Submitted:
29 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Development of M3S cycle populations
2.2. Simple sequence repeat (SSR) genotyping
2.3. Statistical analysis of the SSR data
3. Results
3.1. Diversity statistics and allele frequency
3.2. Analysis of molecular variance (AMOVA)
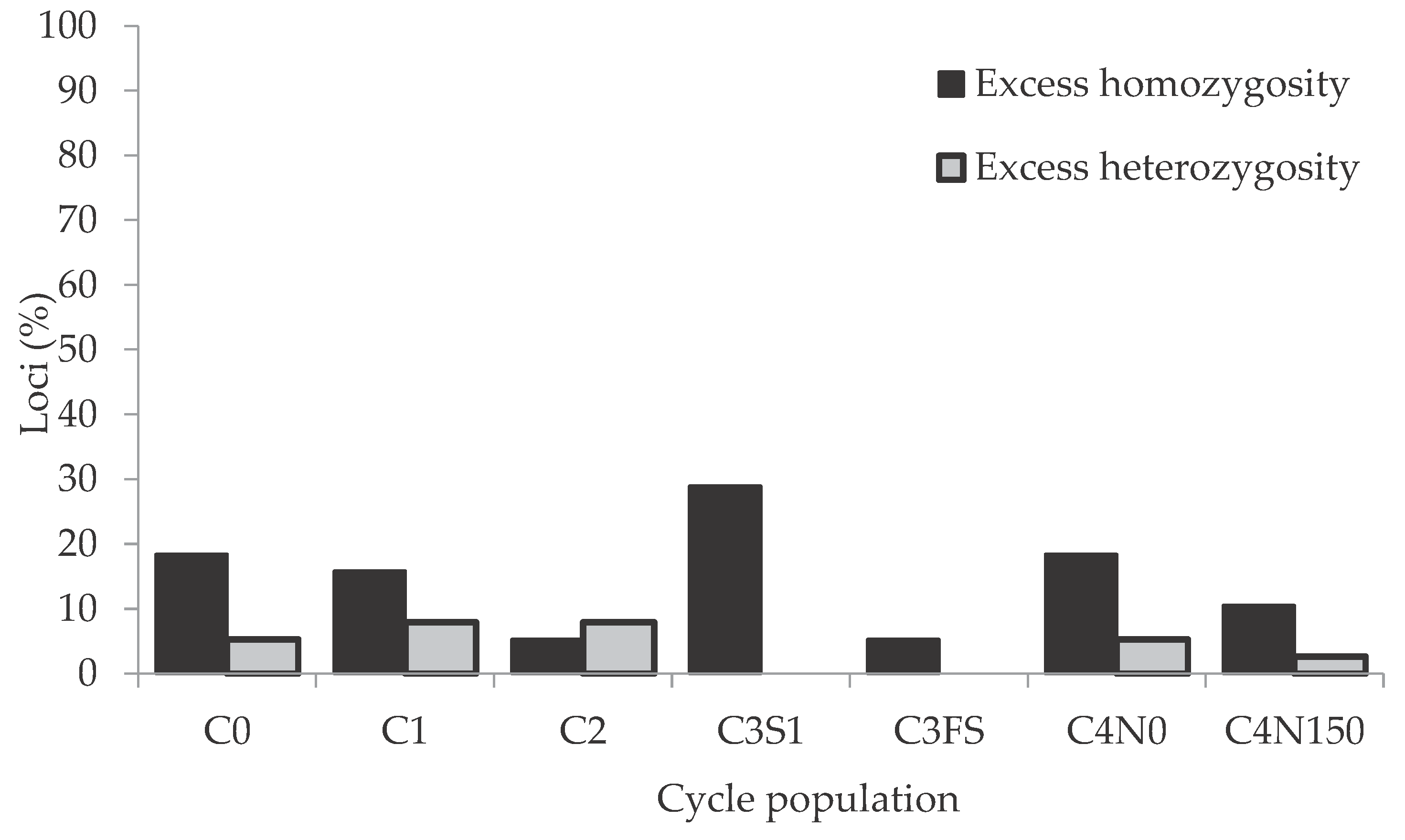
3.3. Hardy-Weinberg (HW) disequilibrium
3.4. Linkage disequilibrium (LD)
4. Discussion
4.1. Diversity statistics and allele frequency
4.2. Hardy-Weinberg (HW) disequilibrium
4.3. Linkage disequilibrium (LD)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Hallauer, A.R.; Darah, L.L. Compendium of recurrent selection methods and their application. Crit. Rev. of Plant. Sci. 1985, 3, 1–33. [Google Scholar] [CrossRef]
- Falke, K.C.; Flachenecker, C.; Melchinger, A.E.; Piepho, H.-P.; Maurer, H.P.; Frisch, M. Temporal changes in allele frequencies in two European F2 flint maize populations under modified recurrent full-sib selection. Theor. Appl. Genet. 2007, 114, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.S.; Mackay, T.F.C. Introduction to quantitative genetics, 4th edn. Longman Sci and Tech, Harlow, 1996.
- Labate, J.A.; Lamkey, K.R.; Lee, M.; Woodman, W. Temporal changes in allele frequencies in two reciprocally selected maize populations. Theor. Appl. Genet. 1999, 99, 1166–1178. [Google Scholar] [CrossRef]
- Pinto, L.R.; Vieira, M.C.L.; de Souza Jr., C.L.; de Souza, A.P. Reciprocal recurrent selection effects on the genetic structure of tropical maize populations assessed at microsatellite loci. Genet. Mol. Biol. 2003, 26, 355–364.
- Šarčević, H.; Pejić, I.; Barić, M.; Kozumplik, V. Originality of M3S maize population and changes in allele frequencies revealed by SSR markers after two cycles of selfed progeny recurrent selection. Euphytica 2008, 161, 97–105. [Google Scholar] [CrossRef]
- Peña-Asin, J.; Álvarez, A.; Ordas, B. Molecular changes during intra and inter recurrent selection of two populations of maize: one adapted and one non adapted to the selection environment. Euphytica 2013, 193, 359–367. [Google Scholar] [CrossRef]
- Weyhrich, R.A.; Lamkey, K.A.; Hallauer, A.R. Hallauer Effective Population Size and Response to S1-Progeny Selection in the BS11 Maize Population. Crop Sci. 1998, 38, 1149–1158. [Google Scholar]
- Ordas, B.; Malvar, R. A.; Diaz, R.; Butron, A. Molecular changes in two maize (Zea mays L.) synthetics after reciprocal selection with two alternative methods. Mol. Breeding 2015, 35, 111. [Google Scholar] [CrossRef]
- Stuber, C.W.; Moll, R.H.; Goodman, M.M.; Schaffer, H.E.; Weir, B.S. Allozyme Frequency Changes Associated with Selection for Increased Grain Yield in Maize (Zea Mays L.). Genetics 1980, 95, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Diaz, O.; Alsirt, A.; Darrah, L.L.; Coe, E.H. Allelic frequency changes in the MoSCSSS maize synthetic in response to bi-directional recurrent selection for rind penetrometer resistance. Maydica 1996, 41, 65–76. [Google Scholar]
- Coque, M.; Gallais, A. Genomic regions involved in response to grain yield selection at high and low nitrogen fertilization from a maize recombinant inbred line population. Theor. Appl. Genet. 2006, 112, 1205–1220. [Google Scholar] [CrossRef]
- Wisser, R.J.; Murray, S.C.; Kolkman, J.M.; Ceballos, H.; Nelson, R. Selection mapping of loci for quantitative disease resistance in a diverse maize population. Genetics 2008, 180, 583–599. [Google Scholar] [CrossRef]
- Romay, M.C.; Butrón, A.; Ordás, A.; Revilla, P.; Ordás, B. Effect of Recurrent selection on the genetic structure of two broad-based Spanish maize populations. Crop Sci. 2012, 52, 1493–1502. [Google Scholar] [CrossRef]
- Beissinger, T.M.; Hirsch, C.N.; Vaillancourt, B.; Deshpande, S.; Barry, K.; Buell, C.R.; Kaeppler, S.M.; Gianola, D.; de Leon, N. A Genome-wide scan for evidence of selection in a maize population under long-term artificial selection for ear number. Genetics 2014, 196(3), 829–840. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.N.; Flint-Garcia, S.A.; Beissinger, T.M.; Eichten, S.R.; Deshpande, S.; Barry, K.; McMullen, M.D.; Holland, J.B.; Buckler, E.S.; Springer, N.; Bue, C.R.; de Leon, N.; Kaeppler, S.M. Insights into the effects of long-term artificial selection on seed size in Maize. Genetics 2014, 198(1), 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, A.O.; Menkir, A.; Gedil, M.; Blay, E.; Ofori, K.; Kling, J.G. Genetic divergence in two tropical maize composites after four cycles of reciprocal recurrent selection. Plant Breeding 2017, 134, 41–47. [Google Scholar] [CrossRef]
- Labate, J.A.; Lamkey, K.R.; Lee, M.; Woodman, W. Molecular genetic diversity after reciprocal recurrent selection in BSSS and BSCB1 maize populations. Crop Sci. 1997, 37, 416–423. [Google Scholar] [CrossRef]
- Hinze, L.L.; Kresovich, S.; Nason, J.D.; Lamkey, K.R. Population genetic diversity in a maize reciprocal recurrent selection program. Crop Sci. 2005, 45, 2435–2442. [Google Scholar] [CrossRef]
- Solomon, K.F.; Martin, I.; Zeppa, A. Temporal genetic structure patterns in tropical maize populations under reciprocal recurrent selection. Euphytica 2010, 176, 239–249. [Google Scholar] [CrossRef]
- Gerke, J.P.; Edwards, J.W.; Guill, K.E.; Ross-Ibarra, J.; McMullen, M.D. The genomic impacts of drift and selection for hybrid performance in maize. Genetics 2015, 201, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Lamkey, C.; Lorenz, A. Relative effect of drift and selection in diverging populations within a reciprocal recurrent selection program. Crop Sci. 2014, 54, 576–585. [Google Scholar] [CrossRef]
- Hallauer, A. L.; Carena, M. J. Recurrent selection methods to improve germplasm in maize. Maydica 2012, 57, 266–283. [Google Scholar]
- Romay, M.C.; Ordás, B.; Revilla, P; Ordás, A. Three cycles of reciprocal recurrent selection in two Spanish maize synthetics. Crop Sci. 2011, 51,1016–1022.
- Ordás, B.; Malvar, R.A.; Revilla, P.; Ordás, A. Effect of three cycles of recurrent selection for yield in four Spanish landraces of maize. Euphytica 2023, 219, 77. [Google Scholar] [CrossRef]
- Sarcevic, H.; Pejic, I.; Baric, M.; Kozumplik, V. Performance and inbreeding depression of an exotic maize population under selfed progeny recurrent selection. Die Bodenkultur 2004, 55, 21–24. [Google Scholar]
- Pejic, I.; Kozumplik, V. Possibility of using local maize genotypes for development of breeding populations (in Croatian). Agriculturae Conspectus Scientificus, 1990, 55, 307–314. [Google Scholar]
- Pejic, I. Heterotic complementarity and genetic diversity of domestic maize germplasm (in Croatian). Agriculturae Conspectus Scientificus 1992, 57, 3–4. [Google Scholar]
- Sabljo, A.; Šarčević, H.; Palaveršić, B.; Buhiniček, I.; Kozumplik, V.; Bukan, M.; Gunjača, J.; Beljo, J.; Tomasović, S.; Ikić, I. Improvement of grain yield and Fusarium stalk rot resistance in the M3S maize population by recurrent selection. Cereal Res. Commun. 2008, 36, 159–160. [Google Scholar]
- Bukan, M.; Šarčević, H.; Gunjača, J.; Buhiniček, I.; Palaveršić, B.; Sabljo, A.; Jambrović, A.; Lewis, R.S.; Kozumplik, V. Evaluation of nitrogen use efficiency in the Maksimir 3 Synthetic maize population. Maydica 2011, 56, 67–75. [Google Scholar]
- Bukan, M.; Šarčević, H.; Buhiniček, I.; Palaveršić, B.; Lewis, R.S.; Kozumplik, V. Stalk rot resistance in Maksimir 3 Synthetic maize population after four cycles of recurrent selection. Genetika 2013, 45(3), 921–928. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes. 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Waples, R.S. Temporal variation in allele frequencies: testing the right hypothesis. Evolution 1989, 43, 1236–251. [Google Scholar] [CrossRef]
- Sprague, G.F.: Eberhart, S.A. Corn breeding. In: Corn and corn improvement, Sprague, G.F.; Dudley, J.W.; Agron. Monogr. ASA, CSSA and SSSA, Madison, WI. 1977, 18, 305–362. [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The principles and practice of statistics in biological research. 3rd edition. W.H. Freeman, New York, (USA), 1995.
- Weir, B.S. Inferences about linkage disequilibrium. Biometrics 1979, 35, 235–254. [Google Scholar] [CrossRef]
- Yeh, F.C.; Boyle, T.J.B. Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg. J. Bot. 1997, 129, 157. [Google Scholar]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Wisser, R.J.; Fang, Z.; Holland, J.B.; Teixeira, J.E.C.; Dougherty, J.; Weldekidan, T.; de Leon, N.; Flint-Garcia, S.; Lauter, N.; Murray, S.C. The genomic basis for short-term evolution of environmental adaptation in maize. Genetics 2019, 213(4), 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Daas, R.R.; Vinayan, M.T.; Patel, M.B.; Phagna, R.K.; Singh, S.B.; Shahi, J.P.; Sarma, A.; Barua, N.S.; Babu, R.; Seetharam, K.; Burgueño, J.A.; Zaidi, P.H. Genetic gains with rapid-cycle genomic selection for combined drought and waterlogging tolerance in tropical maize (Zea mays L.). Plant Genome 2020, 13, e20035. [Google Scholar] [CrossRef]
- Pinto, L.R.; Vieira, M.C.L.; de Souza Jr., C.L.; de Souza, A.P. Genetic diversity assessed by microsatellites in tropical maize populations submitted to a high-intensity reciprocal recurrent selection. Euphytica 2003, 134, 277–286.
- Butrón, A.; Tarrío, R.; Revilla, V.; Ordás, A.; Malvar R. A. Molecular changes in the maize composite EPS12 during selection for resistance to pink stem borer. Theor. Appl. Genet. 2005, 110, 1044–1051. [CrossRef]
- Franzoni, J.; Scapim, C.A.; Beviláqua, M.R.R.; De Fátima Pires da Silva Machado, M.; Pacheco, C.A.P.; Mangolin, C.A. Application of microsatellite markers to evaluate the heterozygosity from the popcorn composite CMS-43 (Zea mays L.) during eight cycles of selection. Plant Breeding. 2012, 131, 479–485. [Google Scholar] [CrossRef]
- Li, L.; Chen, W.; Xiang, K.; Reid, L.M.; Lan, H.; Yang, K.C.; Zhang, M.; Pan, G.T.; Rong, T. The effect of 5 cycles of biparental mass selection on a narrow base maize population based on phenotype, combining ability, and SSR analyses. Maydica 2013, 58(3–4), 238–242.
- Guimarães, A.G.; Amaral Júnior, A.T.; Almeida Filho, J.E.; Pena, G.F.; Vittorazzi, C.; Pereira, M.G. Population structure and impact of recurrent selection on popcorn using EST-SSR markers. Acta. Sci. Agron., 2018, 40, e35218. [Google Scholar] [CrossRef]
- Ledesma, A.; Ribeiro, F.A.S.; Uberti, A.; Edwards, J.; Hearne, S.; Frei, U., Lübberstedt, T. Molecular characterization of doubled haploid lines derived from different cycles of the Iowa Stiff Stalk Synthetic (BSSS) maize population. Front. Plant. Sci. 2023, 14, 1226072. [CrossRef]
- McLean-Rodríguez, F. D.; Elston Costich, D.; Carolina Camacho-Villa, T.; Enrico Pè, M.; Dell’Acqua, M. Genetic diversity and selection signatures in maize landraces compared across 50 years of in situ and ex situ conservation. Heredity 2021, 126, 913–928. [Google Scholar] [CrossRef]
- Crossa, J.; Vargas, M.; Van Eeuwijk, F.A.; Jiang, C.; Edmeades, G.O.; Hoisington, D. Interpreting genotype x environment interaction in tropical maize using linked molecular markers and environmental covariables. Theor. Appl. Genet. 1999, 99, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Van Eeuwijk, F.A.; Crossa, J.; Vargas, M.; Ribaut, J-M. Analyzing QTL-environment interaction by factorial regression, with an application to the CIMMYT drought and low-nitrogen stress program in maize. In: Quantitative genetics, genomics and plant breeding, Kang, M.s., Ed., CABI International, New York, 2002.
- Devey, M.E.; Russell, W.A. Evaluation of recurrent selection for stalk quality in a maize cultivar and effects on other agronomic traits. Iowa State J. Res. 1983, 58, 207–219. [Google Scholar]
- Klenke, J.R.; Russell, W.A.; Guthrie, W.D. Recurrent selection for resistance to European corn borer in a corn synthetic and correlated effects on agronomic traits. Crop Sci. 1986, 26, 864–868. [Google Scholar] [CrossRef]
- Bertin, P.; Gallais, A. Physiological and genetic basis of nitrogen use efficiency. II. QTL detection and coincidences. Maydica 2001, 46, 53–68. [Google Scholar]
- Liu, X.; Zheng, Z.; Tan, Z.; Li, Z.; He, C.; Liu, D.; Zhang, G.; Luo, Y. QTL mapping for controlling anthesis-silking interval based on RIL population in maize. Afr. J. Biotechnol. 2000, 9, 950–955. [Google Scholar]
- Wang, L.; Zhou, Z.; Li, R.; Weng, J.; Zhang, Q.; Li, X.; Wang, B.; Zhang, W.; Song, W.; Li, X. Mapping QTL for flowering time-related traits under three plant densities in maize. Crop J. 2021, 9, 372–379. [Google Scholar] [CrossRef]
- Leng, P.; Khan, S.U.; Zhang, D.; Zhou, G.; Zhang, X.; Zheng, Y.; Wang, T.; Zhao, J. Linkage mapping reveals QTL for flowering time-related traits under multiple abiotics conditions in maize. Int. J. Mol. Sci. 2022, 23, 8410. [Google Scholar] [CrossRef] [PubMed]
- Labate, J.A.; Lamkey, K.R.; Lee, M.; Woodman, W. Hardy–Weinberg and linkage equilibrium estimates in the BSSS and BSCB1 random mated populations. Maydica 2000, 45, 243–255. [Google Scholar]
- Templeton, A. R. Population Genetics and Microevolutionary Theory, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006.
- Pfaffelhuber, P.; Lehnert, A.; Stephan, W. Linkage disequilibrium under genetic hitchhiking in finite populations. Genetics 2008, 179, 527–537. [Google Scholar] [CrossRef]
- Brown, A.H.D.; Allard, R.W. Effect of reciprocal recurrent selection for yield on isozyme polymorphisms in maize (Zea mays L). Crop Sci. 1971, 11, 888–893. [Google Scholar] [CrossRef]
- Zhao, M; Shu, G; Hu, Y.; Cao, G; Wang, Y. Pattern and variation in simple sequence repeat (SSR) at different genomic regions and its implications to maize evolution and breeding. BMC Genomics 2023, 24,136. [CrossRef]

| Cycle population | At | Am | He |
|---|---|---|---|
| C0 | 127 | 3.34±0.242 | 0.5170±0.031 |
| C1 | 130 | 3.42±0.231 | 0.5318±0.025 |
| C2 | 130 | 3.42±0.234 | 0.5171±0.029 |
| C3S1 | 126 | 3.32±0.207 | 0.5135±0.030 |
| C3FS | 123 | 3.24±0.218 | 0.5012±0.030 |
| C4N0 | 129 | 3.39±0.240 | 0.5266±0.024 |
| C4N150 | 125 | 3.29±0.237 | 0.4924±0.028 |
| Allele frequency class |
Cycle population | ||||||
|---|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C3S1 | C3FS | C4N0 | C4N150 | |
| % | |||||||
| 0.00–0.10 | 23 | 28 | 28 | 26 | 29 | 27 | 29 |
| 0.11–0.20 | 25 | 12 | 21 | 22 | 20 | 15 | 20 |
| 0.21–0.30 | 13 | 21 | 15 | 14 | 9 | 17 | 12 |
| 0.31–0.40 | 11 | 11 | 8 | 10 | 13 | 14 | 12 |
| 0.41–0.50 | 8 | 10 | 8 | 11 | 10 | 8 | 4 |
| 0.51–0.60 | 8 | 6 | 8 | 6 | 6 | 4 | 7 |
| 0.61–0.70 | 5 | 2 | 4 | 3 | 6 | 5 | 7 |
| 0.71–0.80 | 4 | 8 | 5 | 3 | 2 | 8 | 4 |
| 0.81–1.00 | 3 | 2 | 3 | 5 | 5 | 2 | 5 |
| Mean allele frequency±SE |
0.29 ±0.021 | 0.29 ±0.020 |
0.29 ±0.021 |
0.29 ±0.021 |
0.29 ±0.021 |
0.29 ±0.020 |
0.29 ±0.022 |
| Bin | Locus | SCP1 | C0 | C1 | C2 | C2 | C3FS | C3FS | C0 | C0 |
|---|---|---|---|---|---|---|---|---|---|---|
| RCP2 | C1 | C2 | C3S1 | C3FS | C4N0 | C4N150 | C4N0 | C4N150 | ||
| 1.01 | phi056 | |||||||||
| 1.03 | phi339017 | |||||||||
| 1.08 | dupssr12 | |||||||||
| 1.11 | phi064 | |||||||||
| 2.00 | phi96100 | |||||||||
| 2.02 | phi098 | |||||||||
| 2.04 | phi083 | |||||||||
| 3.02 | phi036 | |||||||||
| 3.05 | phi053 | |||||||||
| 3.07 | bnlg197 | |||||||||
| 4.01 | phi213984 | |||||||||
| 4.04 | phi308090 | |||||||||
| 4.05 | phi438301 | |||||||||
| 4.11 | phi076 | |||||||||
| 5.00 | nc130 | |||||||||
| 5.01 | bnlg143 | |||||||||
| 5.03 | phi109188 | |||||||||
| 5.07 | phi128 | |||||||||
| 6.00 | phi126 | |||||||||
| 6.03 | phi389203 | |||||||||
| 6.04 | phi452693 | |||||||||
| 6.07 | phi123 | |||||||||
| 7.02 | phi034 | |||||||||
| 7.03 | bnlg572 | |||||||||
| 7.05 | phi082 | |||||||||
| 7.06 | phi116 | |||||||||
| 8.00 | umc1359 | |||||||||
| 8.03 | phi115 | |||||||||
| 8.08 | phi015 | |||||||||
| 8.09 | phi233376 | |||||||||
| 9.01 | phi033 | |||||||||
| 9.02 | umc1033 | |||||||||
| 9.05 | phi236654 | |||||||||
| 9.07 | bnlg128 | |||||||||
| 10.02 | phi96342 | |||||||||
| 10.03 | phi050 | |||||||||
| 10.04 | phi084 | |||||||||
| 10.3 | phi059 |
 Significant Waples neutrality test at the 0.05 probability level.
Significant Waples neutrality test at the 0.05 probability level.| Cycle population |
C0 | C1 | C2 | C3S1 | C3FS | C4N0 | C4N150 |
|---|---|---|---|---|---|---|---|
| C0 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| C1 | 0.0503 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| C2 | 0.0395 | 0.0535 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| C3S1 | 0.0437 | 0.0575 | 0.0143 | <0.0001 | <0.0001 | <0.0001 | |
| C3FS | 0.0702 | 0.0872 | 0.0542 | 0.0750 | <0.0001 | <0.0001 | |
| C4N0 | 0.0598 | 0.0664 | 0.0537 | 0.0728 | 0.0247 | <0.0001 | |
| C4N150 | 0.0705 | 0.0843 | 0.0595 | 0.0739 | 0.0320 | 0.0143 | |
| Mean1 | 0.0560 | 0.0670 | 0.0460 | 0.0560 | 0.057 | 0.0490 | 0.0560 |
| Locus | Bin |
Cycle population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C3S1 | C3FS | C4N0 | C4N150 | Mean | ||
| Fixation index (FIS) | |||||||||
| phi098 | 2.02 | -0.12 | -0.38* | ml1 | 1.00** | -0.21 | -0.27 | 0.53* | 0.09 |
| phi076 | 4.11 | -0.07 | 0.43** | 0.93** | -0.07 | -0.00 | 1.00** | -0.02 | 0.31 |
| bnlg143 | 5.01 | 0.09 | 0.49** | 0.21 | -0.03 | 0.19* | 0.23* | 0.26* | 0.21 |
| phi452693 | 6.04 | 0.34* | 0.50** | -0.21 | -0.03 | -0.19 | 0.30* | 0.15 | 0.13 |
| bnlg572 | 7.03 | 0.30** | 0.86** | 0.23 | 0.91** | 0.59** | 0.90** | 0.77** | 0.65 |
| phi050 | 10.03 | 0.28** | 0.20 | 0.57** | 0.10** | -0.02 | 0.19 | 1.00** | 0.33 |
| Bin | Locus | Bin | Locus | Bin | Locus | Bin | Locus | |
|---|---|---|---|---|---|---|---|---|
| C0 | C3S1 | |||||||
| 3.07 | bnlg197 | 4.04 | phi308090 | 9.01 | phi033 | 9.02 | umc1033 | |
| 3.07 | bnlg197 | 8.00 | umc1359 | C3FS | ||||
| 8.08 | phi015 | 8.09 | phi233376 | 9.07 | bnlg128 | 9.01 | phi033 | |
| C1 | 3.07 | bnlg197 | 1.01 | phi056 | ||||
| 9.07 | bnlg1281 | 9.01 | phi033 | 3.07 | bnlg197 | 6.00 | phi126 | |
| 9.07 | bnlg128 | 5.03 | phi109188 | 3.07 | bnlg197 | 6.04 | phi452693 | |
| 3.07 | bnlg197 | 1.08 | dupssr12 | 8.08 | phi015 | 8.09 | phi233376 | |
| 3.07 | bnlg197 | 3.02 | phi036 | 1.01 | phi056 | 6.00 | phi126 | |
| 3.07 | bnlg197 | 4.11 | phi076 | 7.05 | phi082 | 7.06 | phi116 | |
| 3.07 | bnlg197 | 2.00 | phi96100 | C4N0 | ||||
| 1.08 | dupssr12 | 8.08 | phi015 | 1.08 | dupssr12 | 8.08 | phi015 | |
| 1.08 | dupssr12 | 4.11 | phi076 | 1.08 | dupssr12 | 9.02 | umc1033 | |
| 1.08 | dupssr12 | 4.05 | phi438301 | 8.08 | phi015 | 8.00 | umc1359 | |
| 1.08 | dupssr12 | 2.00 | phi96100 | 1.01 | phi056 | 9.02 | umc1033 | |
| 8.08 | phi015 | 4.05 | phi438301 | 7.05 | phi082 | 7.06 | phi116 | |
| 3.02 | phi036 | 7.06 | phi116 | C4N150 | ||||
| 10.03 | phi050 | 10.04 | phi084 | 3.07 | bnlg197 | 1.08 | dupssr12 | |
| C2 | 3.07 | bnlg197 | 9.02 | umc1033 | ||||
| 3.07 | bnlg197 | 1.08 | dupssr12 | 9.01 | phi033 | 8.03 | phi115 | |
| 3.07 | bnlg197 | 10.03 | phi050 | 4.04 | phi308090 | 4.05 | phi438301 | |
| 3.07 | bnlg197 | 6.03 | phi389203 | |||||
| 3.02 | phi036 | 7.05 | phi082 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





