1. Introduction
Heat Shock Proteins (HSPs), or heat shock proteins, are a class of highly expressive cellular proteins, constituting approximately 1-2% of the proteins present in eukaryotic cells under normal conditions. However, this percentage can increase up to 4-6% when cells are exposed to high temperatures. Their main function is to preserve the integrity of the cell during thermal stress situations.
HSPs act as chaperones, assisting in the correct folding of proteins. This process is essential to ensure that proteins acquire a correct and functional three-dimensional structure. Furthermore, HSPs are involved in cellular signal transduction, contributing to the regulation of cellular responses to stress [
1,
2,
3]. The present work was focused on Docking Study of Heat shock protein HSP 90-alpha and Heat shock cognate 71 kDa protein respectively with Hypericin, a natural compound. HSP90-alpha is a key heat shock protein involved in cellular protection during stressful situations, acting as a molecular chaperone and contributing to the stability and functionality of proteins important for cellular health. HSP90-alpha has been associated with several diseases, including some forms of cancer. Its overexpression has been observed in many types of tumors, and selective inhibitors of HSP90-alpha are being studied as possible anticancer agents[
4,
5]. Heat Shock Cognate 71 kDa Protein (HSC70) is a fundamental protein that plays a crucial role as a molecular chaperone and participates in different aspects of cellular physiology, contributing to the correct folding of proteins and the maintenance of cellular integrity [
6]. The present study was implemented on molecular docking analysis [
7,
8] to predict potential binding sites between hypericin and HSP90 and HSC70 proteins. This can help identify regions of interaction and expected binding strength. Docking analysis was performed by Mcule Database [
9].
2. Material and Methods
HSP90 and HSC70 proteins were performed by Mcule Database [
9]by Autodock Vina [
10]. They were investigated in their Ligand Binding Site pockets:
- -
Heat shock protein HSP 90-alpha ( PDB Code: 3qtf) Binding site center X( 37,0119), Y( 10,6262) Z( 25,0491)
- -
Heat shock cognate 71 kDa protein ( PDB Code: 3fzl) Binding site center x( 18,755), Y( -2,3008) X( 2,9452)
3. Results and Discussion
Heat Shock Proteins (HSPs), also known as heat shock proteins, are a highly expressed class of cellular proteins, comprising about 1-2% of the proteins in eukaryotic cells under normal circumstances. This percentage, however, can escalate to 4-6% when cells encounter elevated temperatures. Their primary role is to maintain cellular integrity in the face of thermal stress.Functioning as chaperones, HSPs play a crucial role in aiding the proper folding of proteins [
1,
2,
3,
4,
5,
6].
This study employs computational methods, specifically molecular docking [
7,
8], to investigate the potential biological interactions between the chaperone proteins HSP90 [
3,
4] and HSC70 [
5] and Hypericin, a natural compound recognized for its anti-tumor properties[
11].
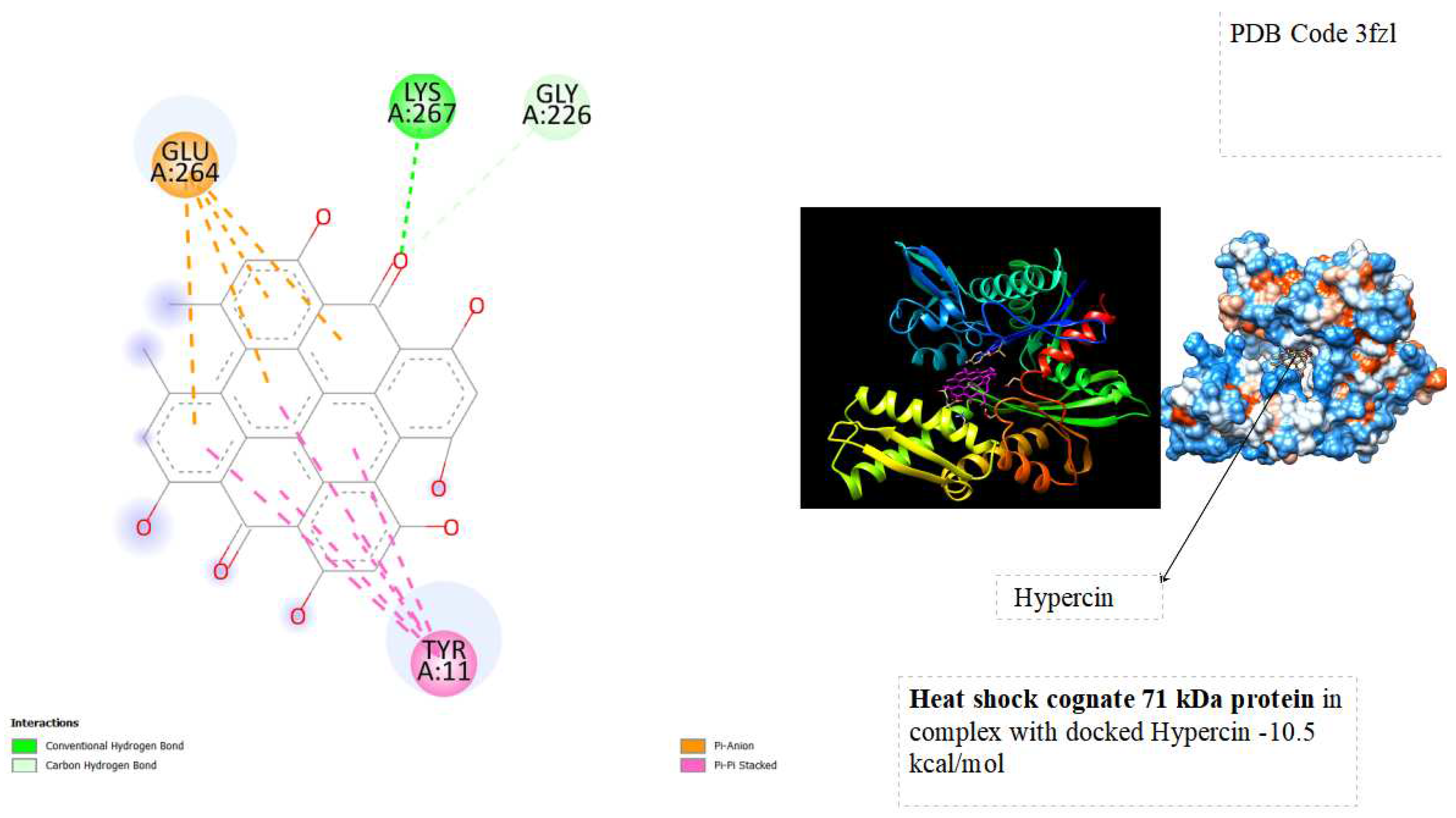
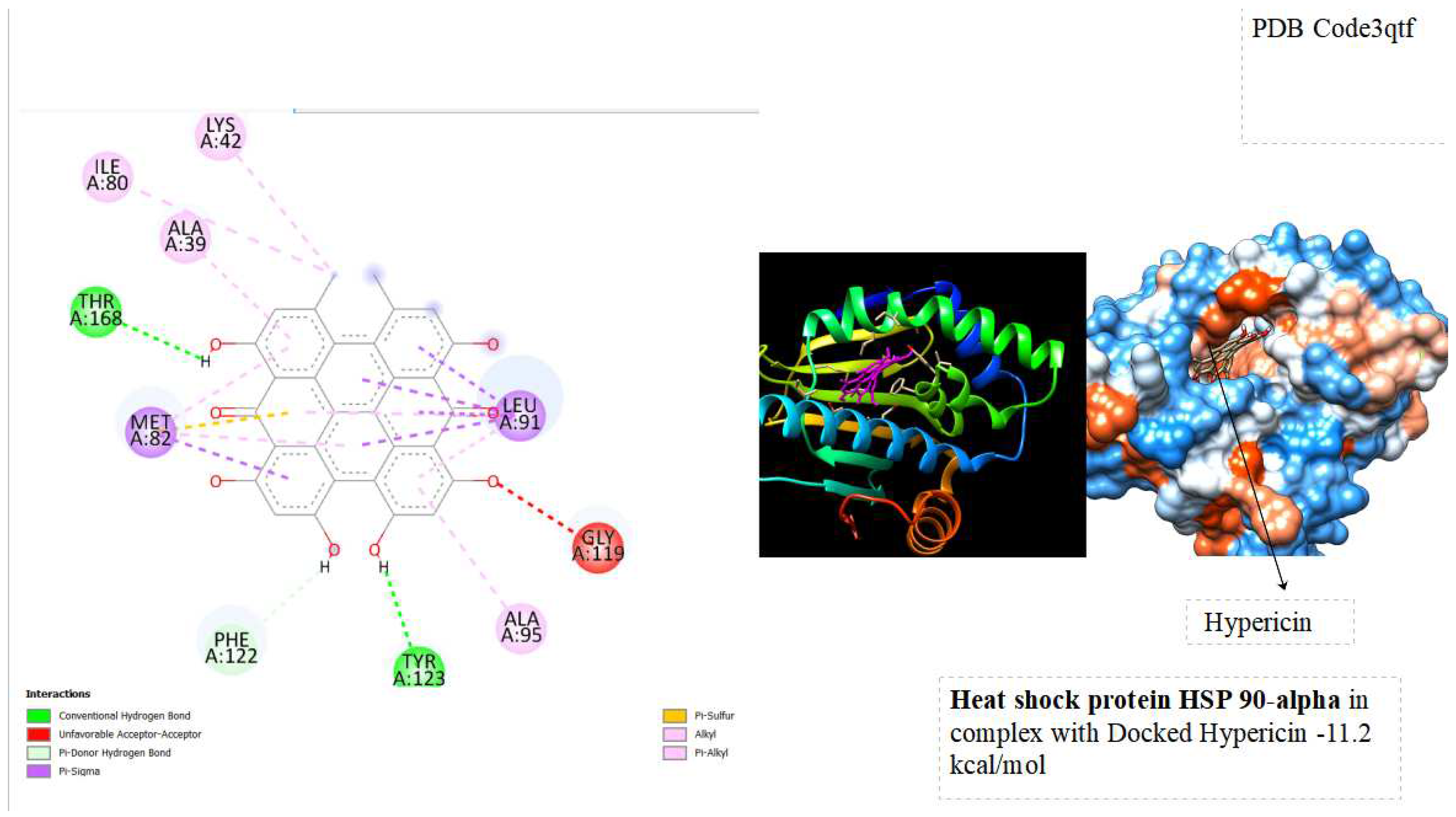
In the docking tests, hypericin exhibited significant binding energy outcomes, with a binding energy of -10.5 kcal/mol for Heat Shock Cognate 71 kDa protein and -11.2 kcal/mol for Heat Shock Protein HSP90-alpha ( See below
Figure 1)
The binding energies of hypericin with Heat Shock Cognate 71 kDa protein (-10.5 kcal/mol) and Heat Shock Protein HSP90-alpha (-11.2 kcal/mol) signify robust and favorable interactions with potential therapeutic implications. The observed low binding energies imply stable and enduring interactions, influencing the overall behavior and function of the proteins. Particularly noteworthy is the high binding energy with heat shock proteins associated with cellular stress responses, like HSP90 and HSC70, suggesting a potential therapeutic impact. Hypericin's interaction with these proteins may contribute to cellular homeostasis, especially in diseases characterized by protein misfolding or cellular stress, such as certain cancers. Furthermore, the binding to molecular chaperones like HSP90 and HSC70 could modulate their activities, influencing the proper folding of client proteins and impacting cellular processes under their regulation. The strong interactions align with hypericin's recognized anti-tumor properties, indicating a potential role in influencing pathways associated with tumor growth or survival. This presents a promising avenue for developing anti-cancer therapeutic strategies. Further exploration of these interactions is essential to unravel specific structural and functional consequences, advancing our understanding of hypericin's potential applications in cellular stress and cancer therapy.
Figure 2.
displays the docking outcomes of Heat shock cognate 71 kDa protein in conjunction with Phypericin within the Ligand Binding Site, as analyzed by Autodock Vina through the Mcule Database. On the left side, 2D diagrams illustrate the residue interactions between the protein and hypericin. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of hypericin.
Figure 2.
displays the docking outcomes of Heat shock cognate 71 kDa protein in conjunction with Phypericin within the Ligand Binding Site, as analyzed by Autodock Vina through the Mcule Database. On the left side, 2D diagrams illustrate the residue interactions between the protein and hypericin. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of hypericin.
4. Conclusion
The study explores the implications of these interactions on the stability and functionality of the proteins, shedding light on the molecular basis of Hypericin's anti-tumor effects. Through a combination of computational simulations and possibly experimental validation, this research seeks to contribute to a deeper understanding of the molecular interplay between Hypericin and key cellular proteins involved in cancer pathways. From docking tests, hypericin was showed excellent results of Binding energy with Heat shock cognate 71 kDa protein ( -10.5 kcal/mol) and with Heat shock protein HSP 90-alpha ( -11.2 kcal/mol). the binding energies suggest that hypericin has a strong affinity for Heat Shock Cognate 71 kDa protein and Heat Shock Protein HSP90-alpha, opening avenues for research into the potential therapeutic applications and cellular consequences of these interactions.
References
- Tkáčová, J., & Angelovičová, M. (2012). Heat shock proteins (HSPs): a review. cell, 17, 18.
- Shrestha, L., Bolaender, A., J Patel, H., & Taldone, T. (2016). Heat shock protein (HSP) drug discovery and development: targeting heat shock proteins in disease. Current topics in medicinal chemistry, 16(25), 2753-2764.
- Tsan, M. F., & Gao, B. (2009). Heat shock proteins and immune system. Journal of Leucocyte Biology, 85(6), 905-910. [CrossRef]
- Birbo, B., Madu, E. E., Madu, C. O., Jain, A., & Lu, Y. (2021). Role of HSP90 in Cancer. International journal of molecular sciences, 22(19), 10317. [CrossRef]
- Barrott, J. J., & Haystead, T. A. (2013). Hsp90, an unlikely ally in the war on cancer. The FEBS journal, 280(6), 1381-1396. [CrossRef]
- Hirschhausen, N., Goetz, F., Peters, G., & Heilmann, C. (2009, September). Human heat shock cognate 71 kDa protein contributes to Staphylococcus aureus internalization by non-professional phagocytes. In INTERNATIONAL JOURNAL OF MEDICAL MICROBIOLOGY (Vol. 299, pp. 56-56). OFFICE JENA, PO BOX 100537, 07705 JENA, GERMANY: ELSEVIER GMBH, URBAN & FISCHER VERLAG.
- Meng, X. Y., Zhang, H. X., Mezei, M., & Cui, M. (2011). Molecular docking: a powerful approach for structure-based drug discovery. Current computer-aided drug design, 7(2), 146-157. [CrossRef]
- Agarwal, S., & Mehrotra, R. J. J. C. (2016). An overview of molecular docking. JSM chem, 4(2), 1024-1028.
- Odhar, H. A., Rayshan, A. M., Ahjel, S. W., Hashim, A. A., & Albeer, A. A. M. A. (2019). Molecular docking enabled updated screening of the matrix protein VP40 from Ebola virus with millions of compounds in the MCULE database for potential inhibitors. Bioinformation, 15(9), 627. [CrossRef]
- Trott, O., & Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry, 31(2), 455-461. [CrossRef]
- Agostinis, P., Vantieghem, A., Merlevede, W., & de Witte, P. A. (2002). Hypericin in cancer treatment: more light on the way. The international journal of biochemistry & cell biology, 34(3), 221-241. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).