1. Introduction
Adipose tissue, in addition to storing energy, secretes a range of cellular components and inflammatory mediators. Tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), leptin, retinol-binding protein, resistin, adiponectin, omentin, apelin, visfatin, and other substances are secreted by different adipose tissue depots with regional heterogeneity (Galic et al., 2010). These secretions have an impact on the metabolism of carbohydrates and lipids, and they also play a significant part in pathological processes that include insulin resistance, type 2 diabetes, atherosclerosis, inflammation, and dysfunction of the vascular endothelium (Galic et al., 2010).
Omentin-1 is a glycoprotein that has emerged as a key player in the complex interplay between adipose tissue and various physiological processes. Omentin-1 is primarily produced by the stromal vascular cells of the visceral adipose tissue (Yang et al., 2006). This adipokine exist in human blood and it is highly expressed in human visceral and epicardial adipose tissues, with lower levels found in other white adipose tissue depots such as subcutaneous WAT (Fain et al., 2005). Omentin-1 is expressed in various cells including endothelial cells, mesothelial cells, vascular cells, intestinal Paneth cells among others; exerting a paracrine, autocrine, and endocrine signaling influence (Watanabe et al., 2017). Importantly and similarly to adiponectin circulating omentin levels are reduced in obese subjects. In fact, individuals with poor glucose regulation have lower serum levels of omentin-1, and this depletion may play a role in the emergence of insulin resistance, type 2 diabetes, obesity, and metabolic syndrome. Indeed, there is a negative correlation between the serum concentration of omentin-1 and the following: body mass index, insulin resistance index, leptin, plasma glucose, fasting insulin, TNFα, and IL-6 (Pan et al., 2010; Herder et al., 2015; Liu et al., 2011; Sperling et al., 2016). This adipokine is downregulated in association with obesity linked metabolic disorders including type 2 diabetes, and insulin resistance (de Souza Batista et al., 2007; Elsaid et al., 2018; Pan et al., 2010; Pan et al., 2019).
Several factors can influence the production and secretion of omentin including obesity, insulin sensitivity, inflammation, genetic factors, and hormones, such as adiponectin and insulin, fibroblast growth factor-21 and dexamethasone may also influence omentin production (Watanabe et al., 2017). Adiponectin, another adipokine, has been linked to omentin, and insulin sensitivity may play a role in omentin regulation. Understanding these factors is essential for unraveling the complex regulatory mechanisms of omentin and its potential implications for metabolic and cardiovascular health. Ongoing research continues to explore the intricate interplay between omentin, adipose tissue, and systemic physiology.
This review highlights the potential therapeutic targets of omentin-1 in metabolic-related disorders. Relevant pre-clinical and clinical studies were summarized and discussed. Pubmed search was performed between 1990-2023 using the words: omentin-1, endothelial function, obesity, type 2 diabetes, inflammation, and oxidative stress.
2. Vascular Effects of Omentin
Omentin has been implicated in several positive effects on vascular function, making it an area of interest in cardiovascular research. Omentin exerts several positive effects on vascular function, including vasodilation, anti-inflammatory actions, and potential anti-atherosclerotic effects (Hiramatsu-Ito et al., 2016).
2.1. Vasodilation
Omentin has been associated with the promotion of vasodilation, which is the relaxation of blood vessels (Yamawaki et al., 2010). This vasodilatory effect is important for maintaining proper blood flow and reducing the resistance within the vascular system (Kazama et al., 2013). Enhanced vasodilation contributes to optimal cardiovascular function (Sena et al., 2014).
2.2. Anti-Inflammatory Actions
Omentin exhibits anti-inflammatory properties. Inflammation plays a crucial role in the development of vascular diseases, including atherosclerosis (Sena et al., 2014). By exerting anti-inflammatory actions, omentin may help mitigate the inflammatory processes within blood vessels, reducing the risk of vascular damage and atherosclerotic plaque formation (Uemura et al., 2015; Askin et al., 2020). Indeed, omentin-1 inhibits TNF- , IL-6 and other inflammatory cytokines ultimately impacting vascular and tissue functions (Kazama et al., 2012; Fernández-Trasancos et al., 2017; Wang et al., 2019). Several studies have described the anti-inflammatory and anti-atherosclerotic effects of omentin through intracellular signaling pathways involving Mitogen-activated protein kinase (p38, JNK, ERK), nuclear factor-κB, and AMP-activated protein kinase/Akt (Watanabe et al., 2017).
2.3. Endothelial Protection
Omentin appears to have protective effects on the endothelium, the inner lining of blood vessels. Endothelial health is essential for maintaining vascular integrity and function (Sena et al., 2014, 2017). In patients with type 2 diabetes, lower levels of omentin-1 have been linked to endothelial dysfunction (Hayashi et al., 2019). Omentin-1 has an endothelial-dependent effect on the vascular reactivity of isolated blood vessels, according to research by Yamawaki and colleagues (2010). Accordingly, we discovered that omentin-1 treatment restored endothelial dysfunction in type 2 diabetes by normalizing ACh-induced relaxation of aortic rings in diabetic Goto-Kakizaki (GK) rats fed a high-fat diet. In arteries without perivascular adipose tissue (PVAT), omentin-1 had no effect on endothelial-independent vasorelaxation. Moreover, the aortas of diabetic GK rats mounted with PVAT showed a reduction in the ET1-induced constrictor response in response to omentin-1 (Leandro et al., 2021). Notably, ex vivo omentin-1 vasorelaxation in aortic rings seems to be successful and mostly unrelated to increased peripheral insulin sensitivity (Yang et al., 2006).
Omentin’s positive influence on endothelial cells contributes to the prevention of endothelial dysfunction, a key factor in the development of cardiovascular diseases (Dong et al. 2021; Liu et al., 2020; Maruyama et al., 2012).
2.4. Nitric Oxide Production
Omentin has been reported to stimulate the production of NO in endothelial cells. Nitric oxide is a signaling molecule with vasodilatory properties. Increased NO production helps regulate blood vessel tone, ensuring proper blood flow and reducing the risk of vascular constriction (Sena et al., 2014). Omentin-1 has been shown in earlier research to protect endothelial cells by inducing NO production and endothelial NO synthase (eNOS) activation (Yamawaki et al., 2010; Qi et al., 2016). Furthermore, omentin-1 was able to raise NO metabolites in the aortas and considerably raise the ratio of p-eNOS to total eNOS, suggesting that omentin-1’s ability to restore endothelial function is caused by an increase in NO bioavailability (Leandro et al., 2021). Vascular oxidative stress was decreased, and nitric oxide (NO) bioavailability was improved by omentin-1 treatment in diabetic GK rats fed (Leandro et al., 2021).
Indeed, omentin-1 can improve endothelial function in normal mice’s arteries that have endothelial dysfunction brought on by high glucose concentrations. This improvement is mediated by AMPK and PPARδ and results in an increase in Akt/eNOS activity and NO production (Liu et al., 2020).
2.5. Anti-Atherogenic Effects
Omentin has an enormous potential against atherosclerotic initiation and progression. Atherosclerosis is a condition characterized by the buildup of fatty deposits (plaque) in the arterial walls, leading to narrowing and hardening of the arteries (Lusis, 2000).
Omentin’s ability to promote vasodilation, reduce inflammation, and protect endothelial cells may contribute to its potential anti-atherogenic properties (Leandro et al., 2021; Watanabe et al., 2016). In addition, omentin-1 as anti-inflammatory, antioxidant and anti-apoptotic properties positively impacting endothelial function and preventing atherosclerosis (Gu et al., 2019; Liu et al., 2011; Watanabe et al., 2016).
Recent research has demonstrated the extensive involvement of omentin in numerous pathophysiological processes, including atherogenesis, obesity, insulin resistance, inflammatory response, and regulation of vascular endothelial function (Du et al., 2016; Ge et al., 2021; Harada et al., 2016; Liu et al., 2020; Varona et al., 2019). It improves insulin sensitivity, lowers inflammation, prevents atherosclerosis, controls the activity of vascular endothelial cells (Leandro et al., 2021; Lin et al., 2021), and protects the cardiovascular system (Greulich et al., 2013).
Omentin-1 has gained attention due to its potential significance in vascular function and its role in metabolic regulation.
3. Metabolic Regulation
Omentin has been implicated in metabolic regulation, particularly in influencing insulin sensitivity and glucose metabolism (Yang et al., 2006; Jialal et al., 2013; Koleva et al., 2013). Omentin is a protein that has been studied for its potential roles in metabolic health and regulation. It has been associated with improved insulin sensitivity, glucose and lipid metabolism.
3.1. Insulin Sensitivity
Omentin has been associated with improvements in insulin sensitivity. Insulin sensitivity refers to how effectively cells respond to insulin’s signaling to uptake glucose from the bloodstream. Enhanced insulin sensitivity is generally considered beneficial for metabolic health as it helps maintain normal blood glucose levels.
Omentin-1 levels have been demonstrated to be lowered in dysmetabolic conditions, including diabetes mellitus (Eimal Latif et al., 2021; Pan et al., 2010; Pan et al., 2019), obesity (de Souza Batista et al., 2007), and impaired glucose tolerance (Cetin Sanlialp et al., 2022). On the other hand, following aerobic exercise (Saremi et al., 2010), hypocaloric weight loss (Moreno-Navarrete et al., 2010), and metformin therapy (Tan et al., 2010), omentin-1 levels are increased. Higher levels of omentin-1 are advantageous for improving insulin-stimulated glucose transport because they activate Akt signaling, which regulates downstream processes like glucose metabolism (Watanabe et al., 2017). Like adiponectin, it increases insulin sensitivity through and increment in insulin mediated glucose uptake in adipose tissue. Omentin-1 is downregulated by glucose/insulin levels (Watanabe et al., 2017).
We have previously demonstrated that omentin-1 could lower insulin resistance in GK rats fed with high-fat diet (Leandro et al., 2021). Prior research indicates that this adipokine plays a significant role in regulating insulin sensitivity. Indeed, chronic omentin-1 infusion into ApoE-/- mice may improve insulin resistance via PPARγ, leading to a decrease in plasma glucose concentration (Yang et al., 2006). The beneficial metabolic effects may indirectly contribute to vascular health by addressing risk factors for cardiovascular diseases.
Omentin-1 has also been associated with beneficial effects against bone metabolic disorders (Xie et al., 2011; Rao et al., 2018). Understanding its role in these processes is essential for exploring its potential therapeutic applications in metabolic disorders.
3.2. Glucose Uptake
Omentin appears to influence glucose uptake in peripheral tissues, such as skeletal muscle and adipose tissue. By promoting glucose uptake, omentin may contribute to the regulation of blood glucose levels. This effect is particularly relevant in the context of insulin resistance, a condition where cells become less responsive to insulin’s actions, leading to elevated blood sugar levels.
3.3. Adiponectin Interaction
Omentin and adiponectin, another adipokine, share structural and functional similarities. Omentin may interact with adiponectin receptors and both adipokines have been linked to improvements in insulin sensitivity (Brunetti et al., 2014). The specific mechanisms through which omentin and adiponectin cooperate in metabolic regulation are still an area of active research.
3.4. Anti-Inflammatory Effects
Chronic inflammation is associated with insulin resistance. Omentin’s anti-inflammatory properties may contribute to improved insulin sensitivity by reducing inflammation in tissues like adipose tissue and the liver (Herder et al., 2015; Michalczyk et al., 2021; Waluga et al., 2019). Inflammation interferes with insulin signaling pathways, and by mitigating inflammation, omentin may help maintain proper insulin responsiveness. Omentin blunts cytokine expression in different cell types (Fernández-Trasancos et al., 2017; Kazama et al., 2012; Kazama et al., 2015; Wang et al., 2019; Yamawaki et al., 2011) and is negatively associated with systemic inflammatory markers such as TNFα and IL-6 (Zabetian-Targhi et al., 2016). Thus, omentin is a biomarker for metabolic health that may function to dampen obesity-related cytokine effects (Shibata et al., 2012; Zhou et al., 2020). Indeed, it is possible that omentin-1 works by blocking the NF-κB pathway and triggering the AMPK- and Akt-dependent pathways (Kataoka et al., 2014). Anti-diabetic medications may have an impact on the level of circulating omentin-1, which is inversely linked to the incidence of type 2 diabetes and certain of its complications, such as cardiomyopathy, retinopathy, and diabetic vascular disease (Okamura et al., 2023).
3.5. Lipid Metabolism
Omentin has been suggested to influence lipid metabolism (Herder et al., 2015; Michalczyk et al., 2021). It may play a role in regulating the breakdown of fats (lipolysis) and lipid storage in adipose tissue (Herder et al., 2015; Michalczyk et al., 2021). By modulating lipid metabolism, omentin could impact insulin sensitivity and overall metabolic health.
3.6. Potential Hormonal Interactions
Omentin’s effects on metabolic regulation may involve interactions with various hormones, including insulin, adiponectin, and others. These interactions contribute to the complex network of signaling pathways that regulate glucose and lipid metabolism (Herder et al., 2015; Michalczyk et al., 2021).
While the evidence suggests a link between omentin and metabolic regulation, it’s crucial to note that research in this field is ongoing, and the precise mechanisms involved are still being uncovered. The potential therapeutic applications of omentin in addressing metabolic disorders, such as insulin resistance and type 2 diabetes, warrant further exploration and investigation.
4. Clinical Implications
Herin are summarize some clinical implications or potential therapeutic applications of omentin in vascular and metabolic disorders.
4.1. Cardiovascular Diseases
Omentin’s positive effects on vascular function, including vasodilation, anti-inflammatory actions, and potential anti-atherosclerotic effects, suggest potential applications in cardiovascular diseases (Biscetti et al., 2019; Biscetti et al., 2020). Therapies aimed at increasing omentin levels or enhancing its activity could be explored for conditions such as atherosclerosis, hypertension, and other cardiovascular disorders (Cetin Sanlialp et al., 2022; Fang et al., 2022; Okamura et al., 2023).
4.2. Metabolic Disorders
Omentin’s involvement in metabolic regulation, insulin sensitivity, and glucose metabolism makes it a potential target for metabolic disorders. Strategies to modulate omentin levels or activity may be considered in the management of insulin resistance, type 2 diabetes (Biscetti et al., 2020), and obesity (Weng et al., 2017; Zhou et al., 2020).
4.2.1. Omentin and obesity
In obesity associated with insulin resistance higher circulating levels of retinol-binding protein 4 (Sun et al., 2013), visfatin (Jacques et al., 2012), chemerin (Weng et al., 2017), vaspin (Feng et al., 2014) and resistin (Fontana et al., 2015) and to lower levels of omentin-1 (Narumi et al., 2014) and adiponectin (Wu et al., 2014) have been reported.
However, adipokine expression levels in specific adipose tissue depots might not necessarily correlate with the adipokine levels in the circulation (Margaritis et al., 2013), suggesting the existence of complex mechanisms that regulate the biology and secretome of the adipose tissue. Importantly, in obesity, omentin-1 plays a significant anti-inflammatory role, most likely through upregulating Th-2 cytokines like IL-13 and IL-14. Increased concentrations of omentin are thought to lower the levels of inflammatory cytokines (Zabetian-Targhi et al., 2016).
4.2.2. Insulin Resistance and Type 2 Diabetes
Improving insulin sensitivity is a key goal in managing insulin resistance and type 2 diabetes. Omentin’s potential to enhance insulin sensitivity suggests that it could be a therapeutic target for individuals with insulin resistance or those at risk of developing type 2 diabetes (Eimal Latif et al., 2021; Zabetian-Targhi et al., 2016; Zhou et al., 2020).
4.2.3. Obesity and Metabolic Syndrome
Omentin’s role in adipose tissue and its potential influence on lipid metabolism may have implications for obesity and metabolic syndrome (Cetin Sanlialp et al., 2022; Liu et al., 2011; Varona et al., 2019; Zhang et al., 2017). Therapeutic approaches that aim to modulate omentin levels or activity could be explored in the context of obesity management and preventing metabolic syndrome-related complications.
4.3. Inflammatory Disorders
Omentin’s anti-inflammatory properties make it relevant in conditions associated with chronic inflammation. This includes inflammatory disorders that can impact vascular health, such as rheumatoid arthritis (Robinson et al., 2017). Omentin-based interventions might be investigated as a complementary approach to address inflammation in these conditions.
4.4. Future Therapeutic Developments
Ongoing research may uncover novel therapeutic strategies, such as the development of omentin-based drugs or interventions that target omentin receptors. These advancements could open new avenues for personalized medicine approaches tailored to individuals with specific vascular and metabolic profiles.
Importantly, while the potential therapeutic applications of omentin are promising, further research, including clinical trials, is needed to establish its efficacy, safety, and optimal modes of administration. The field of omentin research is dynamic, and advancements in understanding its role in health and disease may lead to new therapeutic opportunities in the future.
5. Challenges and Future Directions
While omentin holds promise for its potential benefits in vascular function, there are several challenges and gaps in understanding that researchers face.
Receptor Identification and Signaling Pathways
Identifying the specific receptors through which omentin exerts its effects on vascular cells remains a challenge. Omentin receptors and the complete signaling pathways and downstream effects are not fully elucidated. A more comprehensive understanding of the molecular mechanisms involved is needed.
5.1. Tissue-Specific Effects
Omentin is expressed in various tissues, including adipose tissue, but its effects may be tissue specific. Understanding how omentin functions in different tissues and whether its effects on vascular function vary in different vascular beds is a complex aspect that requires further investigation.
5.2. Interactions with Other Adipokines
Omentin shares similarities with other adipokines, such as adiponectin or apelin. The interactions and potential synergies between omentin and other adipokines in modulating vascular function are not fully understood. Disentangling these interactions is crucial for a more comprehensive view of omentin’s role.
5.3. Dose-Response Relationships
Determining optimal dosage levels for potential therapeutic interventions is challenging. Omentin’s effects may vary based on concentration and understanding the dose-response relationships is essential for designing effective treatments without adverse effects.
5.4. Role in Disease Progression
The context-dependent role of omentin in various disease states is not fully understood. For example, while it may have anti-atherosclerotic effects, its role in advanced stages of vascular diseases or in conditions with chronic inflammation needs further exploration.
5.5. Biomarker Validity
Omentin has been proposed as a potential biomarker for certain metabolic and cardiovascular conditions. However, the validity, specificity, and sensitivity of omentin as a biomarker need to be thoroughly validated in diverse populations and clinical settings.
Initiate longitudinal studies to investigate the association between circulating omentin levels and the development of vascular and metabolic disorders over time. This could provide insights into the predictive value of omentin as a biomarker.
5.6. Limited Clinical Data
While preclinical studies suggest beneficial effects, translating these findings to clinical applications poses challenges. Establishing the safety, efficacy, and feasibility of interventions targeting omentin in humans requires well-designed clinical trials. Conduct well-designed clinical trials to assess the safety and efficacy of interventions targeting omentin in humans. Investigate the potential therapeutic applications of omentin in cardiovascular diseases, metabolic disorders, and other related conditions.
The number of clinical studies investigating omentin’s role in vascular function is relatively limited compared to preclinical research. Expanding the clinical evidence base is critical for understanding its relevance in human health and disease.
Addressing these challenges will contribute to a more comprehensive understanding of omentin’s role in vascular function and facilitate the development of targeted therapeutic interventions. As research progresses, these challenges are likely to be addressed, leading to a clearer picture of omentin’s potential in clinical applications.
Future research on omentin could explore several key areas to deepen our understanding of its role in health and disease.
5.7. Mechanistic Insights
Elucidate the precise molecular mechanisms through which omentin exerts its effects on vascular cells. Identify the specific receptors and downstream signaling pathways involved in omentin-mediated vasodilation, anti-inflammatory actions, and other vascular effects.
5.8. Role in Inflammation
Further investigate omentin’s role in modulating inflammation, both locally within adipose tissue and systemically. Explore how omentin’s anti-inflammatory actions may impact the progression of inflammatory diseases, including those affecting the vasculature.
5.9. Genetic Variations
Explore the impact of genetic variations in the omentin gene on omentin expression and function (Rathwa et al., 2019). Investigate whether specific genetic polymorphisms are associated with altered susceptibility to vascular and metabolic disorders.
5.10. Omentin as a Therapeutic Target
Evaluate the feasibility and efficacy of developing therapeutic interventions that directly target omentin or its receptors. Investigate the potential use of omentin-based drugs or interventions to improve vascular function and metabolic health.
5.11. Sex Differences
Explore potential sex differences in omentin levels and effects on vascular function. Investigate whether omentin’s role varies between males and females, which could have implications for personalized medicine approaches.
As research in the field of omentin continues, addressing these areas of exploration will contribute to a more comprehensive understanding of its physiological functions and potential clinical applications.
6. Conclusion
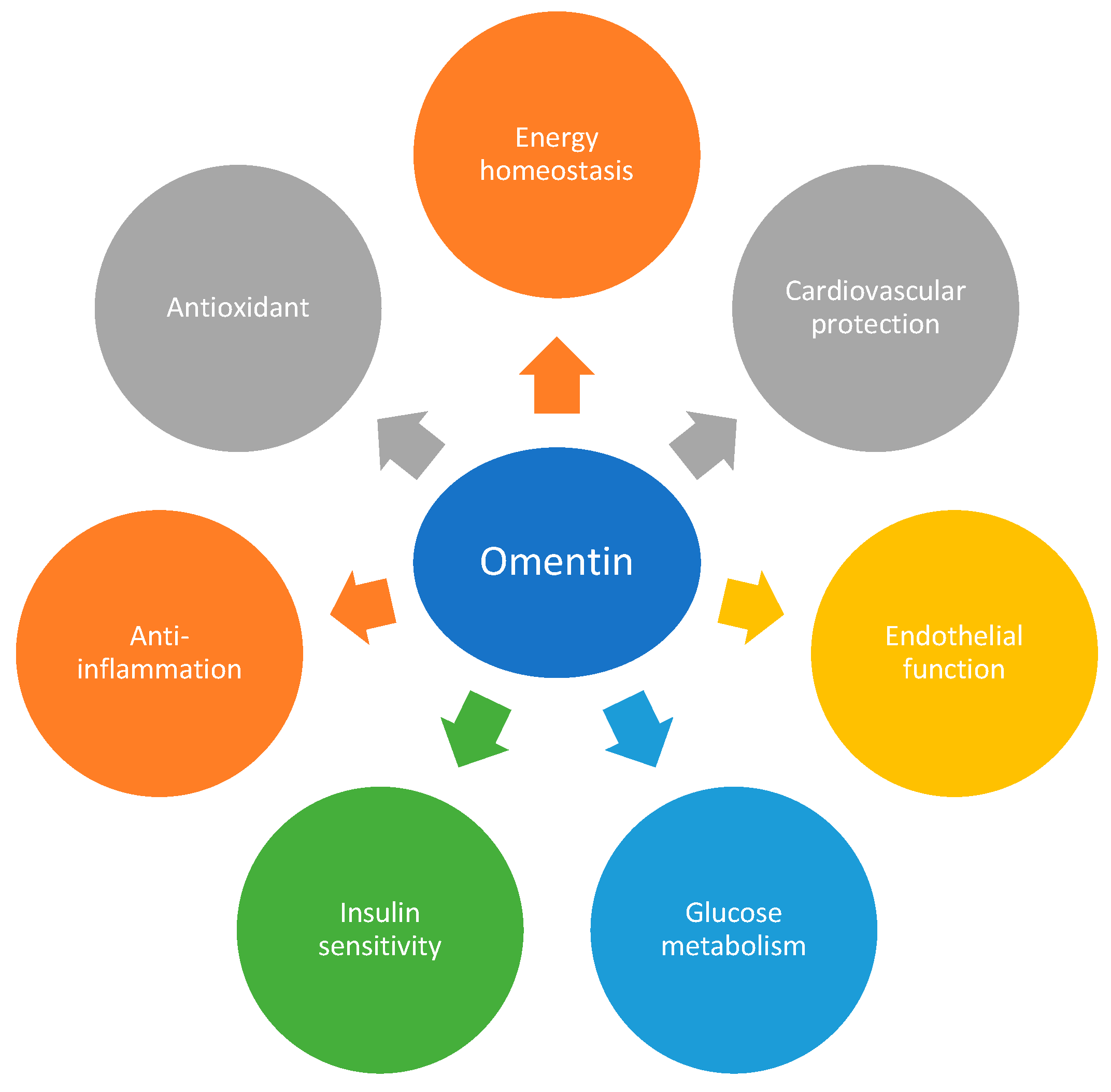
A schematic diagram summarizing the key features of omentin is presented.
Figure 1.
Schematic representation of the beneficial effects of omentin-1.
Figure 1.
Schematic representation of the beneficial effects of omentin-1.
- -
Omentin-1 is an adipocytokine widely expressed in a variety of cells, exhibiting microbial defense, antioxidative, anti-inflammatory, and anti-apoptotic properties.
- -
Omentin-1 exhibits a wide range of therapeutic potential in diabetes by reducing comorbidities linked to type 2 diabetes mellitus, such as vascular diseases and diabetic nephropathy (Senthilkumar et al., 2018; Song et al., 2018). Noteworthy, of the major adipocytokines that inhibit atherosclerosis, omentin-1 has a strong correlation with inflammation, macrophage differentiation, arterial calcification, and plaque formation (Xu et al., 2019).
- -
Omentin-1 inhibits insulin resistance, atherosclerosis, and inflammation through the intracellular signaling pathways of AMP-activated protein kinase/Akt/nuclear factor-κB/mitogen-activated protein kinase (ERK, p38, and JNK).
- -
Omentin-1 may be used as a biomarker for metabolic syndrome, obesity, diabetes, atherosclerosis, ischemic heart disease, and inflammatory diseases.
- -
This review sheds light on how omentin-1 might be used to treat these diseases and serve as a biomarker.
Funding
This work was supported by the Fundação para a Ciência e Tecnologia, Portugal: Reference number: 2022.04526.PTDC. FCT 2023.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Askin, L.; Duman, H.; Ozyıldız, A.; Tanriverdi, O.; Turkmen, S. Association between omentin-1 and coronary artery disease: pathogenesis and clinical research. Curr Cardiol Rev. 2020,16,198-201. [CrossRef]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc Diabetol. 2019,18,74. [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M. M.; Cecchini, A. L.; Angelini, F.; Cina, A.; Iezzi, R.; Filipponi, M.; Santoliquido, A.; Pitocco, D.; Landolfi, R.; Flex, A. Association between omentin-1 and major cardiovascular events after lower extremity endovascular revascularization in diabetic patients: a prospective cohort study. Cardiovasc Diabetol. 2020,19,170. [CrossRef]
- Brunetti, L.; Leone, S.; Orlando, G.; Ferrante, C.; Recinella, L.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol Rep. 2014,66,991-995. [CrossRef]
- Cai, R.C.; Wei, L.; DI J.Z.; Yu, H.Y.; Bao, Y.Q.; Jia, W.P. Expression of omentin in adipose tissues in obese and type 2 diabetic patients. Zhonghua Yi Xue Za Zhi. 2009,89:381-4.
- Cetin Sanlialp, S.; Nar, G.; Nar, R. Relationship between circulating serum omentin-1 levels and nascent metabolic syndrome in patients with hypertension. J Investig Med 2022,70,780-785. [CrossRef]
- De Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; Fried, S. K.; Gong, D.W.; Shuldiner, A.R.; Pollin, T.I.; McLenithan, J.C. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007,56,1655-1661. [CrossRef]
- Dong, Q.; Xing, W.; Li, K.; Zhou, X.; Wang, S.; Zhang, H. Tetrahydroxystilbene glycoside improves endothelial dysfunction and hypertension in obese rats: The role of omentin-1. Biochem Pharmacol. 2021,186,114489. [CrossRef]
- Du, Y.; Ji, Q.; Cai, L.; Huang, F.; Lai, Y.; Liu, Y.; Yu, J.; Han, B.; Zhu, E.; Zhang, J.; Zhou, Y.; Wang, Z.; Zhao, Y. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc Diabetol. 2016,15,90. [CrossRef]
- Eimal Latif, A.H.; Anwar, S.; Gautham, K.S.; Kadurei, F.; Ojo, R.O.; Hafizyar, F.; Muhammad Haroon, D.; Rakesh, F.; Talpur, A.S. Association of plasma omentin-1 levels with diabetes and its complications. Cureus 2021,13,e18203. [CrossRef]
- Elsaid, N.H.; Sadik, N.A.; Ahmed, N.R.; Fayez, S.E.; Mohammed, N.A.E. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J Clin Transl Endocrinol. 2018,13.14-19. [CrossRef]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E., Wolf, R.Y.; Carter, R.A., Tichansky, D.S.; Madan, A.K. Identification of omentin mRNA in human epicardial adipose tissue: Comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond). 2008,32,810-815. [CrossRef]
- Fang, L.; Ohashi, K.; Otaka, N.; Ogawa, H.; Hiramatsu-Ito, M.; Kawanishi, H.; Bando, Y. K.; Shibata, R.; Shimizu, Y.; Kato, K.; Takikawa, T.; Ozaki, Y.; Takefuji, M.; Murohara, T.; Ouchi, N. Omentin attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice. Cardiovasc Res. 2022,6,118,1597-1610. [CrossRef]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta-analysis. Diabetes Res. Clin. Pract. 2014,106,88-94. [CrossRef]
- Fernández-Trasancos, Á.; Agra, R.M.; García-Acuña, J.M.; Fernández, Á.; González-Juanatey, J.R.; Eiras, S. Omentin treatment of epicardial fat improves its anti-inflammatory activity and paracrine benefit on smooth muscle cells. Obesity. 2017,25,1042-1049. [CrossRef]
- Fontana, A.; Spadaro, S.; Copetti, M.; Spoto, B.; Salvemini, L.; Pizzini, P.; Frittitta, L.; Mallamaci, F.; Pellegrini, F.; Trischitta, V.; Menzaghi, C. Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis. Plos one 2015,10, e0120419. [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cel. Endocr. 2010, 316, 129-139. [CrossRef]
- Ge, Q.; Xie, X.; Chen, X.; Huang, R.; Rui, C. X.; Zhen, Q.; Hu, R.; Wu, M.; Xiao, X.; Li, X. Circulating exosome-like vesicles of humans with nondiabetic obesity impaired islet β-cell proliferation, which was associated with decreased Omentin-1 protein cargo. Genes Dis. 2021,9,1099-1113. [CrossRef]
- Greulich, S.; Chen, W.J.; Maxhera, B.; Rijzewijk, L.J.; van der Meer, R.W.; Jonker, J.T.; Mueller, H.; de Wiza, D.H.; Floerke, R.R.; Smiris, K.; Lamb, H.J.; de Roos, A.; Bax, J.J.; Romijn, J.A.; Smit, J.W.; Akhyari, P.; Lichtenberg, A.; Eckel, J.; Diamant, M.; Ouwens, D.M. Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studies. PLoS One. 2013;8:e59697. [CrossRef]
- Gu, N.; Wang, J.; Di, Z.; Liu, Z.; Jia, X.; Yan, Y.; Chen, X.; Zhang, Q.; Qian, Y. The effects of intelectin-1 on antioxidant and angiogenesis in HUVECs exposed to oxygen glucose deprivation. Front Neurol 2019,10,383. [CrossRef]
- Harada, K.; Shibata, R.; Ouchi, N.; Tokuda, Y.; Funakubo, H.; Suzuki, M.; Kataoka, T.; Nagao, T.; Okumura, S.; Shinoda, N.; Kato, B.; Sakai, S.; Kato, M.; Marui, N.; Ishii, H.; Amano, T.; Matsubara, T.; Murohara, T. Increased expression of the adipocytokine omentin in the epicardial adipose tissue of coronary artery disease patients. Atherosclerosis 2016, 251, 299-304. [CrossRef]
- Hayashi, M.; Morioka, T.; Hatamori, M.; Kakutani, Y.; Yamazaki, Y.; Kurajoh, M.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shioi, A.; Shoji, T.; Emoto, M.; Inaba, M. Plasma omentin levels are associated with vascular endothelial function in patients with type 2 diabetes at elevated cardiovascular risk. Diabetes Res. Clin. Pract. 2019,148,160-168. [CrossRef]
- Herder, C.; Ouwens, D. M.; Carstensen, M.; Kowall, B.; Huth, C.; Meisinger, C.; Rathmann, W.; Roden, M.; & Thorand, B. Adiponectin may mediate the association between omentin, circulating lipids and insulin sensitivity: results from the KORA F4 study. Eur J Endocrinol. 2015,172,423-432. [CrossRef]
- Hiramatsu-Ito, M.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kanemura, N.; Kambara, T.; Enomoto, T.; Yuasa, D.; Matsuo, K.; Ito, M.; Hayakawa, S.; Ogawa, H.; Otaka, N.; Kihara, S.; Murohara, T.; Ouchi, N. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2016, 110, 107-117. [CrossRef]
- Jacques, C.; Holzenberger, M.; Mladenovic, Z.; Salvat, C.; Pecchi, E.; Berenbaum, F.; Gosset, M. Proinflammatory actions of visfatin/nicotinamide phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and nampt enzymatic activity. J. Biol. Chem. 2012, 287,15100-15108. [CrossRef]
- Kataoka, Y.; Shibata, R.; Ohashi, K.; Kambara, T.; Enomoto, T.; Uemura, Y.; Ogura, Y.; Yuasa, D.; Matsuo, K.; Nagata, T.; Oba, T.; Yasukawa, H.; Numaguchi, Y.; Sone, T.; Murohara, T.; Ouchi, N. Omentin Prevents Myocardial Ischemic Injury Through AMP-Activated Protein Kinase- and Akt-Dependent Mechanisms. J. Am. Coll. Cardiol. 2014, 63, 2722-2733. [CrossRef]
- Kazama, K.; Okada, M.; Hara, Y.; Yamawaki, H. A novel adipocytokine, omentin, inhibits agonists-induced increases of blood pressure in rats. J Vet Med Sci 2013,75,1029 -1034. [CrossRef]
- Kazama, K.; Okada, M.; Yamawaki, H. Adipocytokine, omentin inhibits doxorubicin-induced h9c2 cardiomyoblasts apoptosis through the inhibition of mitochondrial reactive oxygen species. Biochem Biophys Res Commun. 2015,457,602-607. [CrossRef]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-a-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012,686,116-123. [CrossRef]
- Leandro, A.; Queiroz, M.; Azul, L.; Seiça, R.; Sena, C.M. Omentin: A novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes. Free. Radic. Biol. Med. 2021, 162, 233-242. [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; Zhou, Y., Wang, Z. Omentin-1 Modulates Macrophage Function via Integrin Receptors 3 and 5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [CrossRef]
- Liu, F.; Fang, S.; Liu, X.; Li, J.; Wang, X.; Cui, J.; Chen, T.; Li, Z.; Yang, F.; Tian, J.; Li, H.; Yin, L.; Yu, B. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARδ signaling pathway. Biochem Pharmacol. 2020; 174:113830. [CrossRef]
- Liu, R.; Wang, X.; Bu, P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract 2011,93,21-25. [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407,233-341.
- Margaritis, M.; Antonopoulos, A.S.; Digby, J.; Lee, R.; Reilly, S.; Coutinho, P.; Shirodaria, C.; Sayeed, R.; Petrou, M.; De Silva, R.; Jalilzadeh, S.; Demosthenous, M.; Bakogiannis, C.; Tousoulis, D.; Stefanadis, C.; Choudhury, R.P.; Casadei, B.; Channon, K.M.; Antoniades, C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 2013,127, 2209-2221. [CrossRef]
- Maruyama, S.; Shibata, R.; Kikuchi, R.; Izumiya, Y.; Rokutanda, T.; Araki, S.; Kataoka, Y.; Ohashi, K.; Daida, H.; Kihara, S.; Ogawa, H.; Murohara, T.; Ouchi, N. Fat-derived Factor Omentin Stimulates Endothelial Cell Function and Ischemia-induced Revascularization via Endothelial Nitric Oxide Synthase-dependent Mechanism. J. Biol. Chem. 2012, 287, 408-417. [CrossRef]
- Michalczyk, K.; Niklas, N.; Rychlicka, M.; & Cymbaluk-Płoska, A. The Influence of Biologically Active Substances Secreted by the Adipose Tissue on Endometrial Cancer. Diagnostics (Basel). 2021,11,494. [CrossRef]
- Moreno-Navarrete, J.M.; Catalán, V.; Ortega, F.; Gómez-Ambrosi, J.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Circulating omentin concentration increases after weight loss. Nutr Metab. 2010,7,27. [CrossRef]
- Narumi, T.; Watanabe, T.; Kadowaki, S.; Kinoshita, D.; Yokoyama, M.; Honda, Y.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; Shishido, T.; Miyamoto, T.; Kubota, I. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Eur. Heart J. 2014,35, 221-222. [CrossRef]
- Okamura, Y.; Adachi, K.; Niijima, R.; Kodama, T.; Otani, K.; Okada, M.; Yamawaki, H. Human omentin-1 reduces vascular insulin resistance and hypertension in Otsuka Long-Evans Tokushima Fatty rats. Naunyn Schmiedebergs Arch Pharmacol. 2023. [CrossRef]
- Pan, H.Y.; Guo, L.; Li, Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010,88,29-33. [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Acheampong, K.; Liu, A. Omentin-1 in diabetes mellitus: A systematic review and meta-analysis. PloS One 2019,14,e0226292. [CrossRef]
- Qi, D.; Tang, X.; He, J.; Wang, D.; Zhao, Y.; Deng, W.; Deng, X.; Zhou, G.; Xia, J.; Zhong, X.; Pu, S. Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism. Cell Death Dis. 2016, 7:e2360. [CrossRef]
- Rao, S.S.; Hu, Y.; Xie, P.L.; Cao, J.; Wang, Z.X.; Liu, J.H.; Yin, H.; Huang, J.; Tan, Y. J.; Luo, J.; Luo, M.J.; Tang, S.Y.; Chen, T.H.; Yuan, L.Q.; Liao, E.Y.; Xu, R.; Liu, Z.Z.; Chen, C.Y.; Xie, H. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone Res 2018,6, 9. [CrossRef]
- Rathwa, N.; Patel, R.; Palit, S.P.; Jadeja, S.D.; Narwaria, M.; Ramachandran, A.V.; Begum, R. Circulatory Omentin-1 levels but not genetic variants influence the pathophysiology of type 2 diabetes. Cytokine 2019,119, 144-151. [CrossRef]
- Robinson, C.; Tsang, L.; Solomon, A.; Woodiwiss, A.J.; Gunter, S.; Millen, A.M.; Norton, G.R.; Fernandez-Lopez, M.J.; Hollan, I.; Dessein, P.H. Omentin concentrations are independently associated with those of matrix metalloproteinase-3 in patients with mild but not severe rheumatoid arthritis. Rheumatol Int. 2017,37,3-11. [CrossRef]
- Saremi, A.; Asghari, M.; Ghorbani, A. Effects of aerobic training on serum omentin- 1 and cardiometabolic risk factors in overweight and obese men. J. Sports Sci. 2010,4,1-6. [CrossRef]
- Sena, C. M.; Pereira, A.; Fernandes, R.; Letra, L.; Seiça, R. M. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue. Br J Pharmacol. 2017,174,3514-3526. [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta 2014,1832,2216-2231.
- Senthilkumar, G.P.; Anithalekshmi, M.S.; Yasir, M.; Parameswaran, S.; Packirisamy, R. M.; Bobby, Z. Role of omentin 1 and IL-6 in type 2 diabetes mellitus patients with diabetic nephropathy. Diabetes Metab Syndr. 2018,12,23-26. [CrossRef]
- Shibata, R.; Ouchi, N.; Takahashi, R.; Terakura, Y.; Ohashi, K.; Ikeda, N.; Higuchi, A.; Terasaki, H.; Kihara, S.; Murohara, T. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr. 2012,4,37. [CrossRef]
- Song, J.; Zhang, H.; Sun, Y.; Guo, R.; Zhong, D.; Xu, R.; Song, M. Omentin-1 protects renal function of mice with type 2 diabetic nephropathy via regulating miR-27a-Nrf2/Keap1 axis. Biomed Pharmacother. 2018,107,440-446. [CrossRef]
- Sperling, M.; Grzelak, T.; Pelczyńska, M.; Jasinska, P.; Bogdanski, P.; Pupek-Musialik, D.; Czyzewska, K. Concentrations of omentin and vaspin versus insulin resistance in obese individuals. Biomed. Pharmacother. 2016,83,542-547. [CrossRef]
- Sun, Q.; Kiernan, U.A.; Shi, L.; Phillips, D.A.; Kahn, B.B.; Hu, F.B.; Manson, J.E.; Albert, C.M.; Rexrode, K.M. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease a prospective analysis among women in the Nurses’ Health Study. Circulation 2013,127,1938-1947.
- Tan, B.K.; Adya, R.; Farhatullah, S.; Chen, J.; Lehnert, H.; Randeva, H.S. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes 2010,59,3023-3031. [CrossRef]
- Uemura, Y.; Shibata, R.; Kanemura, N.; Ohashi, K.; Kambara, T.; Hiramatsu-Ito, M.; Enomoto, T.; Yuasa, D.; Joki, Y.; Matsuo, K.; Ito, M.; Hayakawa, S.; Ogawa, H.; Murohara, T.; Ouchi, N. Adipose-derived protein omentin prevents neointimal formation after arterial injury. FASEB J. 2015,29,141-151. [CrossRef]
- Varona, J.F.; Ortiz-Regalón, R.; Sánchez-Vera, I.; López-Melgar, B.; García-Durango, C.; Castellano Vázquez, J.M.; Solís, J.; Fernández-Friera, L.; Vidal-Vanaclocha, F. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch Med Res. 2019,50,20-28. [CrossRef]
- Waluga, M.; Kukla, M.; Kotulski, R.; Zorniak, M.; Boryczka, G.; Kajor, M.; Ciupinska-Kajor, M.; Lekstan, A.; Olczyk, P.; Waluga, E. Omentin, vaspin and irisin in chronic liver diseases. J Physiol Pharmacol 2019,70,277-285. [CrossRef]
- Wang, J.; Gao, Y.; Lin, F.; Han, K.; Wang, X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-kB signaling. Arch Biochem Biophys. 2019,679,108187. [CrossRef]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T. A.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; Watanabe, T. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc. Res. 2016, 110, 118-128. [CrossRef]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Physiol. 2017, 7, 765-781.
- Weng, C.; Shen, Z.; Li, X.; Jiang, W.; Peng, L.; Yuan, H.; Yang, K.; Wang, J. Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am. J. Transl Res. 2017,9, 3096-3104.
- Wu, Z.J.; Cheng, Y.J.; Gu, W.J. Aung; L.H. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism 2014,63, 1157-1166. [CrossRef]
- Xie, H.; Xie, P.-L.; Wu, X.-P.; Chen, S.-M.; Zhou, H.-D.; Yuan, L.-Q.; Sheng, Z.-F.; Tang, S.-Y.; Luo, X.-H.; Liao, E.-Y. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc. Res. 2011, 92, 296-306. [CrossRef]
- Xu, F.; Li, F.X.; Lin, X.; Zhong, J.Y.; Wu, F.; Shan, S.K.; Tan, C.M.; Yuan, L.Q.; Liao, X.B. Adipose tissue-derived omentin-1 attenuates arterial calcification via AMPK/Akt signaling pathway. Aging 2019,11,8760-8776. [CrossRef]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011,408,339-343. [CrossRef]
- Yamawaki, H.; Tsubaki, N.; Mukohda, M.; Okada, M.; Hara, Y. Omenting a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010,393,668-672. [CrossRef]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253-E1261. [CrossRef]
- Zabetian-Targhi, F.; Mirzaei, K.; Keshavarz, S.A.; Hossein-Nezhad, A. Modulatory role of omentin-1 in inflammation: cytokines and dietary intake. J Am Coll Nutr. 2016,35,670-678. [CrossRef]
- Zhang, M.; Tan, X.; Yin, C.; Wang, L.; Tie, Y.; Xiao, Y. Serum levels of omentin-1 are increased after weight loss and are particularly associated with increases in obese children with metabolic syndrome. Acta Paediatr 2017,106,1851-1856. [CrossRef]
- Zhou, H.; Zhang, Z.; Qian, G.; Zhou, J. Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice. Fundam Clin Pharmacol 2020,34,721-735. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).