Submitted:
27 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
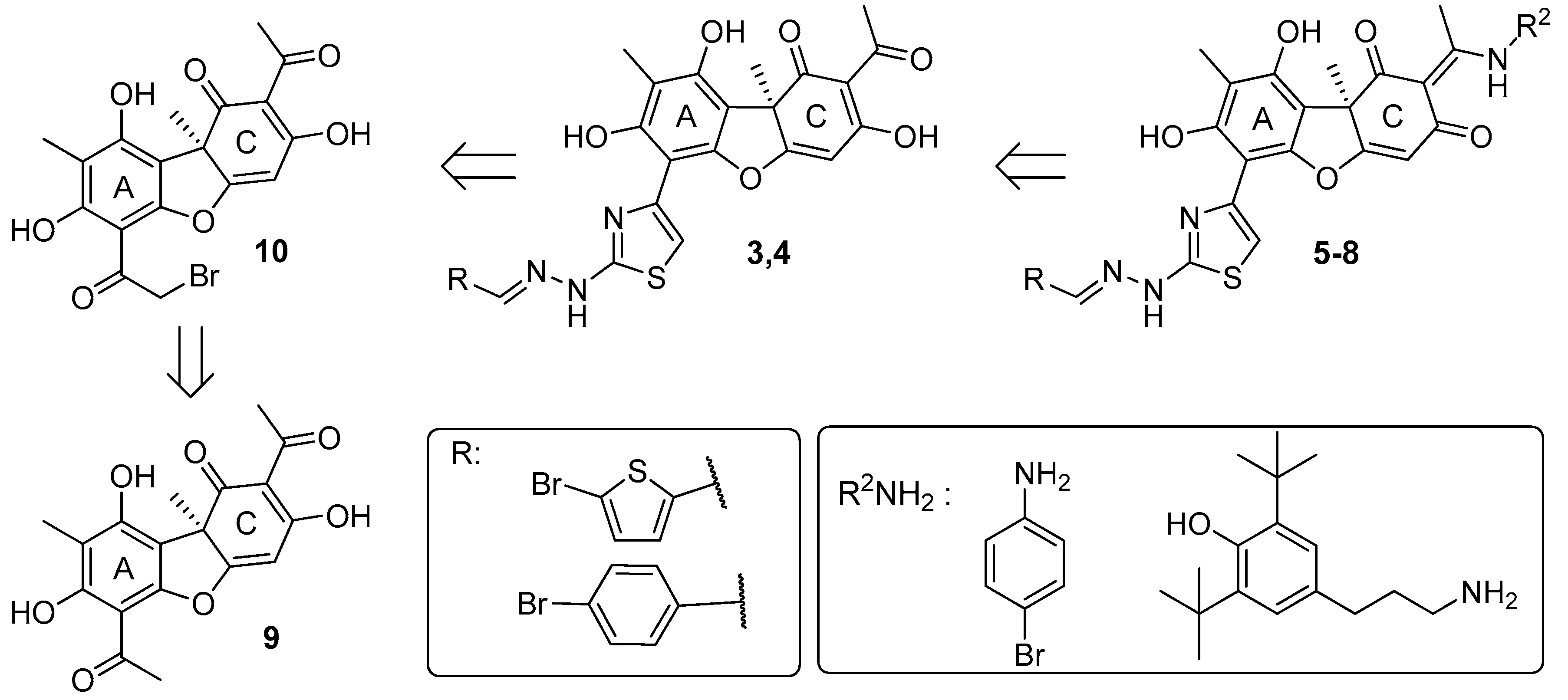
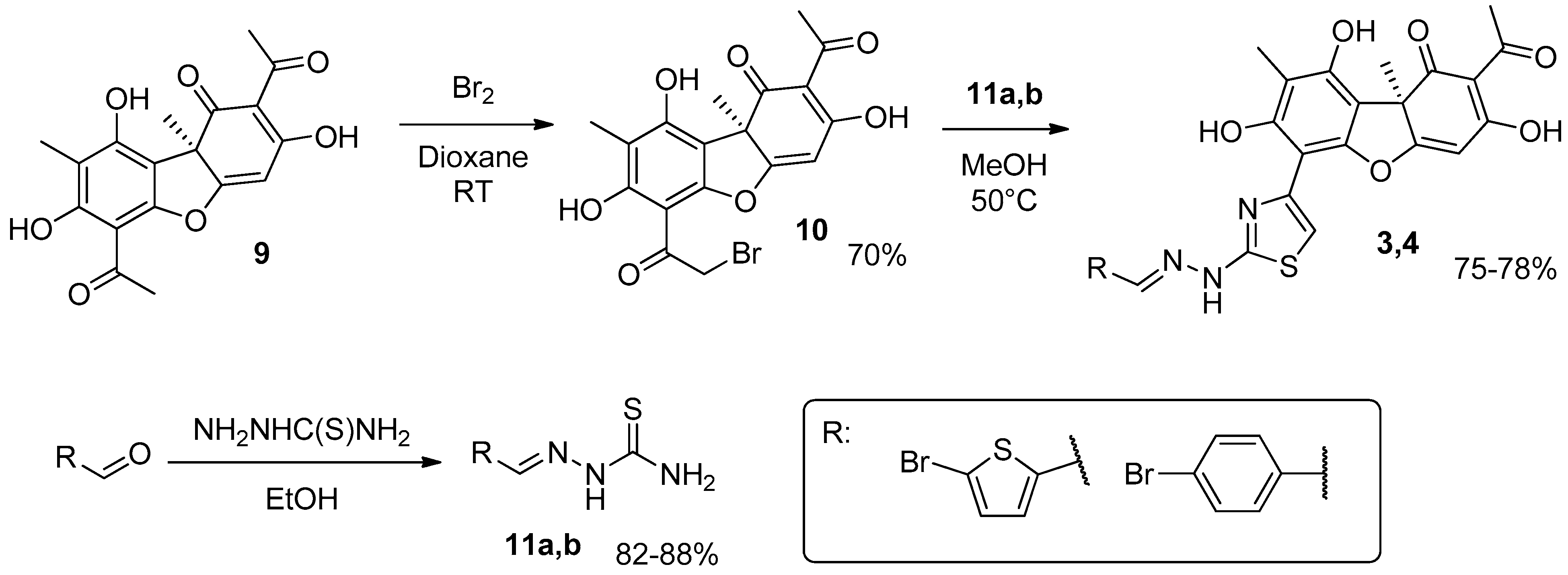
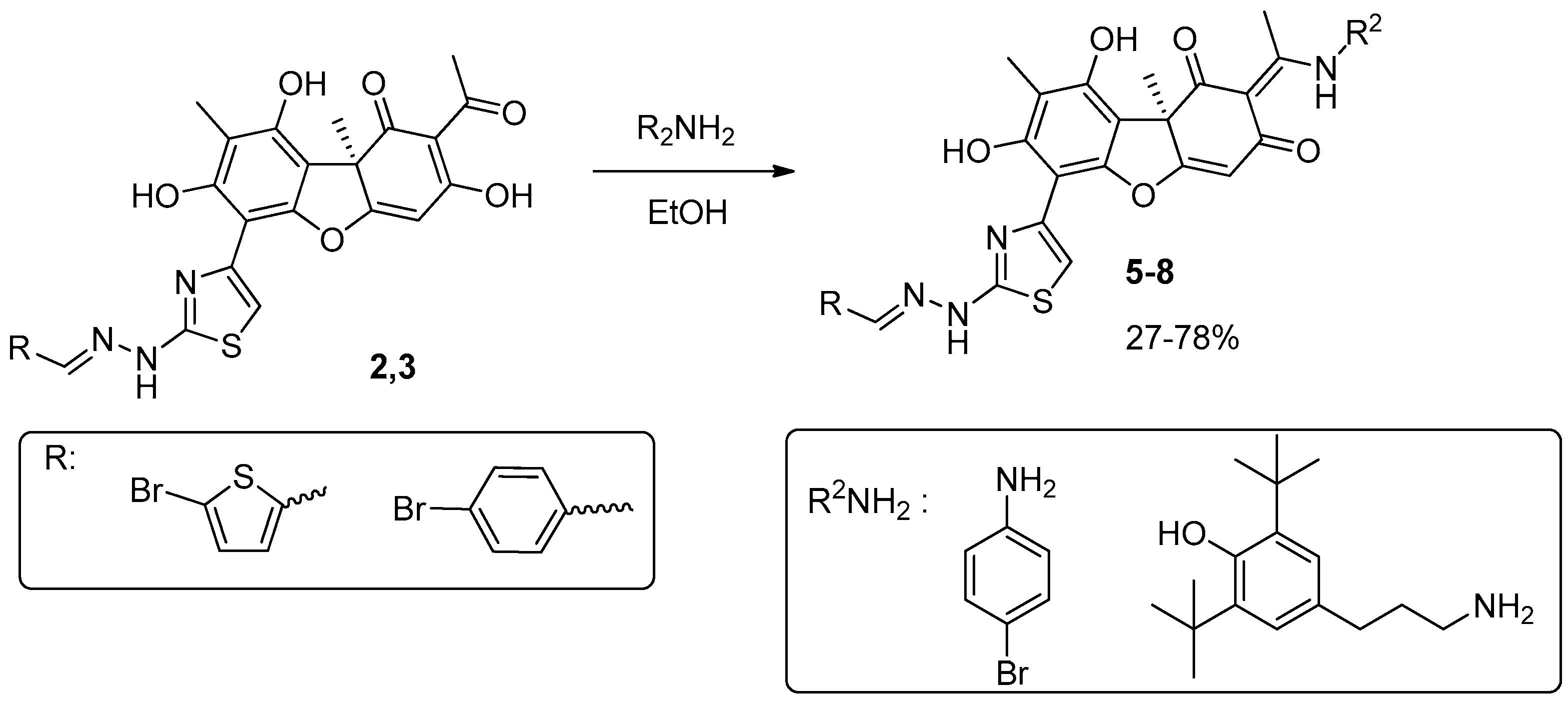
2.1. Chemistry
2.1. Biology
2.1.1. Inhibitory Properties of Usnic Acid Derivatives against Tdp1
2.1.2. The Influence of Usnic Acid Derivatives on the Survival of Various Types of Cells
2.1.3. Sensitizing Properties of Usnic Acid Derivatives In Vitro
2.1.4. Anticancer and Antimetastatic Effects of Compound 7 Used as Monotherapy or in Combination with Topotecan in the Lewis Lung Carcinoma Model
2.1.5. Anticancer Effect of Compound 7 Used as Monotherapy or in Combination with Topotecan in the Krebs-2 Ascitic Carcinoma Model

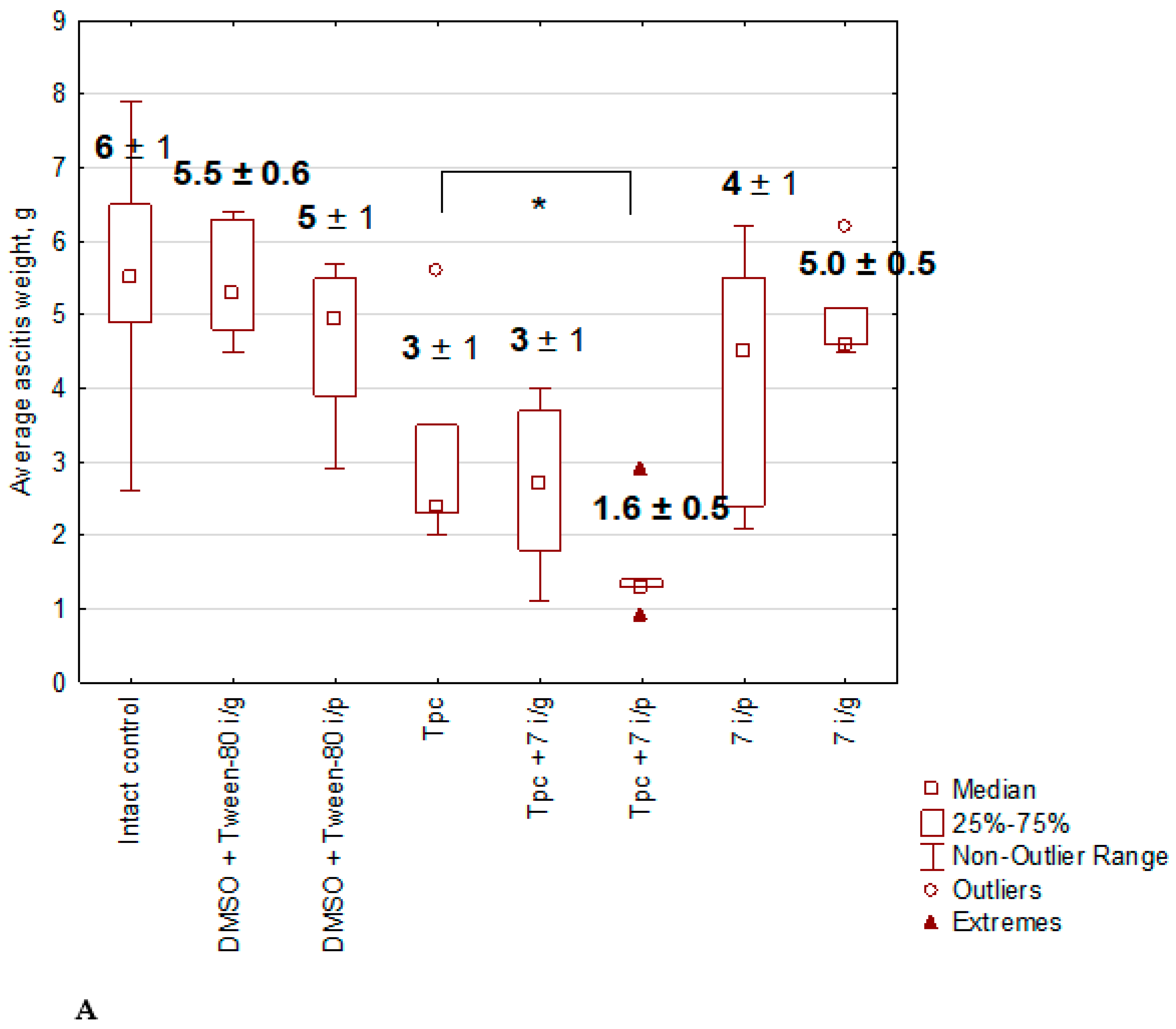
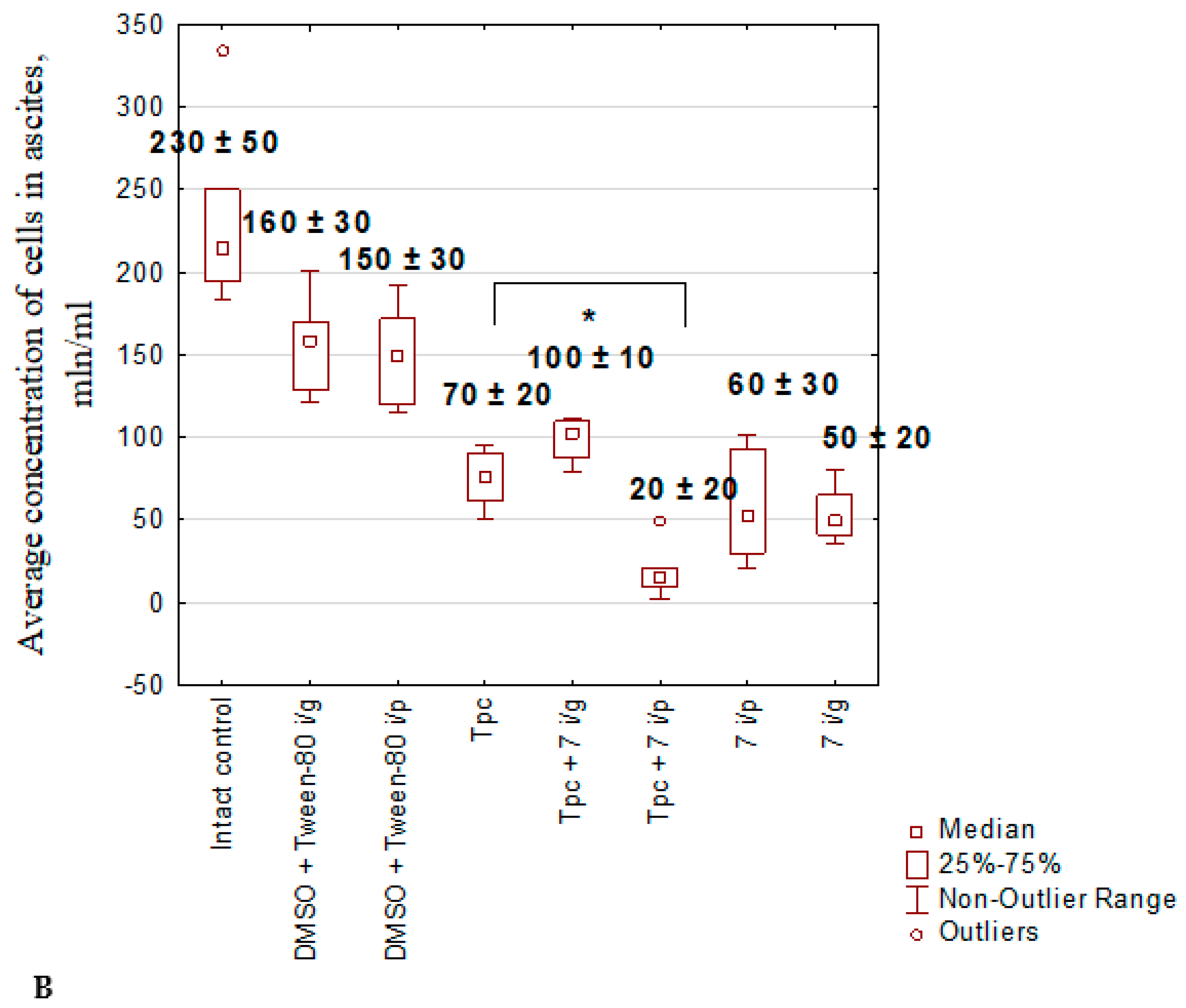
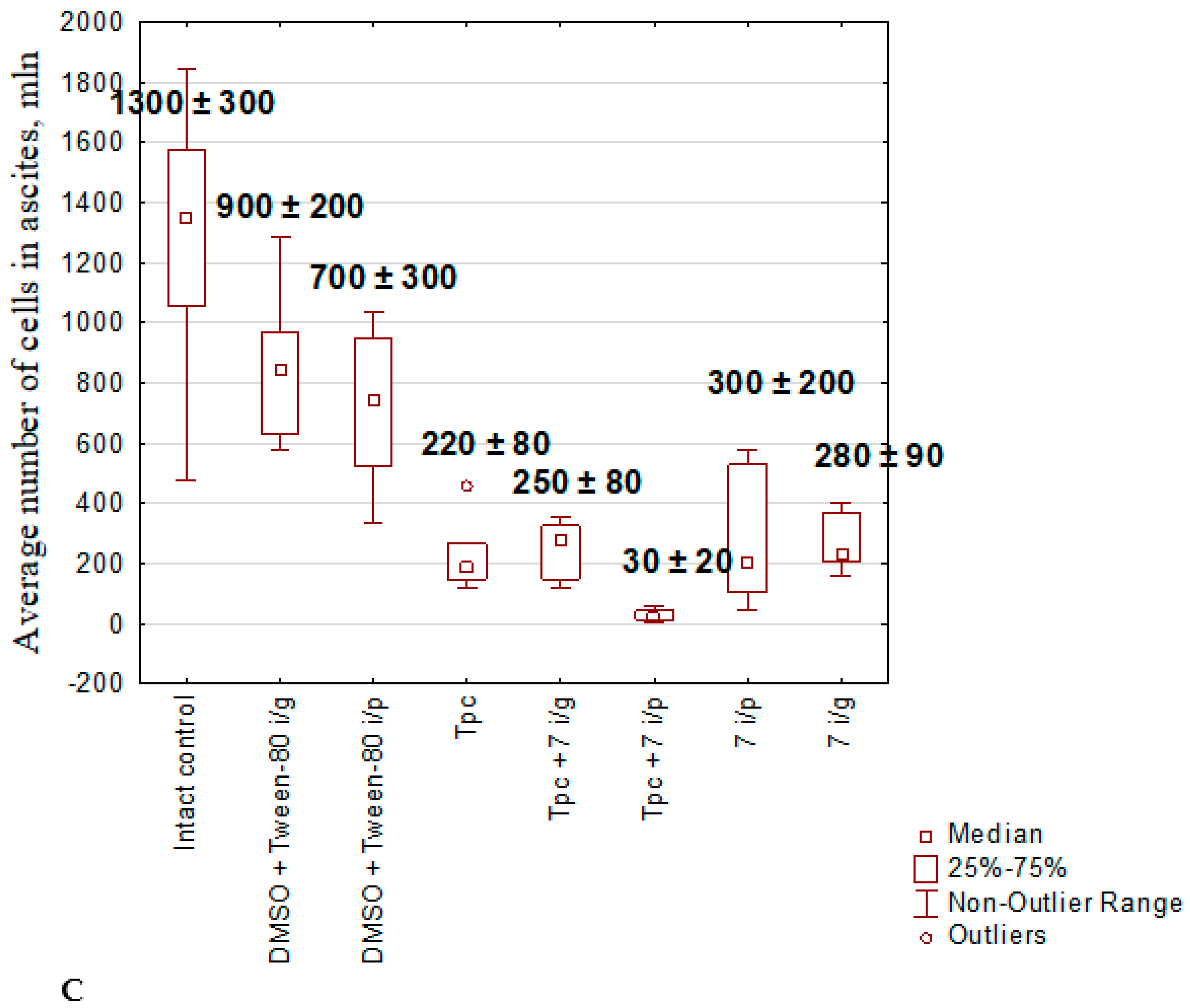
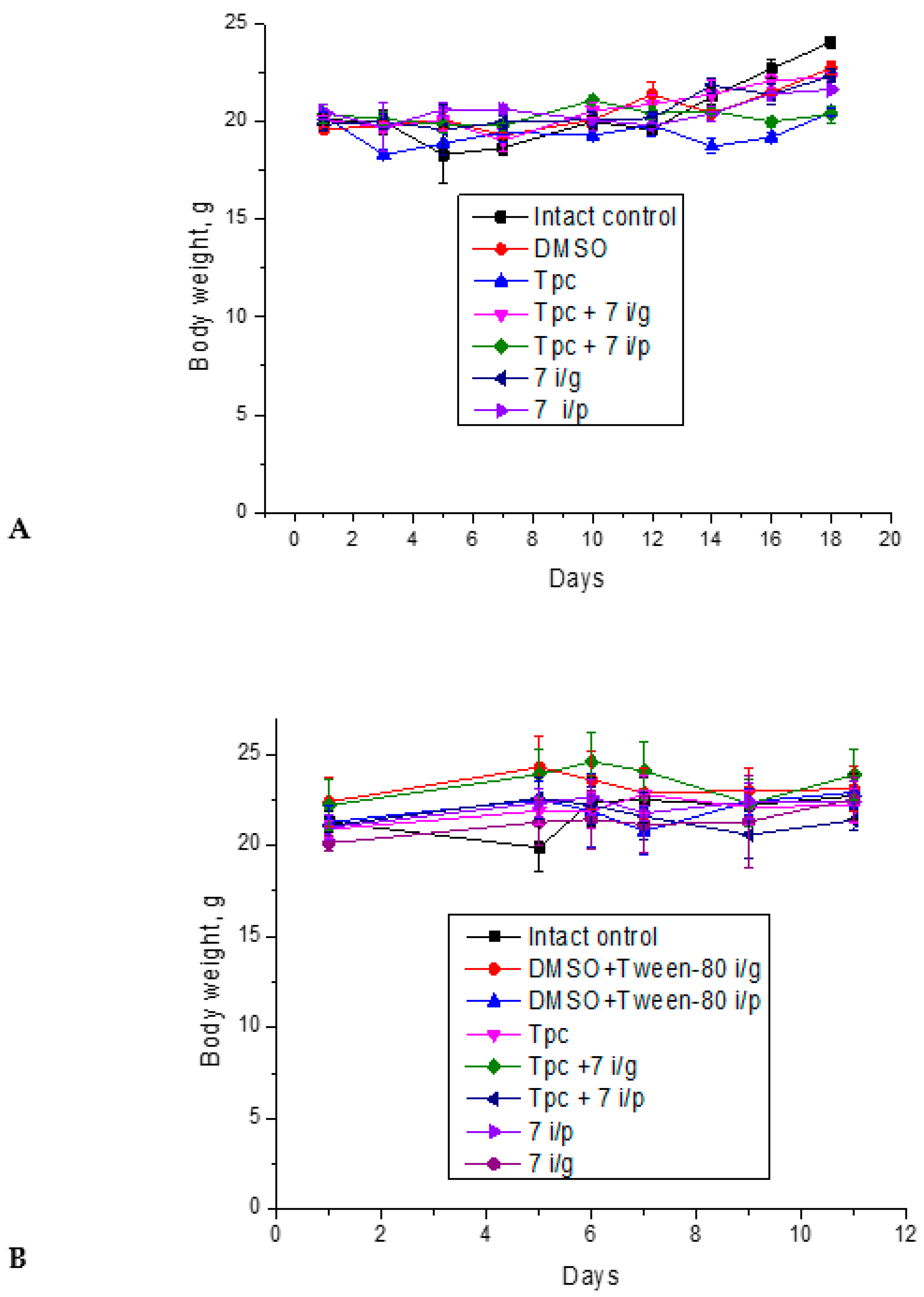
2.1.6. The Toxic Effect of Drugs and Their Combination
2.1.7. Effects of the Test Substances on the Peripheral Blood in Mice with Krebs-2 Carcinoma
3. Conclusion
4. Experimental Section
4.1. Chemistry
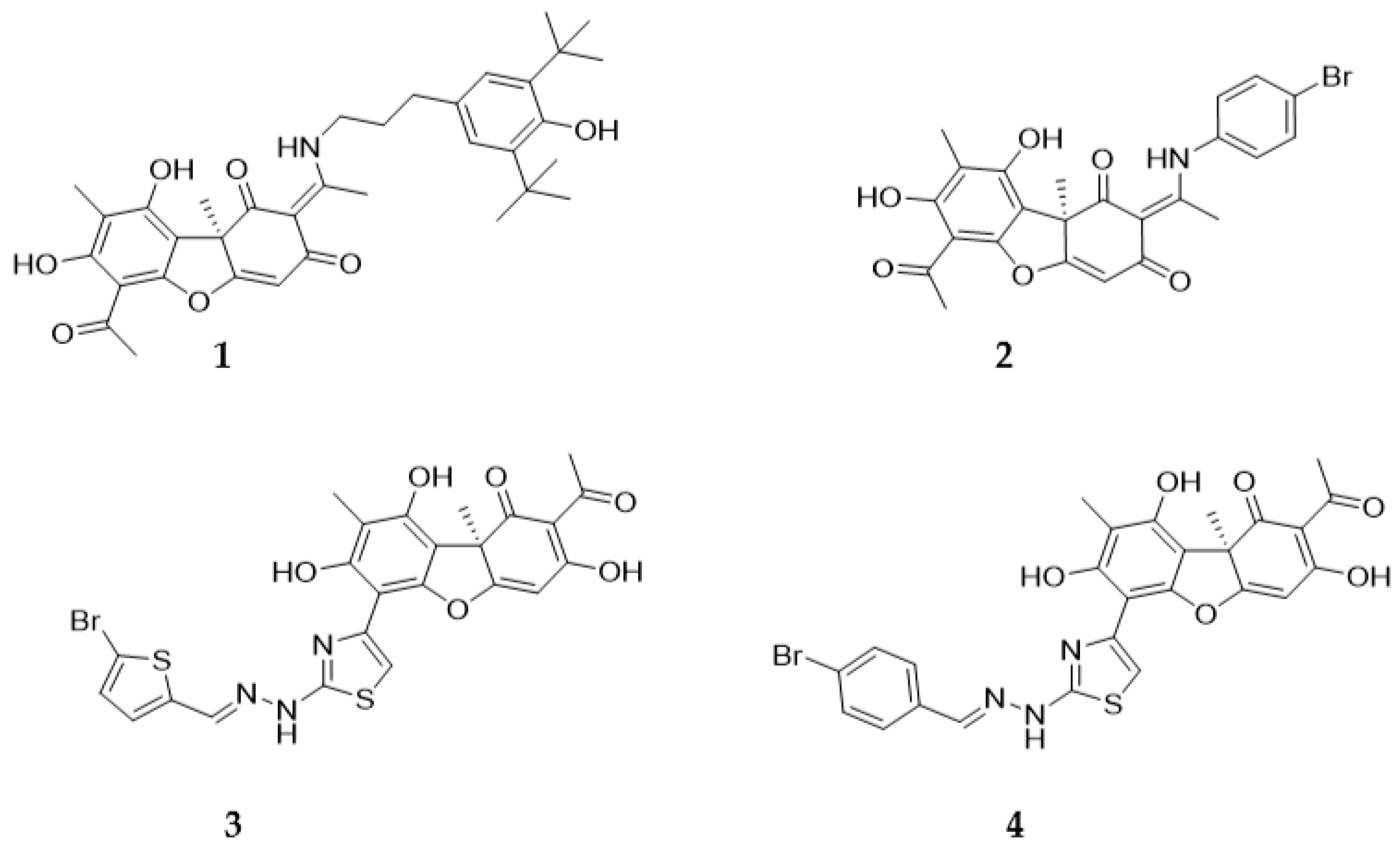
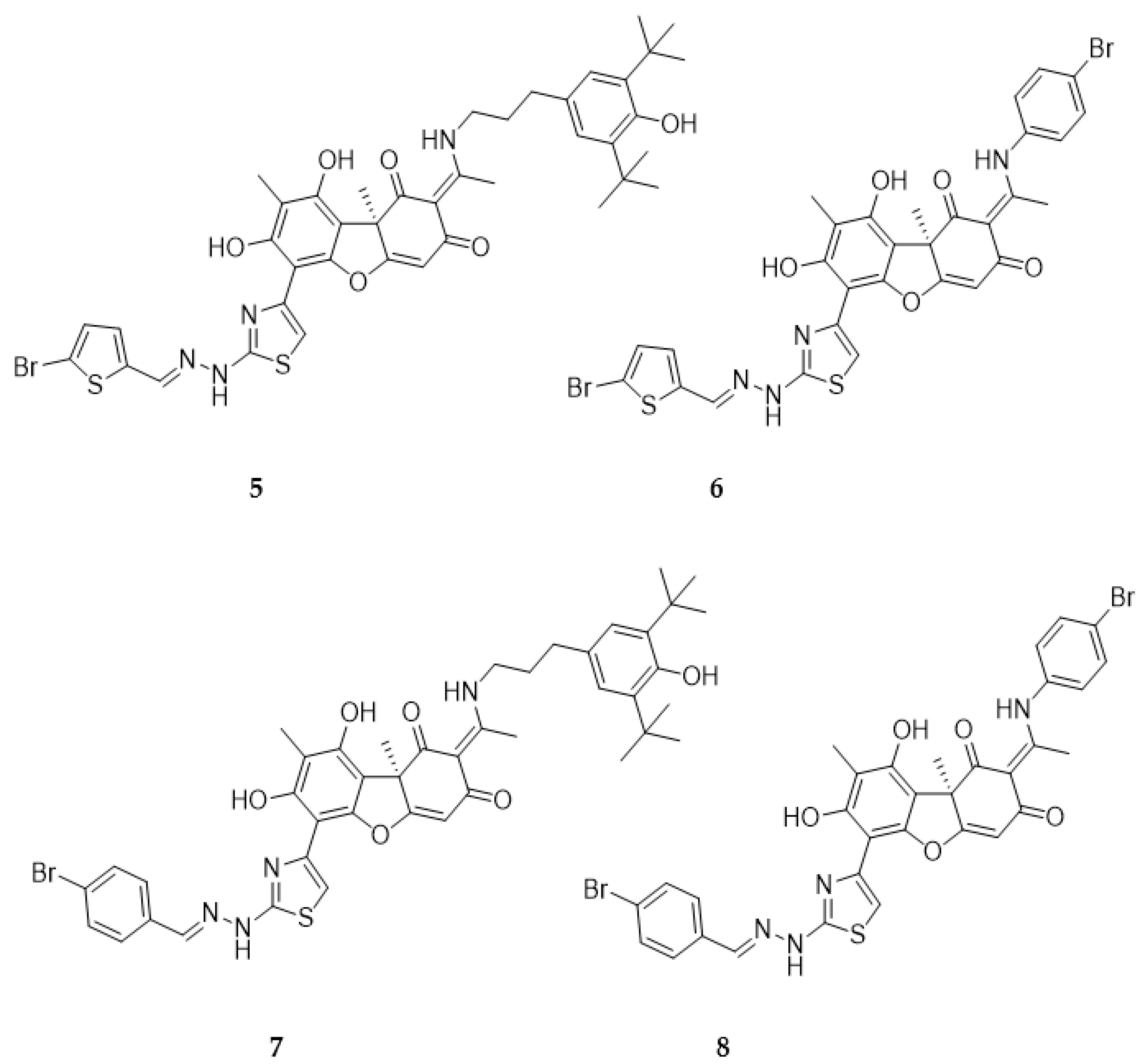
4.1.1. Synthesis of Hybrid Compounds 5-8
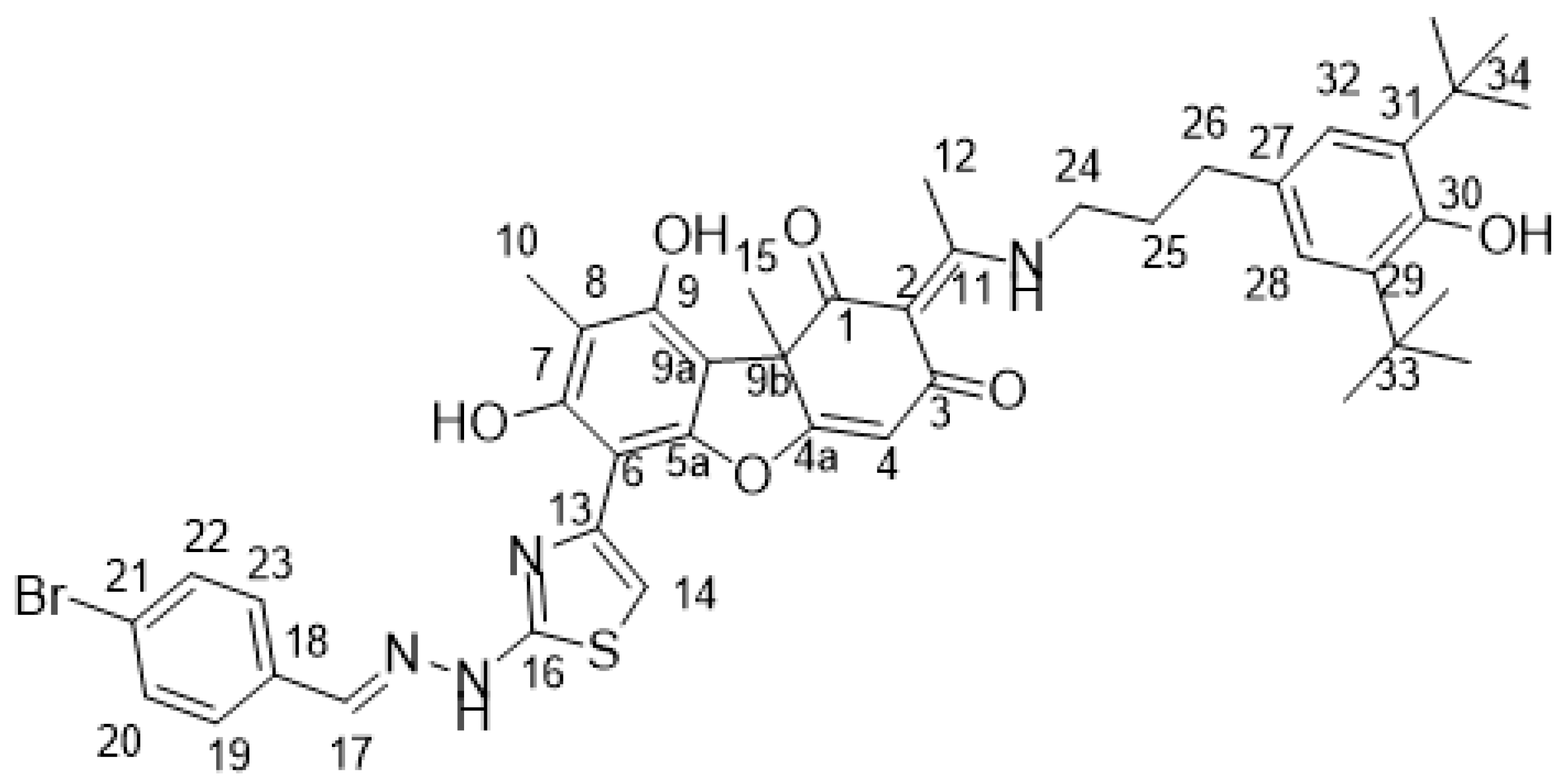
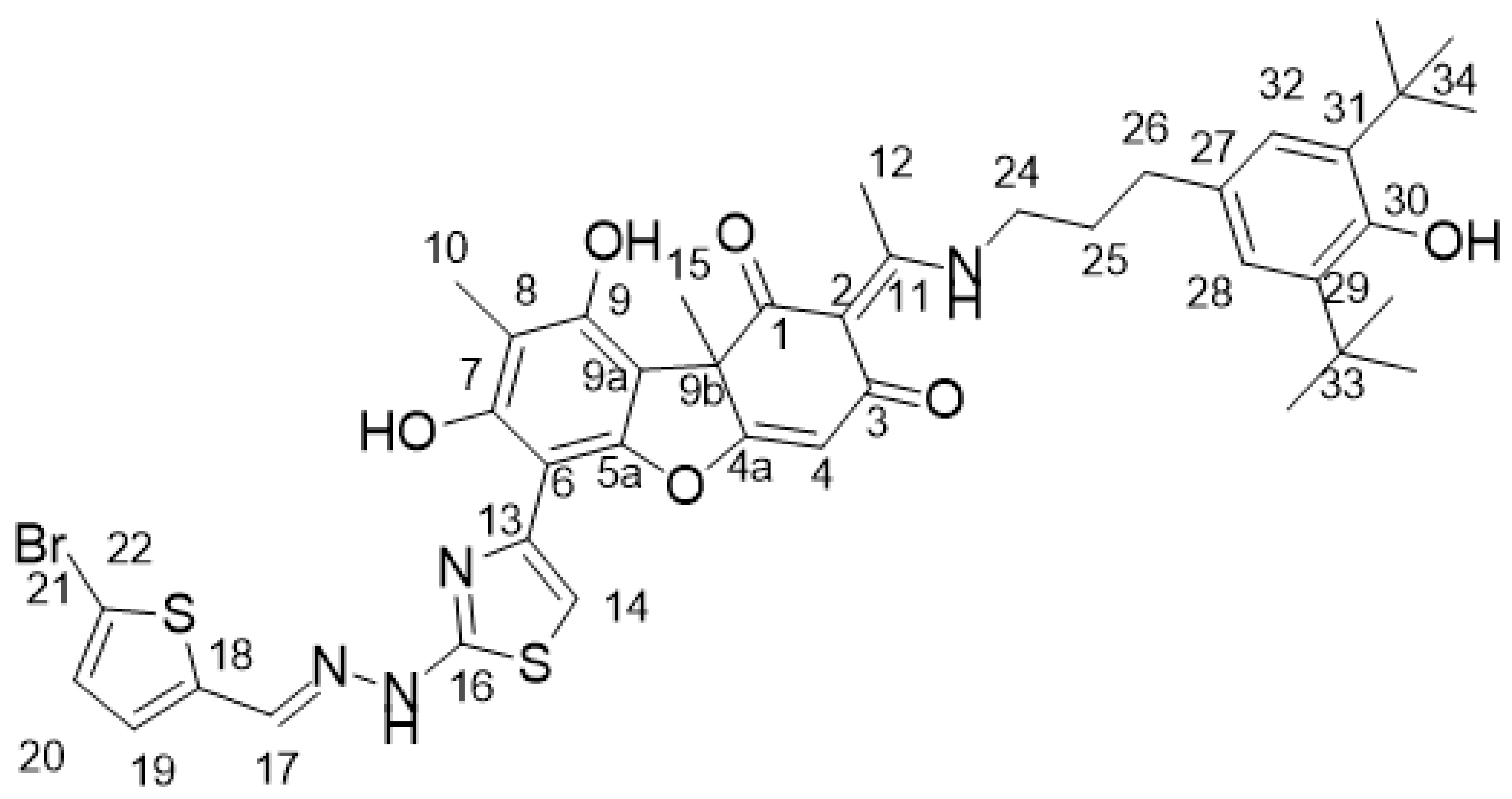
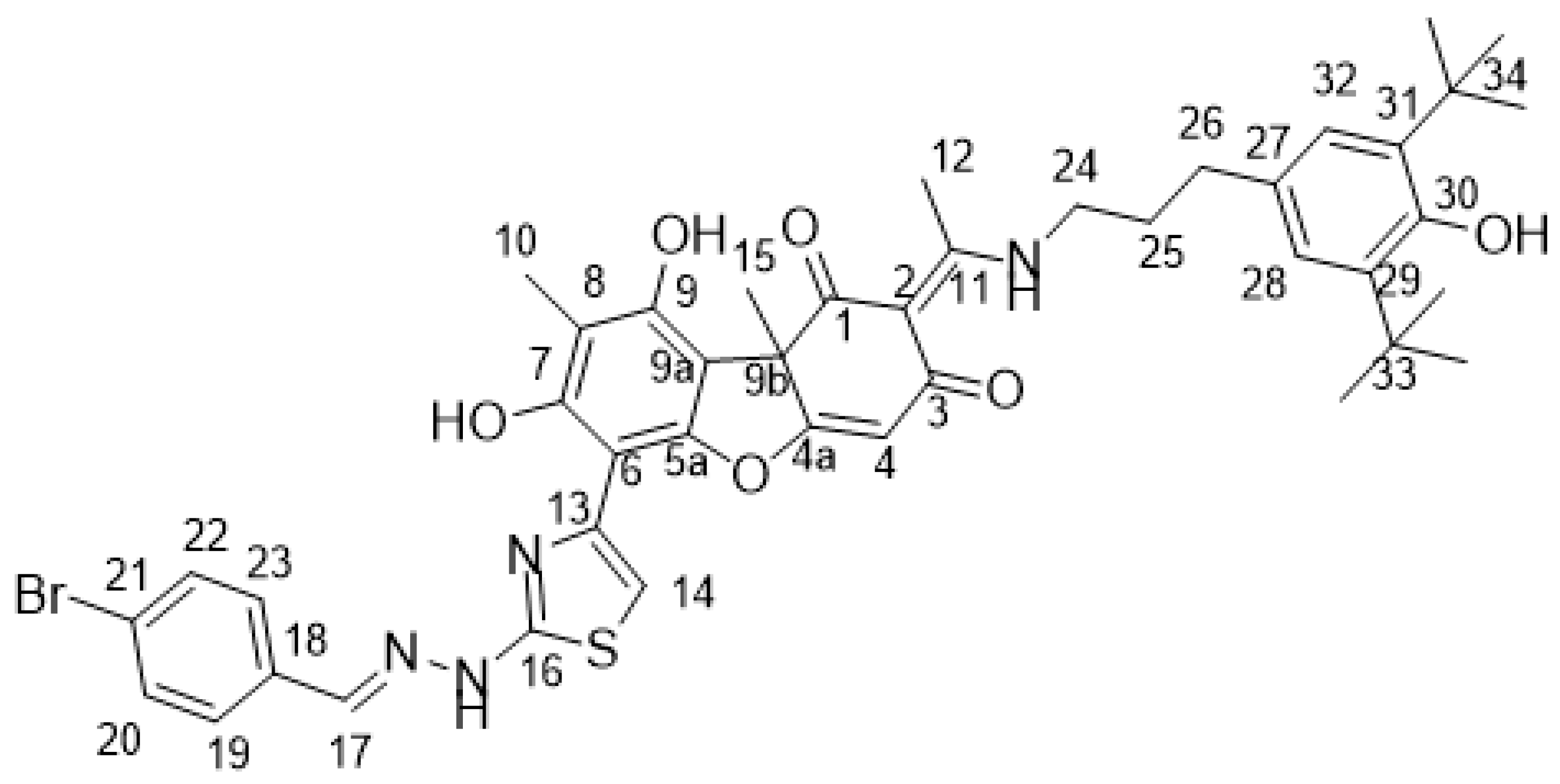

4.2. Biological Assays
4.2.1. Tdp1 Activity
4.2.2. Cytotoxicity Assays
4.2.3. Laboratory Animals and Tumor Models
4.2.4. Antitumor and Antimetastatic Effects of Compound 7 Used as Monotherapy or Combined with Topotecan in the LLC Model
4.2.5. Antitumor Effect of Compound 7 Used as Monotherapy or in Combination with Topotecan in the Krebs-2 Ascitic Carcinoma Model
4.2.6. The Toxic Effect of Drugs and Their Combination
4.2.7. The Effects of the Substances on the Differential Blood Count
4.2.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comeaux, E.Q.; van Waardenburg, R.C. Tyrosyl-DNA phosphodiesterase I resolves both naturally and chemically induced DNA adducts and its potential as a therapeutic target. Drug Metab. Rev., 2014, 46, 494–507. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol., 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Beretta, G.L.; Cossa, G.; Gatti, L.; Zunino, F.; Perego, P. Tyrosyl-DNA phosphodiesterase 1 targeting for modulation of camptothecin-based treatment. Curr. Med. Chem., 2010, 17, 1500–1508. [Google Scholar] [CrossRef]
- Cuya, S.M.; Comeaux, E.Q.; Wanzeck, K.; Yoon, K.J.; van Waardenburg, R.C. Dysregulated human Tyrosyl-DNA phosphodiesterase I acts as cellular toxin. Oncotarget. 2016, 7, 86660–86674. doi: 10.18632/oncotarget.13528.; Katyal, S.; El-Khamisy, S.F.; Russell, H.R.; Li, Y.; Ju, L.; Caldecott, K.W.; McKinnon, P.J. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J, 2007, 26, 4720–4731 doi: 10.1038/sj.emboj.7601869.; Alagoz, M.; Wells, O.S.; El-Khamisy, S.F. Nucleic Acids Res, 2014, 42, 3089–3103. [CrossRef]
- Huang, H.C.; Liu, J.; Baglo, Y.; Rizvi, I.; Anbil, S.; Pigula, M.; Hasan, T. Mechanism-informed Repurposing of Minocycline Overcomes Resistance to Topoisomerase Inhibition for Peritoneal Carcinomatosis. Mol Cancer Ther, 2018, 17, 508–520. [Google Scholar] [CrossRef]
- Nivens, M.C.; Felder, T.; Galloway, A.H.; Pena, M.M.; Pouliot, J.J.; Spencer, H.T. Engineered resistance to camptothecin and antifolates by retroviral coexpression of tyrosyl DNA phosphodiesterase-I and thymidylate synthase. Cancer Chemother. Pharmacol. 2004, 53, 107–115. DOI: 10.1007/s00280-003-0717-6; Barthelmes, H.U.; Habermeyer, M.; Christensen, M.O.; Mielke, C.; Interthal, H.; Pouliot, J.J.; Boege, F.; Marko, D. TDP1 overexpression in human cells counteracts DNA damage mediated by topoisomerases I and II. J. Biol. Chem. 2004, 279, 55618–55625. [CrossRef]
- Meisenberg, C.; Gilbert, D.C.; Chalmers, A.; Haley, V.; Gollins, S.; Ward, S.E.; El-Khamisy, S.F. Clinical and cellular roles for TDP1 and TOP1 in modulating colorectal cancer response to irinotecan. Mol Cancer Ther, 2015, 14, 575–585. [Google Scholar] [CrossRef]
- Dexheimer, T.S.; Antony, S.; Marchand, C.; Pommier, Y. Tyrosyl-DNA phosphodiesterase as a target for anticancer therapy. Anticancer Agents Med Chem. 2008, 8, 381–389. [Google Scholar] [CrossRef]
- Kawale, A.S.; Povirk, L.F. Tyrosyl-DNA phosphodiesterases: rescuing the genome from the risks of relaxation. Nucleic Acids Res. 2018, 46, 520–537. [Google Scholar] [CrossRef]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Tyrosyl-DNA phosphodiesterase inhibitors: Progress and potential. Bioorg Med Chem. 2016, 24, 5017–5027. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Chepanova, A.A.; Dyrkheeva, N.S.; Salakhutdinov, N.F.; Lavrik, O.I. Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1. Int J Mol Sci. 2023, 24, 5781. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotecan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef]
- Koldysheva, E.V.; Men'shchikova, A.P.; Lushnikova, E.L.; Popova, N.A.; Kaledin, V.I.; Nikolin, V.P.; Zakharenko, A.L.; Luzina, O.A.; Salakhutdinov, N.F.; Lavrik, O.I. Antimetastatic Activity of Combined Topotecan and Tyrosyl-DNA Phosphodiesterase-1 Inhibitor on Modeled Lewis Lung Carcinoma. Bull Exp Biol Med. 2019, 166, 661–666. [Google Scholar] [CrossRef]
- Nikolin, V.P.; Popova, N.A.; Kaledin, V.I.; Luzina, O.A.; Zakharenko, A.L.; Salakhutdinov, N.F.; Lavrik, O.I. The influence of an enamine usnic acid derivative (a tyrosyl-DNA phosphodiesterase 1 inhibitor) on the therapeutic effect of topotecan against transplanted tumors in vivo. Clin. Exp. Metastasis 2021, 38, 431–440. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Korchagina, D.V.; Reynisson, J.; et al. Promising New Inhibitors of Tyrosyl-DNA Phosphodiesterase I (Tdp 1) Combining 4-Arylcoumarin and Monoterpenoid Moieties as Components of Complex Antitumor Therapy. Int. J. Mol. Sci. 2019, 21, 126. [Google Scholar] [CrossRef]
- Chernyshova, I.A.; Zakharenko, A.L.; Kurochkin, N.N.; Dyrkheeva, N.S.; Kornienko, T.E.; Popova, N.A.; Nikolin, V.P.; Ilina, E.S.; Zharkov, T.D.; Kupryushkin, M.S.; Oslovsky, V.E.; Drenichev, M.S.; Lavrik, O.I. The Lipophilic Purine Nucleoside-Tdp1 Inhibitor-Enhances DNA Damage Induced by Topotecan In Vitro and Potentiates the Antitumor Effect of Topotecan In Vivo. Molecules 2022, 28, 323. [Google Scholar] [CrossRef]
- Zakharenko, A.; Luzina, O.; Koval, O.; Nilov, D.; Gushchina, I.; Dyrkheeva, N.; Švedas, V.; Salakhutdinov, N.; Lavrik, O. Tyrosyl-DNA Phosphodiesterase 1 Inhibitors: Usnic Acid Enamines Enhance the Cytotoxic Effect of Camptothecin. J Nat Prod. 2016, 79, 2961–2967. [Google Scholar] [CrossRef]
- Dyrkheeva, N.S.; Filimonov, A.S.; Luzina, O.A.; Zakharenko, A.L.; Ilina, E.S.; Malakhova, A.A.; Medvedev, S.P.; Reynisson, J.; Volcho, K.P.; Zakian, S.M.; Salakhutdinov, N.F.; Lavrik, O.I. New Hybrid Compounds Combining Fragments of Usnic Acid and Monoterpenoids for Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibition. Biomolecules 2021, 11, 973. [Google Scholar] [CrossRef]
- Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kuprushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; Patel, J.; Leung, I.K.H.; Chand, R.; Ayine-Tora, D.M.; Reynisson, J.; Volcho, K.P.; Salakhutdinov, N.F.; Lavrik, O.I. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules 2019, 24, 3711. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological Activity of Usnic Acid and Its Derivatives: Part 2. Effects on Higher Organisms. Molecular and Physicochemical Aspects. Russ. J. Bioorganic Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, Y.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. BBA – Bioenergetic. 2019, 1860, 310–316. [Google Scholar] [CrossRef]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Bioorg. Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.T.; Wu, M.; Wang, W.; Agama, K.; Pommier, Y.; An, L.K. Synthesis of 11-aminoalkoxy substituted benzophenanthridine derivatives as tyrosyl-DNA phosphodiesterase 1 inhibitors and their anticancer activity. Bioorg Chem. 2022, 123, 105789. [Google Scholar] [CrossRef]
- Leung, E.; Patel, J.; Hollywood, J.A.; Zafar, A.; Tomek, P.; Barker, D.; Pilkington, L.I.; van Rensburg, M.; Langley, R.J.; Helsby, N.A.; Squire, C.J.; Baguley, B.C.; Denny, W.A.; Reynisson, J.; Leung, I.K.H. Validating TDP1 as an Inhibition Target for the Development of Chemosensitizers for Camptothecin-Based Chemotherapy Drugs. Oncol Ther. 2021, 9(2), 541–556. [Google Scholar] [CrossRef]
- Hu, D.X.; Tang, W.L.; Zhang, Y.; Yang, H.; Wang, W.; Agama, K.; Pommier, Y.; An, L.K. Synthesis of Methoxy-, Methylenedioxy-, Hydroxy-, and Halo-Substituted Benzophenanthridinone Derivatives as DNA Topoisomerase IB (TOP1) and Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Inhibitors and Their Biological Activity for Drug-Resistant Cancer. J Med Chem. 2021, 64, 7617–7629. [Google Scholar] [CrossRef]
- Dyrkheeva, N.S.; Zakharenko, A.L.; Novoselova, E.S.; Chepanova, A.A.; Popova, N.A.; Nikolin, V.P.; Luzina, O.A.; Salakhutdinov, N.F.; Ryabchikova, E.I.; Lavrik, O.I. Antitumor Activity of the Combination of Topotecan and Tyrosyl-DNA-Phosphodiesterase 1 Inhibitor on Model Krebs-2 Mouse Ascite Carcinoma. Mol Biol (Mosk) 2021, 55(2), 312–317. [Google Scholar] [CrossRef]
- Bertram, J.S.; Janik, P. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett 1980, 11, 63–73. doi: 10.1016/0304-3835(80)90130-5.; Zhu, H.; Kauffman, M.E.; Trush, M.A.; Jia, Z.Q.; Li, Y.R. A simple bioluminescence imaging method for studying cancer cell growth and metastasis after subcutaneous injection of Lewis lung carcinoma cells in syngeneic C57BL/6 mice. React Oxyg Species (Apex) 2018, 5, 118–125. [CrossRef]
- Ma, X.M.; Yu, M.W.; Zhang, G.L.; Yu, J.; Cao, K.X.; Sun, X.; Yang, G.W.; Wang, X.M. Comparison of mouse models of Lewis lung carcinoma subcutaneously transplanted at different sites. Acta Lab Anim Sci Sin 2017, 25, 386–390. [Google Scholar]
- Klein, G.; Klein, E. The transformation of a solid transplantable mouse carcinoma into an “ascites tumor.” Cancer Res 1951, 11, 466–469; Patt, H.M.; Blackford, M.E. Quantitative studies of the growth response of the Krebs ascites tumor. Cancer Res 1954, 14, 391–396; Yushok, W.D.; Mallalieu, L.J.; Batt, W.G. Properties of Krebs 2 ascites carcinoma cells: weight, size, specific gravity, and protein content. J Frankl Inst 1956, 262, 507–509. https://doi.org/10.1016/0016-0032(56)90688-3; Parsons, D.F.; Marko, M.; Braun, S.J.; Wansor, K.J. Ascites tumor invasion of mouse peritoneum studied by high-voltage electron microscope stereoscopy. Cancer Res 1982, 42, 4574–4583. [CrossRef]
- Kornienko TE, Zakharenko AL, Ilina ES, Chepanova AA, Zakharova OD, Dyrkheeva NS, Popova NA, Nikolin VP, Filimonov AS, Luzina OA, Salakhutdinov NF, Lavrik OI. Effect of Usnic Acid-Derived Tyrosyl-DNA Phosphodiesterase 1 Inhibitor Used as Monotherapy or in Combination with Olaparib on Transplanted Tumors In Vivo. Mol Biol (Mosk) 2023, 57, 220–231. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
| Compound | IС50 | A-549 | HCT-116 | HEK293A | HeLa | MCF-7 | MRC-5 | T98G |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.14±0.02 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6 | 1.0±0.4 | >100 | 62±8 | >100 | 47±12 | 60±20 | 90±20 | >100 |
| 7 | 0.12±0.01 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 8 | 5±1 | 30±10 | 88±26 | >100 | 30±20 | 44±13 | ||
| 4 | 0.03±0.01 | 3.9±0.2 | 7.0±0.6 | 12±5 | 9.3±2.2 | 7.0±0.7 | 4.0±1.0 |
| Cell line | MRC-5 | HeLa | HEK293A | HCT-116 | А-549 | MCF-7 | T98G | |
|---|---|---|---|---|---|---|---|---|
| Compound | ||||||||
| 5 | doubtfully | doubtfully | doubtfully | no | no | |||
| 6 | doubtfully | no | no | no | no | |||
| 7 | no | yes | no | yes | yes | yes | doubtfully | |
| 8 | no | no | no | no | ||||
| Blood components | Healthy mice | Intact control | DMSO + Tween-80 i/g | DMSO + Tween-80 i/p | Tpc | Tpc+7 i/g | Tpc+7 i/p | 7 i/p | 7 i/g |
|---|---|---|---|---|---|---|---|---|---|
| Leukocytes | 7.5±1.3 | 13.6±1.2 | 12.8±1.5 | 12.3±1.1 | 11.2±0.7 | 11.7±1.1 | 8.9±0.9 | 10.1±0.9 | 10.7±0.8 |
| Erythrocytes | 8.5 ±0.4 | 6±0.6 | 6.2±0.4 | 6.4±0.6 | 7.6±0.4 | 7.5±0.4 | 8.3±0.5 | 7±0.6 | 7.2±0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





