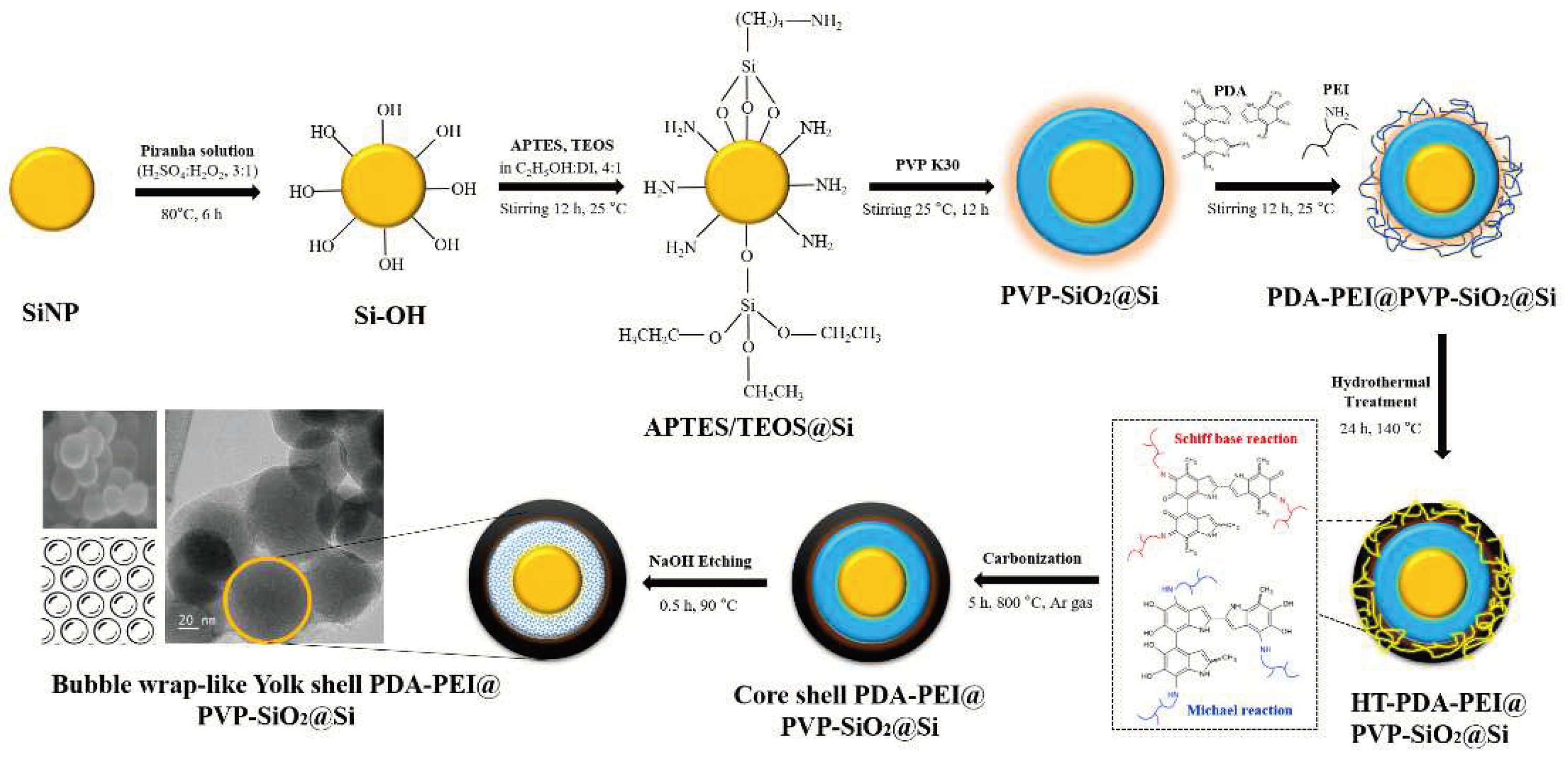
3.1. Role of APTES in the Synthesis of SiO2@Si Shells
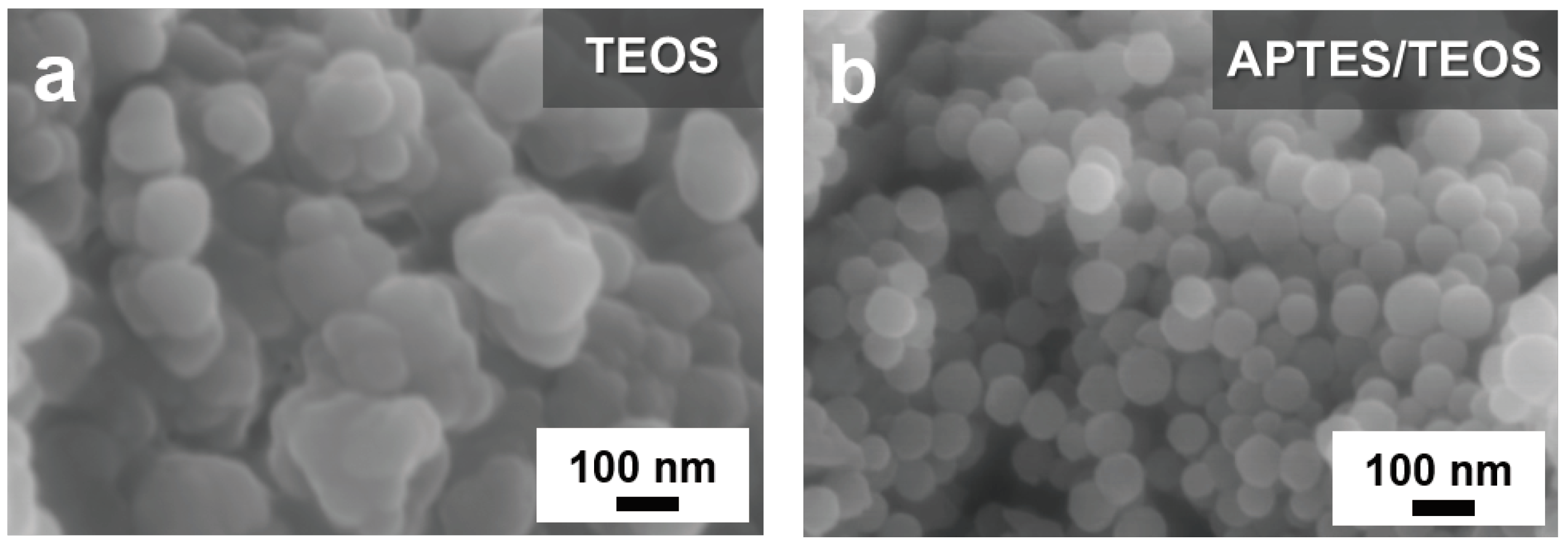
Figure 2 compares the SEM images of the TEOS- and APTES/TEOS-derived SiO
2 shells precursors
:
Figure S1 of the Supplementary Information (SI) presents the FE−SEM image and the FT−IR spectra of Si into Si–OH after the piranha pre-treatment. A thick and uneven coating from the TEOS-derived SiO
2 shell can be observed in
Figure 2a. In contrast, the APTES/TEOS-derived SiO
2 presented in
Figure 2b exhibits a relatively more uniform SiO
2 coating, with less agglomeration of Si–OH nanoparticles. Additionally, a conformal spherical morphology can be observed with particle average diameter of approximately 60 nm. This result is attributed to the self-catalytic activity of APTES regarding the sol–gel SiO
2 particle formation, wherein the formation of siloxane bonds with the abundant silanol groups of Si–OH takes place, even without the presence of an alkali catalyst [
55].
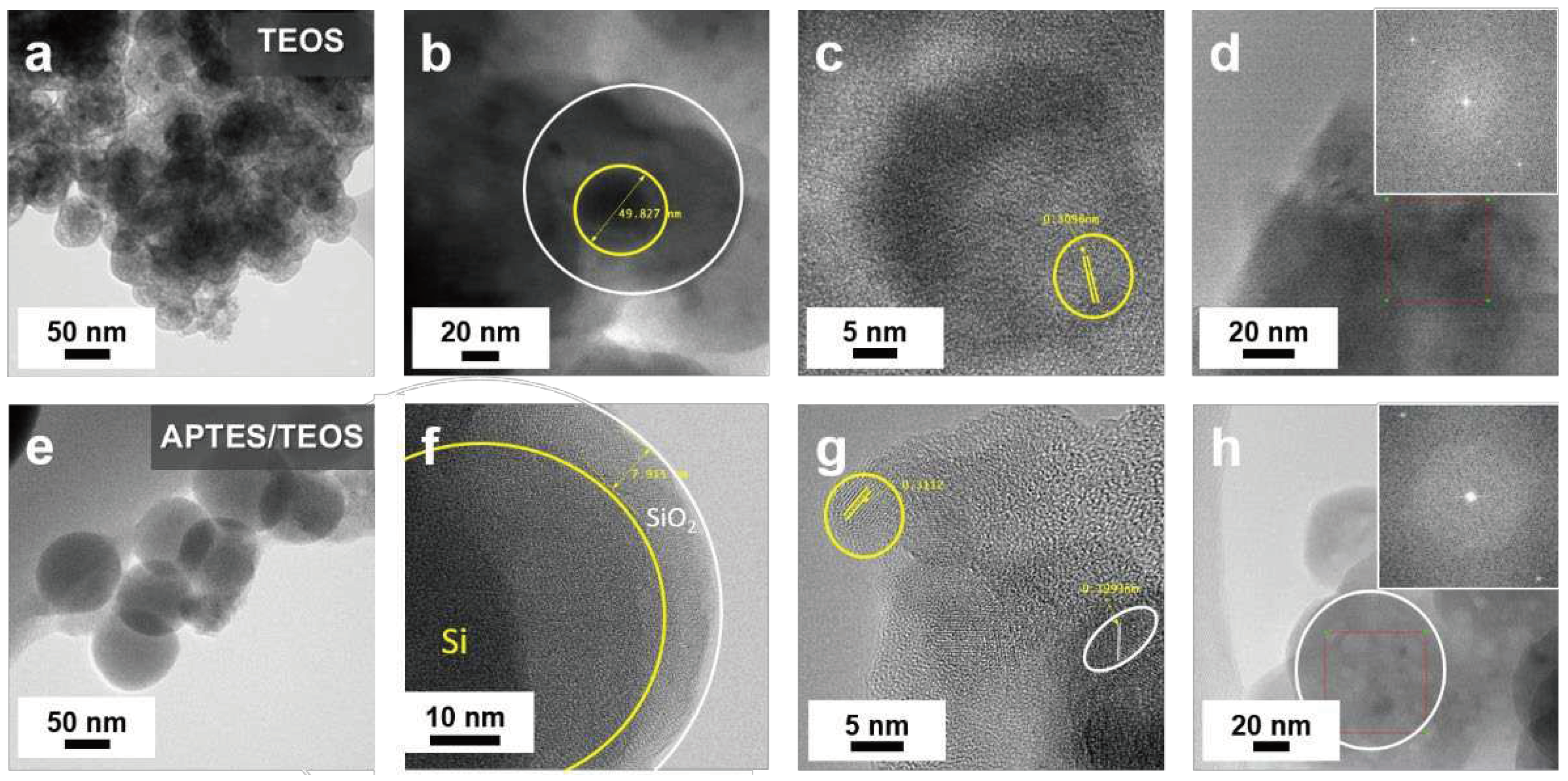
Evidence supporting the self-catalyzing role of APTES in facilitating SiO
2 growth and the successful coating on Si–OH is further supplied by the TEM results of
Figure 3. Notable particle agglomeration of the TEOS−SiO
2@Si with no structural organization can be observed in
Figure 3a. An isolated TEOS−SiO
2@Si particle shown in
Figure 3b portrays an approximately 50 nm Si nanoparticle encircled by a thick SiO
2 coating with evident aggregation characterized by dark patches scattered in the background. The in-plane lattice fringe highlighted in
Figure 3c reveals a 0.3096 nm ordered lattice spacing, which is attributed to the (111) plane of Si [
37]. The inset in
Figure 3d shows that after the SiO
2 sol–gel coating process using TEOS, a corresponding fast Fourier transform (FFT) pattern verified Si crystallinity was sustained.
On the other hand, the role of APTES as a structure-directing agent is highlighted in
Figure 3e, which depicts the APTES/TEOS-derived SiO
2@Si with no visible aggregation of particles, and with uniform spherical morphology. A thin, conformal SiO
2 coating (~8 nm) was observed in an isolated APTES/TEOS−SiO
2@Si nanoparticle shown in
Figure 3f. Two distinct crystal lattice spacings were detected in
Figure 3g, namely (0.3112 and 0.1993) nm, in good agreement with the (111) and (220) planes, respectively, of Si [
31,
37]. Notable bright spots are shown in the inset image in
Figure 3h, indicating that the inherent crystallinity of Si is maintained.
Previous literature citing the co-condensation of TEOS and APTES in EtOH and DI have generally incorporated APTES as a surface-modifier in a post-modification or grafting of amino groups to Si [
35], as a precursor material to SiO
2 [
55], such as in soft-hard template strategies [
47], often with other surfactants (e.g., cetyl trimethyl ammonium bromide, CTAB) [
58].
Figure S2 of the SI illustrates the crucial role of APTES as a self-catalytic, structure-directing agent to TEOS, the hydrolysis and condensation reaction mechanism, and the formation of siloxane networks from APTES and TEOS. Note that although the pristine Si nanoparticles inherently possess a native oxide layer due to inevitable surface oxidation during manufacturing, pre-treatment with piranha solution is generally preferred to optimize the number of surface hydroxyl groups to form bonds with the silanol groups of APTES and TEOS. The −OH groups of piranha-pre-treated Si–OH energetically favored the formation of siloxane bonds in a condensation reaction, resulting in a well-ordered silane layer on the surface of Si in the APTES/TEOS, even without the presence of an alkali catalyst.
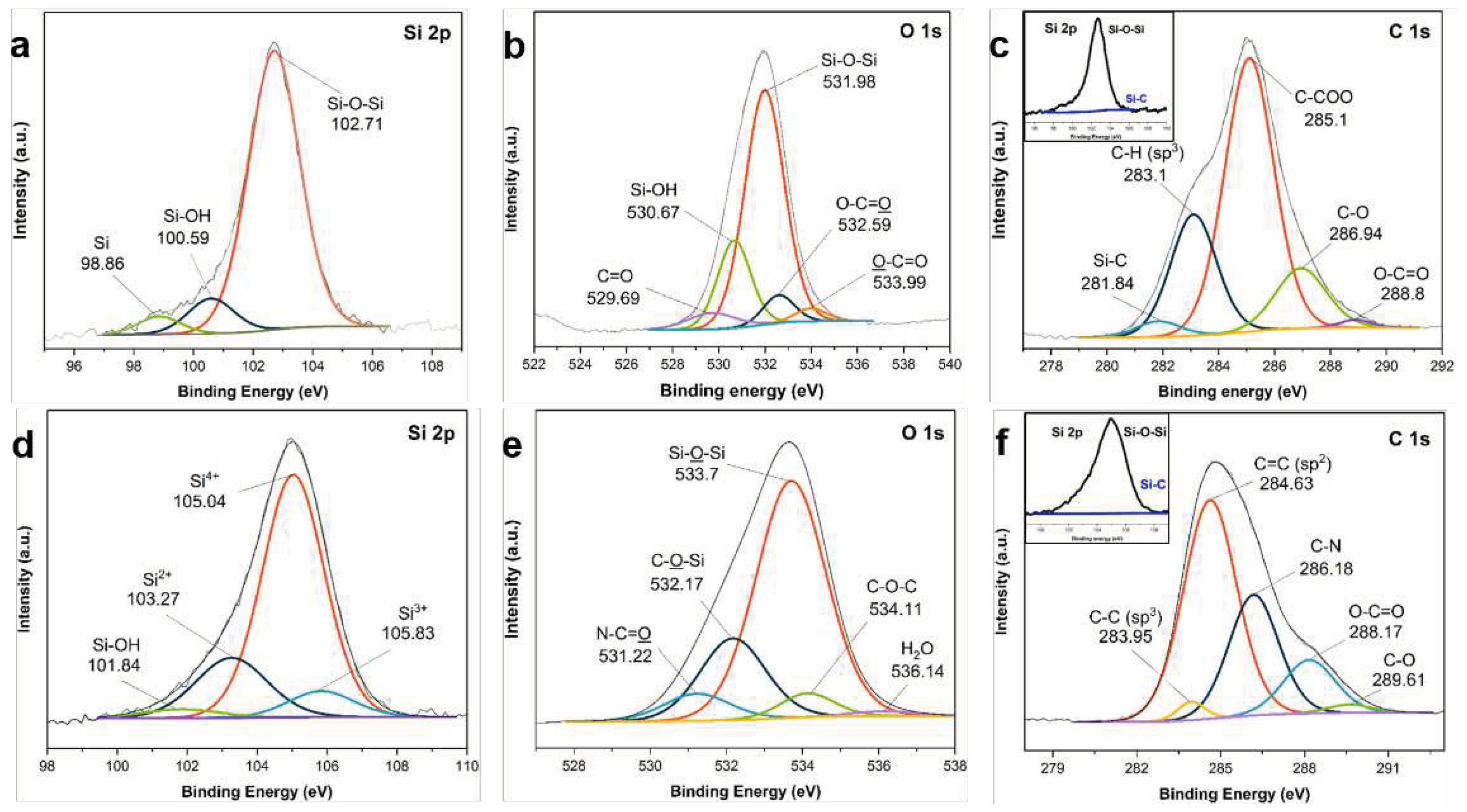
Figure 4 shows the results of XPS analysis conducted to confirm the changes in the elemental composition during SiO
2 synthesis from the two precursors, and subsequent coating with Si nanoparticles. The high-resolution Si 2p spectra of the TEOS-derived SiO
2@Si in
Figure 4a reveals three peaks at 102.71 eV that are attributed to the Si–O–Si band, 100.59 eV related to Si–OH, and a small peak at 98.86 eV corresponding to Si. These peaks also appear in the O 1s scan in
Figure 4b, revealing peaks at 531.98 eV for the Si–O–Si and 530.67 eV for the Si–OH [
59]. The high relative intensities of the Si–O–Si peak at both Si 2p and O 1s scans suggest that the majority of the TEOS silane precursor was successfully converted into SiO
2. The trace amounts of Si–OH are assumed to be from the unreacted hydroxyl groups from piranha pre-treatment. The occurrence of a small Si peak suggests that the SiO
2 coating derived from TEOS precursor resulted in inefficient coating, with bare Si nanoparticles remaining. The C 1s spectra in
Figure 4c reveal peaks corresponding to the different chemical environments of carbon within the organic groups of TEOS: 283.1 for sp
3 C−C, 285.1 eV for the C−COO sub-peak due to the chemical shift of the carbon bonded to an adjacent ester group, the ester group C−O at 286.94 eV, and the ester group sub-peak O−C=O at 288.8 eV, and Si–C peak at 281.84 eV. Furthermore, C 1s scan also identified the O 1s peaks at 532.59 eV for O−C=O and 533.99 eV for O−C=O showing different bonding environment of the O atoms in the ester groups [
60].
Meanwhile,
Figure 4d reveals that neither the Si–OH nor pure Si peaks were detected in the Si 2p scans of the APTES/TEOS-derived SiO
2@Si nanoparticles. This observation indicates that all the Si–OH was successfully condensed into an SiO
2 layer after the reaction. The Si 2p scans reveal that the Si–O–Si peak with relatively high intensity shifts to a higher binding energy of 105.04 eV, due to the generation of a particle charge on the deposited SiO
2 coating. A shift to higher binding energy was observed in the silicon oxides while the non-oxidized chemical species exhibited no shift [
59,
61]. In other reports, the Si 2p peak at 105.04 eV signifies that the Si obtained after APTES/TEOS−SiO
2 coating is in the 4
+ oxidation state, while the broad peaks at (103.27 and 105.83) eV are ascribed to the Si
2+ and Si
3+ oxidation states, respectively, of the amorphous SiO
x [
62]. The relatively small peak of Si–OH at 101.84 eV further supports the construction of SiO
2 coating on Si. Apart from the major peak shifted to 533.7 eV relating to the Si–O–Si band, the O 1s peaks in
Figure 4e that are detected at (532.17, 531.22, and 534.11) eV for C−O−Si, N−C=O, and C−O−C, respectively [
63], align well with the C 1s scans in
Figure 4f, with peaks centered at (284.63, 286.18, 288.17, 283.95, and 289.61) eV for the sp
2 C=C, C−N, O−C=O, sp
3 C−C, and C−O, respectively [
62,
64]. A negligible peak detected at 536 eV was due to the trace amounts of adsorbed H
2O molecules, presumably during sample analysis [
65]. Additionally, the N 1s peaks at 400.07 eV ascribed to C−N confirmed the successful SiO
2 modification into an amino-functionalized SiO
2 coating, due to the chemical composition of APTES [
63].
3.2. Multifaceted Effects of the Proposed Modified Stöber via Hydrothermal Treatment
It has been established that (1) the Si active material needs to be coated uniformly with a thin carbon layer, providing a physical barrier of protection from direct electrolyte contact. Since inevitable Si volume expansion is expected, it is crucial for the SiO2 sacrificial layer to be effectively etched during template removal, to allow the formation of an internal void space. Moreover, the rigid structure of SiO2 coupled with its amorphous character presents another condition that needs to be satisfied. (2) The void space provided by yolk–shell structure is not just for the absorption of Si volume expansion but is also crucial to prevent the formation of cracks in the carbon shell from repetitive volume fluctuations. Furthermore, (3) the carbon coating should be able to withstand the etching process without damage to its mechanical structure. To achieve all three conditions above, PVP K30 polymer was loaded to the APTES/TEOS−SiO2@Si, followed by subsequent PDA carbon coating and PEI crosslinking via the proposed hydrothermal treatment. A control sample was fabricated following the same procedures at RT.
The importance of a hydrothermal treatment in increasing the efficiency and stability of PDA carbon shells has been reported, even after 40 wt.% HF etching for 2 h [
66]. Building on this idea, a stable PDA coating to Si can be constructed with satisfactory structural integrity. It is also important for the proposed fabrication process to not alter the inherent crystallinity of the Si active material during template removal. Therefore, the crystallinity of the Si active material was evaluated during the entire composite fabrication process via XRD analysis;
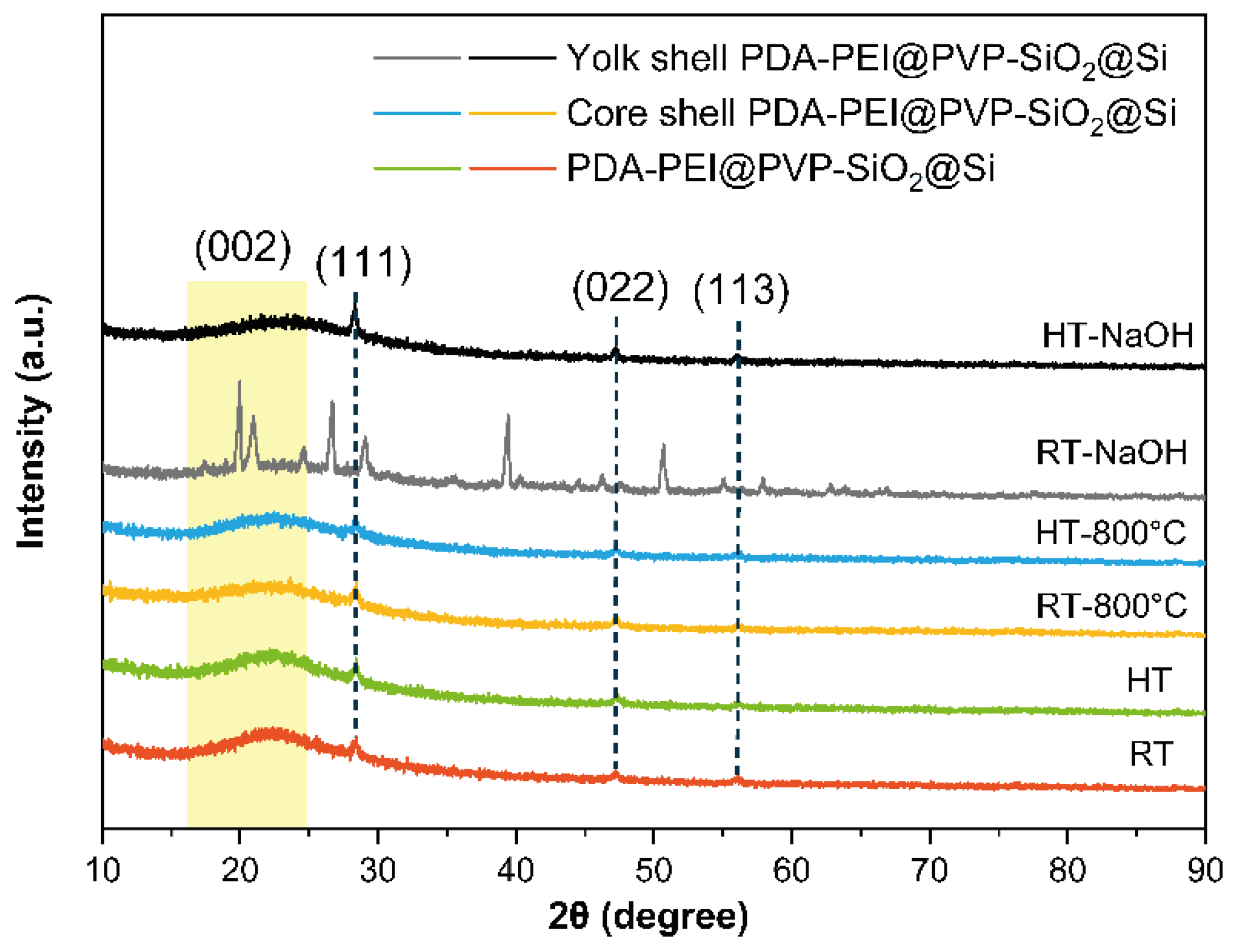
Figure 5 presents the results verifying the Si crystallinity.
The crystallinity of the Si active material during the composite fabrication process was monitored via XRD analysis. For comparison,
Figure S3 of the SI presents the XRD patterns of the pristine Si nanoparticles, TEOS−SiO
2@Si, and APTES/TEOS−SiO
2@Si. As shown in
Figure 5, the crystallinity of Si is well maintained in both synthetic routes. Apart from the broad peak centered at 2ϴ =26.0° that is attributed to the (002) plane of graphitic carbon materials due to the PDA−PEI carbonization, intense diffraction peaks at 2ϴ = (28.4, 47.3, and 56.1)° were well-indexed to the (111), (220), and (311) facets, respectively, of a typical face-centered cubic Si crystal (Reference code 98-065-2265), presented in
Figure S4 of the SI. Representative Si peaks were all observed in the composites with or without the hydrothermal treatment, even after high-temperature pyrolysis. However, after the composite samples without hydrothermal treatment have undergone the etching process, these peaks have disappeared, and multiple intense peaks at various diffraction angles are detected. In contrast, the composite samples synthesized via the proposed hydrothermal route present Si peaks, even after NaOH etching. The XRD results verify the positive effect of hydrothermal treatment in fabricating yolk–shell composites, without altering the Si crystallinity upon template removal.
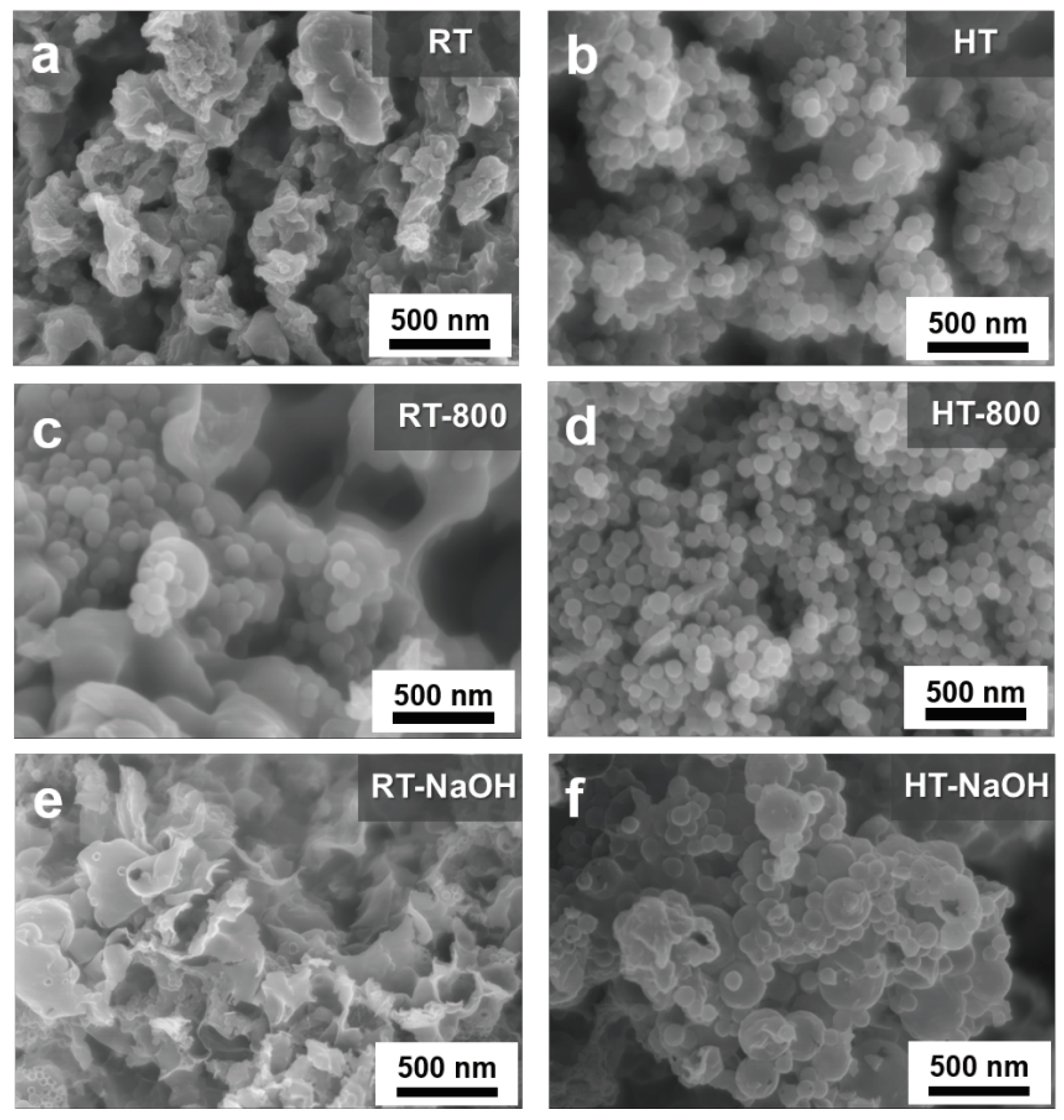
Aside from maintaining Si crystallinity against harsh etching conditions, positive effect on the coating efficiency was also observed in the sample composites prepared via hydrothermal treatment, as compared in
Figure 6:
A thin, sheet-like structure enveloping spherical SiO
2@Si nanoparticles that were aggregated to a large extent was obtained in a one-pot synthetic route at RT, as shown in
Figure 6a. Meanwhile, the composites obtained via the hydrothermal treatment Stöber route illustrated in
Figure 6b exhibit well-dispersed SiO
2 shells coated with PDA−PEI in a uniform manner, with less aggregation. The dramatic difference between the carbon coating efficiency through both routes is evident in comparing
Figure 6c,d after pyrolysis. The uneven carbon coating of RT–composite samples demonstrate exposed APTES/TEOS−SiO
2@Si nanoparticles lumped together above sheet-like PDA−PEI carbon structures. Even with PVP-surface protection during NaOH etching, the composites without hydrothermal treatment exhibit severe damage to the carbon network structure, as shown in
Figure 6e, wherein the PDA−PEI carbon coating and crosslinking structure were compromised as the SiO
2 template was removed. On the other hand, the composite samples prepared with hydrothermal treatment were able to maintain distinguishable PDA carbon coatings with almost no damage to the structure, as shown in
Figure 6f. The TEM images in
Figure S5 of the SI provide a visual insight into the effect of hydrothermal treatment, which resulted in a more complete PDA carbon coating and PEI crosslinking to the APTES/TEOS−SiO
2@Si nanoparticles.
3.3. Significance of PVP K30 Surface Protection during NaOH Etching
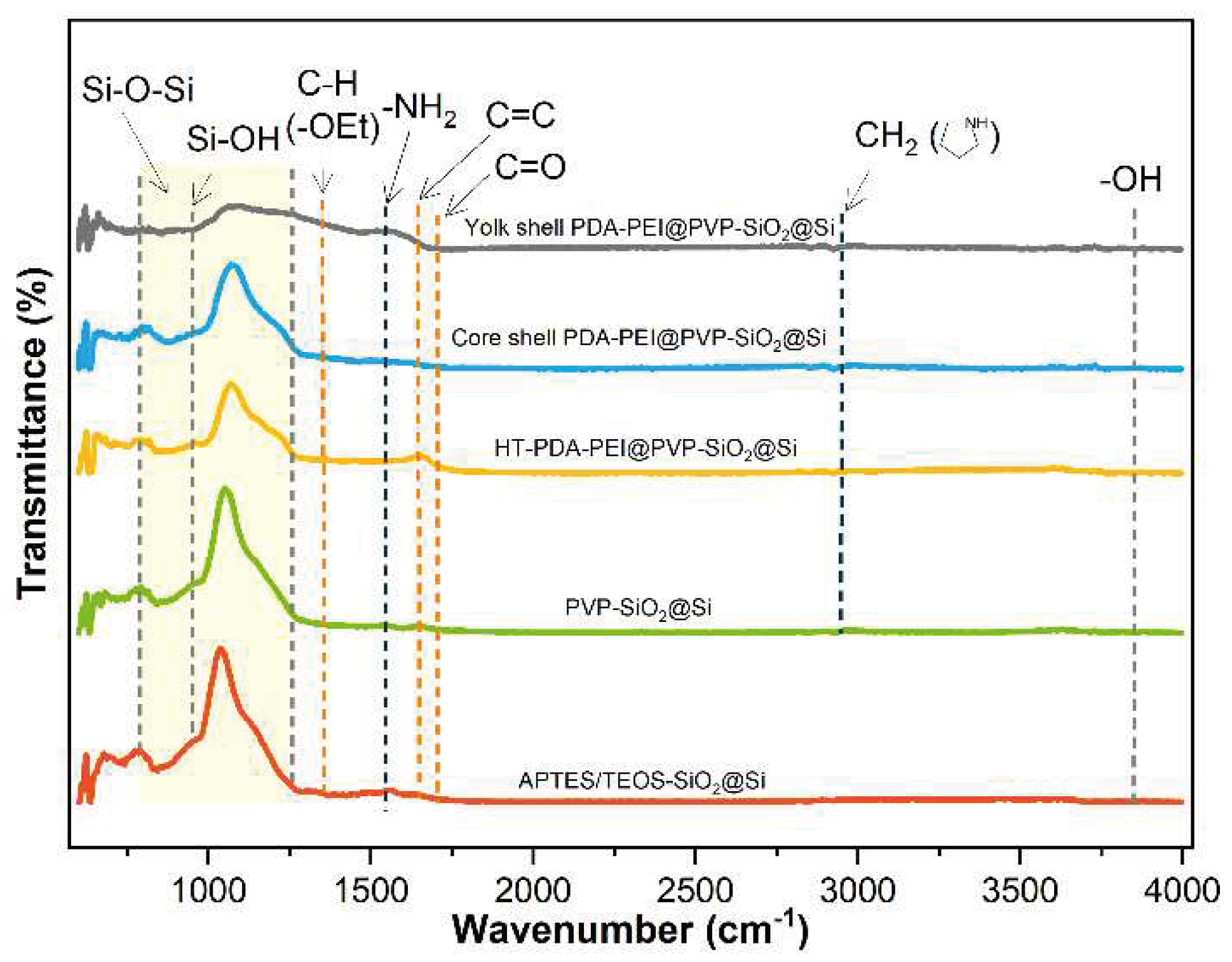
The direct loading of the PVP K30 polymer was studied by comparing the chemical bonds present in the composite samples during the entire fabrication process. FTIR analysis was conducted to verify the successful loading of the PVP K30, and
Figure 7 presents the resultant spectra:
The abundant surficial –OH groups previously detected at ~3,400 cm
−1 after piranha pre-treatment disappeared completely from all sample spectra, which can be interpreted as a result of the successful condensation reactions of the APTES/TEOS. An adsorption band at 1,544 cm
−1 corresponding to −NH
2 groups is attributed to the amino groups present in the APTES precursor solution [
31]. The weak absorption band at 1,348 cm
−1 is attributed to the C−H bending vibration of the unhydrolyzed −OEt groups detected on the APTES/TEOS−SiO
2@Si, which when the PVP K30 is loaded to yield PVP-SiO
2@Si, further reduce in intensity. This observation is attributed to the formation of hydrogen bonds with the SiO
2 surface. Additional vibration peaks detected at 2,925 cm
−1, assigned to the −CH
2 stretching modes in the pyrrolidone ring, C=O stretching band at 1,703 cm
−1, and C=C bond at the PVP polymer backbone at 1,645 cm
−1, provide further evidence of the successful PVP loading into the SiO
2 shells [
56]. An increase in the C=C peak can be attributed to the formation of a graphitic carbon structure during hydrothermal treatment, which is due to the polymerization and crosslinking reactions of PDA and PEI polymers. Meanwhile, a broad and intense absorption band in the range (788−1,095) cm
−1 corresponding to the symmetric and asymmetric stretching of Si–O–Si bands and Si–OH peak at 947 cm
−1 both present for all samples further verifies the complete condensation of APTES/TEOS precursor, and the formation of SiO
2 shell [
44].
Note also that the intensity of the Si–O–Si bands follows a decreasing trend from sol–gel coating to etching. The decrease in Si–O–Si band after PVP K30 is added can be ascribed to the formation of PVP-treated SiO2 shells. Further decrease in the peak intensity is observed after PDA−PEI is added to form a carbon shell to the SiO2, aligned with the occurrence of a strong C=C peak. The thermal treatment at 800 °C increases the SiO2 stability, which is characterized by a slight increase in the Si–O–Si band intensity. Finally, after NaOH etching, the Si–O–Si band intensity is significantly reduced, dissolving the SiO2 template.
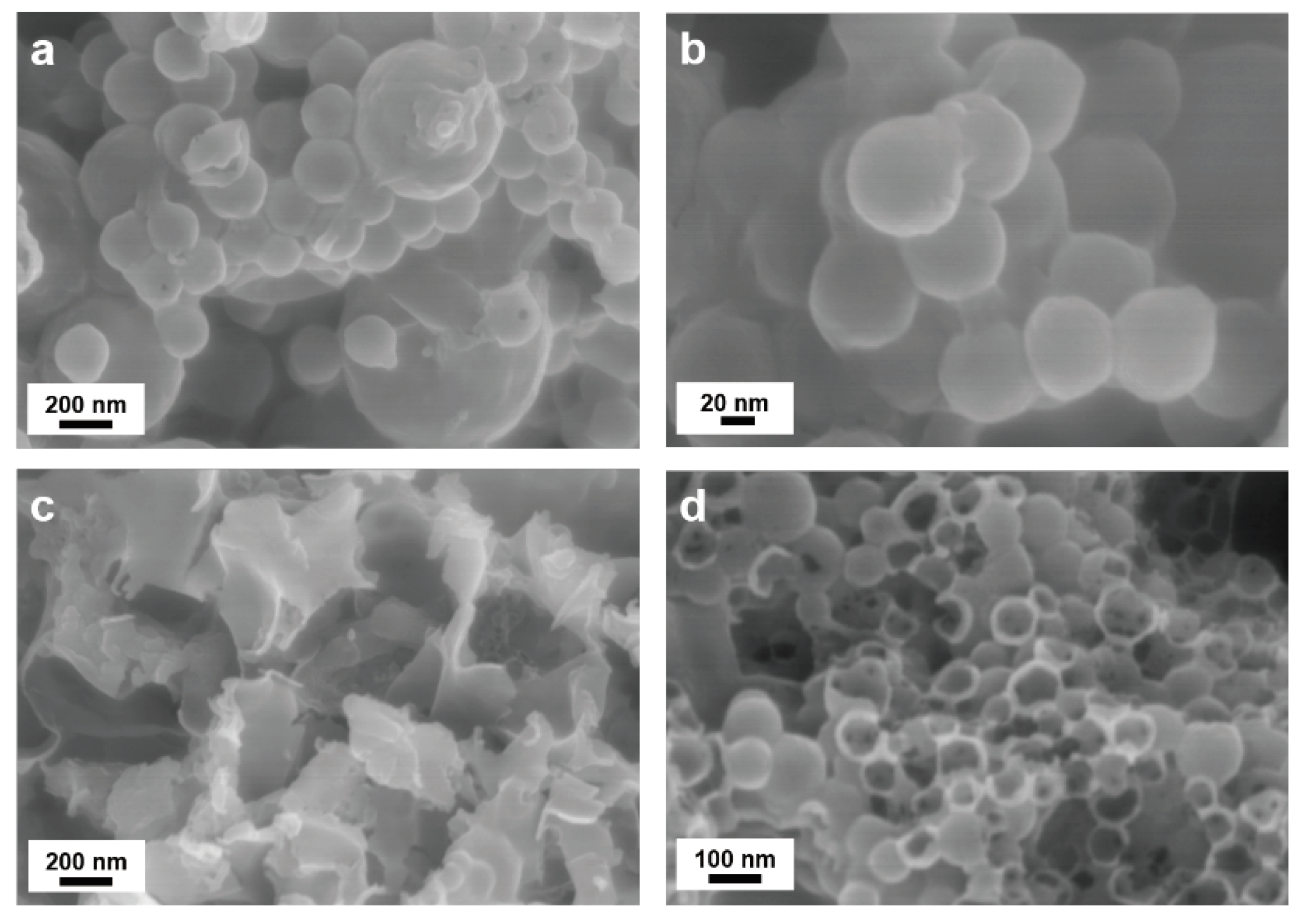
The TEM analysis results shown in
Figure 8 comparing sample composites with and without a PVP K30 surface protection highlight the integrity of carbon coating, and whether the spherical morphologies are maintained after etching. The PVP K30-surface protected composites samples shown in
Figure 8a,b exhibit excellent stability, with no damage to the PDA−PEI carbon network structure, even after NaOH etching. However, a contrasting difference in the morphologies of the composite samples without PVP K30 protection can be observed in
Figure 8c,d, with the majority of PDA carbon shells destroyed, and the sheet-like PEI carbon networks torn apart.
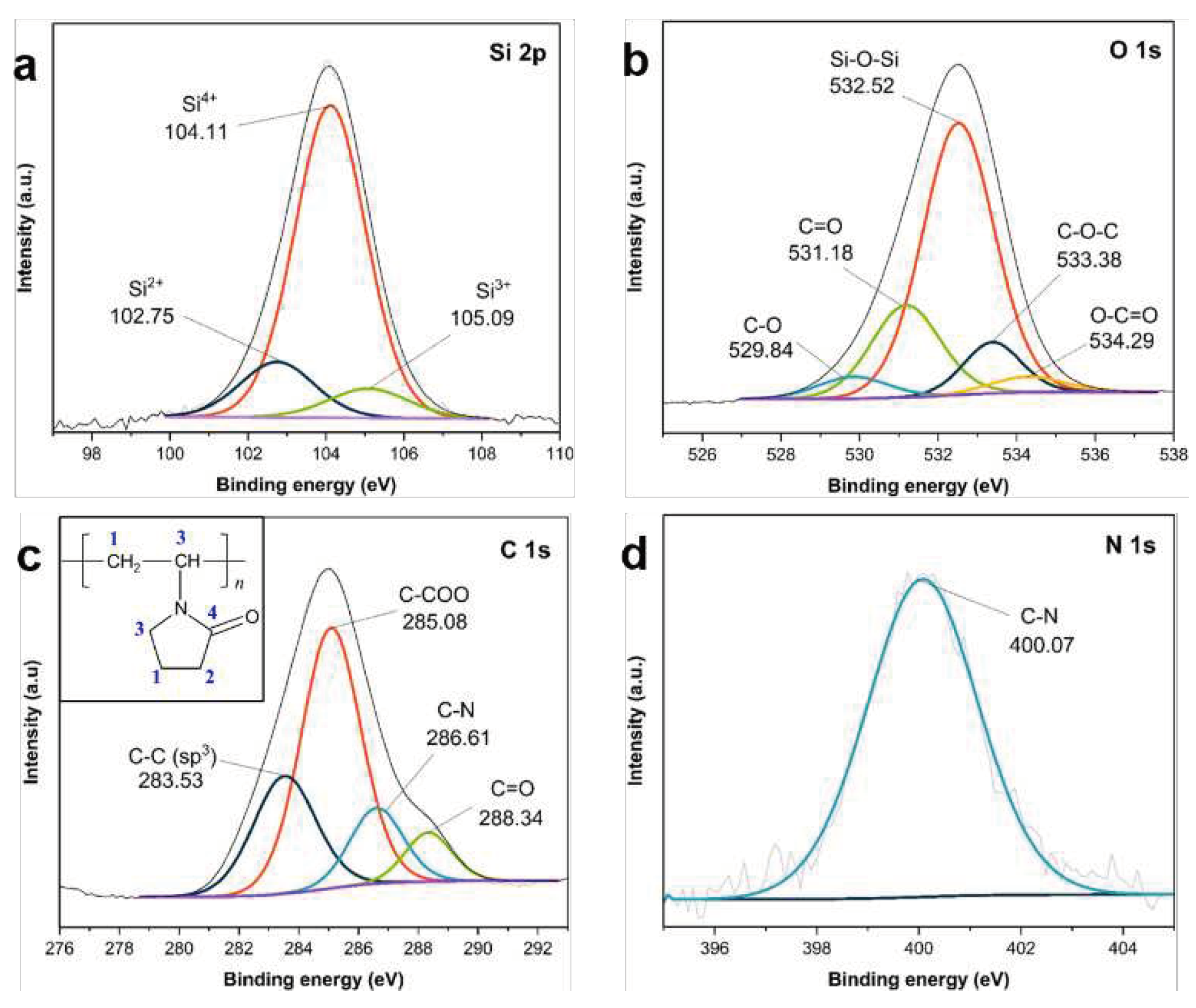
The successful loading of PVP K30 polymer into APTES/TEOS−SiO
2@Si is also verified by the XPS analysis shown in
Figure 9. The high-resolution Si 2p scans in
Figure 9a reveal three peaks at (104.11, 102.75, and 105.09) eV, which are all ascribed to the Si–O–Si band, and the increasing oxidation states of Si into Si
2+, Si
3+ and Si
4+ oxidation states, respectively, related to the synthesis of SiO
2 shells after the condensation of APTES and TEOS [
59,
61]. The O 1s scans in
Figure 9b detect peaks at 532.52 eV with a high relative intensity, which are ascribed to the Si–O–Si band of the siloxane networks, with (531.18, 533.38, and 529.84) eV for the C=O, C−O−C, and C−O bonds, respectively, in the PVP molecular structure [
67]. The small peak at 534.29 eV is attributed to the O−C=O peak initially identified from the carbon-containing organic groups of the silane precursors used [
60]. The C 1s scans in
Figure 9c also reveal peaks at (283.53, 285.08, 286.61, and 288.34) eV which originate from the different carbon atoms of the PVP molecules (see inset in
Figure 9c) [
67]. The peak revealed in the N 1s scan at 400.07 eV in
Figure 9d is assigned to the N atoms of C−N from the APTES structure.
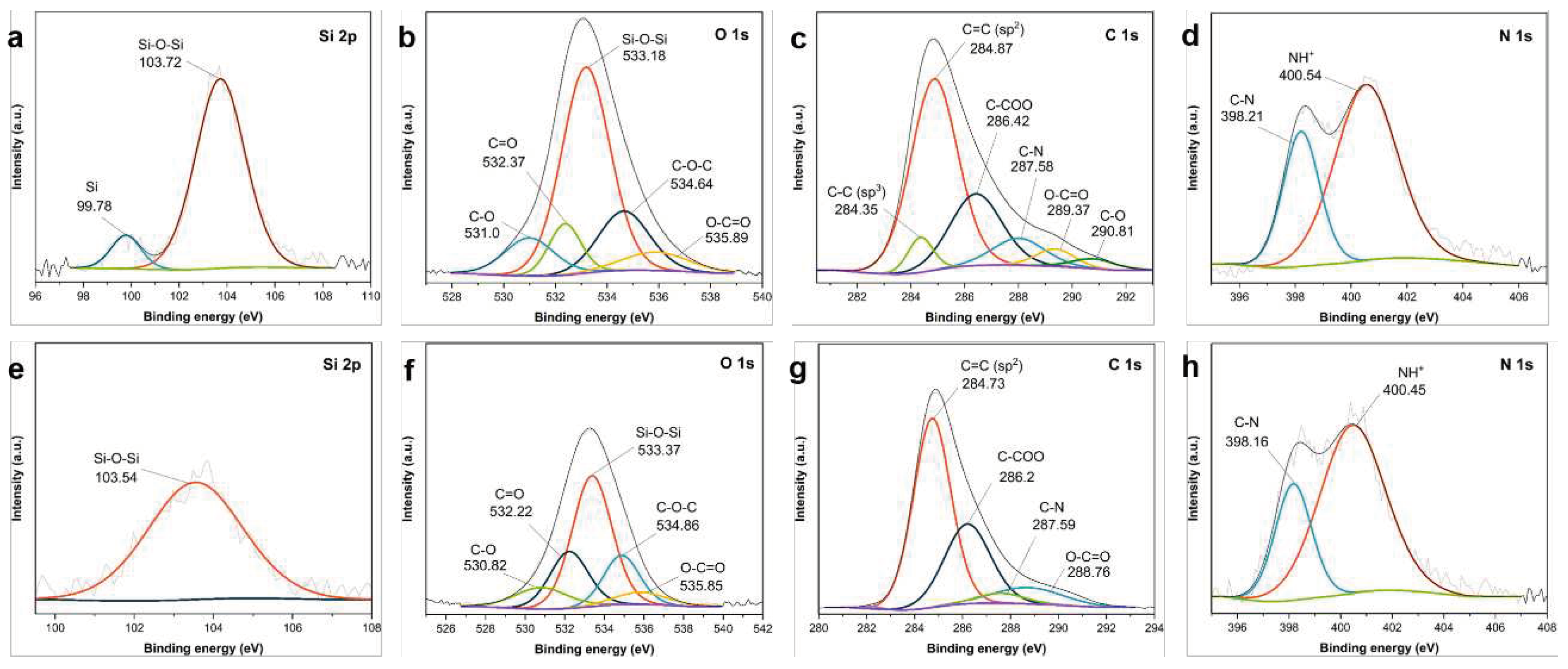
3.4. Characterization of Representative Core–Shell and Yolk–Shell Composites
Representative composites bearing core–shell and yolk–shell structures, including a sample composite with a PVP-surface protected SiO
2 shells and PEI crosslinked structure, were fabricated following the proposed synthetic route. To study the changes in the surface composition of the fabricated composites with a core–shell and a yolk–shell structure, namely, the core–shell PDA−PEI@SiO
2@Si and yolk–shell PDA−PEI@SiO
2@Si, XPS analysis was conducted, and
Figure 10 presents the results:
The high-resolution Si 2p scan of the core–shell PDA−PEI@SiO2@Si composite sample in
Figure 10a reveals two peaks; one at 103.72 eV, which is attributed to the Si–O–Si band with a relatively higher intensity than the peak located at 99.78 eV, which is ascribed to Si [
59]. These peaks have been previously identified, and matched with the cited literature. The O 1s scans in
Figure 10b are deconvoluted into five components in decreasing order of peak intensity: (533.18, 534.64, 532.37, 531.0, and 535.89) eV for the Si–O–Si band, C−O−C, C=O, C−O, and O−C=O, respectively [
60,
63]. Meanwhile, the peaks revealed in the C 1s scan were located at the following six peaks in order of decreasing intensity: (284.87, 286.42, 289.37, 284.35, 289.37, and 290.81) for the sp
2 C=C bonds, C−COO, C−N, sp
3 C−C bonds, O−C=O, and C−O, respectively [
62,
64]. Similar peaks were observed in the yolk–shell PDA−PEI@SiO
2@Si sample, except for the reduced intensities for the O-containing compounds, due to the NaOH etching. For example, the intensity of the Si–O–Si band at 103.54 eV shown in
Figure 10e, as well as the O 1s scans in
Figure 10f, exhibit a similar reduction in peak intensities for Si–O–Si, C−O−C, C=O, O−C=O, and C−O peaks at (533.37, 534.86, 532.22, 535.85, and 530.82) eV, respectively. However, the peaks for sp
3 C−C and C−O bonds in
Figure 10c of the core–shell composite disappear in
Figure 10g after the removal of SiO
2. This observation can be attributed to the destruction of the carbon structures, previously confirmed in
Figure 8 showing TEM images of the composite materials without PVP K30 surface protection. The chemical species detected in the O 1s scans align well with the results of C 1s scans all attributed to the SiO
2 component, to the carbon-containing ligands of the silanes used, and to the polymer chains of PDA carbon coating compound and PEI crosslinks. Two peaks are revealed in the N 1s scan of the core–shell PDA−PEI@SiO
2@Si sample in
Figure 10d centered at (400.54 and 398.21) eV, while these peaks in the yolk–shell PDA−PEI@SiO
2@Si sample are located at (400.45 and 398.16) eV. These peaks were ascribed to the protonated amines due to APTES hydrolysis and C−N bonds, respectively [
63]. The C−N bond that appeared on the O 1s, C 1s, and N 1s scans is attributed to the crosslinking reaction of the amino groups and catechol in oxidized PDA polymer chains with PEI molecules. The formation of the PDA−PEI networks are presumed to be via a Schiff base reaction or a Michael addition reaction mechanism, as illustrated in
Figure S6 of the SI [
68].
The effect of PVP K30 polymers and the role of PEI crosslinked structures in enhancing the durability of the carbon coating layer is further highlighted in the XPS results of the core–shell PDA@SiO
2@Si, when compared to the yolk–shell PDA−PEI@PVP−SiO
2@Si composite sample, as shown in
Figure 11:
Similar peak observations were detected in the composite samples. For example, the Si 2p of the core–shell PDA@SiO2@Si in
Figure 11a detects a similar Si–O–Si band, which also appears in the yolk–shell PDA−PEI@PVP−SiO
2@Si sample in
Figure 11e, only with a lower peak intensity. Likewise, the O 1s scans in
Figure 11b,f in both samples detect similar chemical compositions at very similar binding energies in decreasing order of peak intensities, namely: Si–O–Si, C=O, C−O−C, C−O, and O−C=O. The same observations are made based on the comparison of C 1s scans of both samples illustrated in
Figure 11c,g; with peaks for the sp
2 C=C, C−COO, C−N, O−C=O, and C−O. Lastly, no significant changes are observed in the N 1s scans of the two composites, as shown in
Figure 11d,h. Apart from the obvious reduction in peak intensities of the O-containing groups across all high-resolution scans observed in the PDA−PEI@PVP−SiO
2@Si composite samples due to etching, note that the important sp
3 C−C peak at 284.47 eV and C−O peak at 289.92 eV in the C 1s of the PVP-surfaced protected composite sample shown in
Figure 11g are sustained, in contrast to the non-PVP protected etched composites shown in
Figure 10g. This result further verifies the important role of PVP surface protection and the PEI crosslinking in terms of constructing a carbon coating layer that can withstand the NaOH etching necessary for template removal.
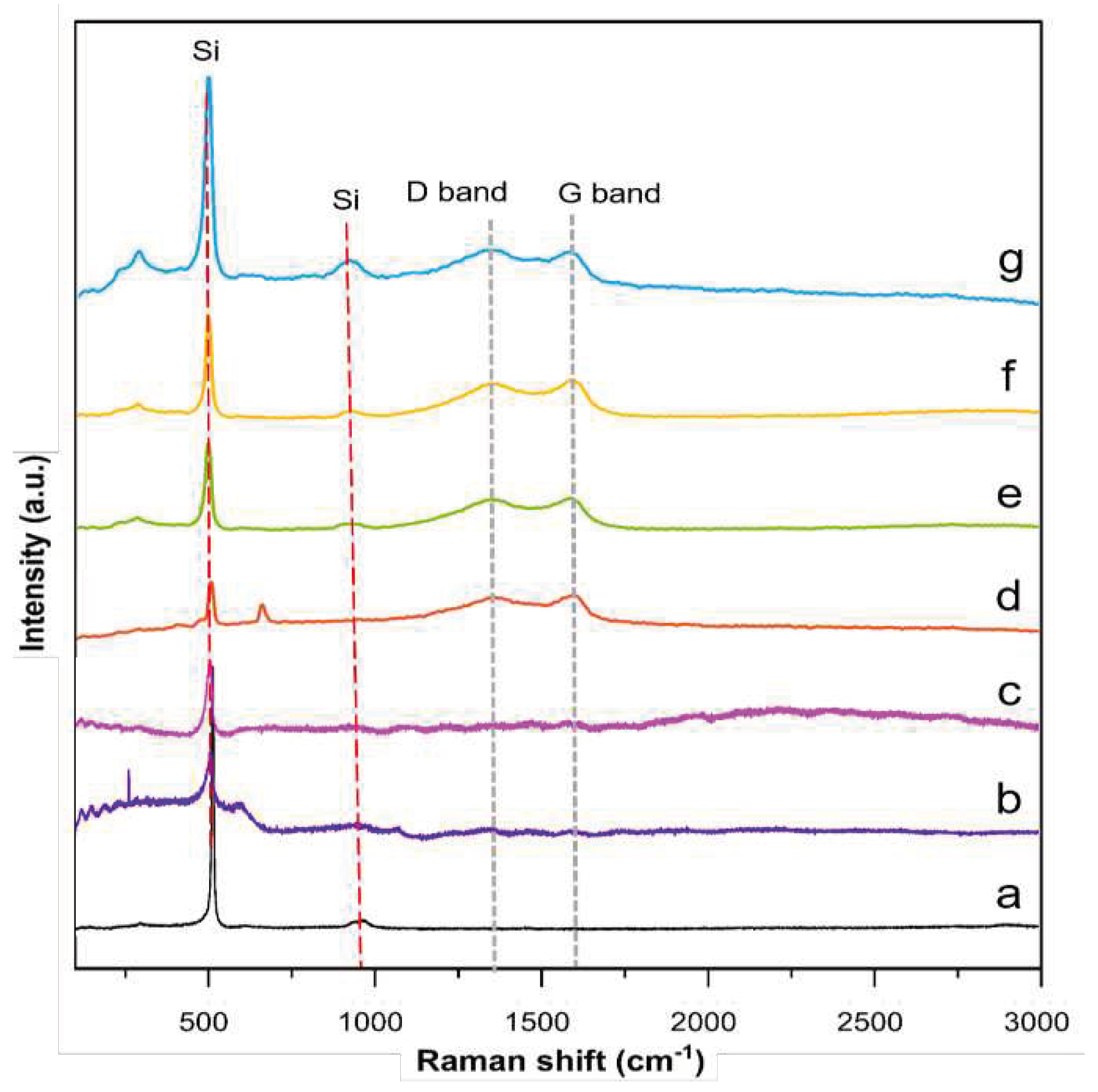
Raman spectroscopy was performed to determine the defect quantity within the carbon coating layer, and identify the degree of graphitization of representative core and yolk–shell composites. Additionally, the TEOS−SiO
2@Si, APTES/TEOS−SiO
2@Si, and PVP-SiO
2@Si samples are also analyzed for reference purposes.
Figure 12 summarizes the recorded spectra of each representative composite and reference sample:
The peaks located at (511 and 918) cm
−1 related to Si appear in all sample curves, suggesting that the Si crystallinity and inherent properties are maintained. The peak attributed to Si exhibits a decrease in intensity after APTES is added to the precursor mix, implying that after the reaction is completed, the Si is coated with a layer of amorphous SiO
x. This decreasing trend in Si peak intensity is further observed after the PVP was loaded onto the SiO
2 shells. Meanwhile, all representative composite samples exhibit a characteristic disordered D band at ~1,350 cm
−1 and ordered G band at ~1,590 cm
−1, typical of graphite and other sp
2-bonded carbons after the pyrolysis of PDA and PEI molecules [
69]. More importantly, second-order vibrations at ~2,400 cm
−1 are shown in the PDA−PEI-containing composites, suggesting that the carbon coating is at least partially graphitic [
70]. The relatively stronger G bands compared to the D bands across all the representative composites provide strong indications that the PDA and PEI molecules are integrated into a crystalline graphitic matrix, as initially predicted via the TEM results shown in
Figure 3. The relative intensity ratio of the disordered carbon to the graphitized carbon (I
D/I
G) is quantified for all composite samples after curve fitting using the Gaussian-Lorentzian model, and are shown in
Figures S7−S10 of the SI, with corresponding I
D/I
G ratios, including the fitting parameter values, summarized in
Table S1 of the SI [
71].
The increase in the I
D/I
G value of the core–shell PDA@SiO
2@Si (0.84) upon the addition of the PEI polymer in the core–shell PDA−PEI@SiO
2@Si (0.85) is ascribed to the increase of sp
2-carbon edge atoms after the co-polymerization of PDA and PEI, and subsequent graphitization during thermal treatment. A similar increase in I
D/I
G value is observed for the yolk–shell PDA−PEI@SiO
2@Si (0.85), which is attributable to the successful carbonization of the polymer coatings. Although the PVP-SiO
2@Si does not show any remarkable variations of the G band peak position or of the I
D/I
G ratio, this lack of variation can be related to the high intensity of Si signals weakening the carbon contribution from the PVP K30 [
72]. The yolk–shell PDA−PEI@PVP−SiO
2@Si (0.86) displays the highest I
D/I
G value among the representative composites, which is attributed to the PDA−PEI graphitization and subsequent carbonization of the PVP K30 polymer chains protecting the SiO
2 shells after thermal treatment.
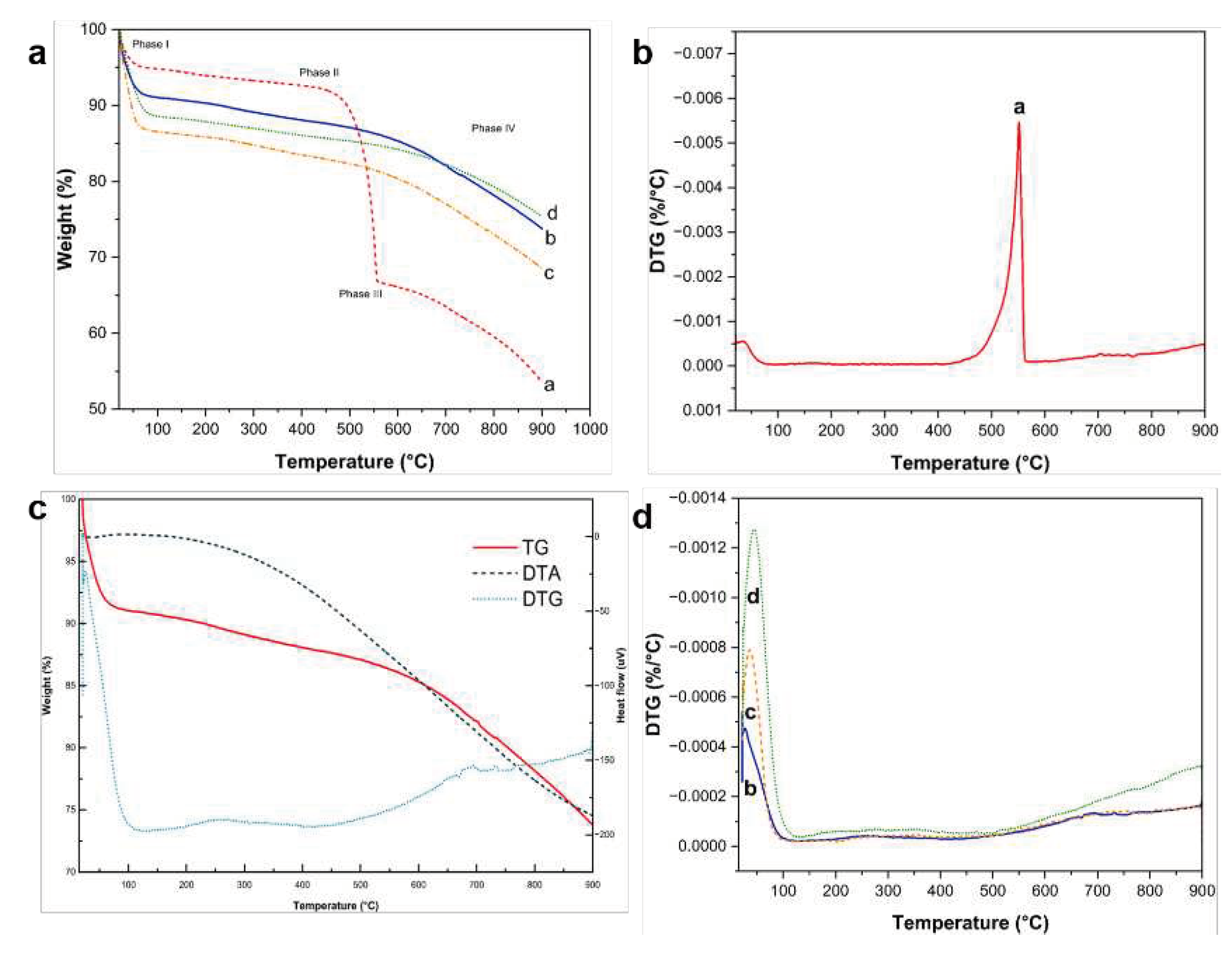
Figure 13 presents the TG/DTA thermograms including the DTG curves of the representative composites during continuous combustion until a temperature cutoff of 800 °C under a N
2 gas-protected environment to prevent the rapid oxidation of Si.
The TG profiles in
Figure 13a can be divided into four phases based on the composition of representative samples. Phase I, marked by the slight decrease in weight of the samples at ~ (50−90) °C, is due to the loss of moisture from physisorbed water on the surface the composites, followed by actual polymer decomposition over a broad range of temperature, until around 220 °C. Phase II is characterized by the gradual weight loss beginning at ~ (400−550) ºC. The core–shell PDA@SiO
2@Si sample exhibits a very sharp decrease in sample weight within this temperature range, due to the rapid degradation of PDA coating without the aid of PEI crosslinks and PVP K30 molecules. This rapid decrease in sample weight is also verified in the DTG profile shown in
Figure 13b. Based on the weight decrease recorded in the TG profiles, the total C content of the core–shell PDA@SiO
2@Si sample was calculated to be approximately 60 wt.%, in good agreement with the ratio during composite fabrication. Another sample weight loss curve is recorded at ~550 °C, which marks the beginning of Phase III due to the oxidation of exposed Si particles vulnerable to oxidation reactions at elevated temperatures [
32]. The oxidation reaction continues during Phase IV until all components in the samples are combusted, except for Si and SiO
x components, at ~700 °C. Interestingly, the remaining composite materials with either PEI crosslinking or a PVP K30 surface protection exhibit good thermal stability, characterized by minimal sample weight loss at temperatures higher than 550 °C, due to the effective PDA carbon coating reinforced with PEI crosslink structures.
The increased thermal stability of the PDA−PEI co-polymerized coating structures is verified in the DT−TGA and DTG curves of the representative composite yolk–shell PDA−PEI@PVP−SiO
2@Si sample presented in
Figure 13c. The TG curve shows a steady and sharp weight loss of ~10 %, which can be associated with the removal of the physisorbed and chemisorbed water. Moreover, the TG curve exhibits a small exothermic peak from ~100 °C, due to moisture loss, a broad endothermic peak centered at (200−400) °C due to polymer decomposition, and a broad exothermic signal starting from 500 °C due to SiO
2 oxidation. The DTG curve shows the removal of water from the small endothermic peak observed at ~100 °C and the actual polymer degradation from the broad endothermic peak between ~ (200 and 400) °C. The decomposition of carbon components is confirmed in a broad exothermic signal from ~ (400−550) °C, while endothermic peaks at ~700 °C [
73] mark the transition to SiO
x.
Apart from the minor loss in sample weight observed at ~ (50−100) °C due to the evaporation of adsorbed water molecules in
Figure 13b, no evident changes were observed in the sample weights at 550 °C for the remaining composite samples in
Figure 13d. Generally, the total combustion of carbon-based compounds is typically observed from ~ (400−550) °C. However, the representative yolk–shell PDA−PEI@PVP−SiO
2@Si composite only displays a slight decrease in sample weight at ~700 °C, suggesting the composite fabrication and design prevent the thermal oxidation of Si. Based on the sample weight loss, the total C content in yolk–shell the PDA−PEI@PVP−SiO
2@Si is calculated to be ~18 % while the Si content of ~73 % is from the combined contributions of SiO
2 from the APTES, TEOS, and pure Si nanoparticles.
3.5. Electrochemical Performance of the Representative Core–Shell and Yolk–Shell Composites
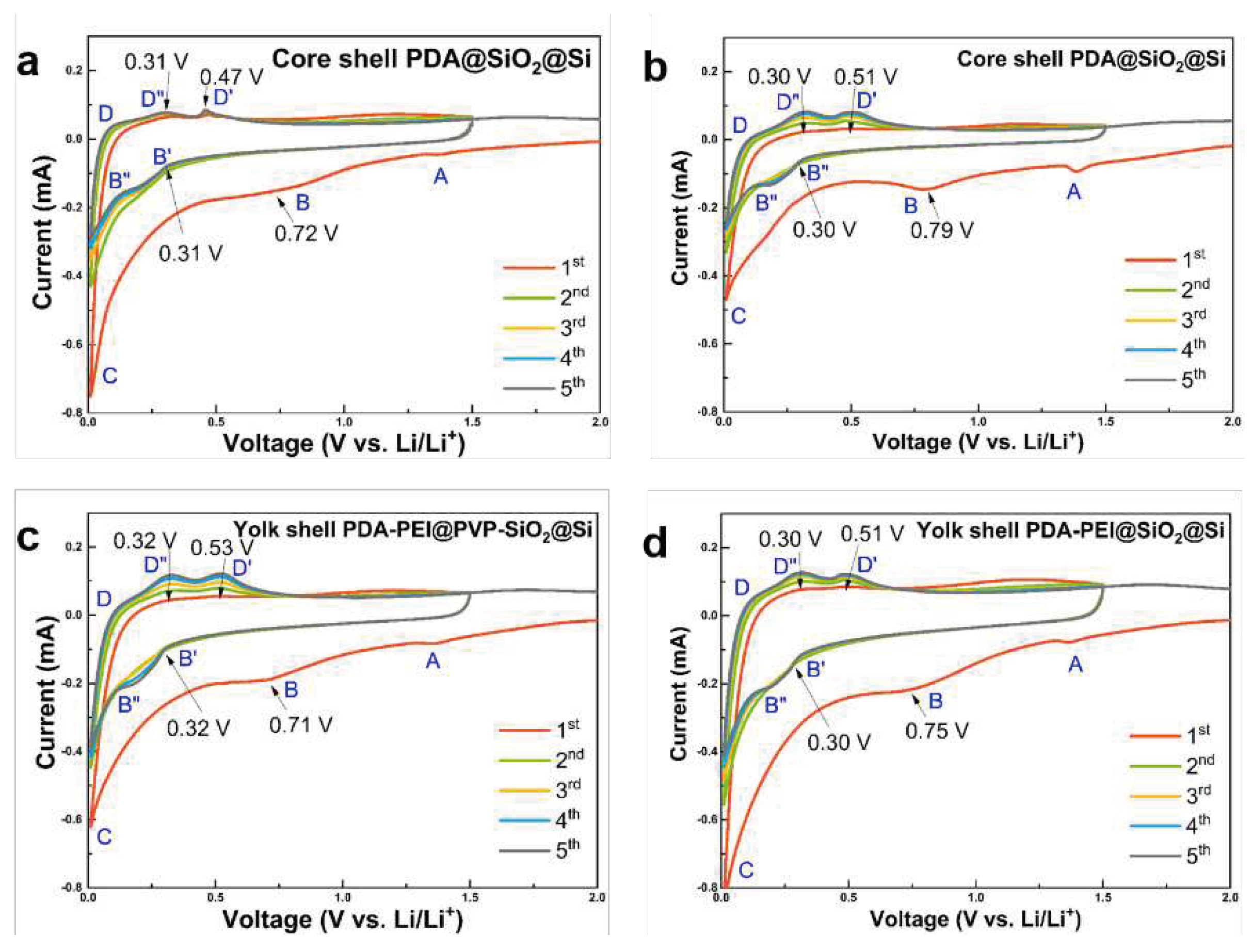
Figure 14 shows the electrochemical performance of the representative yolk and core–shell composite samples that are first characterized by CV.
All composite samples demonstrate two distinct peaks at ((0.30 − 0.32) and (0.72 − 0.79)) V during the first cathodic scan, which are ascribed to the initial electrochemical reactions between the bulk Si and Li
+ atoms, which leads to the formation of irreversible lithiated precipitates.
Table 1 summarizes the Si phase transformations and corresponding chemical reactions [
37]:
The cathodic peaks in the range (0.72 – 0.79) V are attributed to the SEI film formation disappearing in the second cycle, suggesting the stabilization of SEI film after the first cycle. Moreover, two broad oxidation peaks centered at ((0.47 − 0.53) and (0.30 − 0.32)) V in the subsequent anodic scans signify the delithiation processes of Li
4.2Si and the complete delithiation into Li
xSi into amorphous Si, respectively. Although less polarization is observed in the core–shell PDA@SiO
2@Si sample in
Figure 14a, the hydrothermally-fabricated counterparts in
Figure 14b demonstrate electrode activation characterized by gradual, yet steady increase in the intensities of both cathodic and anodic scans in the subsequent cycles. Similar gradual electrode activation is observed in the PVP-surface protected composites in
Figure 14c, compared to the less polarized counterpart in
Figure 14d. Meanwhile, the CV scans of the core–shell PDA−PEI@TEOS−SiO
2@Si composite via RT in
Figure S11 of the SI demonstrate similar peak observations and peak qualities; however, important oxidation peaks during the delithiation process are not clearly manifest, implying the difficulty in retrieving Li
+ from Li
xSi alloyed components during the reaction.
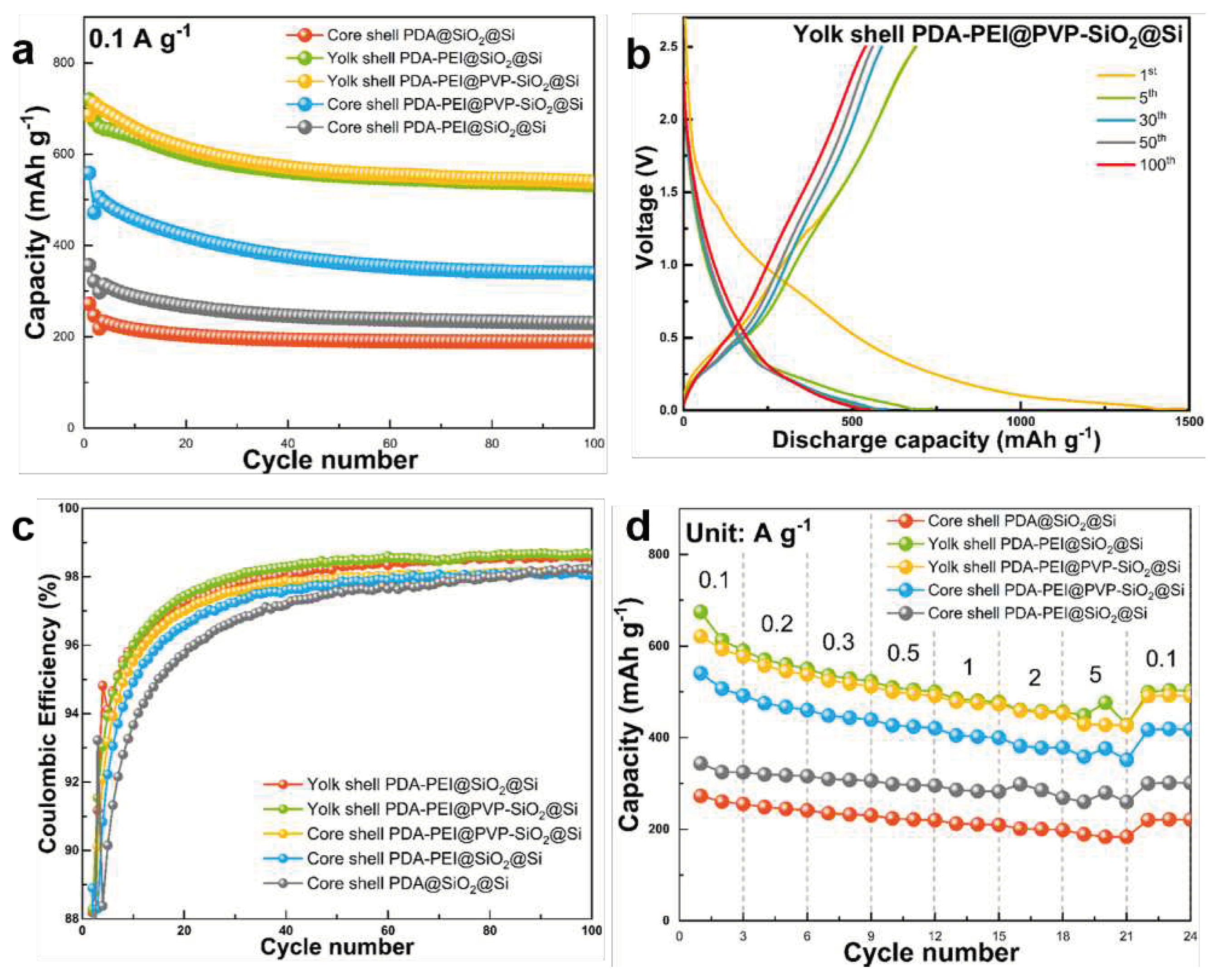
Figure 15 compares the electrochemical performance of the composites in terms of cycling stability, rate performance, and CE stability over 100 cycles.
Figure S12 of the SI also presents the galvanostatic charge and discharge profiles of the other composites.
The influence of the core–shell and yolk–shell structures and PVP surface protection and PDA−PEI carbon crosslinking on the electrochemical performances is evaluated at a low current density of 0.1 A·g
−1 for 100 cycles, as reflected in
Figure 15a. Among the studied composites, the representative yolk–shell PDA−PEI@PVP−SiO
2@Si sample exhibits the highest discharge capacity of 719 mAh·g
−1 with an initial CE (ICE) value of 47.94 %, which after five cycles readily increases to 94 %, and during the subsequent cycles, maintains more than 98 %. The galvanostatic charge and discharge profiles of the representative composite presented in
Figure 15b also show minimal electrode polarization with overlapping profile scans over increasing cycle number. Generally, the low ICE values of the Si-based composite anodes are due to the decomposition reaction at the SEI interface, consuming Li
+ in the process, hence the decreased available reversible Li
+ to participate in electrochemical reactions during the initial cycles. The representative yolk–shell PDA−PEI@PVP−SiO
2@Si sample was able to retrieve 539 mAh·g
−1 discharge capacity after 100 cycles with a CE value of 98 %. Moreover, the CE was stabilized after the initial SEI formation, as shown in
Figure 15c.
Overall, the yolk–shell composite samples demonstrate better cycling performance and CE stability, even without the PVP K30 surface protection. For example, the yolk–shell PDA−PEI@SiO2@Si composite sample without PVP K30 is able to maintain capacity of 531.25 mAh·g−1 after 100 cycles. Meanwhile, even with the help of PVP K30 surface protection, the core–shell PDA−PEI@PVP−SiO2@Si composite sample demonstrates inferior cycling performance with only 339.62 mAh·g−1 of capacity after 100 cycles. This result highlights the role of void spaces in yolk–shell structures that are sufficient to absorb the internal volume changes of Si, and stabilize the cycling performance. With both PDA−PEI@SiO2@Si and PDA@SiO2@Si composites possessing core–shell structures, the difference in the cycling performances and CE values can be attributed to the enhanced electronic conductivity of PDA carbon coating due to PEI crosslinking across the entire electrode structure, which provide continuous pathways for fast electron and ion transport.
The rate capabilities of the composite samples were examined at several current densities ranging (0.1 – 5) A·g
−1, and
Figure 15d summarizes the results. The representative yolk–shell PDA−PEI@PVP−SiO
2@Si electrode exhibits better rate performance than the core–shell composites with or without PVP K30 and PEI crosslinking across all current densities, without the formation of Li dendrites. The specific capacities of the yolk–shell PDA−PEI@PVP−SiO
2@Si electrode are (621.21, 577.46, 537.96, 512.50, 491.53, 472.71, and 453.16) mAh·g
−1 at the current densities of (0.1, 0.2, 0.3, 0.5, 1, 2, and 5) A·g
−1. When the current density is returned to 0.1 A·g
−1, 490.73 mAh·g
−1 is retrieved.
Although the yolk–shell PDA−PEI@SiO
2@Si composite performs slightly better, the sudden increase in capacity from (456.32 to 476.41) mAh·g
−1 at 5 A·g
−1 suggests an open short circuit has taken place due to Li dendritic formations, which typically occur at high current densities. Similar sudden increase in capacities are also observed in the core–shell PDA−PEI@PVP−SiO
2@Si and core–shell PDA−PEI@SiO
2@Si composites. It is also worth noting that when the current density is reduced to 0.1 A·g
−1, the slight increases in capacity observed in other sample composites are all stabilized, implying the ability of the designed composites to recover satisfactory capacities, even after high-rate tests.
Table 2 summarizes the cycling and rate performances of the representative yolk–shell PDA−PEI@PVP−SiO
2@Si composite against the other fabricated composites:
Electrochemical impedance measurements were recorded before and after the 100th cycle to describe the factors that led to the enhancement of Li
+ storage capacity of the representative yolk–shell PDA−PEI@PVP−SiO
2@Si composite.
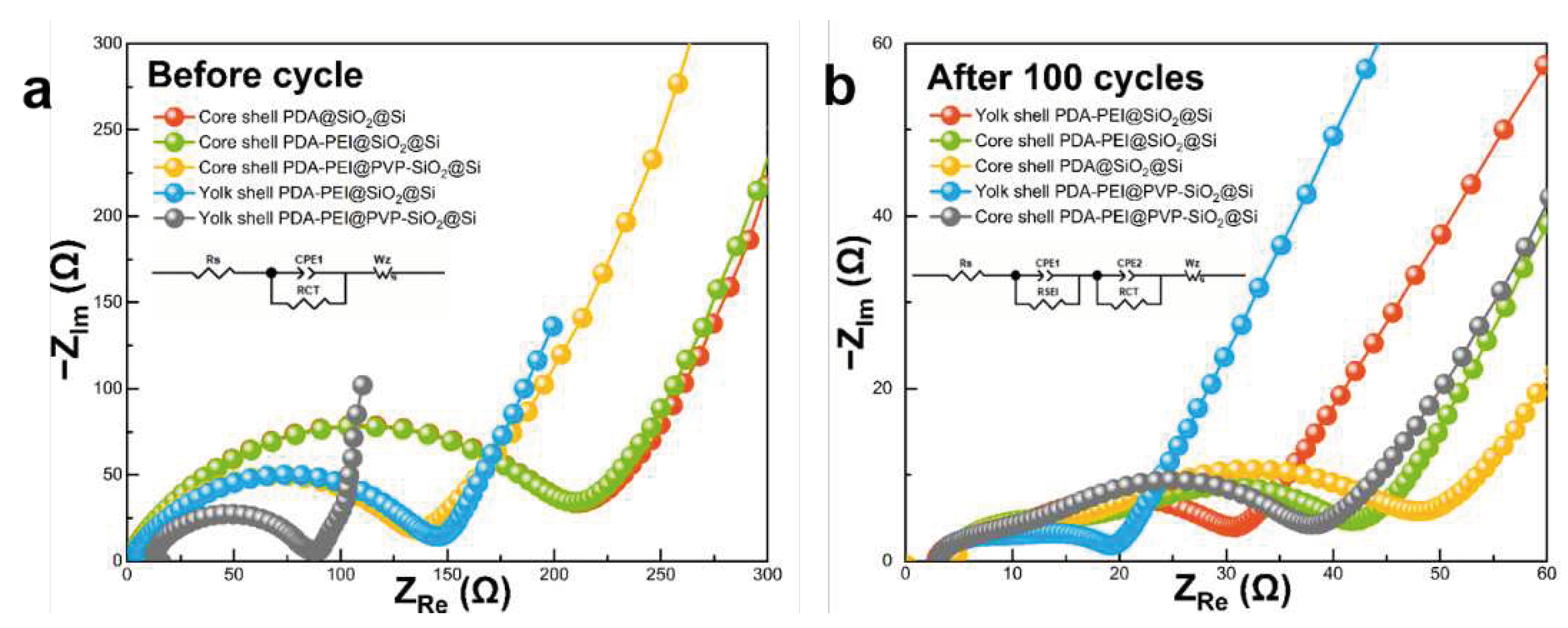
Figure 16 shows the Nyquist plots that were obtained. The insets in
Figure 16a,b show the equivalent circuit models corresponding to Nyquist plots. The resistance value emerging from the interaction of electrolyte solution with the bulk Si denoted as R
s, the resistance of Li
+ as it migrates back and forth between the two electrodes passing the SEI layer as R
SEI, the resistance value during charge transfer as R
CT, and the Warburg impedance denoted as W
z are all considered in the circuit model. Meanwhile, the double-layer capacitance of surface film CPE1 and CPE2 represents the constant phase elements of the cell.
As shown in
Figure 16a, the Nyquist plots of the studied composites before cycling are comprised of a semicircle located in the middle-frequency region, and a slanted sloping line in the low-frequency region. The smaller diameter of the representative yolk–shell PDA−PEI@PVP−SiO
2@Si before cycling signifies lower resistance value (73.76 Ω), and therefore faster charge transfer. The relatively fast charge transfer kinetics of the representative composite can be attributed to the synergy of yolk–shell structure that allows direct contact of the carbon coating layer with the PVP-SiO
2@Si active material. Additionally, the shorter slanted line of the representative composite material implies relatively faster Li
+ diffusion rates. The synergistic effect of a yolk–shell structure combined with PVP K30 surface protection can also be observed in the yolk–shell PDA−PEI@SiO
2@Si (139.60 Ω) and core–shell PDA−PEI@PVP−SiO
2@Si (140.21 Ω), with the PVP K30 showing comparable R
CT values, yet much lower than in the core–shell PDA@SiO
2@Si (211.16 Ω) and core–shell PDA−PEI@SiO
2@Si (209.23 Ω). The thick layer of the amorphous SiO
2 coating present in the core–shell composites prevent the direct contact between the Si active material and conductive PDA carbon coating. Therefore, a drastic increase in Li
+ tortuosity is observed in the core–shell composites as the Li
+ needs to migrate from the PDA coating, passing through the electronically insulating SiO
2 layer, before it reaches the Si active material.
After 100 cycles, the Nyquist plots in
Figure 16b verify the formation of a stable SEI layer. The Nyquist plots of the fabricated electrodes demonstrate two semicircles; one in the high-frequency region, and the other in the middle-frequency region, which are attributed to the R
SEI and R
CT, respectively, followed by a slanted line in the low-frequency region. The smallest diameter of the semicircle in the high-frequency zone observed in the representative composite translates to the lowest R
SEI value (6.30 Ω). This is due to the formation of a mechanically stable SEI layer thanks to the PDA coating, which prevents excessive electrolyte decomposition. The yolk–shell PDA−PEI@SiO
2@Si also demonstrates a relatively lower R
SEI (8.13 Ω) value compared to its core–shell counterparts, further supporting the importance of yolk–shell structure. However, the larger R
CT value of the yolk–shell PDA−PEI@SiO
2@Si (19.28 Ω) compared to the representative composite (9.71 Ω) emphasizes the importance of constructing yolk–shell structures with PVP K30 surface protection. The core–shell composites PDA−PEI@PVP−SiO
2@Si (9.51 Ω) with PVP K30 exhibit lower R
SEI values than the PDA−PEI@SiO
2@Si (10.77 Ω) sample. The stabilized SEI formation in the core–shell PDA−PEI@PVP−SiO
2@Si can be explained by acknowledging the influence of PVP K30 polymer chains after being loaded inside amorphous SiO
2 shells. Assuming a crack formation in the PDA carbon coating due to expansion of the lithiated Si and SiO
2 components, the PVP K30 polymer chains embedded within SiO
2 shells provide a secondary barrier that prevents direct contact with the Si active materials. In addition, the flexibility of PVP K30 polymer chains aids SEI stable formation by providing a buffer against the rigid and dense SiO
2 layer susceptible to crack formation during repetitive volume fluctuations. The contribution of the embedded PVP K30 polymer chains within SiO
2 also help increase the conductivity of the amorphous SiO
2 seeds, hence the slight improvement in the R
CT of the core–shell PDA−PEI@PVP−SiO
2@Si (23.32 Ω), compared to the core–shell PDA−PEI@SiO
2@Si (28.01 Ω). On the other hand, the lack of PEI component in the core–shell PDA@SiO
2@Si results in higher R
SEI (10.84 Ω) coupled with thick SiO
2 shells that block Li
+ migration, thereby increasing the tortuosity (32.50 Ω).
Table 3 summarizes the parameters acquired from the Nyquist plots of the fabricated composites before and after 100 lithiation/delithiation processes.