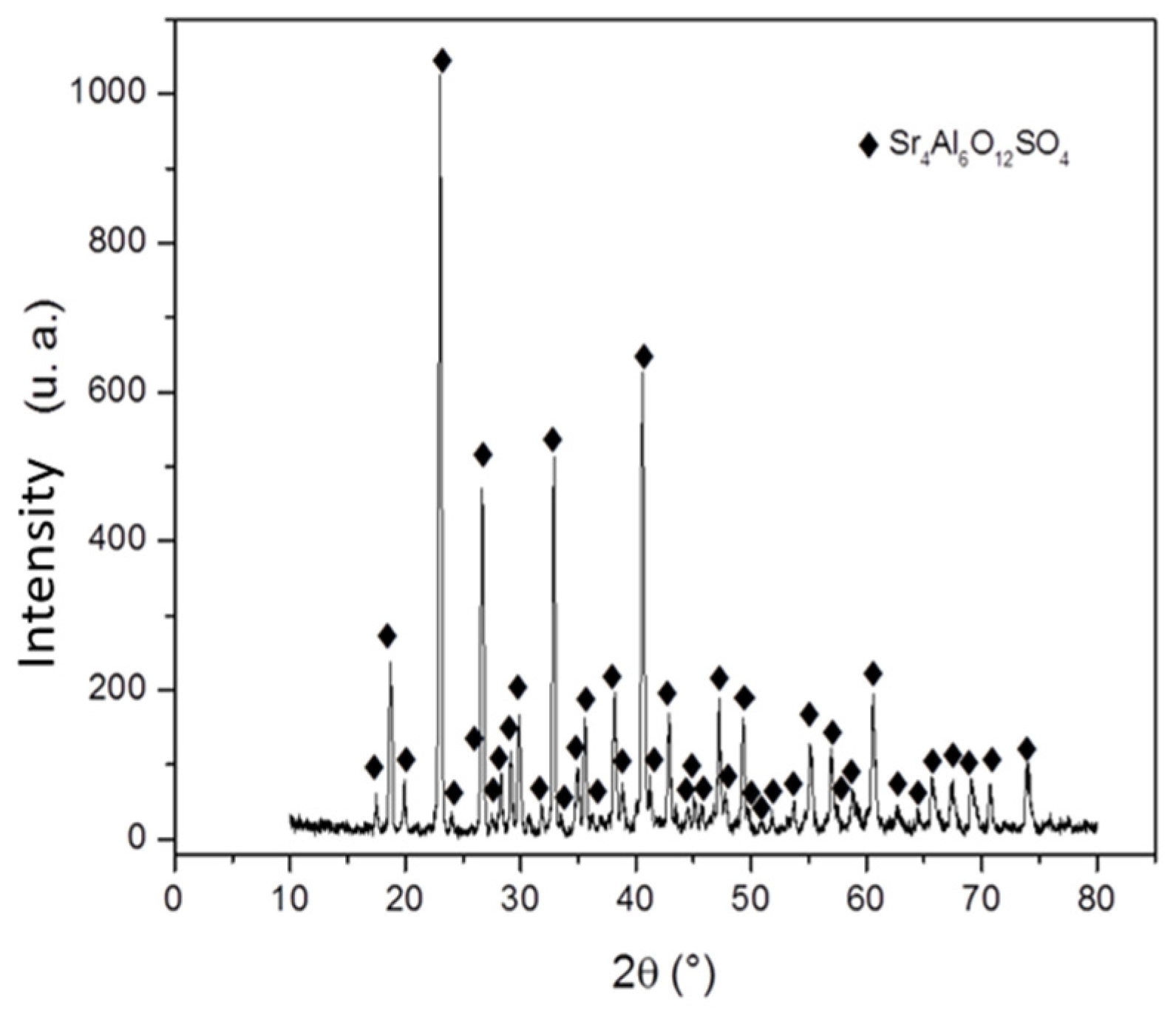
3.2. Static immersion test results
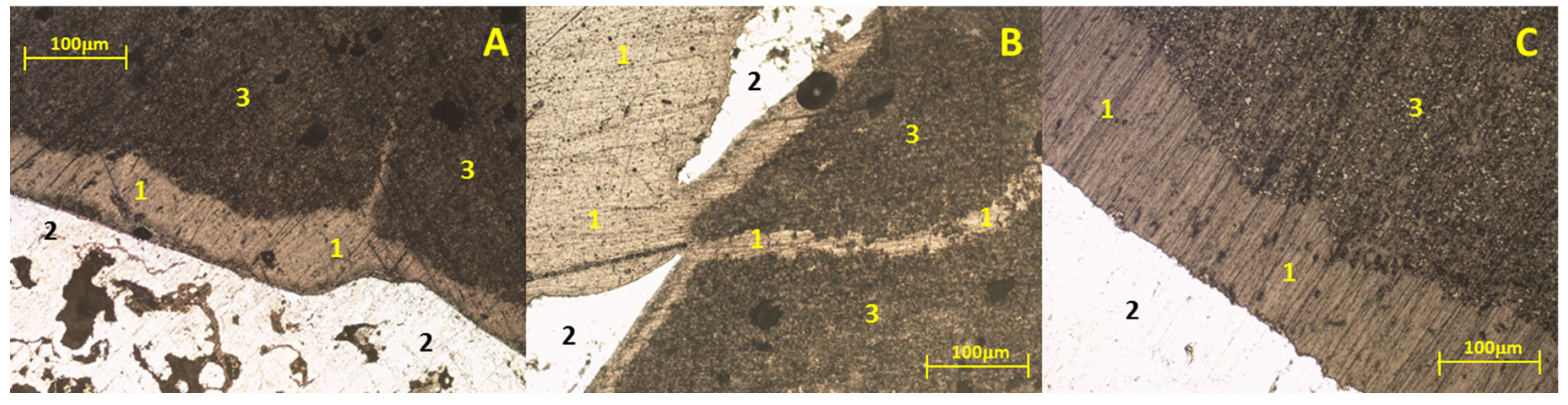
Figure 2 presents micrographs of the study samples after static immersion tests in Al–Si alloys at 800°C and isotherms of 25h (A), 50h (B), and 100h (C) as experimental conditions.
It is worth mentioning that, due to the physical conditions of the study samples (low mechanical properties), the metallographic preparation was complicated, bringing with it, in some cases, the introduction of resin between areas of the study sample and the metal. It can be seen in the three micrographs that under these experimental conditions, there is no chemical interaction between the ceramic substrate Sr
4Al
6O
12SO
4 and the Al–Si alloy; that is, there was no formation of reaction products (analyzing the metal surface).
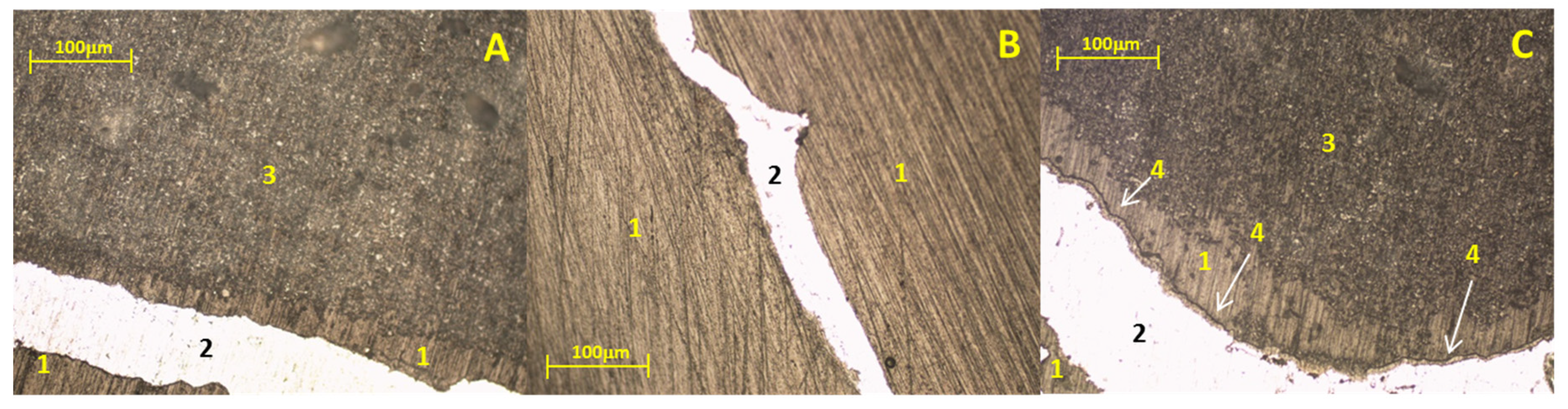
Figure 3 presents micrographs of study samples after static immersion tests in Al–Si alloys at 900°C and isotherms of 25h (A), 50h (B), and 100h (C) as experimental conditions.
Micrographs A and B show no chemical interaction between the ceramic substrate Sr
4Al
6O
12SO
4 and the Al–Si alloy; that is, there was no formation of reaction products. However, in micrograph C a thin line of reaction products adhered to the metal surface can be seen. Due to the limitation of the characterization technique, it is impossible to determine the chemical composition of the registered reaction products. To complement the results,
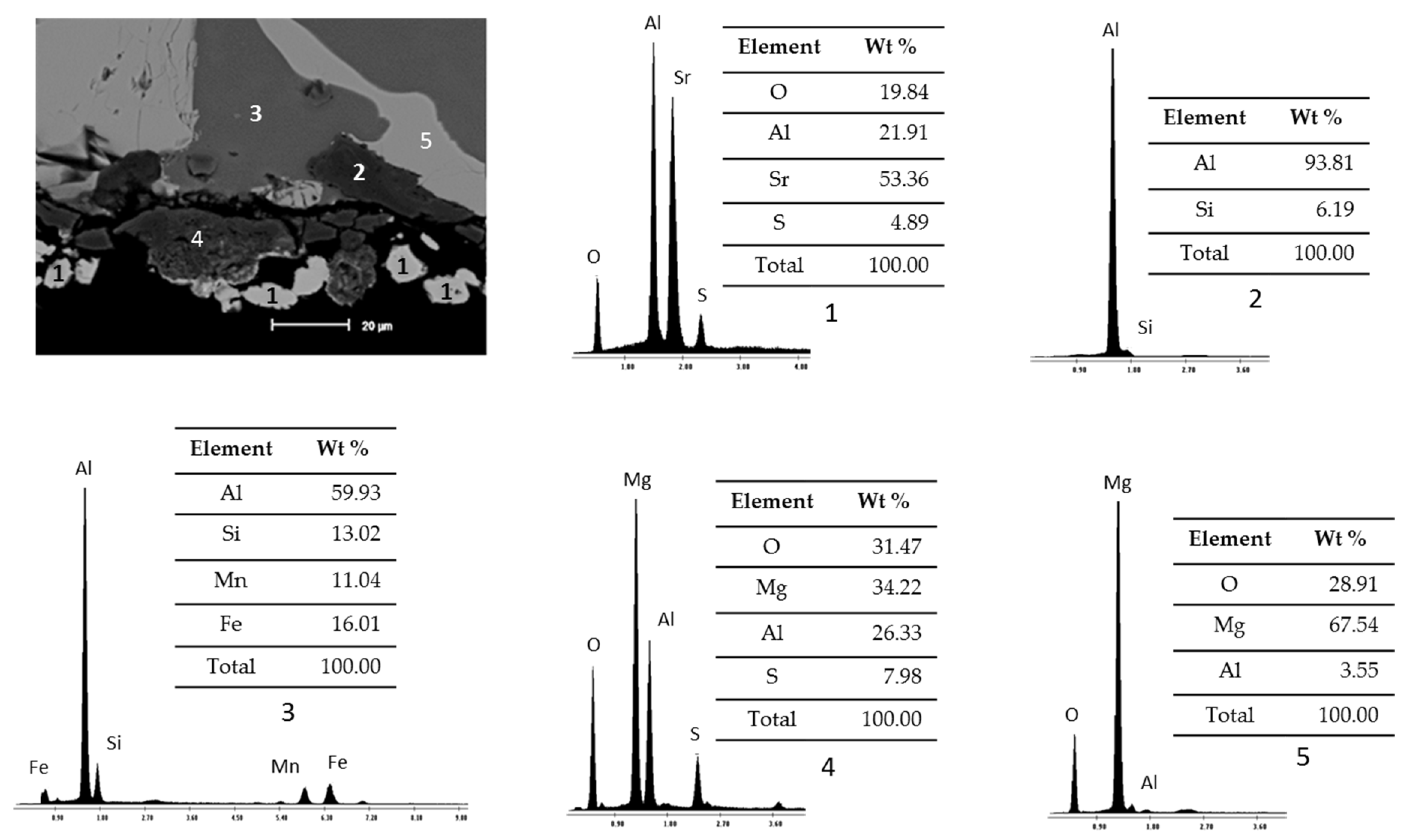
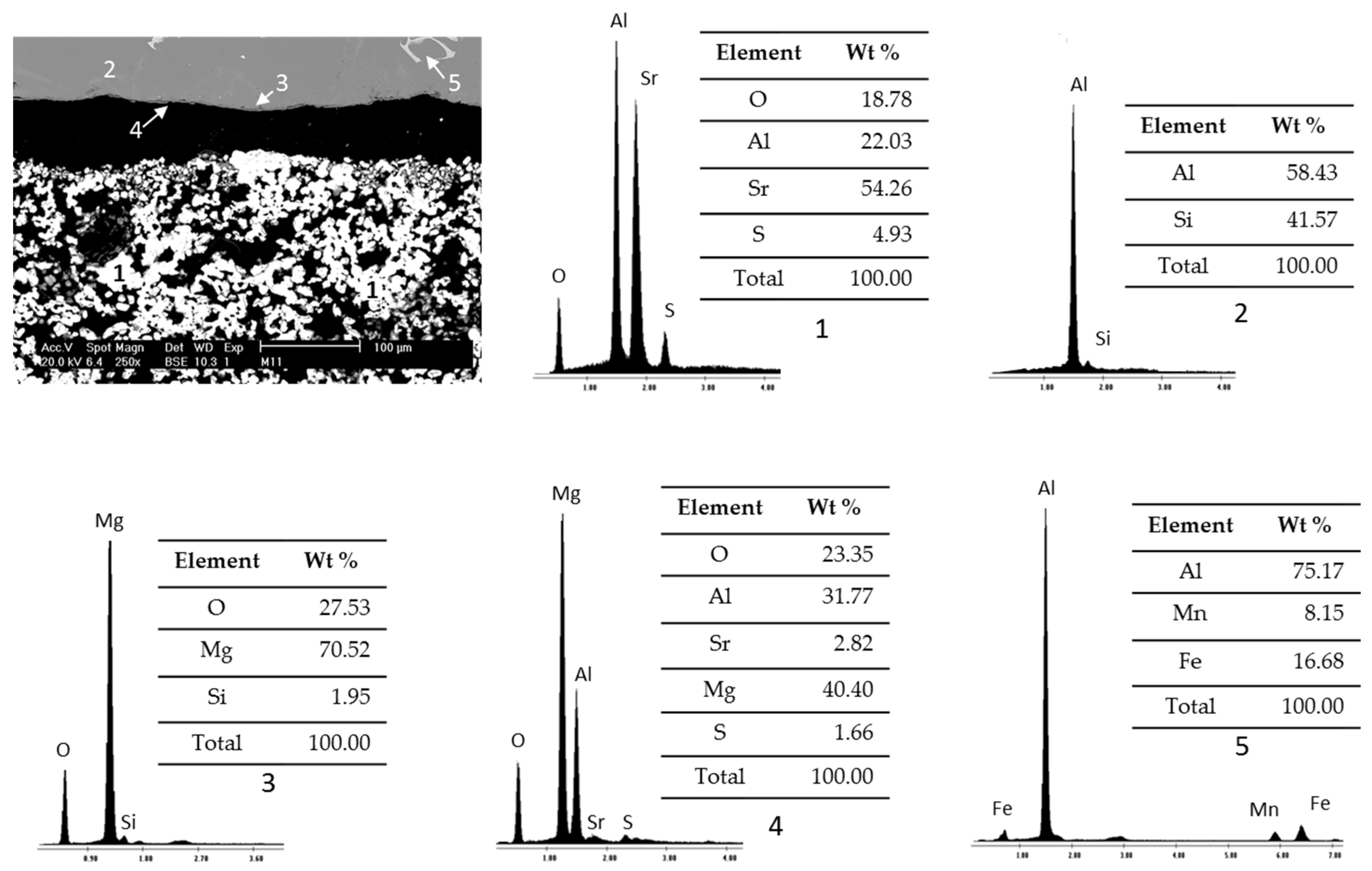
Figure 4 presents SEM micrographs and EDS analysis of study samples after static immersion tests in Al–Si alloys at 900°C and 100h as experimental conditions.
The micrograph presents the visual analysis of an area of the study sample and the EDS spectra present the specific chemical analysis of different areas. According to the percentages of the registered chemical elements, the particles identified with the number 1 are related to the ceramic substrate Sr4Al6O12SO4, and the areas identified with numbers 2 and 3 correspond to the Al–Si alloy (one with higher purity than the other). In addition, reaction products are presented due to the chemical interaction between the ceramic substrate Sr4Al6O12SO4 and the Al–Si alloys, such as areas of spinels (MgAl2O4) identified with number 4 and intermetallics of the alloy (MgO) identified with number 5.
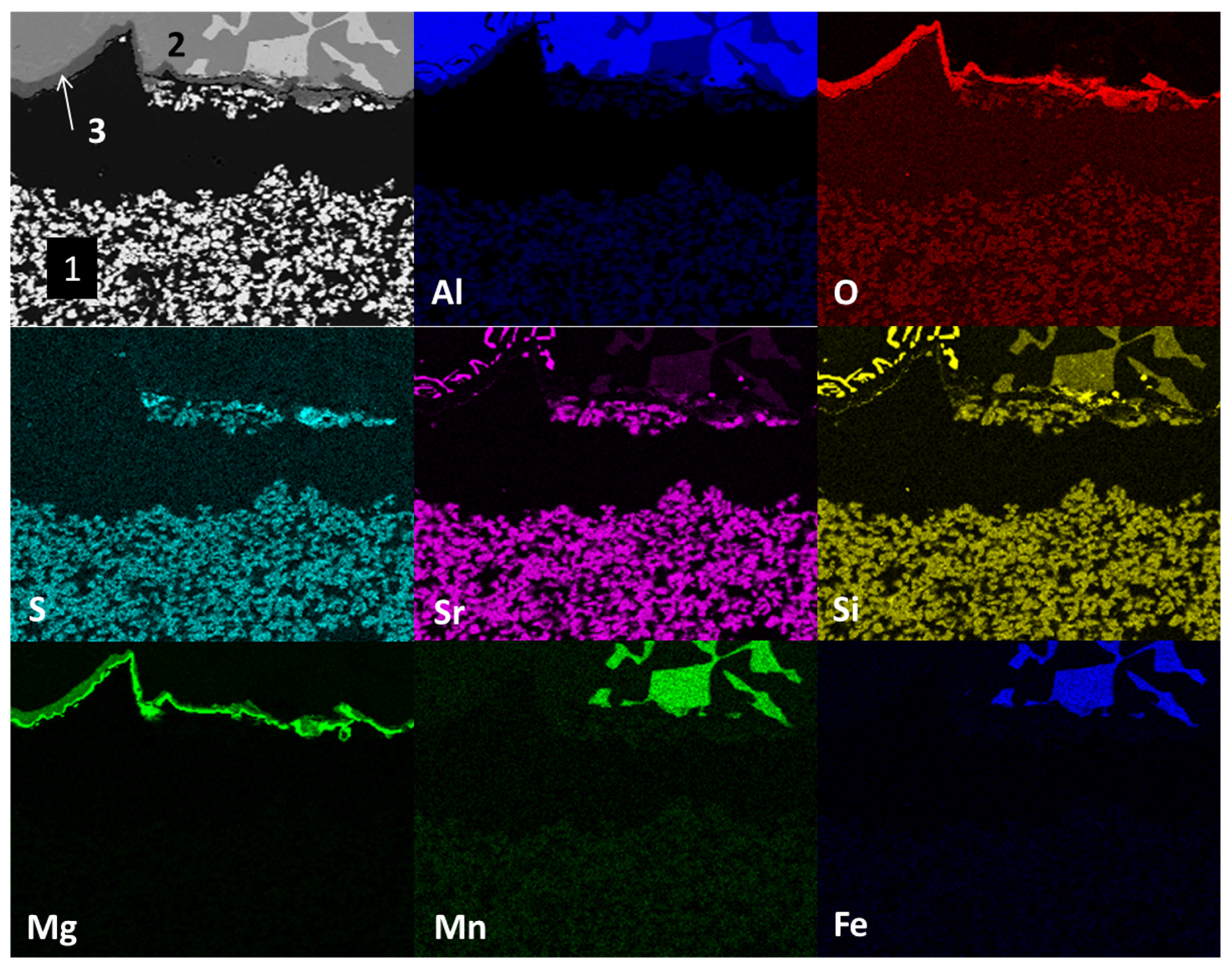
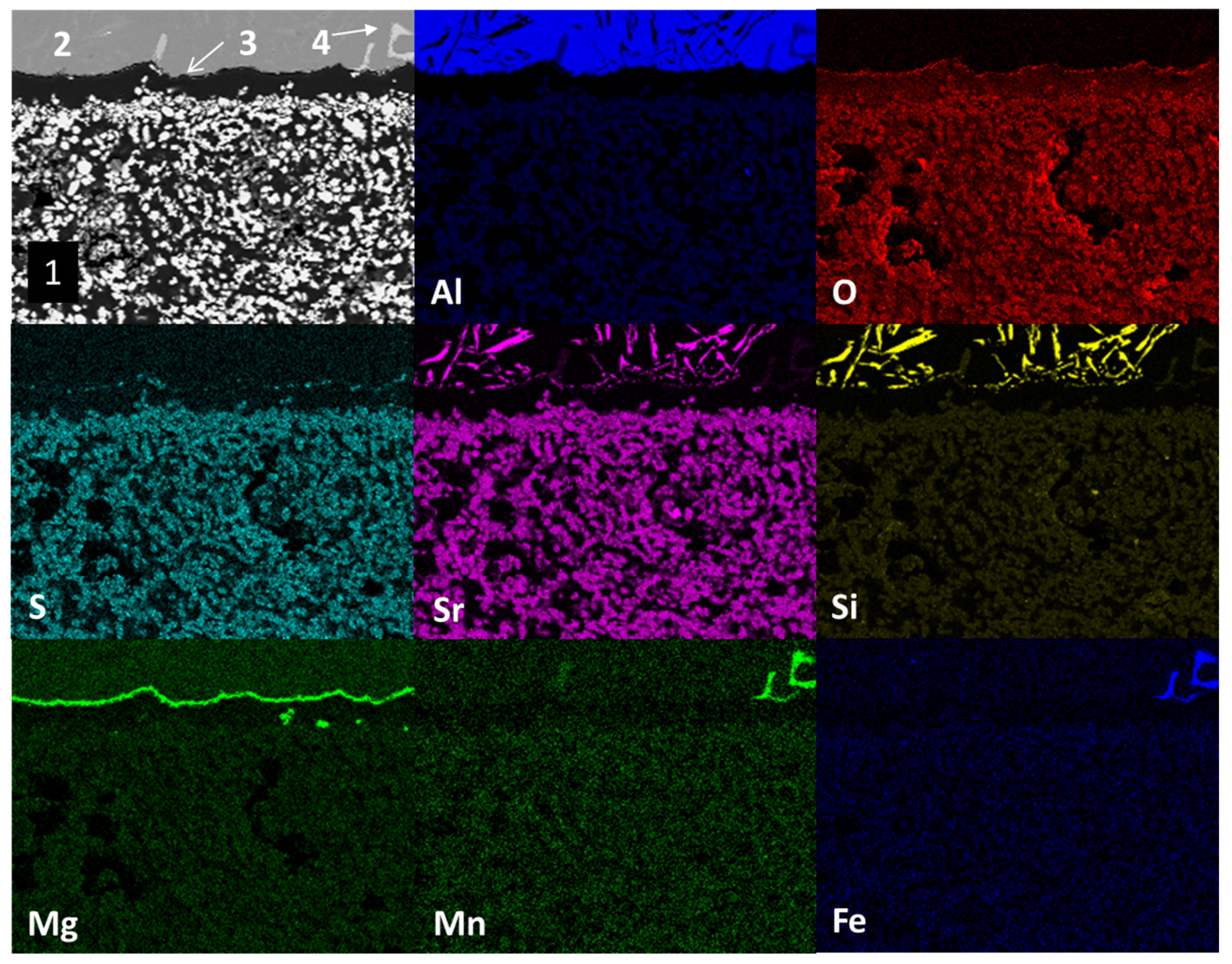
Figure 5 shows the mapping by chemical element (SEM) present in study samples after the static immersion test in Al–Si alloys at 900°C for 100h as experimental conditions. The main micrograph (upper left corner) presents the section of the study sample analyzed. Three main areas are observed: at the bottom, agglomerates of white particles (1); at the top, a solid section of mainly light gray color (2) and attached to the bottom of section 2 a thin dark gray line (3). According to the distribution of chemical elements, the area identified with the number 1 (light-colored agglomerates) corresponds to the ceramic substrate Sr
4Al
6O
12SO
4, the light gray solid section identified with the number 2 corresponds to the Al–Si alloy (the light within section 2 corresponds to manganese and iron intermetallics present in the alloy) and the thin dark gray line identified with the number 3 corresponds to reaction products of the type spinel (MgAl
2O
4) and magnesium oxide (MgO).
This phenomenon, where magnesium diffuses to the metal–ceramic interface, has been reported in the literature, increasing its content in this area [
20]. On the other hand, strontium diffusion from the ceramic substrate towards the metal alloy; and silicon, manganese and iron from the metal alloy towards the ceramic substrate are observed. The above results from of the chemical interaction process between the ceramic substrate and the alloy in Al–Si alloys (corrosion). The mapping of iron and manganese elements shows the distribution of intermetallics within aluminum.
Based on the results presented, two interaction mechanisms are proposed between the ceramic substrate Sr4Al6O12SO4 with Al–Si alloys (900°C–100h) to form reaction products:
According to mechanism number 1, it can be said that ceramic substrate Sr4Al6O12SO4, upon contact with the Al–Si alloy at 900°C and 100h, decomposes into three parts: strontium (Sr), alumina (Al2O3), and trioxide sulfur (SO3). At the same time, the magnesium present in the alloy (<1%) is oxidized, giving rise to magnesium oxide (MgO). Finally, alumina and magnesium oxide (products of reactions 1 and 2) react with each other to form spinel (MgAl2O4).
According to mechanism number 2, it can be said that the ceramic substrate Sr
4Al
6O
12SO
4, upon contact with the Al–Si alloy at 900°C and 100h, decomposes into four parts: strontium (Sr), alumina (Al
2O
3), magnesium oxide (MgO) and sulfur trioxide (SO
3). At the same time, the magnesium from the alloy is oxidized and reacts with alumina to produce spinel (MgAl
2O
4).
Figure 6 presents the micrographs of study samples after static immersion tests in Al–Si alloys at 1000°C and isotherms of 25h (A), 50h (B), and 100h (C) as experimental conditions.
It can be seen in the micrograph identified with the letter A, apparently there is no chemical interaction between the ceramic substrate Sr
4Al
6O
12SO
4 and the Al–Si alloy. However, a thin dark line can be observed at the border on the ceramic substrate as if it were burned. In micrographs B and C, at the boundary of the metallic phase, a thin line of reaction products can be seen which increases in thickness as a function of exposure time. To complement the results,
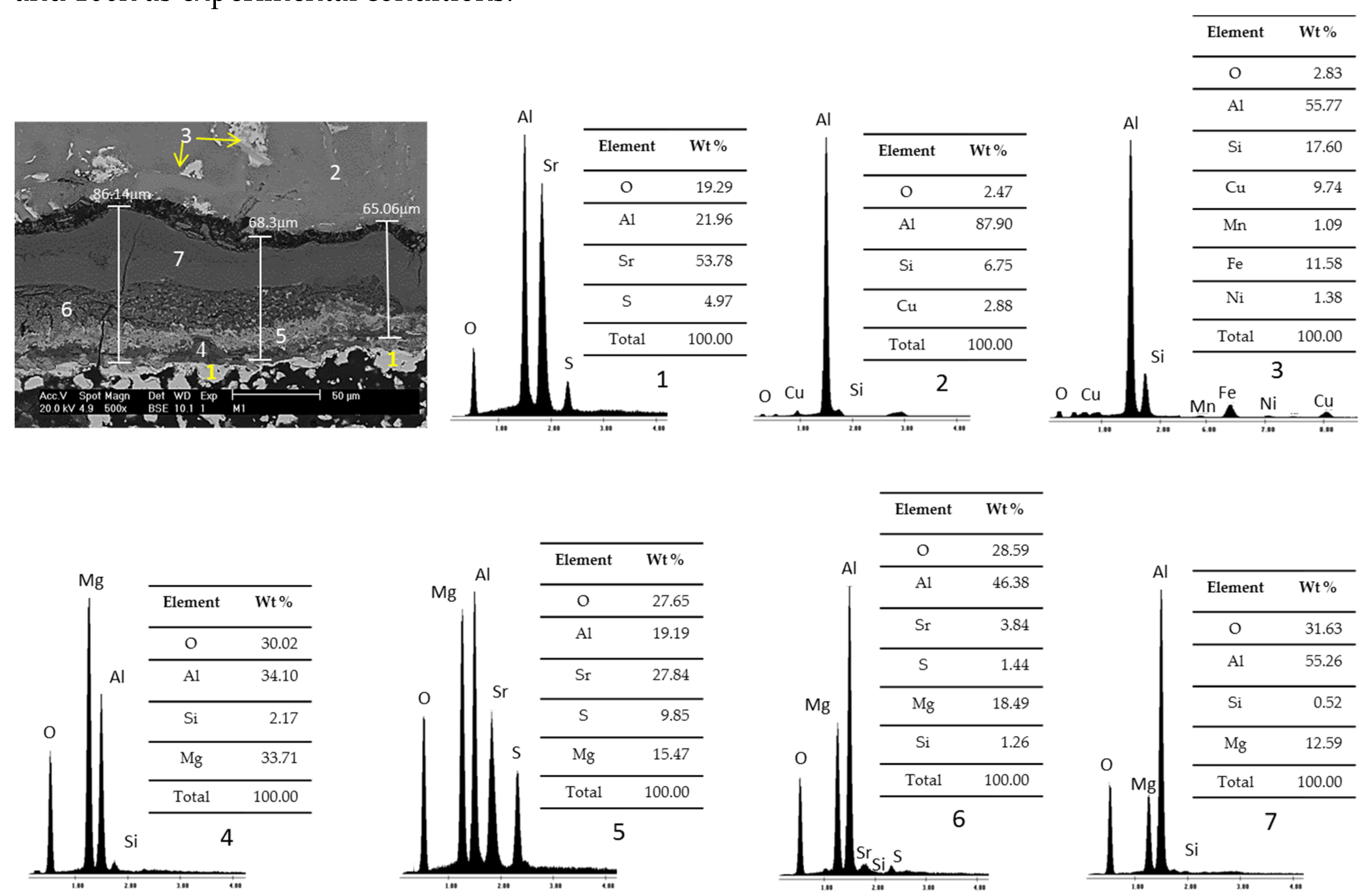
Figure 7 presents SEM micrographs and EDS analysis of study samples after static immersion tests in Al–Si alloys at 1000°C and 100h as experimental conditions.
According to the percentages of the registered chemical elements, the particles identified with the number 1 are related to the ceramic substrate Sr
4Al
6O
12SO
4, the area identified with the number 2 corresponds to the Al–Si alloy, the particles identified with the number 3 correspond to intermetallics mainly iron. In addition, a corrosion layer is observed between the alloy and the ceramic substrate, between 65 to 87μm thick, generating reaction products, mostly spineras (MgAl
2O
4) in different magnesium concentrations (4, 5, 6, and 7). On the other hand, cracking can be observed through the corrosion layer due to the difference between the thermal expansion coefficients of the phases present [
24].
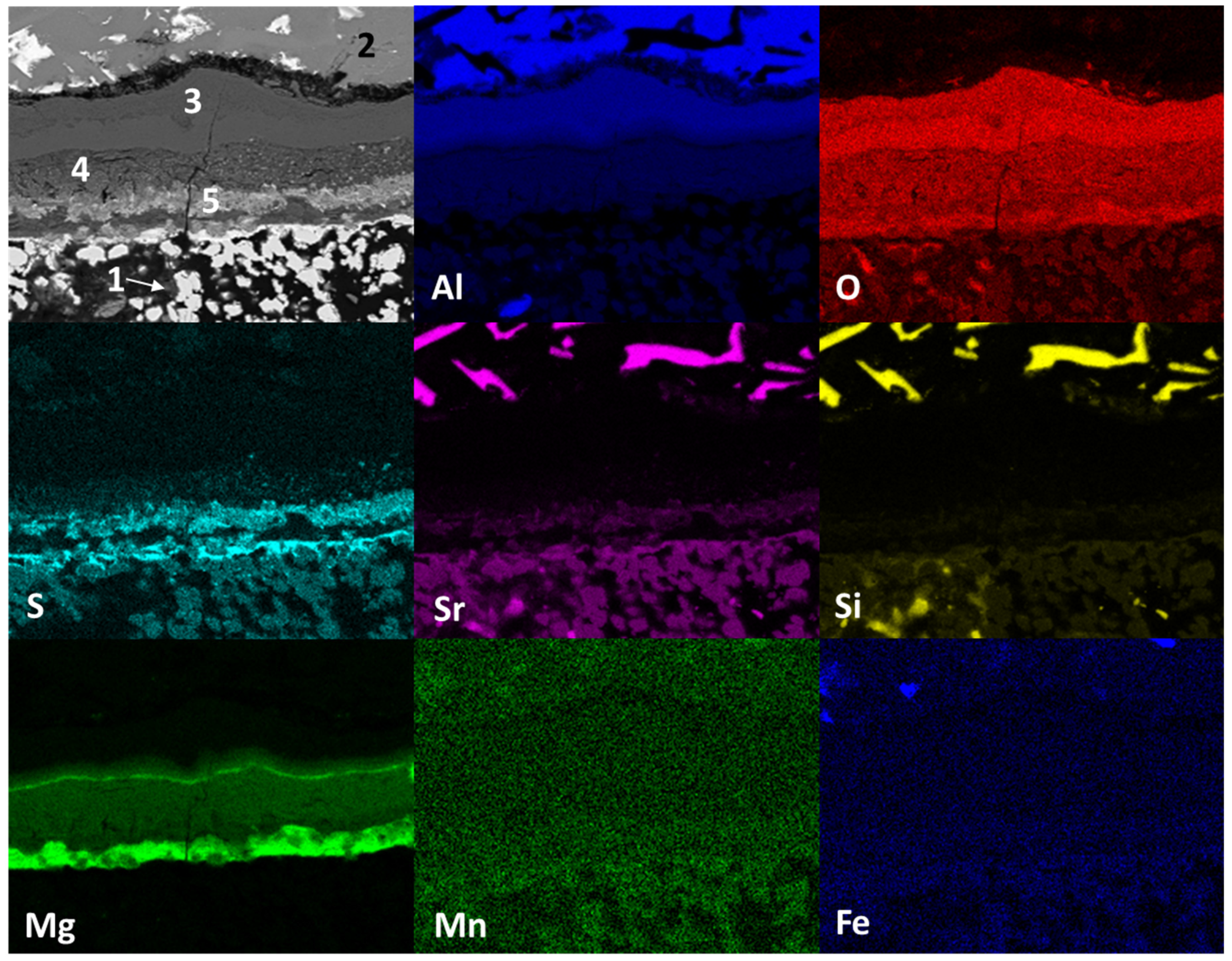
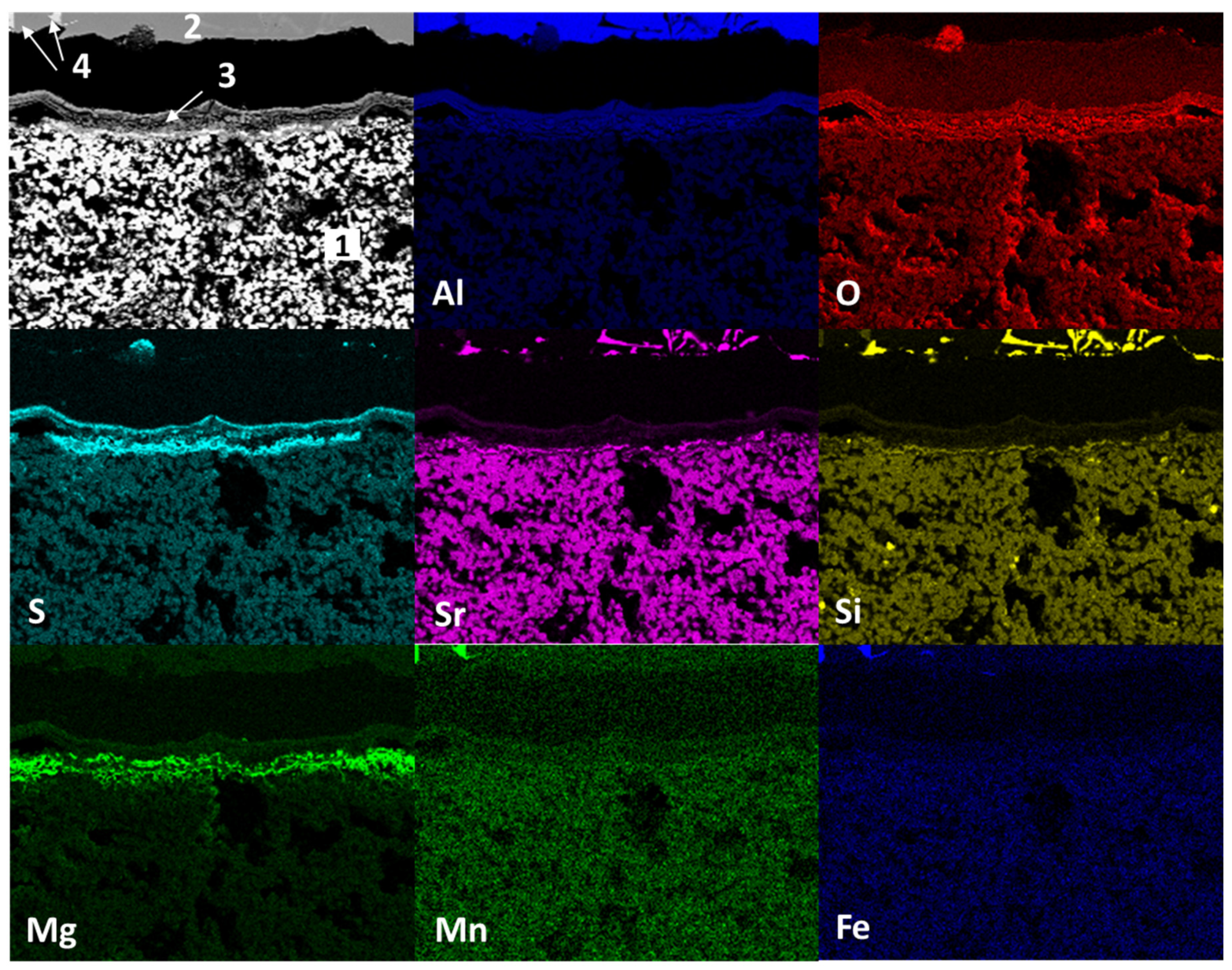
Figure 8 shows the mapping by chemical element (SEM) present in the study sample after static immersion tests in Al–Si alloys at 1000°C for 100h as experimental conditions. The main micrograph (upper left corner) presents study sample analyzed. Three main areas are observed: in the lower part, agglomerates of white particles (1); in the upper part, a solid section of light gray color (2); and between these sections, there is a zone of reaction products. According to the distribution of chemical elements, the area identified with the number 1 (light-colored agglomerates) corresponds to the ceramic substrate Sr
4Al
6O
12SO
4, the light gray solid section identified with the number 2 corresponds to the Al–Si alloy, within the reaction zone, is identified with the number 3 the alumina phase (Al
2O
3), with the number 4 the spinel (MgAl
2O
4) and magnesium oxide (MgO) phases are identified, with the number 5 the presence of sulfates is identified, possibly strontium (SrSO
4). On the other hand, strontium diffusion from the ceramic substrate towards the metal alloy and silicon, manganese and iron from the metal alloy towards the ceramic substrate are observed. This reaction results from the chemical interaction process between the ceramic substrate and the aluminum silicon–alloy (corrosion).
Based on the previous results, the following interaction mechanism is proposed between the ceramic substrate Sr
4Al
6O
12SO
4 with Al–Si alloys (1000°C–100h) to form reaction products:
According to the proposed mechanism, it can be said that sections of the ceramic substrate Sr4Al6O12SO4, upon contact with the Al–Si alloy at 1000°C and 100h, decompose into three parts: strontium (Sr), alumina (Al2O3), and strontium sulfate (SrSO4). At the same time, the magnesium present in the alloy (<1%) is oxidized, giving rise to magnesium oxide (MgO). Alumina and magnesium oxide react to produce spinel (MgAl2O4).
3.3. Wettability test results
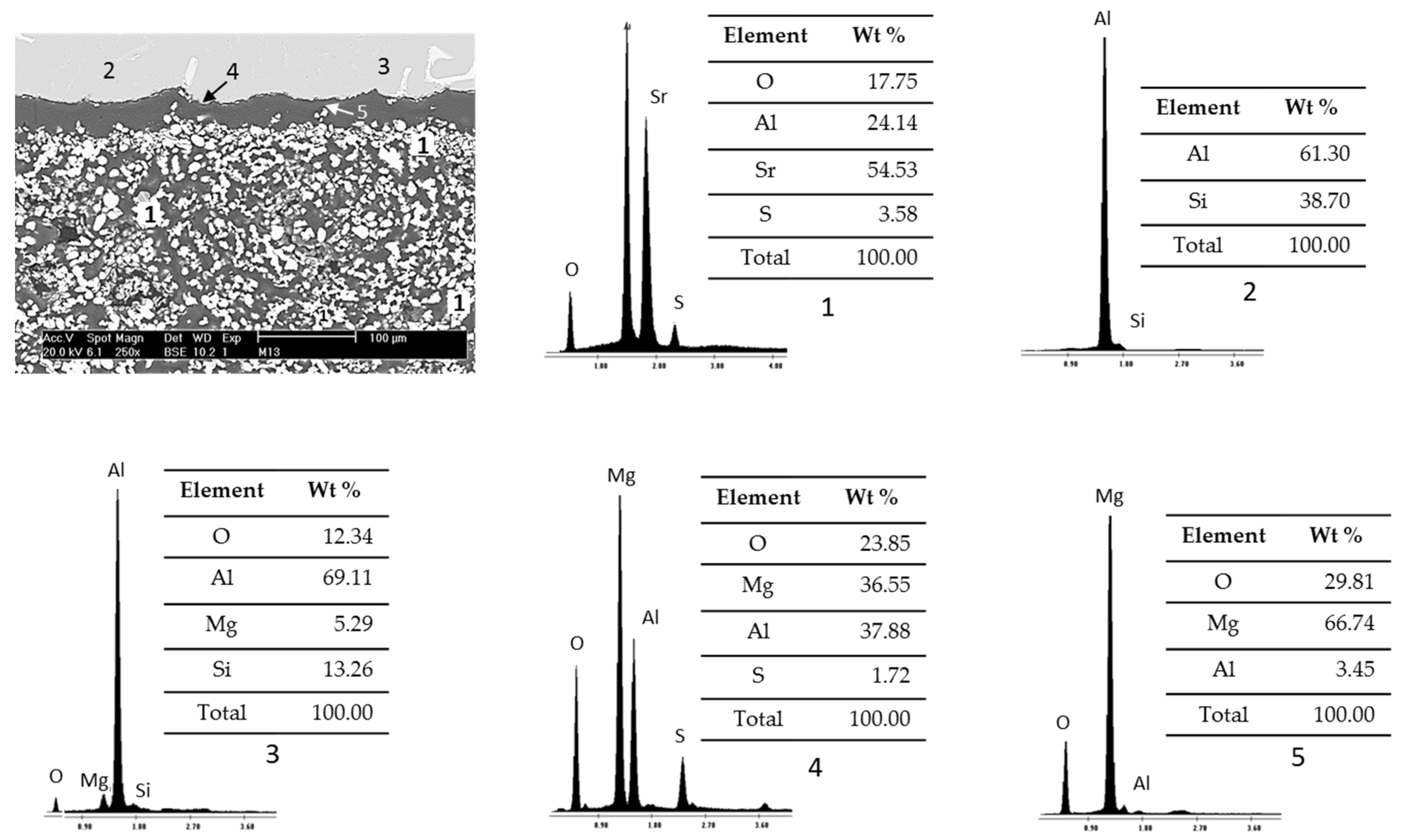
Figure 9 presents SEM micrographs and EDS analysis of study samples after wettability tests with Al–Si alloys at 900°C and 2h as experimental conditions.
According to the percentages of the chemical elements recorded, the particle agglomerates located in the lower area of the micrograph were identified with the number 1 and correspond to the ceramic substrate Sr4Al6O12SO4, the areas identified with numbers 2 and 3 correspond to the Al–Si alloy, reaction products are presented due to the chemical interaction between the ceramic substrate Sr4Al6O12SO4 and the Al–Si alloy, such as a thin line of spinel (MgAl2O4) in the boundary with the metal identified with the number 4 and isolated sections of magnesium oxide (MgO) identified with the number 5.
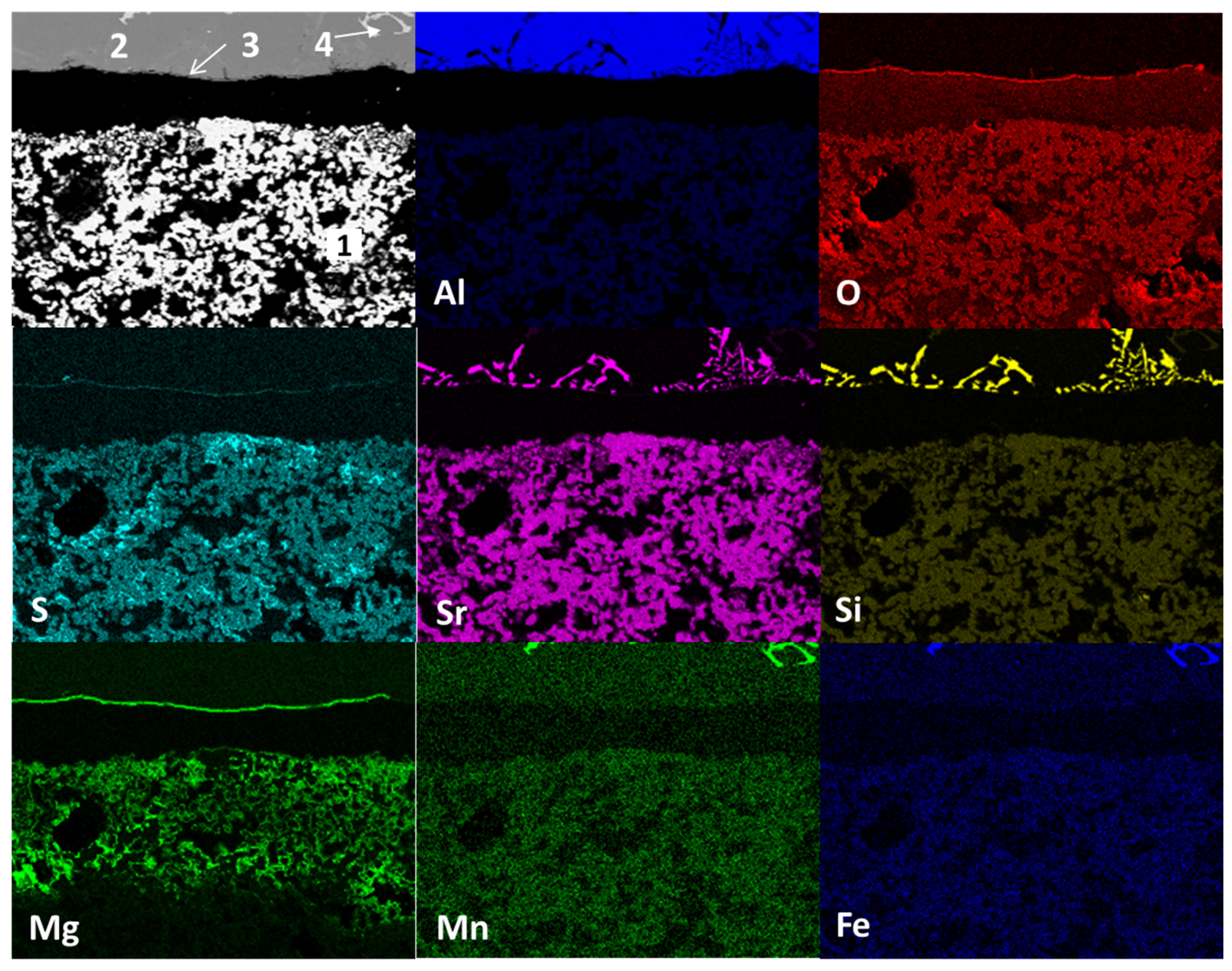
Figure 10 shows the mapping by chemical element (SEM) present in the study sample after wettability tests with Al–Si alloys at 900°C and 2h as experimental conditions. The main micrograph (upper left corner) presents the study sample section analyzed. Four main areas are observed: in the lower part, agglomerates of white particles (1); in the upper part, a solid section of dark gray color (2), attached to the border of section 2, there is a thin line of products of reaction (3) and within the metallic zone there are small light gray sections (4). According to the distribution of chemical elements, the area identified with the number 1 (agglomerates of light-colored hemispherical particles) corresponds to the ceramic substrate Sr
4Al
6O
12SO
4, the dark gray solid section identified with the number 2 corresponds to the Al–Si alloy, the line identified with the number 3 corresponds to the spinel (MgAl
2O
4), and magnesium oxide (MgO) phases. The number 4 identified the presence of manganese and iron intermetallics within the metal alloy, although the latter was not recorded in EDS.
On the other hand, strontium diffusion from the ceramic substrate towards the metal–alloy, and silicon, manganese and iron from the metal–alloy towards the ceramic substrate are observed. The above is the result of the chemical interaction process between the ceramic substrate and the Al–Si alloy (corrosion).
Figure 11 presents SEM micrographs and EDS analysis of study samples after wettability tests with Al–Si alloys at 1000°C and 2h as experimental conditions. According to the percentages of the chemical elements recorded, hemispherical and agglomerated particles in the lower part of the micrograph were identified with the number 1 and correspond to the ceramic substrate Sr
4Al
6O
12SO
4, the dark gray solid area identified with the number 2 corresponds to the alloy Al–Si, there are reaction products due to the chemical interaction between the ceramic substrate and the Al–Si alloy, such as a thin line of magnesium oxide (MgO) identified with the number 3 and spinel (MgAl
2O
4) identified with the number 4 on the border with the metal alloy.
On the other hand, intermetallics are present in the alloy, formed by Fe and Mn and identified with the number 5.
The above can be corroborated with the chemical element mapping study on the sample after wettability tests with Al–Si alloys at 1000°C for 2h as experimental conditions. The results are presented in
Figure 12. The main micrograph (upper left corner) presents the study sample section analyzed using chemical element mapping (SEM) technique. Four main areas are observed: in the lower part, agglomerates of white particles (1); in the upper part, a solid section of dark gray color (2), attached to the border of section 2, there is a thin line of products of reaction (3) and within the metallic zone there are small light gray sections (4). According to the distribution of chemical elements, the area identified with the number 1 (agglomerates of light-colored hemispherical particles) corresponds to the ceramic substrate Sr
4Al
6O
12SO
4, the dark gray solid section identified with the number 2 corresponds to the Al–Si alloy. , the line identified with the number 3 corresponds to the spinel (MgAl
2O
4) and magnesium oxide (MgO) phases and with the number 4 the presence of manganese and iron intermetallics is identified within the metal alloy. Strontium diffusion from the ceramic substrate towards the metal–alloy, and silicon, manganese and iron from the metal–alloy towards the ceramic substrate are observed. The above is the result of the chemical interaction process between the ceramic substrate and the Al–Si alloy (corrosion).
Figure 13 presents SEM micrographs and EDS analysis of study samples after wettability tests with Al–Si alloys at 1100°C and 2h as experimental conditions. According to the percentages of the chemical elements recorded, hemispherical and agglomerated particles in the lower part of the micrograph were identified with the number 1 and correspond to the ceramic substrate Sr
4Al
6O
12SO
4, the dark gray solid area identified with the number 2 corresponds to the alloy Al–Si, a reaction zone is observed between the ceramic substrate and the alloy whose thickness is around 32 to 35μm. The products formed are magnesium oxide (MgO), identified with the number 3; spinel (MgAl
2O
4), identified with the number 4; and alumina (Al
2O
3), identified with the number 5. This last reaction product exists in a greater proportion and was only presented at 1100°C and 2h as experimental conditions.
The above can be corroborated with the chemical element mapping (SEM) study on the sample after wettability tests with Al–Si alloys at 1100°C for 2h as experimental conditions. The results are presented in
Figure 14. The main micrograph (upper left corner) presents the section of the study sample analyzed. Four main zones are observed: in the lower part, agglomerates of white particles (1); in the upper part, a solid section of dark gray color (2); a zone of reaction products (3); and within the metallic zone there are small light gray sections (4). According to the distribution of chemical elements, the area identified with the number 1 (agglomerates of light-colored hemispherical particles) corresponds to the ceramic substrate Sr
4Al
6O
12SO
4, the dark gray solid section identified with the number 2 corresponds to the Al–Si alloy, in the lower part of the area identified with number 3, due to the high concentration of magnesium, the presence of magnesium oxide (MgO) was recorded and a little higher up there is the presence of the spinel phase (MgAl
2O
4). The rest of the reaction zone and the main product are alumina (Al
2O
3). The number 4 identifies the presence of manganese and iron intermetallics within the metal alloy, although these phases were not recorded in the EDS analyses. On the other hand, the strontium and sulfur diffusion from the ceramic substrate towards the metal–alloy, and silicon, manganese and iron from the metal–alloy towards the ceramic substrate are observed. This reactions results of the chemical interaction process between the ceramic substrate and the Al–Si alloy (corrosion).
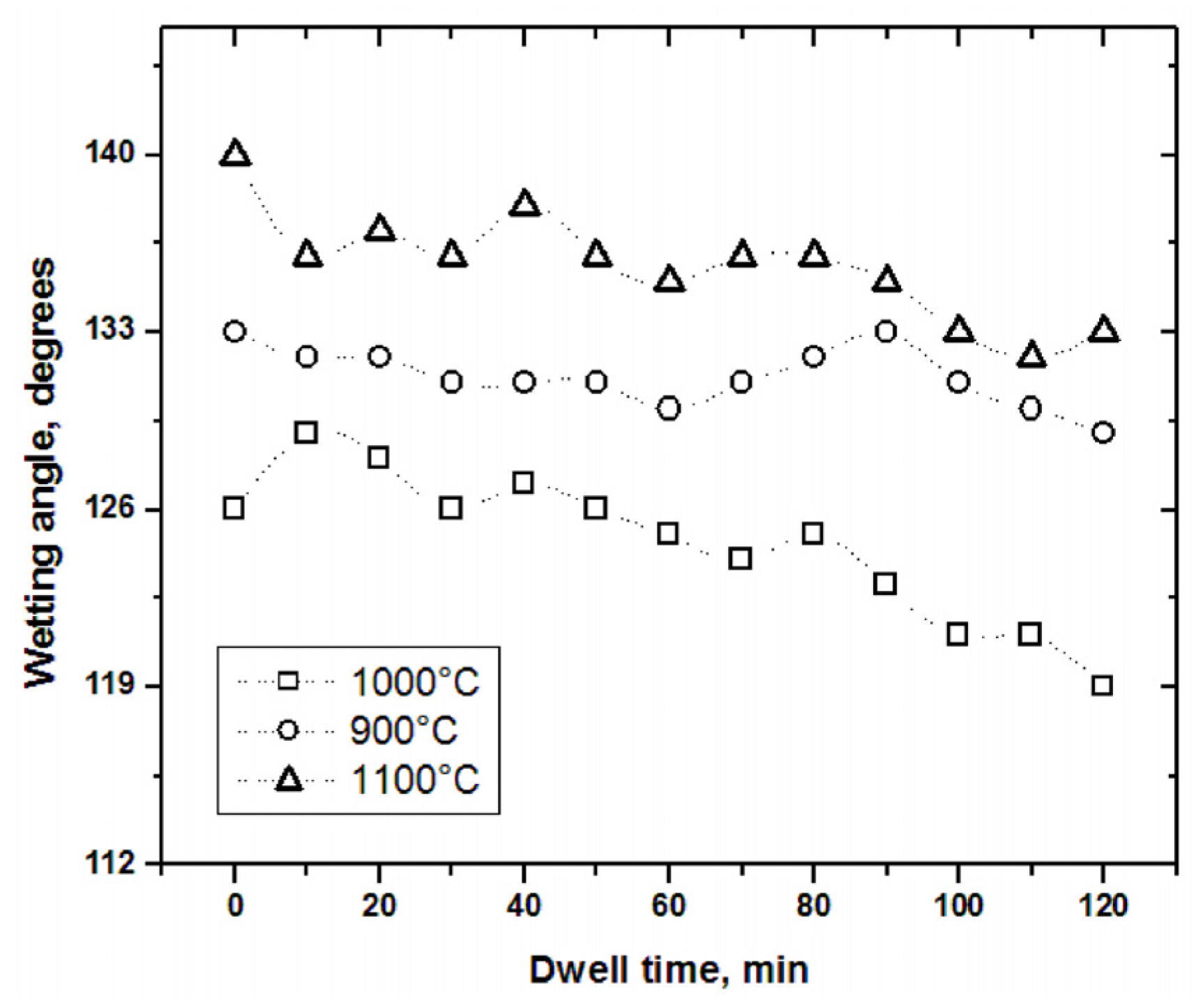
Figure 15 presents the results referring to the wetting angles recorded in the wettability tests of samples tested at 900, 1000, and 1100°C for 2h in contact with Al–Si alloys as experimental conditions.
As a first analysis, it is worth highlighting that all data are graphed within the zone of non-wettable materials (above 90°) in the three samples. On the other hand, the variation of the wetting angles can be observed with respect to the increase in test time. The sample exposed to 1100°C recorded the highest average wetting angle (≈135.61°), which means that it is the sample with the least affectation by the liquid metal in the tests. This behavior may be due to the formation of a considerable layer of reaction products on the surface of the ceramic substrate, consisting mainly of alumina of around 35µm thickness, which acts as a protective shield for the sample and prevents the rest of the material from corrosion. The samples at 900°C and 1000°C presented very thin layers of reaction products, mainly spinel, less than 5µm thick. The sample tested at 1000°C reported the lowest average wetting angle (≈124.57°).