Submitted:
27 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
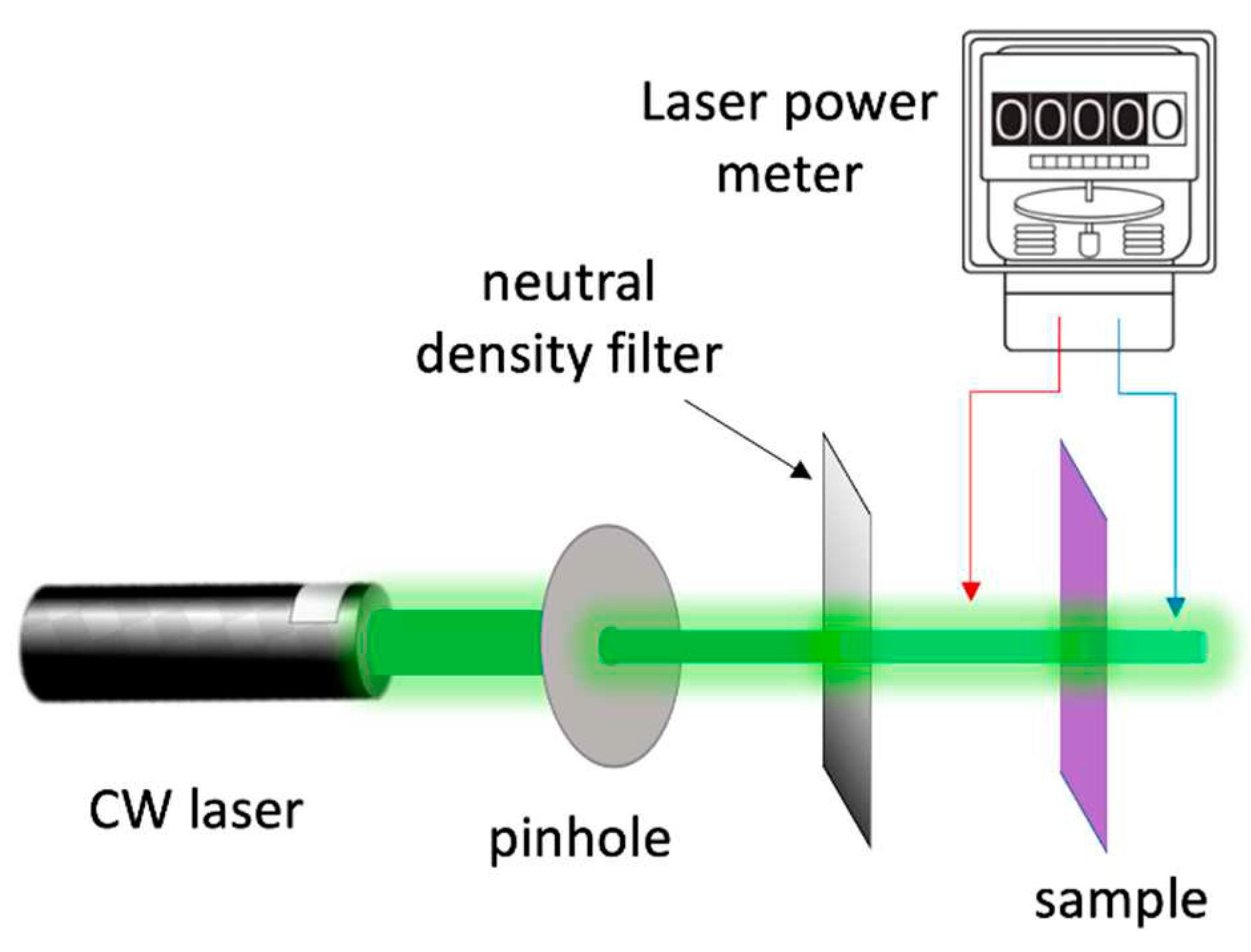
2. Materials and Methods
2.1. Materials
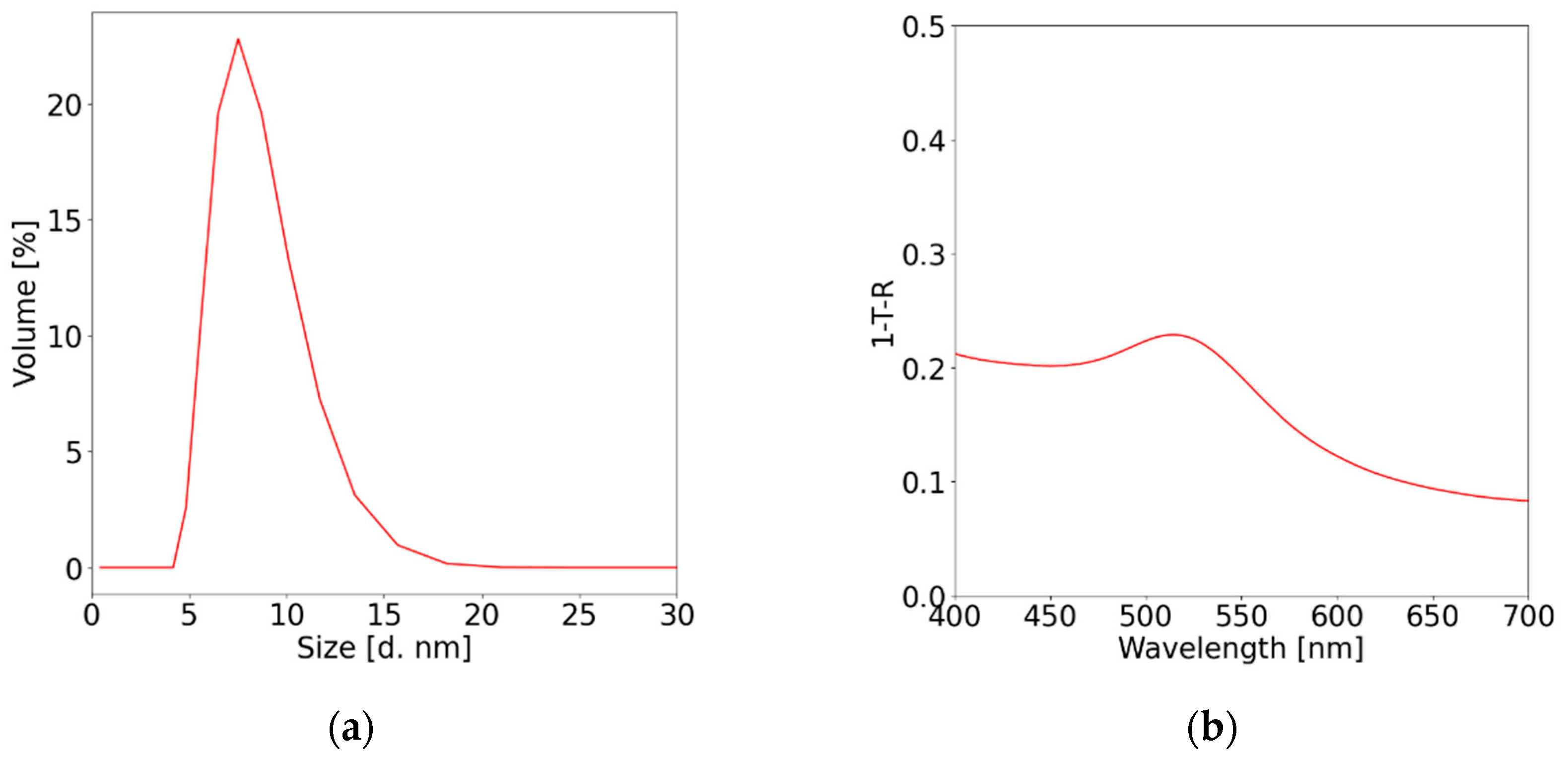
2.2. Gold nanoparticles (Au NPs) synthesis
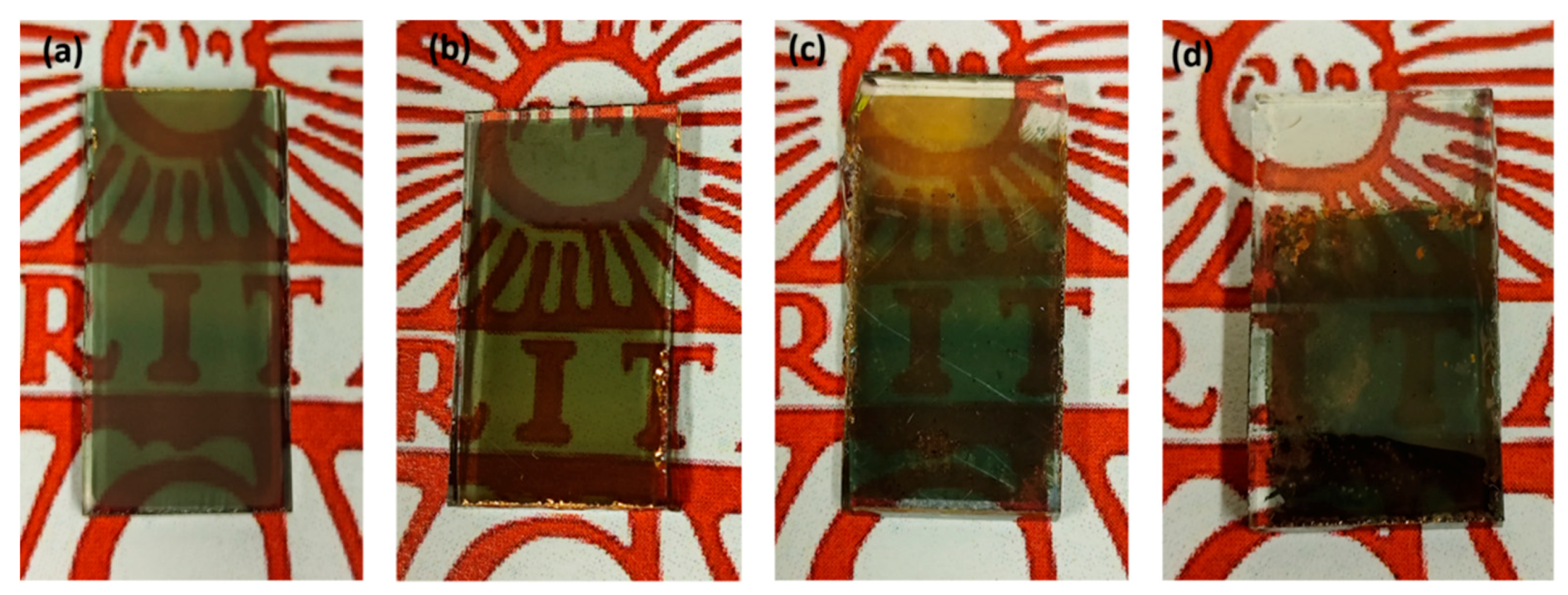
2.3. Device fabrication
2.4. Characterisation
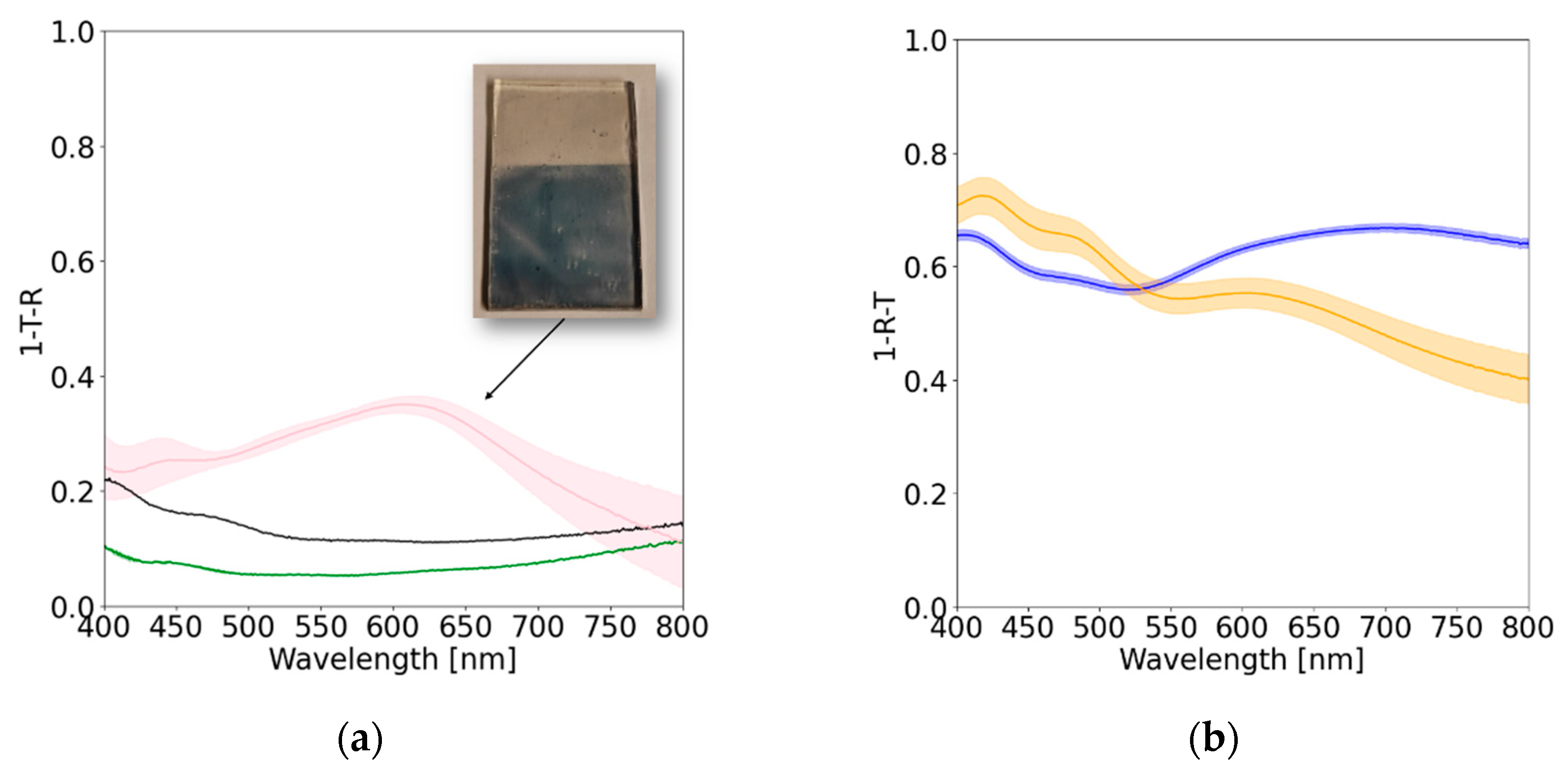
3. Results and discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Brongersma, M. L.; Halas, N. J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotech. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Salamin, Y.; Ma, P; Baeuerle, B. ; Emboras, A.; Fedoryshyn, Y.; Heni, W.; Cheng, B’; Josten, A.; Leuthold, J. 100 GHz Plasmonic Photodetector. ACS Photon. 2018, 5, 3291–3295. [Google Scholar] [CrossRef]
- Zou, X.; Vadell, R. B.; Cai, B.; Geng, X.; Dey, A.; Liu, Y.; Gudmundsson, A.; Meng, J.; Sá, J. Ultrafast infrared-to-visible photon upconversion on plasmon/TiO2 solid films. J. Phys. Chem. Lett. 2023, 14, 6255–6262. [Google Scholar] [CrossRef]
- Brongersma, M. L. Plasmonic Photodetectors, Photovoltaics, and Hot-Electron Devices. Proc. IEEE 2016, 104, 2349–2361. [Google Scholar] [CrossRef]
- Vadell, R. B.; Zou, X.; Drillet, M.; Corvoysier, H.; Silveira, V. R.; Konezny, S. J.; Sá, J. Carrier dynamics in solution-processed CuI as a p-type semiconductor: the origin of negative photoconductivity. J. Phys. Chem. Lett. 2023, 14, 1007–1013. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/ metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photon. 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Geng, X.; Abdellah, M.; Vadell, R. B.; Folkenant, M.; Edvinsson, T.; Sá, J. Direct plasmonic solar cell efficiency dependence on spito-OMeTAD Li-TFSI content. Nanomaterials 2021, 11, 3329. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Lee, J.; Singh, N.; Krämer, S.; Stucky, G. D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotech. 2013, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Lee, J.; Singh, N.; Krämer, S.; Stucky, G. D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotech. 2013, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Ueno, K.; Misawa, H. Plasmon-induced ammonia synthesis through nitrogen photofixation with visible light irradiation. Angew. Chem. Int. Ed. 2014, 53, 9802–9805. [Google Scholar] [CrossRef]
- Zhang, N.; Han, C.; Fu, X.; Xu, Y. J. Funtion-oriented engineering of metal-based nanohybrids for photoredox catalysis: exerting plasmonic effect and beyond. Chem 2018, 4, 1832–1861. [Google Scholar] [CrossRef]
- . Vadell, R. B.; Sekar, P.; Patehebieke, Y.; Zou, X.; Kaul, N.; Broqvist, P.; Lindblad, R.; Lindblad, A.; Arkhypchuk, A.; Walletin, C.-J.; Sá, J. Single-Electron Transfer Reactions on Surface-Modified Gold Plasmons. Mater. Today Chem. 2023, 34, 101783. [Google Scholar] [CrossRef]
- Brongersma, M. L.; Shalaev, V. M. The Case for Plasmonics. Science 2010, 328, 440–441. [Google Scholar] [CrossRef]
- Coronado, E. A.; Encina, E. R.; Stefani, F. D. Optical properties of metallic nanoparticles: manipulating light, heat and forces at the nanoscale. Nanoscale 2011, 3, 4042–4059. [Google Scholar] [CrossRef] [PubMed]
- Hartland, G. V. Optical Studies of Dynamics in Noble Metal Nanostructures. Chem. Rev. 2011, 111, 3858–3887. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Gallardo, O. A.; Berdakin, M.; Fauenheim, T.; Sánchez, C. G. Plasmon-induced hot-carrier generation differences in gold and silver nanoclusters. Nanoscale 2019, 11, 8604–8615. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Tikhodeev, S. G.; Gippius, N. A.; Kuhl, J.; Giessen, H. Waveguide-plasmon polaritons: strong coupling of photonic and electronic resonances in a metallic photonic crystal slab. Phys. Rev. Lett. 2003, 91, 183901. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Liang, E.; Cai, G.; Hu, W.; Fan, C.; Xue, Q. Dual-band perfect absorption and field enhancement by interaction between localized and propagating surface plasmons in optical metamaterials. J. Opt. 2011, 13, 075005. [Google Scholar] [CrossRef]
- Zeng, P.; Cadusch, J.; Chakraborty, D.; Smith, T. A.; Roberts, A.; Sader, J. E.; Davis, T. J.; Gómez, D. E. Photoinduced electron transfer in the strong coupling regime: waveguide-plasmon polaritons. Nano Lett. 2016, 16, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, C.; Zeltzer, G.; Ruiz, R.; Wangperawong, A.; Roelofs, K. E.; Bent, S. F. Strong coupling of plasmon and nanocavity modes for dual-band, near-perfect absorbers and ultrathin photovoltaics. ACS Photon. 2016, 3, 456–463. [Google Scholar] [CrossRef]
- Shi, X.; Ueno, K.; Oshikiri, T.; Sun, Q.; Sasaki, K.; Misawa, H. Enhanced water splitting under modal strong coupling conditions. Nat. Nanotech. 2018, 13, 953–958. [Google Scholar] [CrossRef]
- Pérot, A. & Fabry, C. On the Application of Interference Phenomena to the Solution of Various Problems of Spectroscopy and Metrology. Astrophys. J. 1899, 9, 87–115. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N. G.; Puntes, V. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–5463. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Lai, J.; Song, X.; Yang, X.; Hagfeldt, A.; Sun, L. One plus one greater than two: high-performance inverted planar perovskite solar cells based on a composite CuI/CuSCN hole-transporting layer. J. Mater. Chem. A 2018, 6, 21435–21444. [Google Scholar] [CrossRef]
- Archer, D.G.; Wang, P. The Dielectric Constant of Water and Debye-Hückel Limiting Law Slopes. J. Phys. Chem. Ref. Data 1990, 19, 371–411. [Google Scholar] [CrossRef]
- Pattanasattayavong, P.; Mottram, A. D.; Yan, F.; Anthopoulos, T. D. Study of the Hole Transport Processes in Solution-Processed Layers of the Wide Bandgap Semiconductor Copper(I) Thiocyanate (CuSCN). Adv. Funct. Mater. 2015, 25, 6802–6813. [Google Scholar] [CrossRef]
- Hawe, P.; Silveira, V. R. R.; Vadell, R. B.; Lewin, E.; Sá, J. Plasmon-Mediated Oxidation Reaction on Au/p-Cu2O: The Origin of Hot Holes. Physchem 2021, 1, 163–175. [Google Scholar] [CrossRef]
- Collier, C. T.; Hesse, E.; Taylor, L.; Ulanowski, Z.; Penttilä, A.; Nousiainen, T. Effects of surface roughness with two scales on light scattering by hexagonal ice crystals large compared to the wavelength: DDA results. J. Quant. Spectrosc. Radiat. Transf. 2016, 182, 225–239. [Google Scholar] [CrossRef]
- Wu, F.; Finkelstein-Shapiro, D.; Wang, M.; Rosenkampff, I.; Yartsev, A.; Pascher, T.; Nguyen-Phan, T. C.; Cogdell, R.; Börjesson, K.; Pullerits, T. Optical cavity-mediated exciton dynamics in photosynthetic light harvesting 2 complexes. Nat. Commun. 2022, 13, 6864. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Lee, M.-W.; Chou, P.-T.; Scholes, G. D.; Schatz, G. C.; Hsu, L.-Y. Can Nanocavities Significantly Enhance Resonance Energy Transfer in a Single Donor–Acceptor Pair? J. Phys. Chem. C 2021, 125, 18119–18128. [Google Scholar] [CrossRef]
- Silveira, V. R.; Vadell, R. B.; Sá, J. Photoelectrocatalytic conversion of nitrates to ammonia with plasmon hot-electrons. J. Phys. Chem. C 2023, 127, 5425–5431. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M. A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Wang, Z. L.; El-Sayed, M. A. Electron dynamics in gold and gold–silver alloy nanoparticles: The influence of a nonequilibrium electron distribution and the size dependence of the electron– phonon relaxation. J. Chem. Phys. 1999, 111, 1255–1264. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M. A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. B 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Tagliabue, G.; DuChene, J. S.; Abdellah, M.; Habib, A.; Gosztola, D. J.; Hattori, Y.; Cheng, W.-H.; Zheng, K.; Canton, S. E.; Sundararaman, R.; Sá, J.; Atwater, H. A. Ultrafast Hot-Hole Injection Modifies Hot-Electron Dynamics in Au/p-GaN Heterostructures. Nat. Mater. 2020, 19, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Ratchford, D. C.; Dunkelberger, A. D.; Vurgaftman, I.; Owrutsky, J. C. Quantification of efficient plasmonic hot-electron injection in gold nanoparticle-TiO2 films. Nano Lett. 2017, 17, 6047–6055. [Google Scholar] [CrossRef] [PubMed]
- Pattanasattayavong, P.; Promarak, V.; Anthopoulos, T. D. Electronic properties of Copper(I) Thiocyanate (CuSCN). Adv. Electron. Mater. 2017, 3, 1600378. [Google Scholar] [CrossRef]

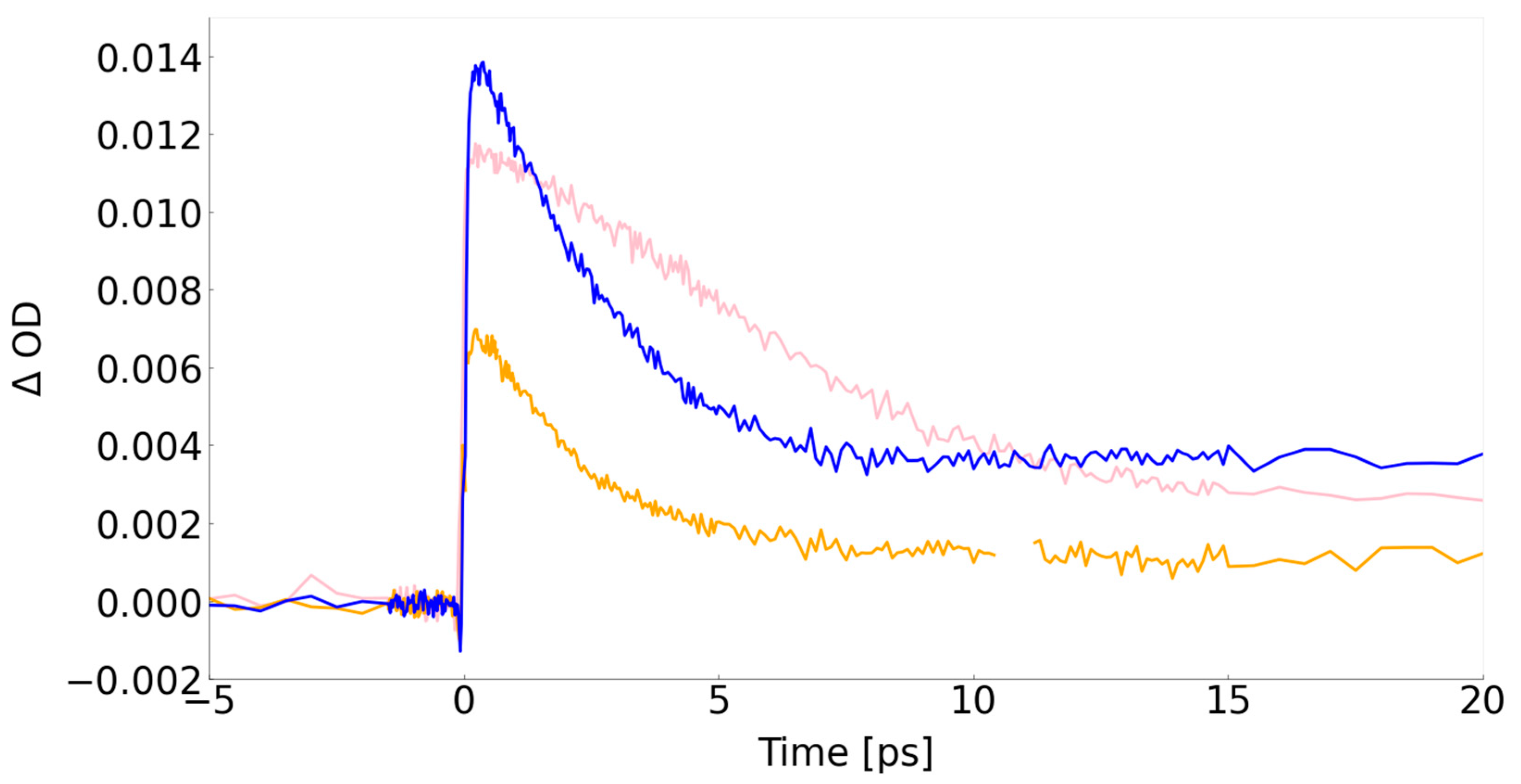
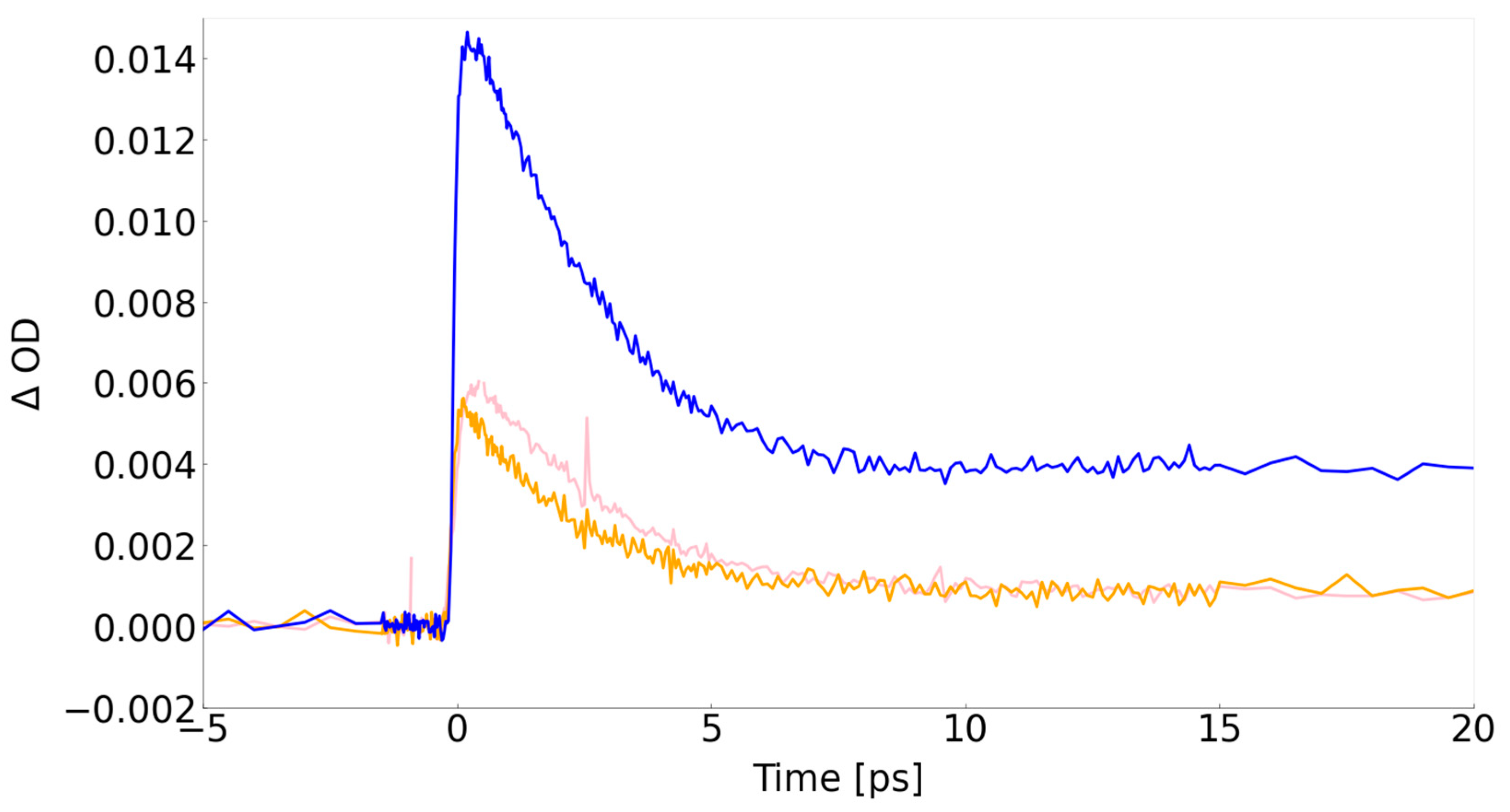
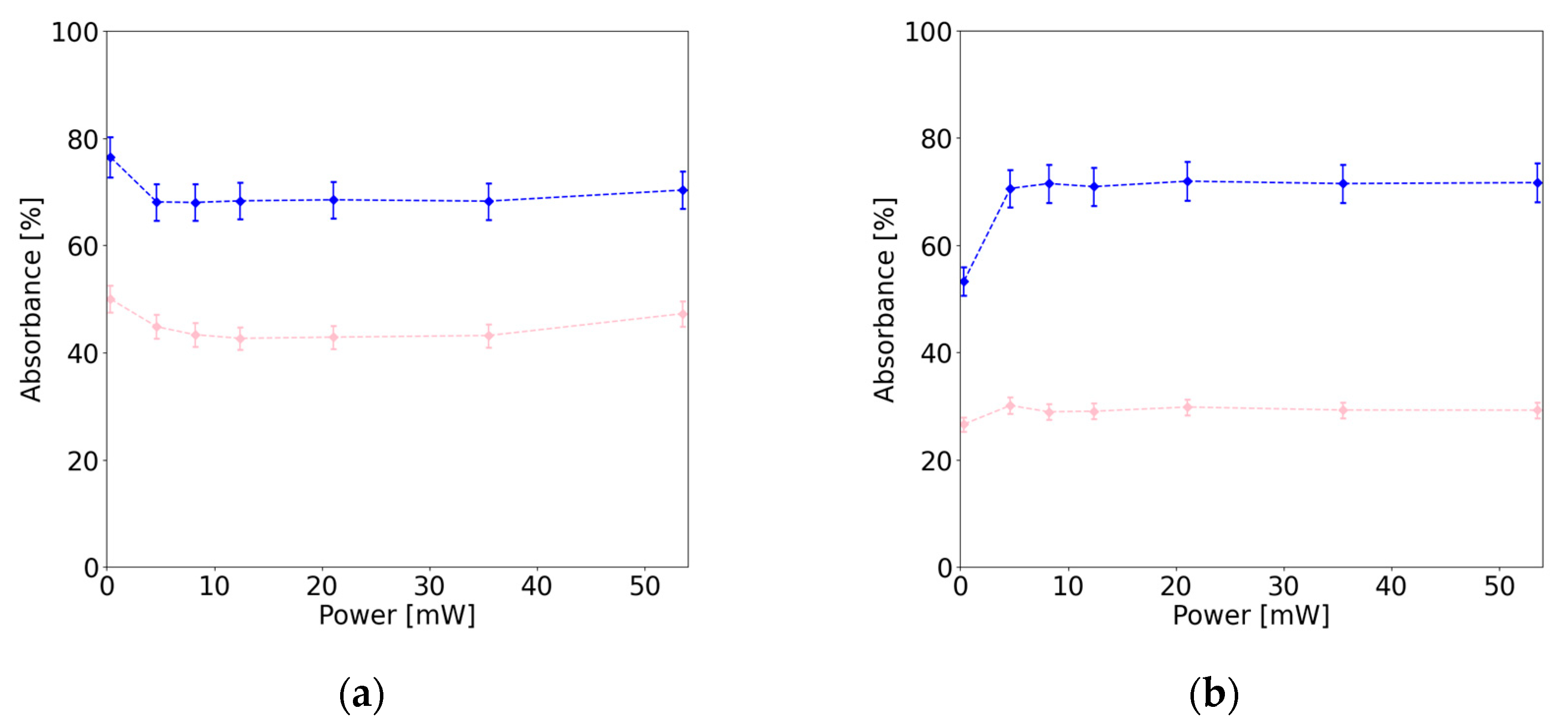
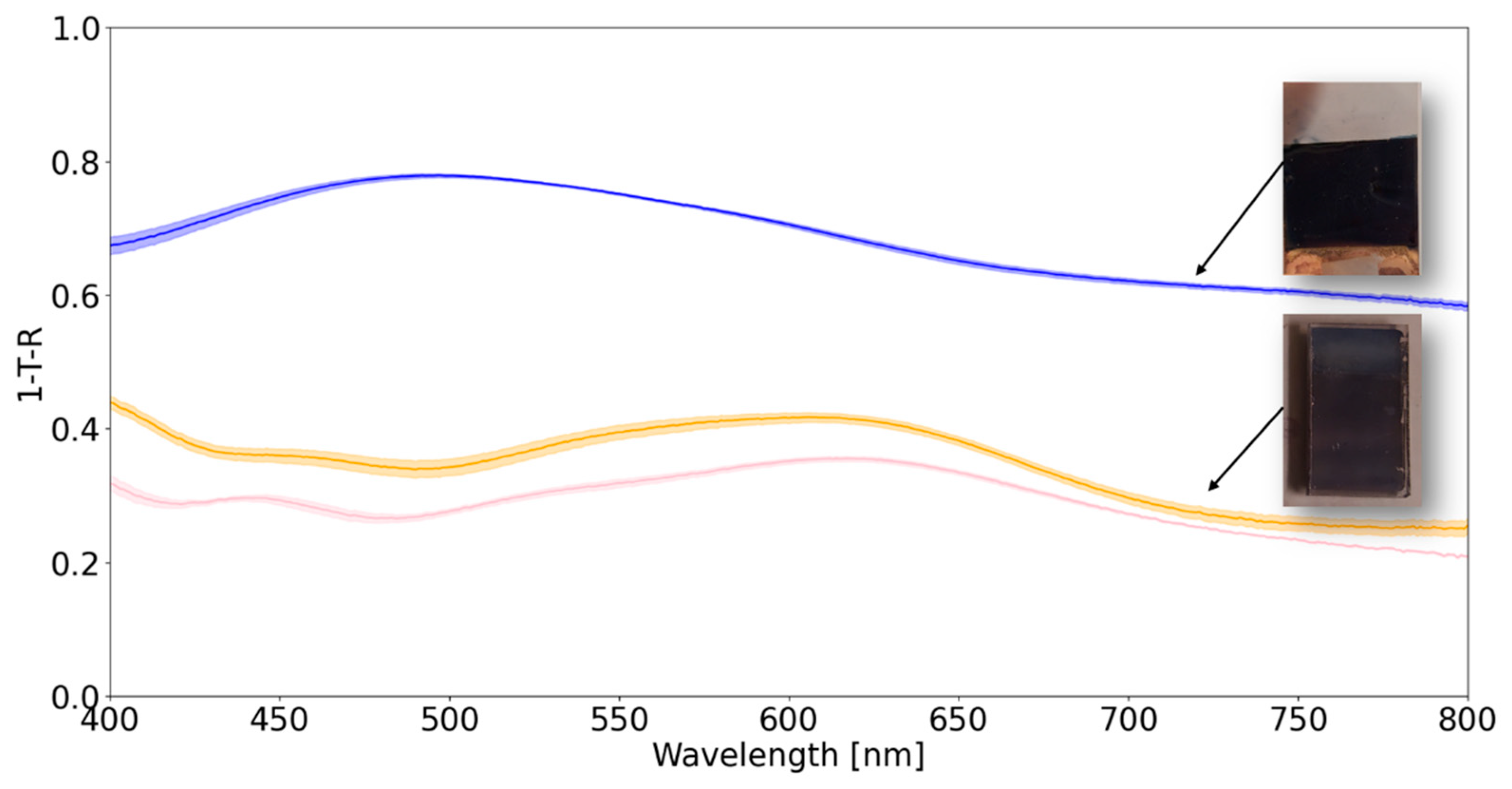
| Sample | Resonant excitation (ps) |
Non-resonant excitation (ps) |
|---|---|---|
| FTO/Au NPs | 4.0 ± 0.1 | --[1] |
| FTO/Au film/CuSCN/Au NPs | 2.2 ± 0.1 | 2.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





