Submitted:
25 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Enzymes Used for Biomass Conversion, Degradation, and Hydrolysis
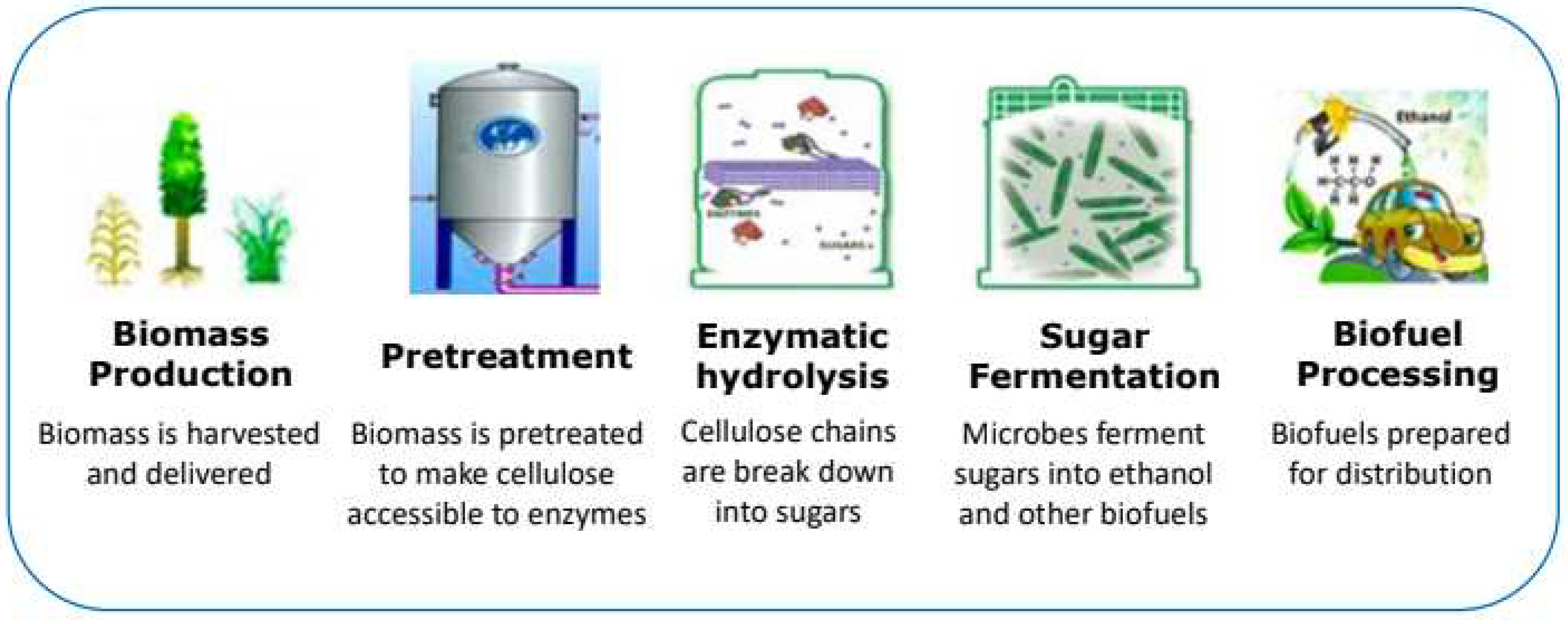
3. Mechanism of Treatment of Biomass:
3.1. Pretreatment of Biomass Wastes
3.2. Enzyme Production
3.3. Enzymatic Hydrolysis
3.4. Fermentation and Further Processing
4. Application of Biomass Waste Management for Treating Contaminated Wastewater
5. Challenges and Future Prospects
6. Conclusion
Author Contributions
Data Availability Statement
Acknowledgments
Conflict of Interest
References
- Alam, S.; Bhardwaj, L.K.; Mallick, R.; Rai, S. Estimation of Heavy Metals and Fluoride Ion in Vegetables Grown Nearby the Stretch of River Yamuna, Delhi (NCR), India. Indian Journal of Environmental Protection 2023, 43, 64–73. [Google Scholar]
- Akcil, A.; Karahan, A.G.; Ciftci, H.; Sagdic, O. Biological treatment of cyanide by natural isolated bacteria (Pseudomonas sp.). Minerals engineering 2003, 16, 643–649. [Google Scholar] [CrossRef]
- Akhter, M.; Wal Marzan, L.; Akter, Y.; Shimizu, K. Microbial bioremediation of feather waste for keratinase production: an outstanding solution for leather dehairing in tanneries. Microbiology insights 2020, 13, 1178636120913280. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Salem, S.R. Degradation of castor oil and lipase production by Pseudomonas aeruginosa. American-Eurasian J Agric Environ Sci 2009, 5, 556–563. [Google Scholar]
- Anzenbacher, P.; Anzenbacherova, E. Cytochromes P450 and metabolism of xenobiotics. Cellular and Molecular Life Sciences CMLS 2001, 58, 737–747. [Google Scholar] [CrossRef]
- Arslan, N.P.; Yazici, A.; Komesli, S.; Esim, N.; Ortucu, S. Direct conversion of waste loquat kernels to pigments using Monascus purpureus ATCC16365 with proteolytic and amylolytic activity. Biomass Conversion and Biorefinery 2021, 11, 2191–2199. [Google Scholar] [CrossRef]
- Awad, G.; Mohamed, E.F. Immobilization of P450 BM3 monooxygenase on hollow nanosphere composite: application for degradation of organic gases pollutants under solar radiation lamp. Applied Catalysis B: Environmental 2019, 253, 88–95. [Google Scholar] [CrossRef]
- Bansal, N.; Kanwar, S.S. Peroxidase (s) in environment protection. The Scientific World Journal 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.M.; Chellappan, S.; Beena, P.S.; Sukumaran, R.K.; Elyas, K.K.; Chandrasekaran, M. Lipase from marine Aspergillus awamori BTMFW032: production, partial purification and application in oil effluent treatment. New Biotechnology 2011, 28, 627–638. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Arica, M.Y. Immobilization of tyrosinase on modified diatom biosilica: Enzymatic removal of phenolic compounds from aqueous solution. Journal of hazardous materials 2013, 244, 528–536. [Google Scholar] [CrossRef]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U.; Parajuli, N. Microbial enzymes used in bioremediation. Journal of Chemistry 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Bhange, K.; Chaturvedi, V.; Bhatt, R. Feather degradation potential of Stenotrophomonas maltophilia KB13 and feather protein hydrolysate (FPH) mediated reduction of hexavalent chromium. 3 Biotech 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, L.K. Evaluation of Bis (2-ethylhexyl) Phthalate (DEHP) in the PET Bottled Mineral Water of Different Brands and Impact of Heat by GC–MS/MS. Chemistry Africa 2022, 5, 929–942. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Jindal, T. Persistent organic pollutants in lakes of Grovnes Peninsula at Larsemann Hill area, East Antarctica. Earth Systems and Environment 2020, 4, 349–358. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Sharma, A. (2021). Microplastics (MPs) in Drinking Water: Uses, Sources & Transport.
- Bhardwaj, L.K. Occurrence of Microplastics (MPs) in Antarctica and Its Impact on the Health of Organisms. Maritime Technology and Research 2023, 6, 265418. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Sharma, A. Estimation of physico-chemical, trace metals, microbiological and phthalate in PET bottled water. Chemistry Africa 2021, 4, 981–991. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Sharma, S.; Jindal, T. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in the lake water at Grovnes Peninsula Over East Antarctica. Chemistry Africa 2021, 4, 965–980. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Jindal, T. Contamination of Lakes in Broknes peninsula, East Antarctica through the Pesticides and PAHs. Asian-Journal of Chemistry 2019, 31, 1574–1580. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Jindal, T. Polar Ecotoxicology: Sources and Toxic Effects of Pollutants. New Frontiers in Environmental Toxicology 2022, 9–14. [Google Scholar]
- Bhardwaj, L.K.; Kumar, D.; Kumar, A. (2023). Phytoremediation Potential of Ocimum Sanctum: A Sustainable Approach for Remediation of Heavy Metals. [CrossRef]
- Bhardwaj, L.K.; Sharma, S.; Jindal, T. Estimation of Physico-Chemical and Heavy Metals in the Lakes of Grovnes & Broknes Peninsula, Larsemann Hill, East Antarctica. Chemistry Africa 2023, 1–18. [Google Scholar]
- Bilal, M.; Iqbal, H.M. Ligninolysis potential of ligninolytic enzymes: a green and sustainable approach to bio-transform lignocellulosic biomass into high-value entities. Alternative Energy Resources: The Way to a Sustainable Modern Society.
- Boyer, A.; Pagé-BéLanger, R.; Saucier, M.; Villemur, R.; Lépine, F.; Juteau, P.; Beaudet, R. Purification, cloning and sequencing of an enzyme mediating the reductive dechlorination of 2, 4, 6-trichlorophenol from Desulfitobacterium frappieri PCP-1. Biochemical Journal 2003, 373, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Duquesne, S.; Bordes, F.; Sandoval, G.; Marty, A. Lipases: an overview. Lipases and phospholipases: methods and protocols 2012, 3-30.
- Castro, A.M.; Castilho, L.R.; Freire, D.M. An overview on advances of amylases production and their use in the production of bioethanol by conventional and non-conventional processes. Biomass Conversion and Biorefinery 2011, 1, 245–255. [Google Scholar] [CrossRef]
- Chakraborty, J.; Das, S. Molecular perspectives and recent advances in microbial remediation of persistent organic pollutants. Environmental Science and Pollution Research 2016, 23, 16883–16903. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Chowdhary, P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environmental Science: Processes & Impacts 2015, 17, 326–342. [Google Scholar]
- Chaudhuri, G.; Dey, P.; Dalal, D.; Venu-Babu, P.; Thilagaraj, W.R. A novel approach to precipitation of heavy metals from industrial effluents and single-ion solutions using bacterial alkaline phosphatase. Water, Air, & Soil Pollution 2013, 224, 1–11. [Google Scholar]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renewable and Sustainable Energy Reviews 2021, 139, 110689. [Google Scholar] [CrossRef]
- Chung, D.; Cha, M.; Guss, A.M.; Westpheling, J. Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proceedings of the National Academy of Sciences 2014, 111, 8931–8936. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, R.W.; Charles, M. Applications of lactase and immobilized lactase. In Immobilized enzymes for food processing; CRC Press. 2019. pp. 153–173.
- Dai, Z.; Liu, L.; Duan, H.; Li, B.; Tang, X.; Wu, X.; Liu, G.; Zhang, L. Improving sludge dewaterability by free nitrous acid and lysozyme pretreatment: Performances and mechanisms. Science of the Total Environment 2023, 855, 158648. [Google Scholar] [CrossRef]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: measurement methods and comparison. Critical reviews in biotechnology 2010, 30, 302–309. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biorefineries for biofuel upgrading: a critical review. Applied energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Ebbs, S. Biological degradation of cyanide compounds. Current opinion in Biotechnology 2004, 15, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Falade, A.O.; Nwodo, U.U.; Iweriebor, B.C.; Green, E.; Mabinya, L.V.; Okoh, A.I. Lignin peroxidase functionalities and prospective applications. MicrobiologyOpen 2017, 6, e00394. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.S.; Chen, P.C.; Wen, F.S.; Hsiao, W.Y.; Lee, C.M. Nitrile hydratase from Mesorhizobium sp. F28 and its potential for nitrile biotransformation. Process Biochemistry 2008, 43, 1391–1397. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: present status and future prospects. Bioresource Technology 2016, 215, 334–345. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.S.; Lee, D.J. Enzymes and enzymatic mechanisms in enzymatic degradation of lignocellulosic biomass: A mini-review. Bioresource Technology 2023, 367, 128252. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angewandte Chemie International Edition 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Hassan, S.W.; Abd El Latif, H.H.; Ali, S.M. Production of cold-active lipase by free and immobilized marine Bacillus cereus HSS: application in wastewater treatment. Frontiers in microbiology 2018, 9, 2377. [Google Scholar] [CrossRef] [PubMed]
- Hirota-Mamoto, R.; Nagai, R.; Tachibana, S.; Yasuda, M.; Tani, A.; Kimbara, K.; Kawai, F. Cloning and expression of the gene for periplasmic poly (vinyl alcohol) dehydrogenase from Sphingomonas sp. strain 113P3, a novel-type quinohaemoprotein alcohol dehydrogenase. Microbiology 2006, 152, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renewable and Sustainable Energy Reviews 2022, 154, 111822. [Google Scholar] [CrossRef]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Ji, D.; Mao, Z.; He, J.; Peng, S.; Wen, H. Characterization and genomic function analysis of phenanthrene-degrading bacterium Pseudomonas sp. Lphe-2. Journal of Environmental Science and Health, 55.
- Joutey, N.T.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: involved microorganisms and genetically engineered microorganisms. Biodegradation-life of science 2013, 1, 289–320. [Google Scholar]
- Khan, M.N.; Luna, I.Z.; Islam, M.M.; Sharmeen, S.; Salem, K.S.; Rashid, T.U.; Zaman, A.; Haque, P.; Rahman, M.M. Cellulase in waste management applications. In New and future developments in microbial biotechnology and bioengineering (pp. 237-256). Elsevier.
- Khatami 2016, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnology and Applied Biochemistry 2022, 69, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Belbahri, L.; Woodward, S.; Ellouz, M.; Dhouib, A.; Sayadi, S.; Mechichi, T. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. Journal of Hazardous Materials.
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and function of phage encoded depolymerases. Frontiers in microbiology 2020, 10, 2949. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Kalia, M.; Gupta, R. Pectin methylesterases: a review. Journal of Bioprocessing & Biotechniques 2015, 5, 1. [Google Scholar]
- Kumar, A.; Sharma, S. (2019). Microbes and enzymes in soil health and bioremediation (pp. 353-366). Singapore: Springer.
- Leong, H.Y.; Chang, C.K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: a solution to global issues. Biotechnology for Biofuels 2021, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, L.; Wang, H.; Li, J.; Cheng, H.; Ma, Q. Process optimization of polyphenol oxidase immobilization: Isotherm, kinetic, thermodynamic and removal of phenolic compounds. International Journal of Biological Macromolecules 2021, 185, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Guengerich, F.P.; Ma, L.; Li, S.; Zhang, W. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. Journal of Biological Chemistry 2020, 295, 833–849. [Google Scholar] [CrossRef]
- Littlechild, J.A. Archaeal enzymes and applications in industrial biocatalysts. Archaea 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bilal, M.; Duan, X.; Iqbal, H.M. Mitigation of environmental pollution by genetically engineered bacteria—current challenges and future perspectives. Science of The Total Environment 2019, 667, 444–454. [Google Scholar] [CrossRef]
- Madhavan, A.; Arun, K.B.; Binod, P.; Sirohi, R.; Tarafdar, A.; Reshmy, R.; Awasthi, M.K. Sindhu, R. Design of novel enzyme biocatalysts for industrial bioprocess: Harnessing the power of protein engineering, high throughput screening and synthetic biology. Bioresource Technology 2021, 325, 124617. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: properties and applications. BioResources 2009, 4. [Google Scholar] [CrossRef]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Mander, P.; Cho, S.S.; Choi, Y.H.; Panthi, S.; Choi, Y.S.; Kim, H.M.; Yoo, J.C. Purification and characterization of chitinase showing antifungal and biodegradation properties obtained from Streptomyces anulatus CS242. Archives of pharmacal research 2016, 39, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; de Eugenio, L.I.; Nieto-Domínguez, M.; Prieto, A.; Martínez, M.J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresource Technology 2021, 324, 124623. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, N.P.; Jackson, B.; Joseph Raj, A.; Sevanan, M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochemistry research international 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Nicell, J.A. Enzymatic treatment of waters and wastes. In Chemical Degradation Methods for Wastes and Pollutants; CRC Press. 2003. pp. 395–441.
- Nimkande, V.D.; Bafana, A. A review on the utility of microbial lipases in wastewater treatment. Journal of Water Process Engineering 2022, 46, 102591. [Google Scholar] [CrossRef]
- Pandey, K.; Singh, B.; Pandey, A.K.; Badruddin, I.J.; Pandey, S.; Mishra, V.K.; Jain, P.A. Application of microbial enzymes in industrial waste water treatment. International Journal of Current Microbiology and Applied Sciences 2017, 6, 1243–1254. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Pérez-Prior, M.T.; González-Sánchez, M.I.; Valero, E. Removal of aromatic compounds from wastewater by hemoglobin soluble and immobilized on Eupergit® CM. Environmental Engineering & Management Journal (EEMJ).
- Phale, P.S.; Sharma, A.; Gautam, K. (2019). Microbial degradation of xenobiotics like aromatic pollutants from the terrestrial environments. In Pharmaceuticals and personal care products: waste management and treatment technology (pp. 259-278). Butterworth-Heinemann.
- Presečki, A.V.; Blažević, Z.F.; Vasić-Rački, Đ. Complete starch hydrolysis by the synergistic action of amylase and glucoamylase: impact of calcium ions. Bioprocess and biosystems engineering 2013, 36, 1555–1562. [Google Scholar] [CrossRef]
- Sahal, S.; Khaturia, S.; Joshi, N. Application of Microbial Enzymes in Wastewater Treatment. Genomics Approach to Bioremediation: Principles 2023, Tools, and Emerging Technologies, 209-227.
- Savla, N.; Pandit, S.; Mathuriya, A.S.; Gupta, P.K.; Khanna, N.; Babu, R.P.; Kumar, S. Recent advances in bioelectricity generation through the simultaneous valorization of lignocellulosic biomass and wastewater treatment in microbial fuel cell. Sustainable Energy Technologies and Assessments 2021, 48, 101572. [Google Scholar]
- Sandhya, S. Biodegradation of azo dyes under anaerobic condition: role of azoreductase. Biodegradation of azo dyes 2010, 39–57. [Google Scholar]
- Saraswat, R.; Verma, V.; Sistla, S.; Bhushan, I. Evaluation of alkali and thermotolerant lipase from an indigenous isolated Bacillus strain for detergent formulation. Electronic Journal of Biotechnology 2017, 30, 33–38. [Google Scholar] [CrossRef]
- Schenk, G.; Mateen, I.; Ng, T.K.; Pedroso, M.M.; Mitić, N.; Jafelicci Jr, M.; Marques, R.F.; Gahan, L.R.; Ollis, D.L. Organophosphate-degrading metallohydrolases: Structure and function of potent catalysts for applications in bioremediation. Coordination Chemistry Reviews 2016, 317, 122–131. [Google Scholar] [CrossRef]
- Sharma, S. Bioremediation: features, strategies and applications. Asian Journal of Pharmacy and Life Science 2012, 2231, 4423. [Google Scholar]
- Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzyme research 2011, 2011. [Google Scholar]
- Singh, B. Review on microbial carboxylesterase: general properties and role in organophosphate pesticides degradation. Biochem Mol Biol 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: industrial progress in 21st century. 3 Biotech 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.G.W.; Rodrigues, C.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: a review. Biomass and Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Sjoblad, R.D.; Bollag, J.M. (2021). Oxidative coupling of aromatic compounds by enzymes from soil microorganisms. In Soil biochemistry.
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Marine Pollution Bulletin 2021, 166, 112185. [Google Scholar] [CrossRef]
- Sondhi, S.; Sharma, P.; George, N.; Chauhan, P.S.; Puri, N.; Gupta, N. An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to biobleach softwood pulp. 3 Biotech 2015, 5, 175–185. [Google Scholar] [CrossRef]
- Tachibana, S.; Naka, N.; Kawai, F.; Yasuda, M. Purification and characterization of cytoplasmic NAD+-dependent polypropylene glycol dehydrogenase from Stenotrophomonas maltophilia. FEMS microbiology letters 2008, 288, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Toesch, M.; Schober, M.; Faber, K. Microbial alkyl-and aryl-sulfatases: mechanism, occurrence, screening and stereoselectivities. Applied microbiology and biotechnology 2014, 98, 1485–1496. [Google Scholar] [CrossRef]
- Wang, D.; Li, A.; Han, H.; Liu, T.; Yang, Q. A potent chitinase from Bacillus subtilis for the efficient bioconversion of chitin-containing wastes. International journal of biological macromolecules 2018, 116, 863–868. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Liang, X.; Wei, M.; Liang, F.; Feng, D.; Xu, C.; Xian, M.; Zou, H. Production and waste treatment of polyesters: Application of bioresources and biotechniques. Critical Reviews in Biotechnology 2022, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Cao, X.; Liu, Y.; Xue, S. Insights into the molecular mechanism of dehalogenation catalyzed by D-2-haloacid dehalogenase from crystal structures. Scientific Reports 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Xue, F.; Ya, X.; Tong, Q.; Xiu, Y.; Huang, H. Heterologous overexpression of Pseudomonas umsongensis halohydrin dehalogenase in Escherichia coli and its application in epoxide asymmetric ring opening reactions. Process Biochemistry 2018, 75, 139–145. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, P.K.; Yadav, D.; Yadav, K.D.S. Pectin lyase: a review. Process Biochemistry 2009, 44, 1–10. [Google Scholar] [CrossRef]
- Zanuso, E.; Gomes, D.G.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Enzyme immobilization as a strategy towards efficient and sustainable lignocellulosic biomass conversion into chemicals and biofuels: current status and perspectives. Sustainable Energy & Fuels 2021, 5, 4233–4247. [Google Scholar]
- Zhang, H.; Han, L.; Dong, H. An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: Experimental and modeling studies. Renewable and sustainable energy reviews 2021, 140, 110758. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. In-depth biochemical identification of a novel methyl parathion hydrolase from Azohydromonas australica and its high effectiveness in the degradation of various organophosphorus pesticides. Bioresource Technology 2021, 323, 124641. [Google Scholar] [CrossRef]
| S. No. | Name of Enzyme | Sources | Application of Enzymes | References |
|---|---|---|---|---|
| 1 | Alkylsulfatase | Pseudomonas C12B | Surfactant degradation | Toesch et al. 2014 |
| 2 | Azoreductase | Intestinal microflora | Removal of azo dyes | Sandhya 2010 |
| 3 | α-Amylase | Bacillus subtilis | Glucose production and hydrolysis of starch | Kolusheva and Marinova 2007; Presečki et al. 2013 |
| 4 | Glucoamylase | |||
| 5 | Cellulase | Trichoderma harzianum, Trichoderma viride | Hydrolysis of cellulose in sludges & municipal solid waste (MSW) to produce alcohol, sugars, and energy | Champagne and Li 2009; Khan et al. 2016; Pandey et al. 2017 |
| 6 | Cellobio-hydrolase | |||
| 7 | Cellobiose | |||
| 8 | Exo-1,4-b-D-glucosidase | |||
| 9 | Chitinase | Streptomyces anulatus CS242 | Production of N-acetyl glucosamine from shellfish waste through bioconversion | Mander et al. 2016 |
| 10 | Chloro-peroxidase | Caldariomyces fumago | Oxidation of phenolic compounds | Sjoblad and Bollag 2021 |
| 11 | Cyanidase | Pseudomonas sp. | Cyanide decay | Akcil et al. 2003 |
| 12 | Cyanide hydratase | Fusarium lateritium | Cyanide hydrolysis | Ebbs 2004 |
| 13 | Depolymerase | Bacteriophase | Bacterial exopolysaccharide | Knecht et al. 2020 |
| 14 | Haemoglobin | Blood | Removal of aromatic amines and phenols | Pérez-Prior et al. 2014 |
| 15 | L-Galactono-lactone oxidase | Candida norvegensis | Conversion of galactose from L-ascorbic acid | Nicell 2003 |
| 16 | Laccase | Pleurotus (P. ostreatus, P. pulmonarius) and Trametes (T. versicolor, T. hirsuta) | Decolorization of kraft, removal of phenols, bleaching of paper pulp, binding of phenols and aromatic amines with humas, detoxification of wastewater | Khatami et al. 2022 |
| 17 | Lactases | Bacterial | Dairy waste processing and production of value-added products | Coughlin and Charles 2022 |
| 18 | Lignin peroxidase (LiP) | Phanerochaete chrysosporium | Removal of phenols and aromatic compounds decolorization of Kraft bleaching effluents | Falade et al. 2017 |
| 19 | Lipase | Various sources | Improved sludge dewatering | Nimkande and Bafana (2022); Di et al. 2023 |
| 20 | Lyzozyme | Bacterial | ||
| 21 | Manganese peroxidase (MnP) | Phanerochaete chrysosporium | Decolorization of synthetic dyes, removal of phenolic contaminants, removal of endocrine disruptive chemicals (EDC), degradation of chlorinated alkanes and alkenes, degradation of chlorinated dioxins | Bansal and Kanwar 2013 |
| 22 | Nitrile hydratase | Mesorhizobium sp. | Removal of acrylonitrile | Feng et al. 2008 |
| 23 | Parathion hydrolase | Azohydromonas australica | Hydrolyzation of organophosphate pesticides | Zhao et al. 2021 |
| 24 | Pectin Lyase | Clostridium beijerinckii | Pectin degradation | Yadav et al. 2009 |
| 25 | Pectinmethylesterase | Aspergillus niger | Kohli et al. 2015 | |
| 26 | Phosphatase | Escherichia coli C90 | Removal of heavy metals | Chaudhuri et al. 2013 |
| 27 | Phosphoesterases | Aspergillus sydowii CBMAI 935 | Removal of chlorpyrifos, diazinon, parathions | Soares et al. 2021 |
| 28 | Polyphenol oxidase | Mushroom (Agaricus bisporus) | Removal of phenolic compounds | Li et al. 2021 |
| 29 | Proteases | Bacillus licheniformis, Aspergillus niger, Chlorella vulgaris | Hydrolyze or breakdown the protein molecules in meat, biodegradation of the industrial sludge | Karn and Kumar 2015; Arslan et al. 2021 |
| 30 | Tyrosinase | Mushroom (Agaricus bisporus) | Removal of phenolic compounds | Bayramoglu et al. 2013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





