3.1. Phase structure and microstructure of alloy specimens
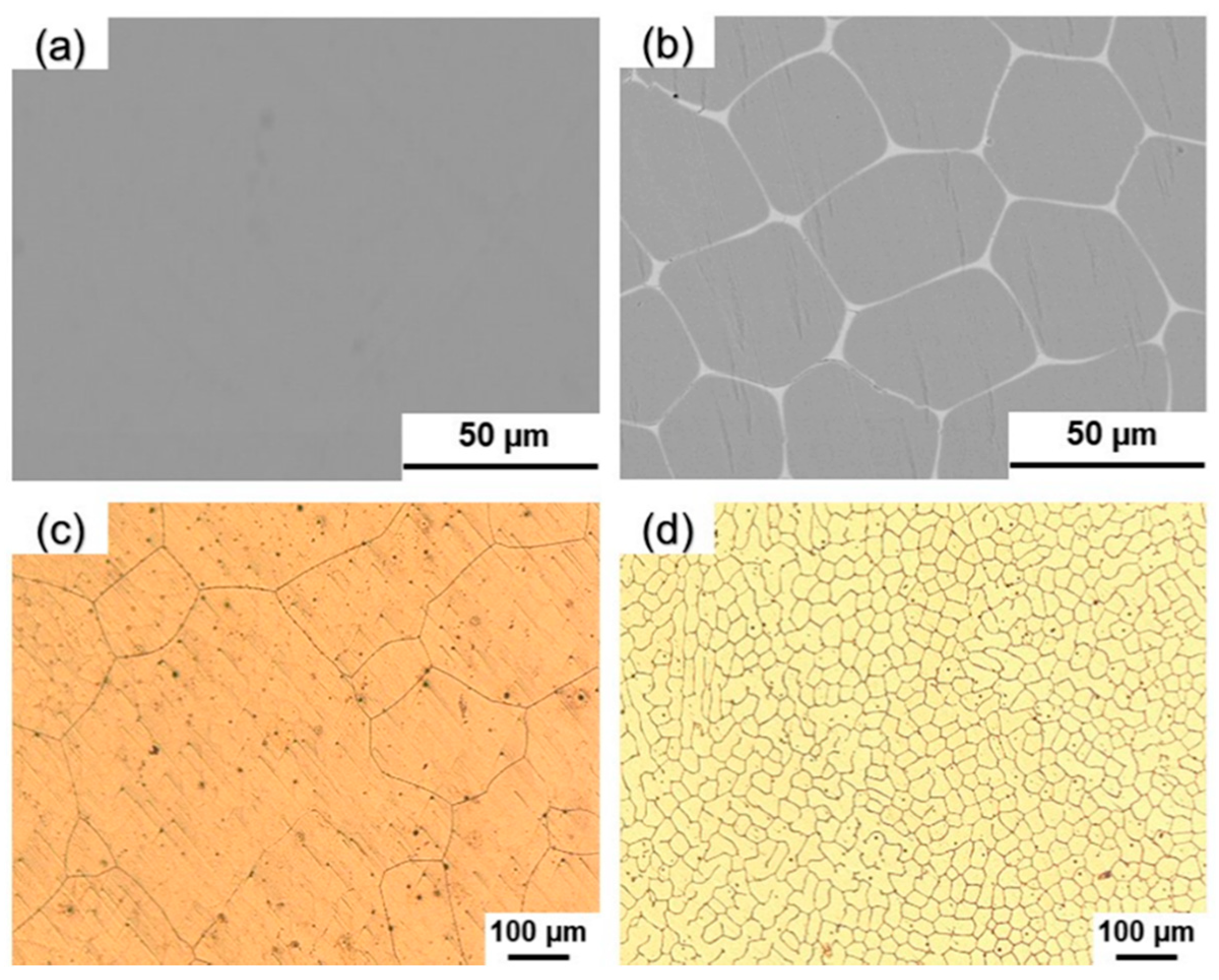
Figure 1 shows the SEM map and optical microscope (OM) metallographic microstructure of the alloy specimens. From
Figure 1a, it can be seen that the surface of the 0Y alloy is smooth and there is no intermetallic phase generation; from
Figure 1b, it can be seen that the 1Y alloy grain size is uniform, and the difference between grey and bright white color is presented within the grains and at the grain boundaries, respectively, and the polarization of bright white intermetallic phase can be observed at the grain boundaries; from
Figure 1c, it can be seen that the morphology of the 0Y alloy specimen is similar to a cellular structure, with obvious grain boundaries, large grain size, between 100 μm -200 μm, and uneven distribution; from
Figure 1d, it can be seen that, compared with the 0Y alloy, the grain size of 1Y alloy has been significantly refined, with fine and uniformly distributed grains, the grain size is 40 μm -50 μm, and the polarization of the intermetallic phase can be clearly observed at the grain boundaries.
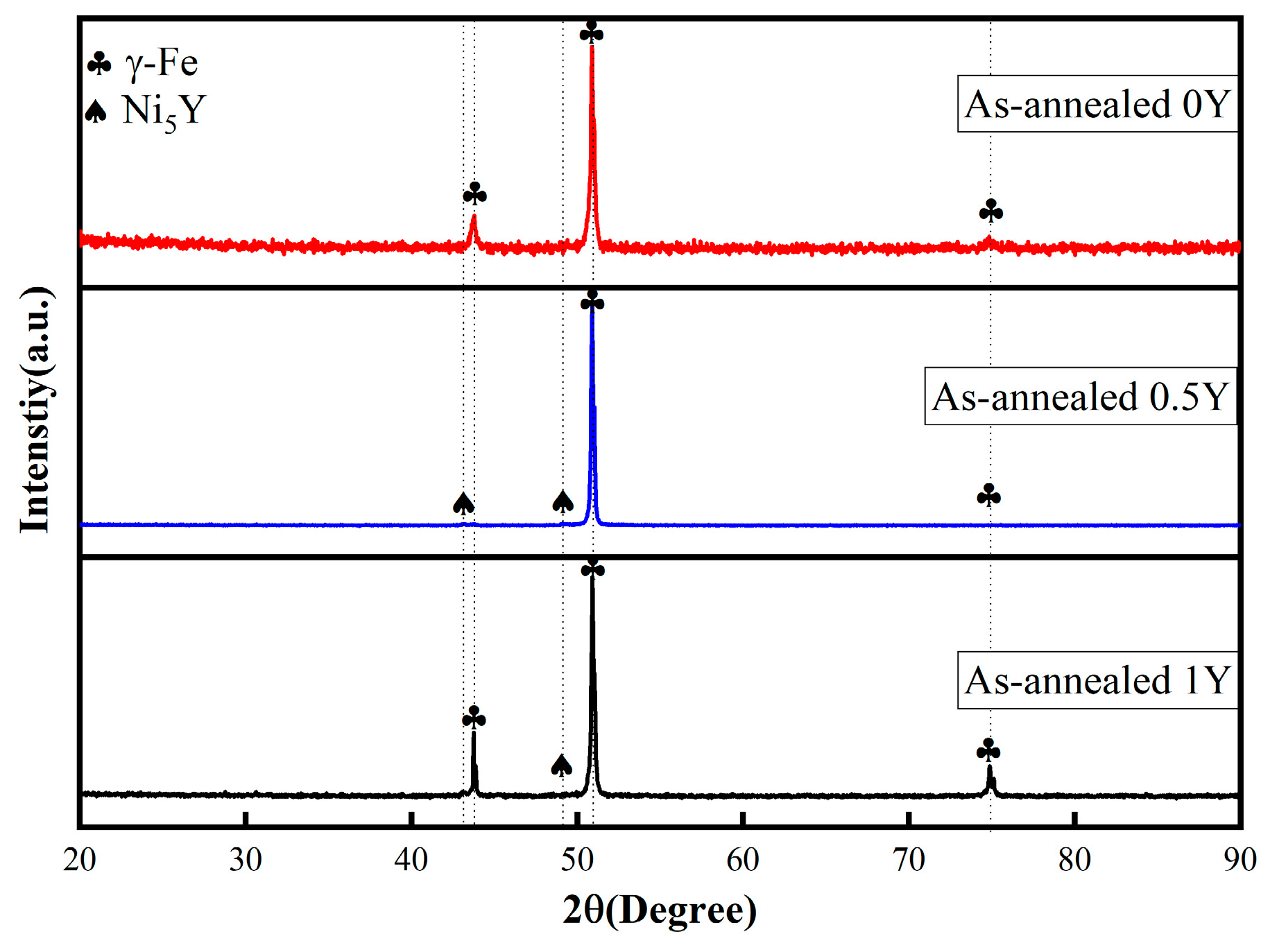
Figure 2 shows the XRD patterns of the alloy specimens, from which it can be seen that the 0Y alloy is a stable austenitic structure of γ-Fe phase, in addition to the characteristic peaks of the γ-Fe phase in both the 0.5 Y alloy and the 1 Y alloy, the characteristic peaks of Ni5Y intermetallic compounds have appeared, and due to the intermetallic phase precipitation at grain boundaries, and the content of small, the characteristic peaks of the intensity of the lower, combined with the results of
Figure 1, it can be concluded that the Y element is easy to precipitate Ni
5Y phase when added to the alloy due to its high activity and small solid solubility in austenite.
With the increase of Y content, the Ni
5Y phase precipitated in the alloy also increases, and the addition of Y element makes the grain size of the alloy undergo the phenomenon of refinement, which is attributed to the effect of the addition of rare earth elements on the thermodynamic and kinetic processes of alloy solidification. Rare earths, as a kind of surface-active elements, can reduce the interfacial tension during grain nucleation, thus reducing the critical size nucleation work and increasing the nucleation rate. And the formation of a large number of Ni
5Y phase polarization in the alloy at the alloy grain boundaries hindered the growth of the alloy, thus playing the role of grain refinement [
17].
3.2. Oxidation kinetics
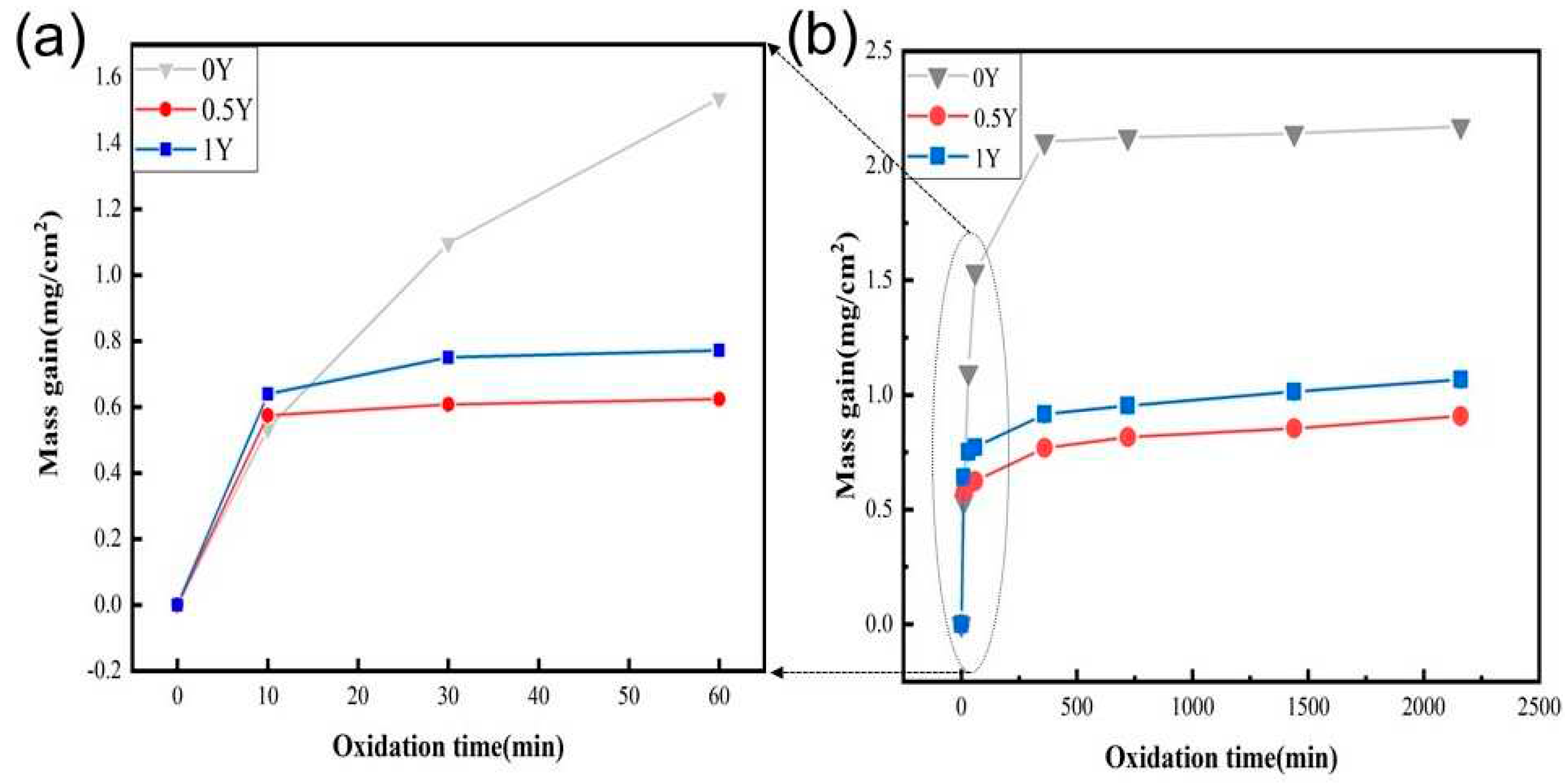
Figure 3 shows the high-temperature oxidation kinetic curves of alloy specimens oxidized in air at 1000°C for 2160 min, from which it can be seen that the mass of three alloys, 0Y, 0.5Y, and 1Y, which have different Y contents, all increase with oxidation time during high-temperature oxidation, and compared with the 0Y alloys, the 0.5Y and 1Y alloys have smaller mass increases, while the 0Y alloys have the largest mass increases. The above results show that the three alloys have the largest mass increase. The above results show that the three alloys have the same oxidation behavior, the initial oxidation stage for 0Y alloy is 0-60 min, the second stage of oxidation is 10-360 min, and the third stage of oxidation is after 360 min, while for 0.5Y and 1Y alloys, the initial stage of oxidation is 0-10 min, the second stage of oxidation is 10-360 min, and the third stage of oxidation is after 360 min. 360 min later. In the initial stage of oxidation, the mass increase are linear growth with the extension of oxidation time, this stage is mainly the formation of oxide film, the oxidation rate is faster, the oxidation is mainly controlled by the chemical reaction; in the second stage of oxidation, the mass increase is slow, mainly the formation of the oxide film is transformed into the growth of the oxide film, the alloy surface forms a layer of thinner Cr
2O
3 oxide film, the oxygen is diffused to the interface through the oxide layer, while the element in the alloy matrix diffusion, at the same time the elements within the alloy matrix to the reaction interface diffusion, the material oxidation rate by the chemical reaction and diffusion rate jointly controlled by the oxidation of the third stage, mainly by the matrix elements outward diffusion and oxygen atoms to the inward diffusion of the control. When the oxidation time reaches 2160 min, the best high-temperature oxidation resistance is 0.5Y alloy, whose oxidized weight gain is about 0.91 (mg/cm
2), the worst high-temperature oxidation resistance is 0Y alloy, whose oxidized weight gain is about 2.17 (mg/cm
2), and 1Y alloy's high-temperature oxidation resistance is a little bit poorer than 0.5Y alloy, but they are all better than that of 0Y alloy.
In other words, all the Y-added alloys have better high temperature oxidation resistance than the non-Y-added alloys. In addition, the reaction time of the initial oxidation stage of the Y-added alloys is greatly reduced compared with that of the Y-free alloys, which suggests that the addition of trace amounts of rare earth Y to the alloys can promote the rapid formation of dense oxide film on the surface of the alloys and improve the high-temperature oxidation resistance of the alloys. According to the reports in the literature [
18,
19], it is speculated that there are several reasons, (1) rare earth Y elements doped into the alloy in the form of precipitation phase in the alloy, due to the presence of Ni
5Y phase, so that the alloy grain in the process of growing up to inhibit the growth of the alloy, the alloy grain size refinement, the alloy grain boundaries increased, and in the process of oxidation diffusion in alloys is generally dominant is the grain boundaries of the diffusion. Alloy grain boundaries increase, Cr element diffusion channels increase, making the alloy to form a dense protective oxide film Cr
2O
3 film time is greatly reduced, so the alloy in the oxidation of the early stage of rapid oxidation, and in the oxidation of the late oxidation rate tends to flatten. (2) Rare earth Y element in the high temperature oxidation process with the oxidation process, rare earth Y element activity is high, easy to oxidize. According to the dynamic polarization theory, Y ions from the matrix polarization in the oxide grain boundaries, due to the atomic radius of the rare earth ions is larger, polarization in the oxide grain boundaries can prevent the other cations in the alloy to the outside of the spread and the oxygen negative ions of the diffusion of the inward, so that the alloy of the oxidation layer thickness is reduced, reduce the oxidation rate of the alloy.
3.3. Surface morphology and tissue composition analysis of oxidation products
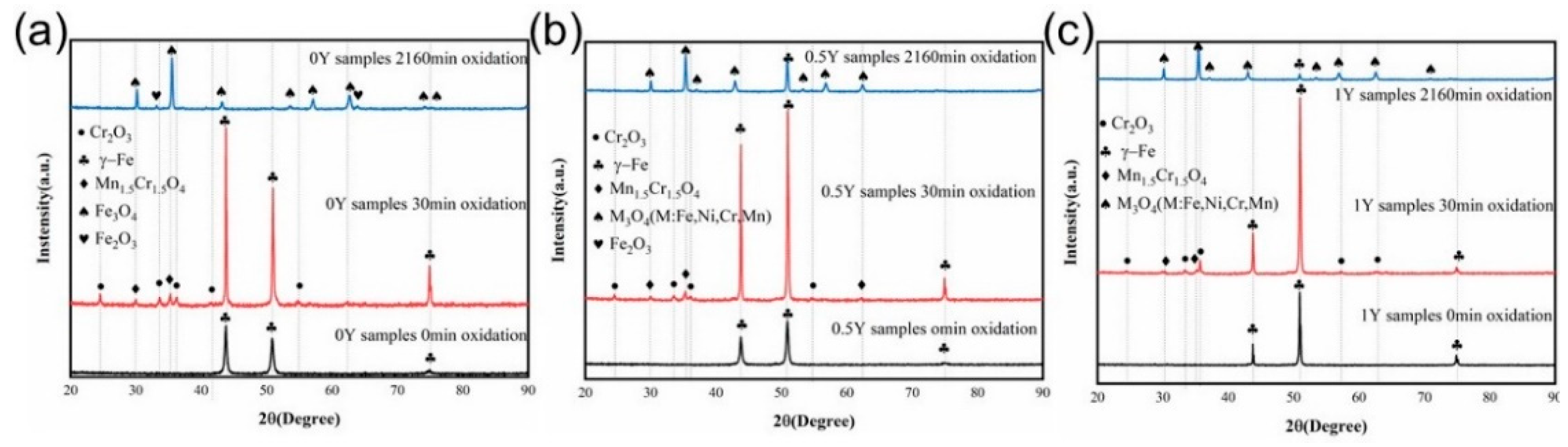
Figure 4 shows the XRD spectra of the surface oxide film of 47Fe-36Ni-15Cr-1.5Mn-xY alloy specimens after high-temperature oxidation, and it can be seen that, after oxidizing the 47Fe-36Ni-15Cr-1.5Mn-xY (0, 0.5 wt.%, and 1 wt.%) alloys at 1000 ℃ for 30 min and 2160 min, the three alloys after oxidation showed similar XRD data spectra, and compared with the specimens before oxidation, the characteristic peaks of γ-Fe were enhanced after 30 min of oxidation, while the characteristic peaks of γ-Fe were weakened or even disappeared after 2160 min of oxidation. Compared with the samples before oxidation, the characteristic peak of γ-Fe is enhanced after 30 min of oxidation, and the characteristic peak of γ-Fe is weakened or even disappeared after 2160 min of oxidation, which indicates that in the initial stage of high-temperature oxidation, the oxide film has not yet been completely formed, and the high temperature is conducive to the birth of γ-Fe austenite phase grains, making the increase in the degree of crystallinity, and thus the characteristic peak of the γ-Fe austenite phase becomes stronger, and the thicker and more stabilized grains are grown on the surface of the alloys with the prolongation of oxidation time. With the extension of oxidation time, a thick and stable oxide film grows on the surface of the alloy, which makes the γ-Fe characteristic peak weaker. From
Figure 4a, it can be seen that the oxidation products of 0Y alloy are mainly composed of Cr
2O
3 and Mn
1.5Cr
1.5O
4 after 30 min of oxidation. With the increase of oxidation time, the alloy oxides are mainly composed of Fe
3O
4 and Fe
2O
3 type oxides. In
Figure 4b,c, it can be seen that in 0.5Y and 1Y alloys after 30 min of oxidation, the oxidation products are mainly Cr
2O
3 and Mn
1.5Cr
1.5O
4, while after 2160 min of oxidation, the oxidation products are mainly composed of M (Fe, Ni, Cr, Mn)
3O
4, which the addition of the surface Y does not have much effect on the type of oxides generated.
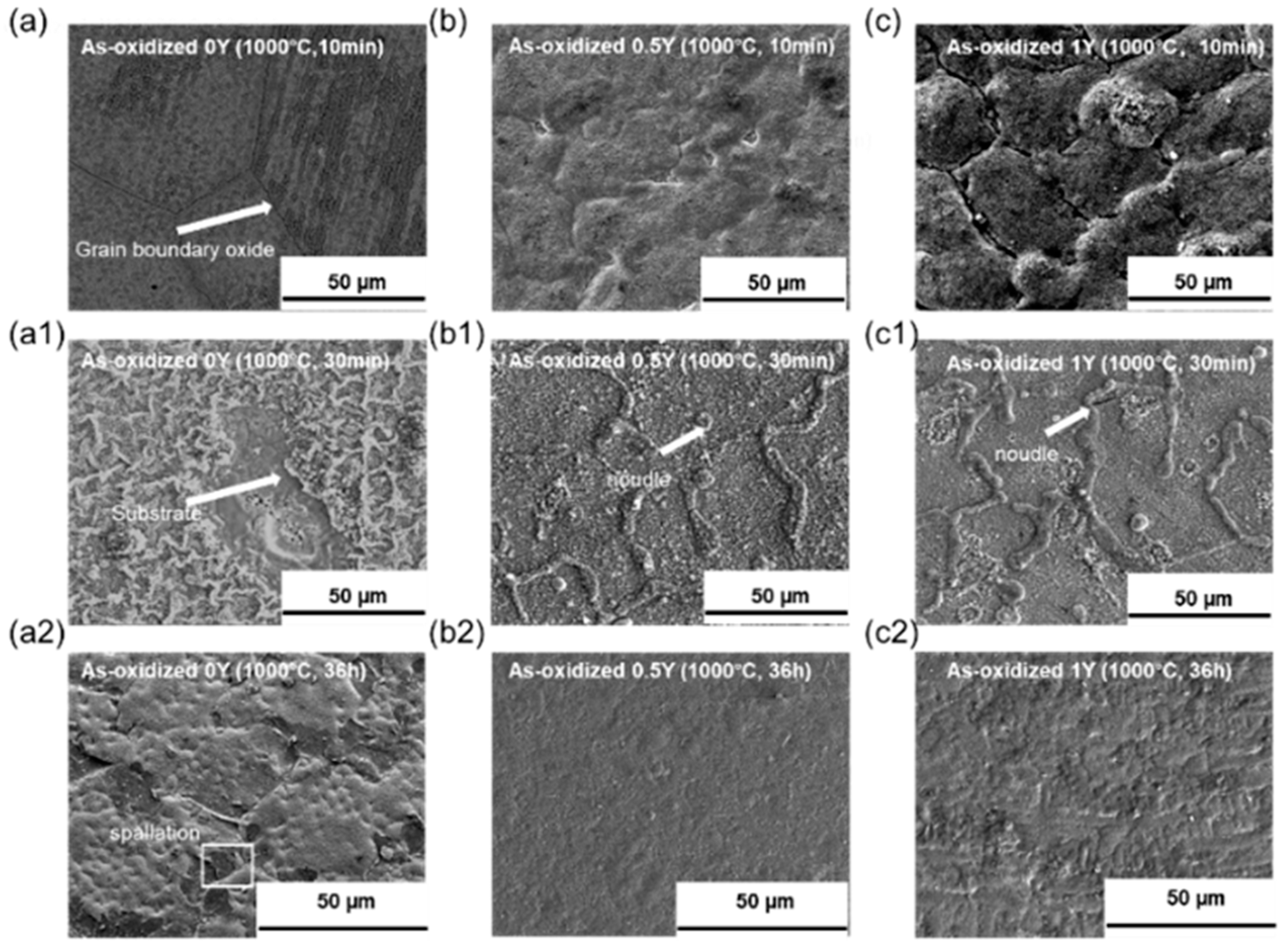
Figure 5 shows the electron microscope scans of the surface morphology of the oxide films of Fe-Ni-Cr-xY alloys after oxidation in air at 1000 °C for 10 min, 30 min and 2160 min. From
Figure 5a–c, it can be seen that grain boundary oxides and spherical oxides were formed in all three alloy samples. The grain boundary oxides are formed rapidly in the grain boundary region, and the grain boundary becomes a diffusion channel for metal cations to combine with oxygen negative ions in the environment. The formation of grain boundary oxides is caused by the rapid diffusion of metal ions through the grain boundary region. The generation of the oxide layer begins when the alloy is exposed to high temperature air, and at the initial stage of oxidation, the oxides begin to nucleate, and the oxygen negative ions react with the alloy metal cations at the interface between the metal and the air. As the exposure time grows, the oxide nucleation ends and begins to grow outward. As the oxide grows, the oxide size gradually increases and the reaction rate increases. Successive lateral growth of the oxide will result in the formation of a continuous oxide layer by collision of the oxide particles with each other. When the surface of the alloy is completely covered by the oxide, the growth rate of the oxide begins to decrease, and the oxide layer changes to grow upward, and this change in growth direction will further thicken the oxide film of the alloy. The diffusion mechanism of metal ions in the alloy is mainly metal ions along the short-circuit diffusion and lattice diffusion. While in the short-circuit diffusion process, the ion diffusion rate is faster and the oxide growth rate is higher. Compared with the 0Y alloy, from the surface of the oxide film after 10 min of oxidation, it can be seen that the surface of the Y-containing alloy generates fluffy bulging oxides and cracking occurs at the grain boundaries, and the amount of oxide generated is more than that of the Y-free alloy, and with the increase of Y content, the amount of oxide generated is more. From
Figure 5a1–c1, it can be seen that the oxide film on the surface of the 0Y alloy gradually becomes thicker at 30 min of oxidation, and local flaking occurs, whereas the oxide film on the surface of the 0.5Y and 1Y alloys becomes denser, and the surface of the oxide film begins to form a tumulus-like oxide and the continuous oxide co-exists with the grain-boundary oxides on the surface of the samples, and the cracks at the grain-boundaries are closed by the subsequently generated oxide film; From
Figure 5a2–c2, it can be seen that after oxidizing for 2160 min, the surface of 0Y alloy formed a thick and uneven oxide layer, and the local spalling phenomenon occurred, in 0.5Y alloy, the surface oxide film was dense and the surface was smooth and flat, and the surface oxide film of 1Y alloy was rough and showed an uneven morphology.
From the metallographic diagram of
Figure 1d, it is concluded that with the addition of Y the grain size of the alloy decreases and the grain boundary coefficient of the alloy increases, which results in an increase in the number of channels for the outward diffusion of metal cations in the alloy, and due to the large number of outward diffusion of the metal cations an oxide film is formed on the surface of the alloy. It has been reported in the literature [
20] that at the early stage of oxidation, due to the high activity of rare-earth elements, rare-earth oxides are preferentially generated on the surface of the alloys to provide nucleation sites for the nucleation and growth of other metal oxides, which is consistent with the conclusion that the weight gain of Y-containing alloys at the early stage of oxidation is higher than that of Y-free alloys in the oxidation kinetic curves of
Figure 3.
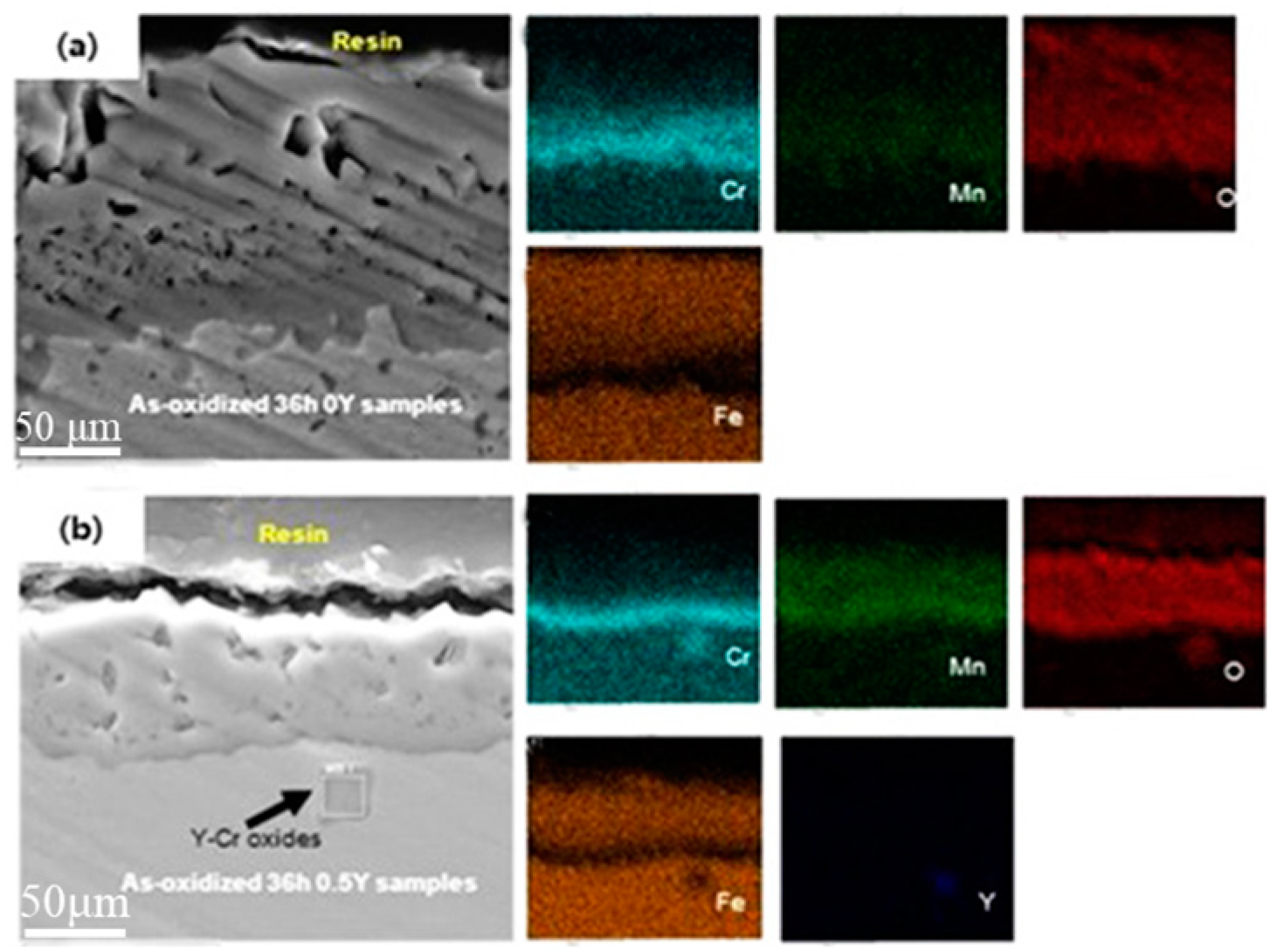
Figure 6 shows the cross-sectional SEM images and corresponding EDS patterns of the 47Fe-36Ni-15Cr-1.5Mn-xY alloy after oxidation at 1000 ℃ for 2160 min. As can be seen in
Figure 6a, the surface of the 0Y alloy formed a continuous coverage of oxide film, which mainly consisted of Fe-rich oxides in the outer layer (with thickness of about 12 μm), and Mn
1.5Cr
1.5O
4 oxides and protective Cr
2O
3 oxides in the inner layer (with thickness of about 5.2 μm). (thickness of about 5.2 μm) of protective Cr
2O
3 oxide. From
Figure 6b, it can be observed that the outer layer of 0.5 Y alloy is mainly Fe-rich oxide layer (thickness of about 7.4 μm), and the inner layer is mainly protective Cr
2O
3 layer (thickness of about 2.1 μm). 0Y alloy has the thickest thickness of the oxide layer, and the thickness of Cr-rich layer and Fe-rich oxide layer is thicker than that of the 0.5 Y alloy, and the large number of pores in the cross-sectional morphology suggests that the oxide layer is relatively loose and cannot be efficiently removed from the surface of the alloy. This shows that the oxide layer is relatively loose and cannot effectively prevent the diffusion of metal cations or oxygen negative ions during the high-temperature oxidation process, and cannot play a good anti-temperature oxidation effect. On the contrary, the cross-section morphology of 0.5 Y alloy has fewer holes, good densification, the appearance of Y, Cr mixed oxides, and the cross-section of the oxide film composition has not changed much, but the overall thickness is reduced by about 50% compared to 0 Y alloy, this is due to the rare earth Y elements added into the alloy, its solubility is low, in the alloy melting process is easy to be in the alloy at the alloy grain boundaries in the form of precipitation phase exists in the alloy. During high-temperature oxidation, these precipitated phases are present inside the grains and at the grain boundaries, leading to the formation of coarser inclusions. Rare earth-rich Y precipitates are preferentially oxidized over Cr. The occurrence of internal oxidation in Y-containing alloys is unavoidable due to the high oxygen diffusivity of rare earth-containing oxides, which can provide a large number of short-circuit diffusion pathways for oxygen inward [
21]. The internally oxidized oxides mainly consist of oxides of Fe, Ni and Cr encapsulated with trace amounts of Y-containing oxides, and the appearance of rare earth precipitation phases leads to severe internal oxidation of the alloys.
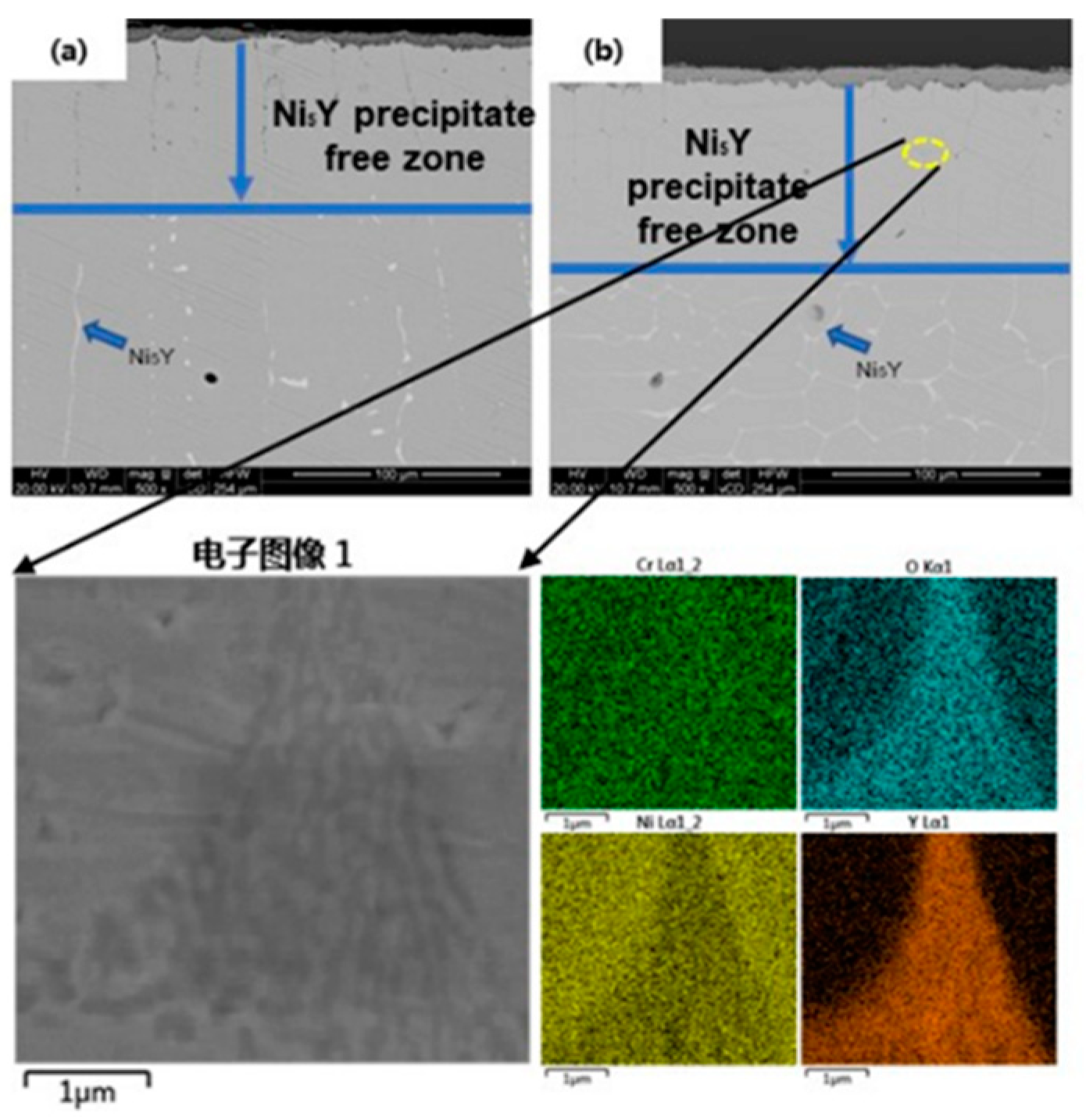
Figure 7 shows the cross-sectional high magnification SEM images and corresponding EDS patterns of 0.5 Y and 1 Y alloys after oxidation at 1000°C for 2160 min. From
Figure 7a, it can be found that three kinds of regions appear in the Y-containing alloys after oxidation, the oxidized layer region, the region of Ni
5Y-phase-free region, and the region of Ni
5Y-phase-enriched region. Comparing the two Y-containing alloys, the degree of internal oxidation of the 0.5 Y alloy is slightly shallower than that of the 1Y alloy, which is due to the fact that the thickness of the surface oxide layer of the alloy decreases as a whole when the rare earth elements are added, which reduces the stress of the growth of the surface oxide film at the late stage of oxidation of the alloy and avoids the phenomenon of flaking off of the oxide film layer. And in the Y-containing alloys without Ni
5Y phase grain boundaries in the region of the rare earth Y-rich wedge-shaped oxides. These oxides can play a "pinning effect" on the outer oxide layer, and the wedge-shaped oxides can significantly improve the bonding force between the alloy oxide film and the substrate, and enhance the spalling resistance between the oxide film layer and the substrate [
22].