Submitted:
28 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
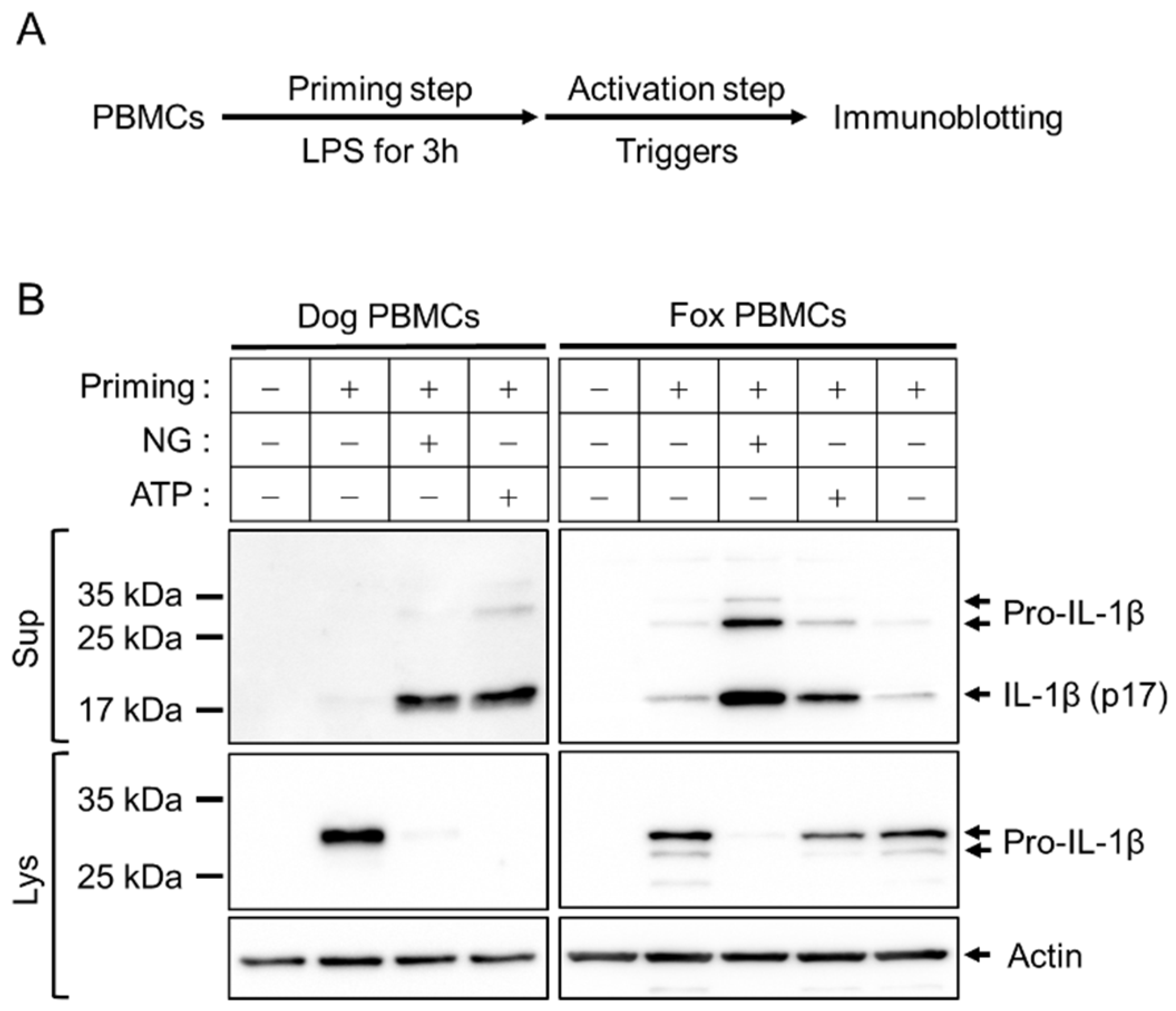
2.1. Cell preparation
2.2. Inflammasome activation and inhibition
2.3. Immunoblotting
2.4. Assay for IL-1β secretion
2.5. Statistical analysis
3. Results
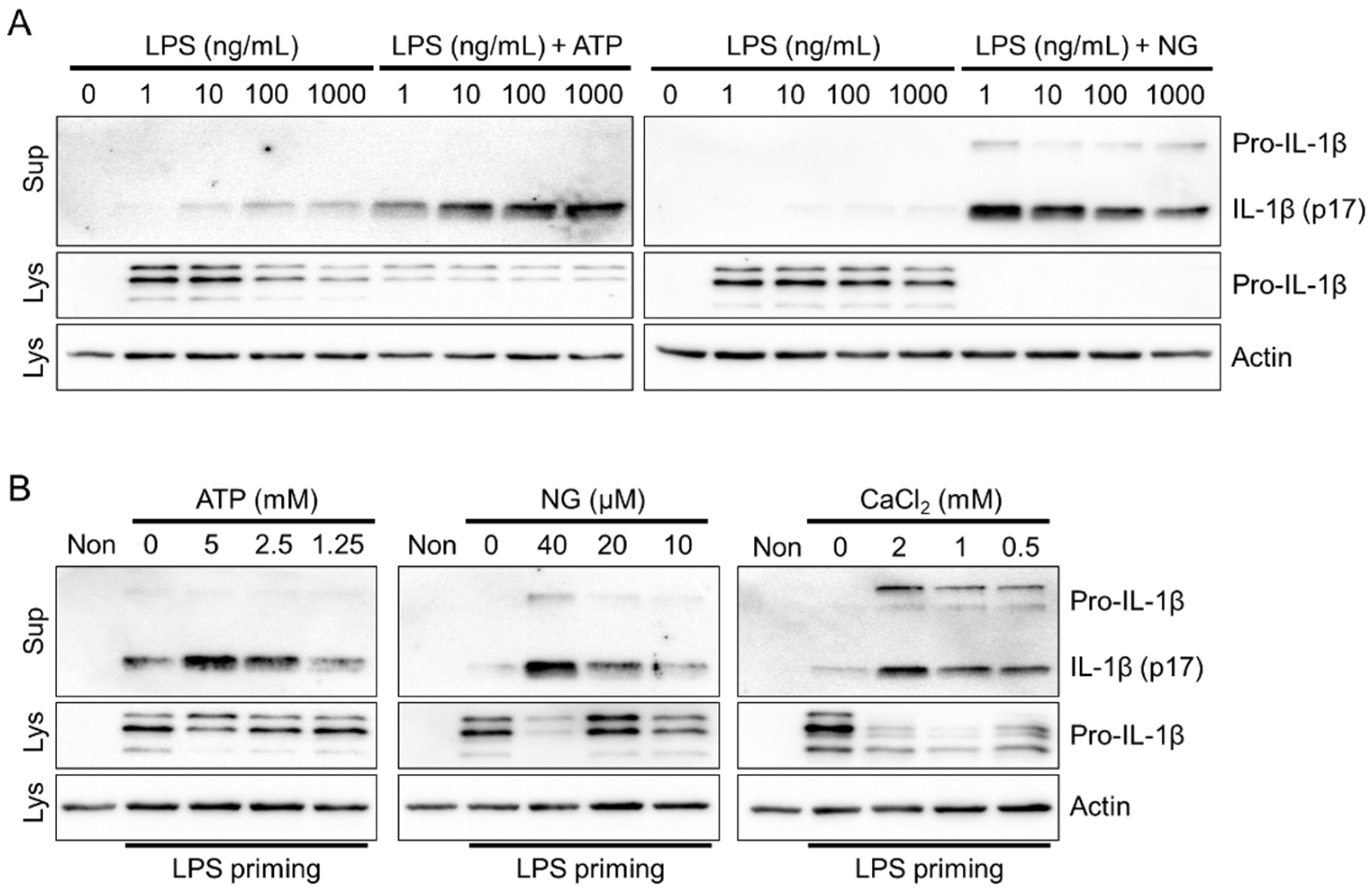
3.1. Optimization of inflammasome priming and activation in fox PBMCs
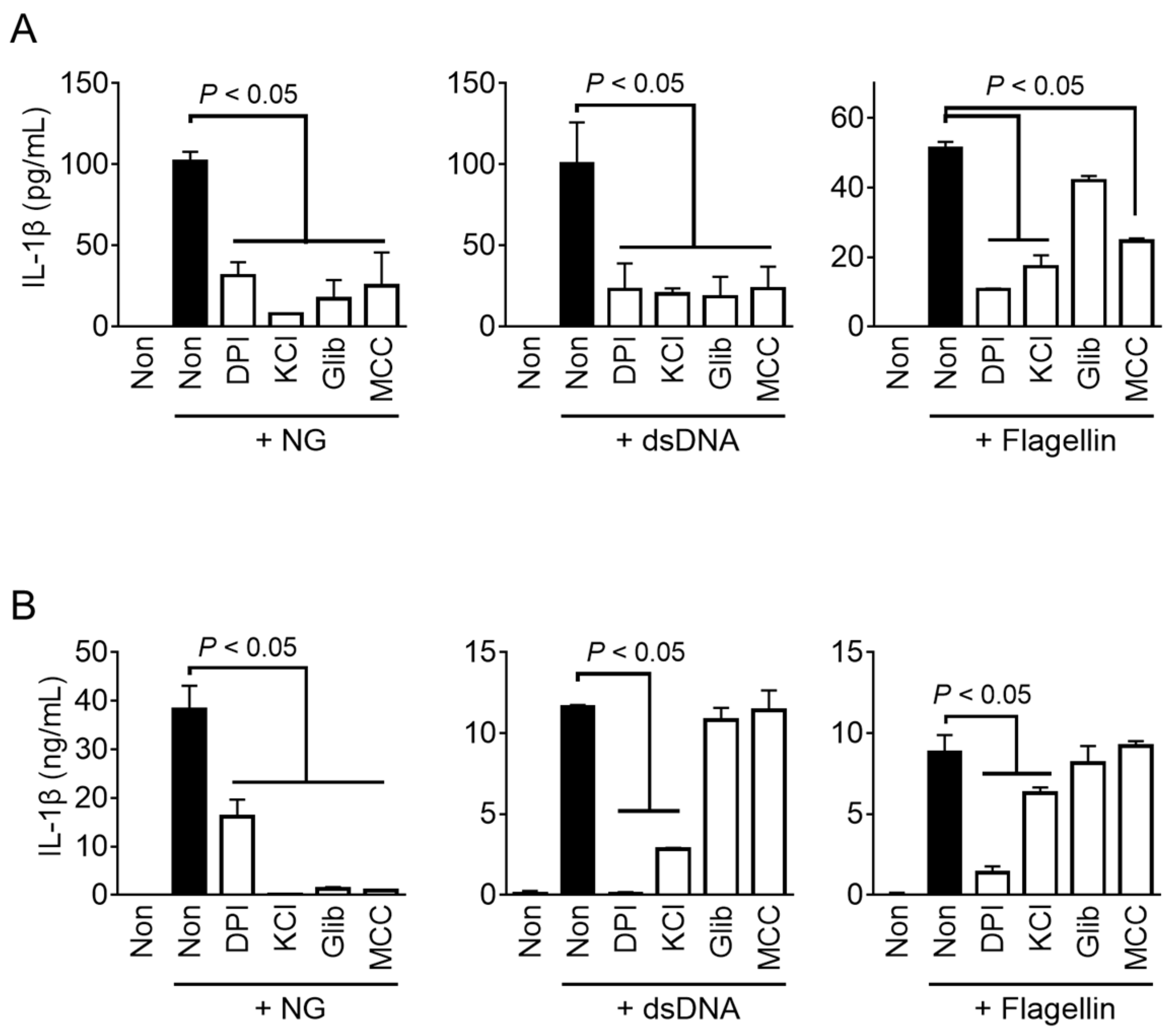
3.2. NLRP3 inflammasome triggers in fox PBMCs
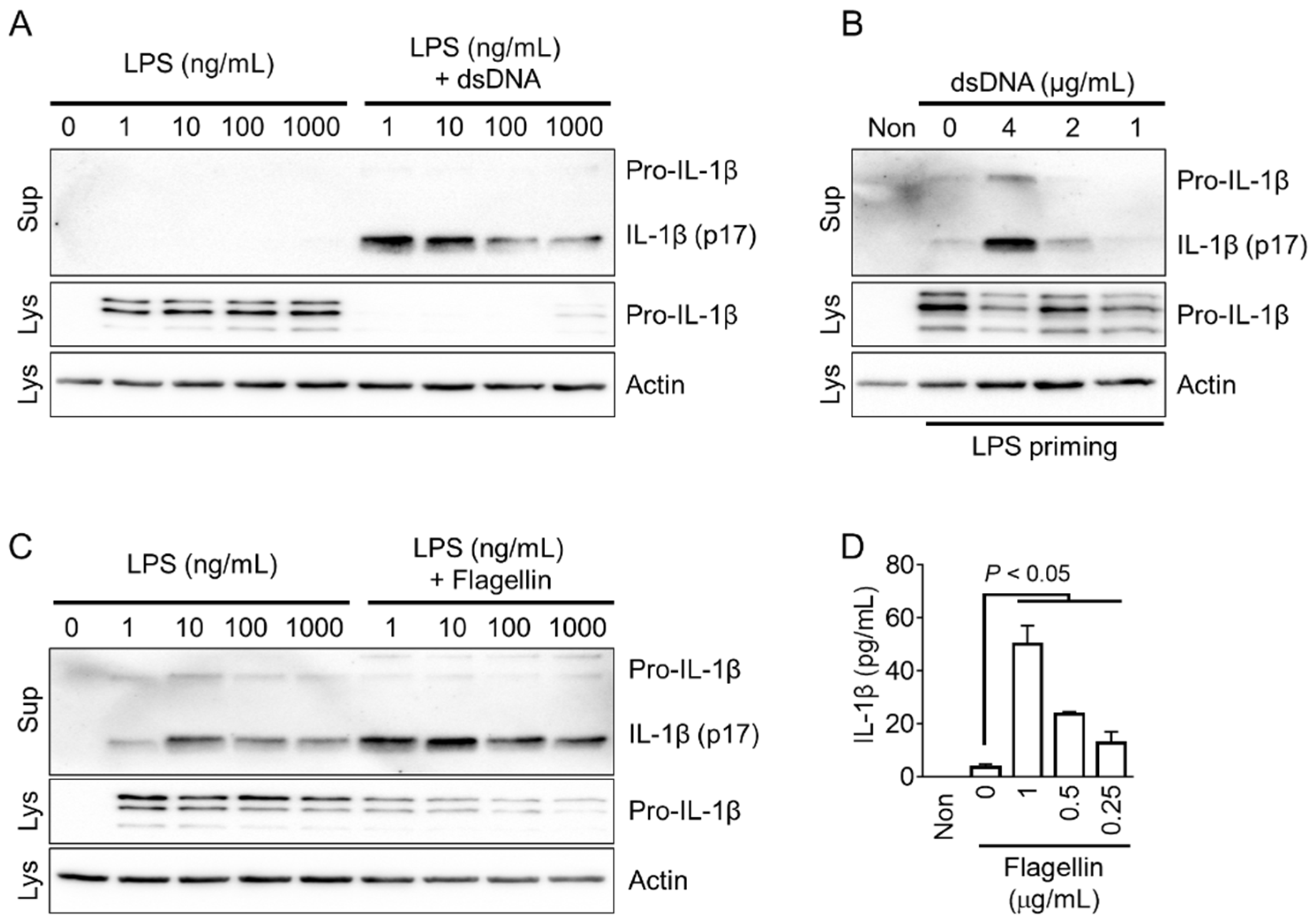
3.3. AIM2 and NLRC4 inflammasome triggers in fox PBMCs
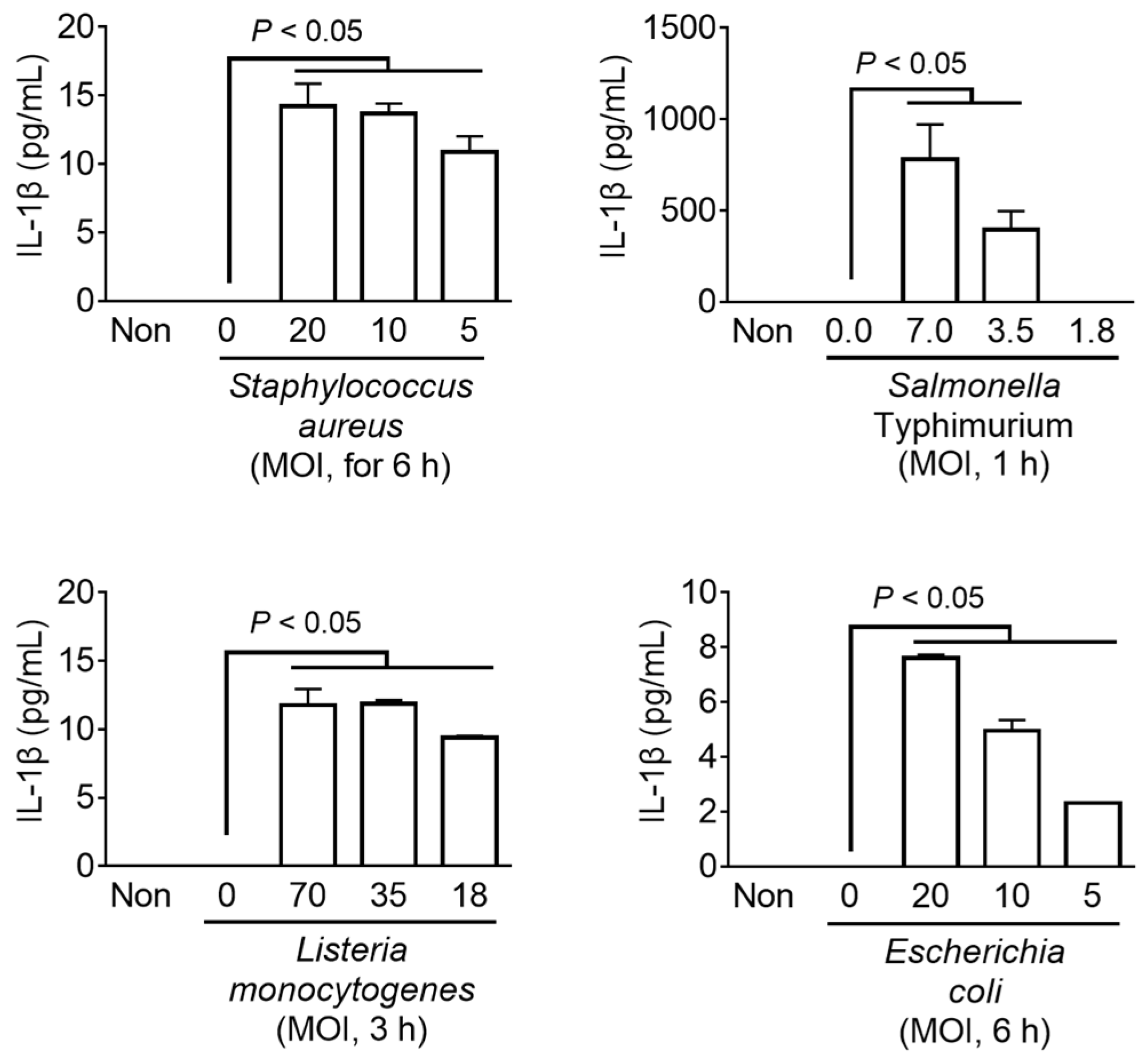
3.4. Bacterial inflammasome triggers in fox PBMCs
3.5. Mechanistic studies of fox inflammasomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunological reviews 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature reviews. Immunology 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Lee, G.S. Korean Red Ginseng, a regulator of NLRP3 inflammasome, in the COVID-19 pandemic. Journal of ginseng research 2022, 46, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological reviews 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.M.; Coll, R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. Journal of leukocyte biology 2020, 108, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.; Lee, H.; Lee, E.; Lee, G.S. Characterization of equine inflammasomes and their regulation. Veterinary research communications 2020, 44, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Deets, K.A.; Vance, R.E. Inflammasomes and adaptive immune responses. Nature immunology 2021, 22, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.N.; Han, S.H.; Kim, B.H.; Kryukov, A.P.; Kim, S.; Lee, B.Y.; Kwak, M. Insights into Korean red fox (Vulpes vulpes) based on mitochondrial cytochrome b sequence variation in East Asia. Zoological science 2012, 29, 753–760. [Google Scholar] [CrossRef]
- Cho, H.K.; Shin, Y.J.; Shin, N.S.; Chae, J.S. Efficient distribution of oral vaccines examined by infrared triggered camera for advancing the control of raccoon dog rabies in South Korea. The Journal of veterinary medical science 2020, 82, 1685–1692. [Google Scholar] [CrossRef]
- Digby, Z.; Tourlomousis, P.; Rooney, J.; Boyle, J.P.; Bibo-Verdugo, B.; Pickering, R.J.; Webster, S.J.; Monie, T.P.; Hopkins, L.J.; Kayagaki, N.; et al. Evolutionary loss of inflammasomes in the Carnivora and implications for the carriage of zoonotic infections. Cell reports 2021, 36, 109614. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Chen, V.C.; Rozario, P.; Ng, W.L.; Kong, P.S.; Sia, W.R.; Kang, A.E.Z.; Su, Q.; Nguyen, L.H.; Zhu, F.; et al. Bat ASC2 suppresses inflammasomes and ameliorates inflammatory diseases. Cell 2023, 186, 2144–2159.e2122. [Google Scholar] [CrossRef]
- Goh, G.; Ahn, M.; Zhu, F.; Lee, L.B.; Luo, D.; Irving, A.T.; Wang, L.F. Complementary regulation of caspase-1 and IL-1beta reveals additional mechanisms of dampened inflammation in bats. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 28939–28949. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.; Aldridge, J.R., Jr.; Webster, R.G.; Magor, K.E. Association of RIG-I with innate immunity of ducks to influenza. Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Poeck, H.; Bscheider, M.; Gross, O.; Finger, K.; Roth, S.; Rebsamen, M.; Hannesschlager, N.; Schlee, M.; Rothenfusser, S.; Barchet, W.; et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nature immunology 2010, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, G.; Han, B.C.; Lee, S.H.; Lee, G.S. Maltol, a Natural Flavor Enhancer, Inhibits NLRP3 and Non-Canonical Inflammasome Activation. Antioxidants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Yu, J.W.; Juliana, C.; Solorzano, L.; Kang, S.; Wu, J.; Datta, P.; McCormick, M.; Huang, L.; McDermott, E.; et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature immunology 2010, 11, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Mueller, J.L.; Vitari, A.C.; Misaghi, S.; Fedorova, A.; Deshayes, K.; Lee, W.P.; Hoffman, H.M.; Dixit, V.M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology 2009, 187, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Abellan, A.; Angosto-Bazarra, D.; Martinez-Banaclocha, H.; de Torre-Minguela, C.; Ceron-Carrasco, J.P.; Perez-Sanchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nature chemical biology 2019, 15, 560–564. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature immunology 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature medicine 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.; Kwon, S.; Kim, P.H.; Kwon, H.M.; Lee, E.; Lee, G.S. Triggers of NLRC4 and AIM2 inflammasomes induce porcine IL-1beta secretion. Veterinary research communications 2018, 42, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, H.; Woo, H.M.; Lee, E.; Lee, G.S. Characterization of porcine NLRP3 inflammasome activation and its upstream mechanism. Veterinary research communications 2014, 38, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Brunette, R.L.; Young, J.M.; Whitley, D.G.; Brodsky, I.E.; Malik, H.S.; Stetson, D.B. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. The Journal of experimental medicine 2012, 209, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.D.; Smith, A.D.; Chen, C.; Urban, J.F., Jr. An in-depth comparison of the porcine, murine and human inflammasomes; lessons from the porcine genome and transcriptome. Veterinary microbiology 2017, 202, 2–15. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




