thresholds for human consumption.
With regard to the acceptable satisfaction threshold (Sat) and corruption threshold (Cor) criteria for E. coli, 50.83% (n=122) of the 240 samples contained the maximal number (M) of CFU g-1 tolerated and 57.08% (n=137) were above the satisfaction threshold (Sat). Of these, 53.28% (73/137) were declared corrupted for E. coli (contamination level higher than Cor); i.e. 30.42% (73/240) if considering all the samples.
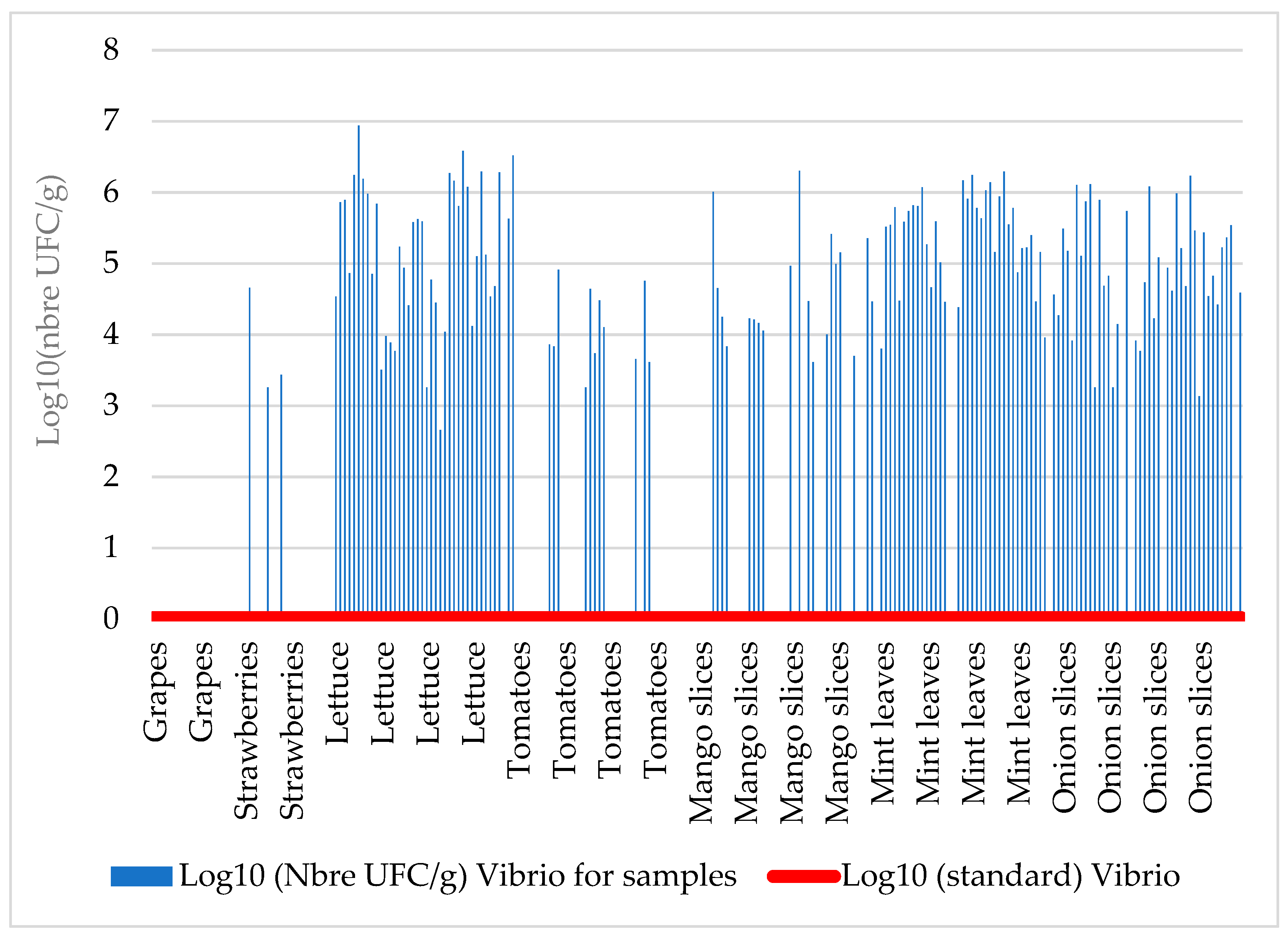
Vibrio spp. contamination reveals that 60.41% (n=145) of the samples were corrupted and the contamination level ranged from 455 to 8,730,000 CFU g
-1 (
Table 5). Thus, the bacterial loads in these samples were above the acceptable thresholds (i.e absence in the sample) (
Figure 3).
Table 5 shows details of density intervals for each targeted bacterium in each analyzed matrix. It also gives an idea of the levels of high contamination of these products in comparison with the maximum standard threshold (M).
Analysis of the standard for consumption according to the sample collection sites showed that E. coli and Vibrio spp. germs were predominant in samples collected at Keur Massar market (90.91% and 87.27% respectively; n=55). Kermel market was ranked in second place with proportions of corrupted samples of 54.28% for E. coli against 80% for Vibrio spp. (n=35) following by Tilène market (61.82% for both E. coli and Vibrio spp. n=55), Castor market (44% and 52% for E. coli and Vibrio spp. respectively; n=25), Cheikh Anta Diop street (22.5% and 47.5% for E. coli and Vibrio spp. respectively ; n=40) and Dakar Down-Town (40% and 15% for E. coli and Vibrio spp. respectively ; n=20) (table 4). In contrast, none sample collected to Gueule Tapée’s market were contaminated with targeted germs (n=10).
3.3. Metagenomic analysis
3.3.1. Bacterial communities
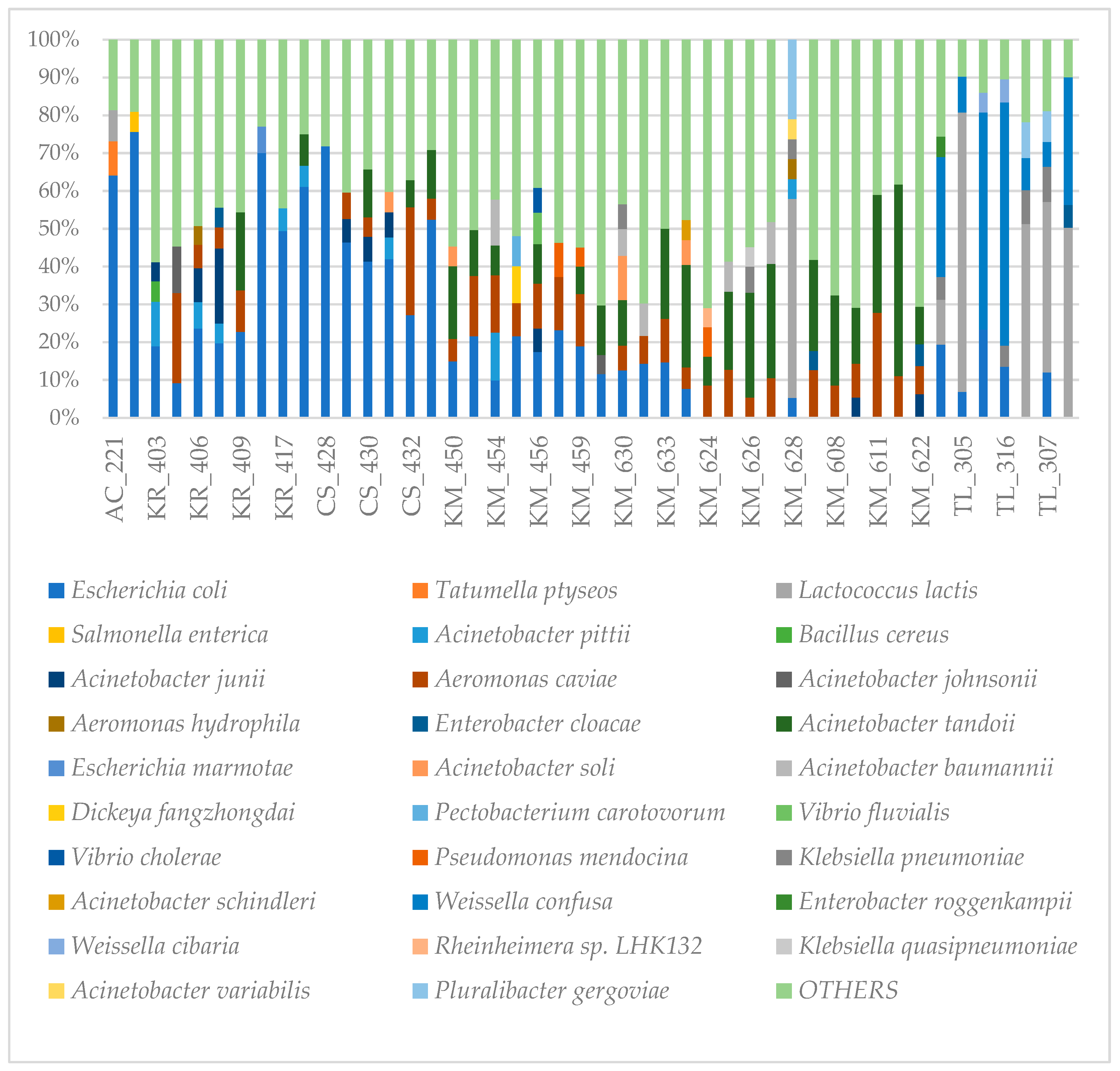
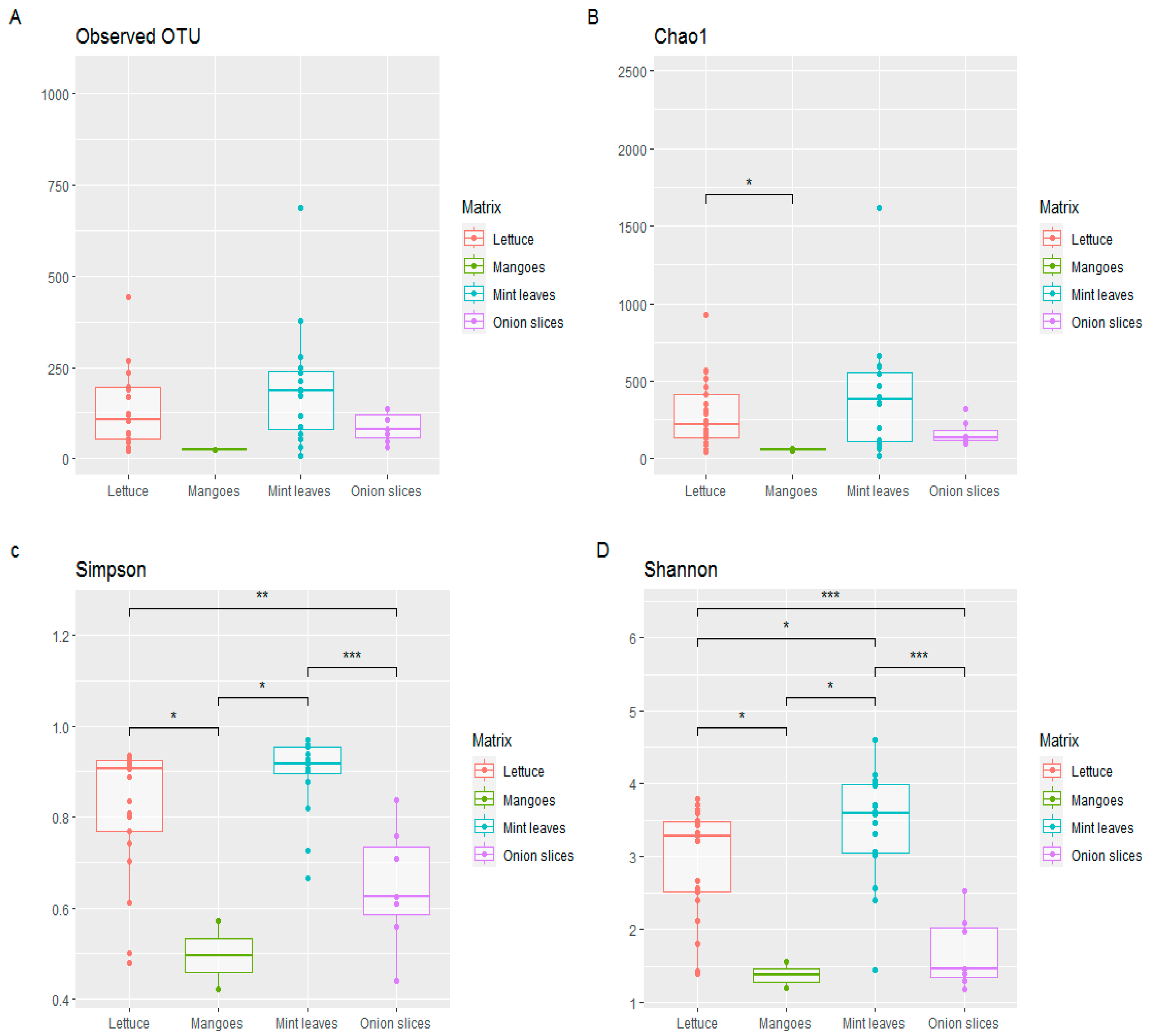
Given the the high contamination level of many products, we selected 46 samples for metagenomic analysis. These samples included mango slices (n=2), onion slices (n=7), lettuce (n=21) and mint leaves (n=16). The bacterial diversity for each sample using the relative abundance of each species were depicted in
Figure 4.
Details of metagenomic analysis showed a predominance of E. coli in 34 samples in which the relative abundance was greater than 5% (for all mangoes and lettuce samples; n=6 and n=5 for mint leaves and onion slices respectively) with the highest abundance noted in a mango slice sample (75.6%). Interestingly, pathogenic serotype E. coli O157:H7 were found in 2/46 samples (4.35%), albeit in low abundance. The microbial community in onion slice samples was dominated by Lactococcus lactis found in 71.43% (n=5) of the samples, with the highest abundance being 73.8%. As for the mint leaf samples, the bacterial community was diverse and dominated by species classified as « OTHERS ».
Salmonella spp. was found in 43/46 (93.47%) samples with a maximum of 79 reads obtained. For Salmonella enterica subsp. enterica, the following serovars were found: Senftenberg, Kentucky, Stanleyville, Enteritidis, Saintpaul, Thyphimurium, Cubana among others. Vibrio spp. was found in 31/46 (67.39%) samples. The highest abundance (560 reads) was found in lettuce sample.
3.3.2. Alpha diversity
Figure 5 shows a heterogeneous alpha diversity from one matrix to another, with the same profiles for all the diversity parameters studied (ranged from mint leaves, lettuce, onions to mango slices, respectively the highest to the lowest alpha diversity).
High diversity was noted in the mint leaves and lettuce samples, particularly for the Shannon and Simpson indexes. The Wilcox test carried out on the alpha diversity metrics showed significant differences from one matrix to another (p-value=0.05). For the other diversity metrics (Observed OTUs and Chao1 index) the difference was not significant, so diversity is almost the same for all matrices combined, with the exception of that observed with the Chao1 index between lettuce and mango slices, showing a significant difference between the microbial communities.
3.3.3. Beta diversity
Dissimilarity was noted between the samples. Metrics such as the Bray-Curtis dissimilarity and the Jaccard distance indicate the presence of three clusters according to the community observed between the matrices when visualized using Principal Coordinates Analysis (
Figure 6).
The samples from mango slices matrix were included in the cluster formed by the lettuce samples. Clearly, we can distinguish two different large clusters: the first grouping together the mango slice, mint leaves and lettuce matrices, and the second entirely comprising onion slice samples. This observation shows that the microbial communities of samples in the first cluster are closer compared to samples in the second cluster.
4. Discussion
This study aimed to assess
E. coli,
Salmonella spp. and
Vibrio spp. contamination level in fresh fruits and vegetables sold on stalls in streets and open-air markets of Dakar, the capital city of Senegal. Our findings show a heterogeneity in microbiological quality status of the analyzed samples. The proportions of corrupted samples varied depending on the bacterial species and the type of sample. The levels of contamination observed were above the critical threshold with more than 6.0 10
6 and 8.73 10
6 CFU g
-1 for
E. coli and
Vibrio spp. respectively. A previous study related to microbiological quality of mango slices sold in Dakar (Senegal) also reports high level of
E. coli contamination [
12]. Similar results have been reported by other authors, particularly in the fourth-line products [
17], fruits and vegetables [
12]. In this study, we did not detect
Salmonella spp. in any of the samples. Similar findings was reported in ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market [
34].
The high prevalence of
E. coli and
Vibrio spp. could indicate fecal contamination of the products. The most unsatisfactory microbiological quality samples were mint leaves and lettuce. This could be caused by the use of untreated wastewater to grow these products [
14,
35,
36,
37,
38,
39,
40,
41,
42,
43]. Additionally, some farmers directly use raw fecal matter as organic fertilizers for horticultural production [
1,
14,
44], which could be a potential source of contamination of fruits and vegetables. Indeed, several studies reported that the type of watering plays an important role in the level of contamination when the used water directly contact the edible part of the plant instead of being poured at the he roots[
1,
14,
42].
All of the sources of contamination mentioned above are associated with the primary production phases. Moreover, although less contaminated than mint leaves and lettuce, other products such as onion slices and mangoes slices could also be corrupted by fecal contamination associated with poor hand hygiene during post-harvest handling. In particular, this concerns equipment for common use, such as multipurpose knives, rinsing and humidification water, contact surfaces of worktops where products are placed during the minimal processing for sale [
3,
14,
17,
19]. In addition, the high contamination of product may be due to the environmental hygiene of the open-air markets and streets of Dakar. These include the high attendance of visitors and traffic density [
12].
The relatively low prevalence of E. coli and Vibrio spp. in tomatoes can be explained by the difference in texture between tomato and the other products investigated. The smooth skin of tomatoes, compared to the folds of lettuce and mint leaves, could limit bacterial adhesion and proliferation. The absence of contamination observed in the grape samples can be explained by the smooth texture of the fruit and by the fact that the fruit is not in contact with the soil during cultivation and harvesting. Most of the time, it is imported and is delivered in primary packaging, which limits the potential handled contaminants.
Our metagenomic analysis further depicts the microbial community colonizing the analyzed fruits and vegetables. The high bacterial diversity noted in the different matrices analyzed could underline the impact of agricultural inputs, farming practices and handling of products sold in the streets and markets. Members of
Enterobacteriaceae were widely present and included a wide range of important enteric foodborne pathogens, which represent a strong threat to public health and food safety
[45,46,47]. Moreover, the application of poultry manure and other incomplete compost to the crops can also result in contamination with enteric bacteria in feces [
21].
The predominance of certain enteric species in the metagenomic analysis, in this case
E. coli (in 100% of the samples sequenced), suggests a contamination of fecal origin and confirms the results obtained in bacterial culture [
48]. The high prevalence of
Salmonella spp. in the metagenomic analysis (93.47%), besides supporting fecal contamination, poses limits concerning the microbiological approach in which no sample was positive to
Salmonella spp. This confirms the sensitivity of sequencing, which have the capacity to detect bacteria present in quantity below the threshold for positive culture [
49]. Beata Kowalska et
al. in their meta-analysis review found that the average prevalence of
Salmonella spp. in lettuce was 4.1%, with values ranging from 0.1% for Japan to 50% for Burkina Faso
[50], figures that are below the results obtained in this study. These prevalences are lowest comparing to those found in this study.
Regarding the sources of contamination, data from the literature show that some pathogens including
Escherichia coli O157: H7,
Listeria monocytogenes, and
Salmonella spp. are commonly isolated from animal feces including poultry and cattle, which are mostly used as fertilizers for horticultural products [
51,
52,
53]. It was shown a few years ago that
E. coli O157: H7 can be transmitted to lettuce through the soil and irrigation water, and can persist throughout the life cycle of the plant and be transmitted to consumers [
51,
52,
53]. The plant morphology (for example mint leaves) may influence the contamination rate due to close contact with the soil.
The high prevalence of
E. coli,
Vibrio spp. and
Salmonella spp. observed in this study indicate a significant health risk associated with the consumption of these products analyzed. Given that most of these products are eaten raw, the possibility of foodborne epidemics is a reality, especially for children and immunocompromised people, who are the most vulnerable population group [
55,
56,
57].
5. Conclusion
The main objective of this study was to assess the level of contamination by E. coli, Salmonella spp. and Vibrio spp. of lettuce, tomatoes, mango and onion slices, mint leaves, strawberries and grapes sold on stalls in the streets and open-air markets of Dakar. Our findings show a high prevalence of the three bacterial species suggesting a fecal contamination that might originate from the use of contaminated water for growing these products or from poor hand hygiene during post-harvest handling.
This study reports a very high rate of contamination of fruit and vegetables by the targeted germs, as well as the first use of the NGS approach in Senegal to describe the bacterial community in these products. Our results stress the need to raise awareness among the actors involved in the value chain of horticultural products, for good agricultural and hygiene practices in order to better integrate health safety and hygiene into their practice. Regarding the diversity of potential origins of contamination, these results represent a call to extend the investigation we conducted to a national scale while increasing the number of sites and of products analyzed.
Author Contributions
Conceptualization and design, A.A.M.D.; methodology, A.A.M.D. and M.M.; sampling, S.S., S.D.T.; lab analysis, S.S., S.D.T. and S.C.; resources, A.A.M.D.; data curation and analysis, S.S., S.D.T.; writing—original draft preparation, S.S., S.D.T., A.A.M.D.; writing—review and editing, S.S., S.D.T., A.A.M.D., Y.D.; supervision, A.A.M.D., M.M., funding acquisition, A.A.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This project was carried out with the financial assistance of the International Development Research Center (IDRC) and the Ministry of Higher Education, Research and Innovation (MESRI) through the FIRST, as part of the SGCI2 financing program.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- O.O. Alegbeleye, I. Singleton, et A. S. Sant’Ana, « Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review », Food Microbiol, vol. 73, p. 177-208, août 2018. [CrossRef]
- J. Li et al., « Identification of human pathogenic Enterocytozoon bieneusi, Cyclospora cayetanensis, and Cryptosporidium parvum on the surfaces of vegetables and fruits in Henan, China », International Journal of Food Microbiology, vol. 307, p. 108292, oct. 2019. [CrossRef]
- B. Mercanoglu Taban et A. K. Halkman, « Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? », Anaerobe, vol. 17, no 6, p. 286-287, déc. 2011. [CrossRef]
- W. K. Bosu, « An overview of the nutrition transition in West Africa: implications for non-communicable diseases », Proc Nutr Soc, vol. 74, no 4, p. 466-477, nov. 2015. [CrossRef]
- M. S. Nnyepi, N. Gwisai, M. Lekgoa, et T. Seru, « Evidence of nutrition transition in Southern Africa », Proc Nutr Soc, vol. 74, no 4, p. 478-486, nov. 2015. [CrossRef]
- J. S. León, L.-A. Jaykus, et C. L. Moe, « Food Safety Issues and the Microbiology of Fruits and Vegetables », in Microbiologically Safe Foods, John Wiley & Sons, Ltd, 2009, p. 255-290. doi: 10.1002/9780470439074.ch12.
- A. N. Olaimat et R. A. Holley, « Factors influencing the microbial safety of fresh produce: a review », Food Microbiol, vol. 32, no 1, p. 1-19, oct. 2012. [CrossRef]
- M. Uyttendaele et al., « Microbial Hazards in Irrigation Water: Standards, Norms, and Testing to Manage Use of Water in Fresh Produce Primary Production », Comprehensive Reviews in Food Science and Food Safety, vol. 14, no 4, p. 336-356, 2015. [CrossRef]
- K. Abrahale, S. Sousa, G. Albuquerque, P. Padrão, et N. Lunet, « Street food research worldwide: a scoping review », Journal of Human Nutrition and Dietetics, vol. 32, no 2, p. 152-174, 2019. [CrossRef]
- E. Baraka, M. S. Willis, et B. A. Ishimwe, « What Kigali’s open-air markets reveal about achieving food and nutrition security: the role of African indigenous crops », Agric Food Secur, vol. 11, no 1, p. 17, 2022. [CrossRef]
- B. Desye, A. H. Tesfaye, C. Daba, et G. Berihun, « Food safety knowledge, attitude, and practice of street food vendors and associated factors in low-and middle-income countries: A Systematic review and Meta-analysis », PLOS ONE, vol. 18, no 7, p. e0287996, juill. 2023. [CrossRef]
- M. Kasse, M. Cisse, A. Toure, M. Ducamp-Collin, et A. Guisse, « Qualité microbiologique des tranches de mangues (Mangifera indica L.) vendues à Dakar (Sénégal) », Int. J. Bio. Chem. Sci, vol. 8, no 4, p. 1611, janv. 2015. [CrossRef]
- J. de S. Mata et al., « Technological tools for assessing children’s food intake: a scoping review », J Nutr Sci, vol. 12, p. e43, 2023. [CrossRef]
- Codex alimentarius, FAO, et WHO, « Normes alimentaires internationales ». Accessed on November 16 2023. [Online]. Available sur: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/fr/.
- M. E. M. Nikiema et al., « Characterization of virulence factors of Salmonella isolated from human stools and street food in urban areas of Burkina Faso », BMC Microbiol, vol. 21, no 1, p. 338, déc. 2021. [CrossRef]
- M. Reynes, « La valorisation des fruits tropicaux : conservation et transformation des fruits à petite échelle », Fruits et légumes tropicaux. Consulté le: 16 novembre 2023. [En ligne]. Disponible sur: https://agritrop.cirad.fr/548479/.
- L. A. Anin, P. D. Y. A. Yapi, Y. T. Monnet, M.-A. Y. Yapi, C. L. Soro, et K. A. K. Kouadio, « Evaluation Microbiologique Et Origines De La Contamination Des Produits De 4ème Gamme Vendus Sur Les Marchés D’abidjan, Cote D’ivoire », European Scientific Journal, ESJ, vol. 12, no 36, Art. no 36, déc. 2016. [CrossRef]
- E. Badosa, N. Chico, M. Pla, D. Parés, et E. Montesinos, « Evaluation of ISO enrichment real-time PCR methods with internal amplification control for detection of Listeria monocytogenes and Salmonella enterica in fresh fruit and vegetables », Letters in Applied Microbiology, vol. 49, no 1, p. 105-111, juill. 2009. [CrossRef]
- L. R. Beuchat et J. H. Ryu, « Produce handling and processing practices », Emerg Infect Dis, vol. 3, no 4, p. 459-465, 1997. [CrossRef]
- Safe Food for a healthy life, « Foodborne Pathogens Associated with Fresh Fruits and Vegetables ». Accessed on November 16 2023. [Online] Available on: http://pew.org/2yJDqTs.
- G. I. Balali, D. D. Yar, V. G. Afua Dela, et P. Adjei-Kusi, « Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World », Int J Microbiol, vol. 2020, p. 3029295, mai 2020. [CrossRef]
- S. Jedrzejewski, T. K. Graczyk, A. Slodkowicz-Kowalska, L. Tamang, et A. C. Majewska, « Quantitative Assessment of Contamination of Fresh Food Produce of Various Retail Types by Human-Virulent Microsporidian Spores », Appl Environ Microbiol, vol. 73, no 12, p. 4071-4073, juin 2007. [CrossRef]
- T. C. Mahopo, C. N. Nesamvuni, A. E. Nesamvuni, M. de Bryun, J. van Niekerk, et R. Ambikapathi, « Operational Characteristics of Women Street Food Vendors in Rural South Africa », Frontiers in Public Health, vol. 10, 2022, Accessed on November 16 2023. [Online]. Available on: https://www.frontiersin.org/articles/10.3389/fpubh.2022.849059.
- M. E. M. Nikiema et al., « Contamination of street food with multidrug-resistant Salmonella, in Ouagadougou, Burkina Faso », PLoS One, vol. 16, no 6, p. e0253312, 2021. [CrossRef]
- N. P. Steyn, D. Labadarios, et J. H. Nel, « Factors which influence the consumption of street foods and fast foods in South Africa-a national survey », Nutrition Journal, vol. 10, no 1, p. 104, oct. 2011. [CrossRef]
- M. Ait Hou, C. Grazia, et G. Malorgio, « Food safety standards and international supply chain organization: A case study of the Moroccan fruit and vegetable exports », Food Control, vol. 55, p. 190-199, sept. 2015. [CrossRef]
- ISO 7218:2007, « Microbiologie des aliments: Exigences générales et recommandations », ISO. Accessed on: November 16 2023. [Online]. Available on: https://www.iso.org/fr/standard/36534.html.
- J. Sakai et al., « An identification protocol for ESBL-producing Gram-negative bacteria bloodstream infections using a MinION nanopore sequencer », Journal of Medical Microbiology, vol. 68, no 8, p. 1219-1226, août 2019. [CrossRef]
- H. S. Tunsjø, I. F. Ullmann, et C. Charnock, « A preliminary study of the use of MinION sequencing to specifically detect Shiga toxin-producing Escherichia coli in culture swipes containing multiple serovars of this species », Sci Rep, vol. 13, no 1, Art. no 1, mai 2023. [CrossRef]
- Thukral, « A review on measurement of Alpha diversity in biology », Agricultural Research Journal, vol. 54, p. 1, janv. 2017. [CrossRef]
- Denise Lynch, « Visualizing microbiome data with Custom Plots », One Codex. Accessed on: November 16 2023. [Online]. Available on: http://docs.onecodex.com/en/articles/4633959-visualizing-microbiome-data-with-custom-plots.
- Carlo Ricotta, « Of beta diversity, variance, evenness, and dissimilarity », Ecology and Evolution - Wiley Online Library. Accessed on: November 16 2023. [Online]. Available on. [CrossRef]
- D. Lynch, « Beta Diversity | One Codex Docs », One Codex. Accessed on: November 16 2023. [Online]. Available on: http://docs.onecodex.com/en/articles/4150649-beta-diversity.
- H. Zhang, E. Yamamoto, J. Murphy, et A. Locas, « Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market », Int J Food Microbiol, vol. 335, p. 108855, déc. 2020. [CrossRef]
- A. Allende et J. Monaghan, « Irrigation Water Quality for Leafy Crops: A Perspective of Risks and Potential Solutions », Int J Environ Res Public Health, vol. 12, no 7, p. 7457-7477, juill. 2015. [CrossRef]
- L. Benjamin et al., « Occurrence of generic Escherichia coli, E. coli O157 and Salmonella spp. in water and sediment from leafy green produce farms and streams on the Central California coast », International Journal of Food Microbiology, vol. 165, no 1, p. 65-76, juill. 2013. [CrossRef]
- I. Castro-Ibáñez, M. I. Gil, J. A. Tudela, et A. Allende, « Microbial safety considerations of flooding in primary production of leafy greens: A case study », Food Research International, vol. 68, p. 62-69, févr. 2015. [CrossRef]
- X. Fernandez-Cassi, N. Timoneda, E. Gonzales-Gustavson, J. F. Abril, S. Bofill-Mas, et R. Girones, « A metagenomic assessment of viral contamination on fresh parsley plants irrigated with fecally tainted river water », Int J Food Microbiol, vol. 257, p. 80-90, sept. 2017. [CrossRef]
- X. Fernandez-Cassi et al., « Metagenomics for the study of viruses in urban sewage as a tool for public health surveillance », Sci Total Environ, vol. 618, p. 870-880, mars 2018. [CrossRef]
- G. Kisluk et S. Yaron, « Presence and Persistence of Salmonella enterica Serotype Typhimurium in the Phyllosphere and Rhizosphere of Spray-Irrigated Parsley », Appl Environ Microbiol, vol. 78, no 11, p. 4030-4036, juin 2012. [CrossRef]
- M. E. Qureshi, R. Q. Grafton, M. Kirby, et M. A. Hanjra, « Understanding irrigation water use efficiency at different scales for better policy reform: a case study of the Murray-Darling Basin, Australia », Water Policy, vol. 13, no 1, p. 1-17, févr. 2011. [CrossRef]
- M. Steele, A. Mahdi, et J. Odumeru, « Microbial assessment of irrigation water used for production of fruit and vegetables in Ontario, Canada », J Food Prot, vol. 68, no 7, p. 1388-1392, juill. 2005. [CrossRef]
- K. Warriner, A. Huber, A. Namvar, W. Fan, et K. Dunfield, « Recent advances in the microbial safety of fresh fruits and vegetables », Adv Food Nutr Res, vol. 57, p. 155-208, 2009. [CrossRef]
- FAO, Éd., Paying farmers for environmental services. in The state of food and agriculture, no. 2007. Rome: FAO, 2007.
- M. J. Jang, S. Y. Kim, S. C. Ricke, M. S. Rhee, et S. A. Kim, « Microbial ecology of alfalfa, radish, and rapeseed sprouts based on culture methods and 16S rRNA microbiome sequencing », Food Research International, vol. 144, p. 110316, juin 2021. [CrossRef]
- K. G. Mladenović et al., « Enterobacteriaceae in food safety with an emphasis on raw milk and meat », Appl Microbiol Biotechnol, vol. 105, no 23, p. 8615-8627, déc. 2021. [CrossRef]
- M. Qiu et al., « Dynamic Changes of Bacterial Communities and Microbial Association Networks in Ready-to-Eat Chicken Meat during Storage », Foods, vol. 11, no 22, p. 3733, nov. 2022. [CrossRef]
- A. C. Sánchez-Alfonso et al., « Microbial indicators and molecular markers used to differentiate the source of faecal pollution in the Bogotá River (Colombia) », Int J Hyg Environ Health, vol. 225, p. 113450, avr. 2020. [CrossRef]
- G. M. Ibrahim et P. M. Morin, « Salmonella Serotyping Using Whole Genome Sequencing », Front Microbiol, vol. 9, p. 2993, 2018. [CrossRef]
- B. Kowalska, « Fresh vegetables and fruit as a source of Salmonella bacteria », Ann Agric Environ Med., vol. 30, no 1, p. 9-14, mars 2023. [CrossRef]
- J. W. Buck et R. Walcott, « Recent trends in microbiological safety of fruits and vegetables », Online. Plant Health Progress, vol. 121, janv. 2003.
- M. Mostafidi, M. R. Sanjabi, F. Shirkhan, et M. T. Zahedi, « A review of recent trends in the development of the microbial safety of fruits and vegetables », Trends in Food Science & Technology, vol. 103, p. 321-332, sept. 2020. [CrossRef]
- K. Söderqvist, « Is your lunch salad safe to eat? Occurrence of bacterial pathogens and potential for pathogen growth in pre-packed ready-to-eat mixed-ingredient salads », Infect Ecol Epidemiol, vol. 7, no 1, p. 1407216, déc. 2017. [CrossRef]
- K. Bhunia, « Foodborne Microbial Pathogens », 2018. [CrossRef]
- T. Bintsis, « Foodborne pathogens », AIMS Microbiol, vol. 3, no 3, p. 529-563, juin 2017. [CrossRef]
- Scientific Committee on Food, « Risk Profile on the Microbiological Contamination of Fruits and Vegetables Eaten Raw », avr. 2002, [Online]. Available on: https://food.ec.europa.eu/system/files/2020-12/sci-com_scf_out125_en.pdf.
- E. Todd, « Food-Borne Disease Prevention and Risk Assessment », Int J Environ Res Public Health, vol. 17, no 14, p. 5129, juill. 2020. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).