1. Introduction
Celiac disease (CD) is an autoimmune condition that arises from gluten sensitivity in genetically predisposed individuals [
1,
2,
3]. It is a complex disorder in which genetic, environmental, and immunological factors converge [
4,
5]. Tolerance to dietary antigens is linked to the activation of regulatory T cells and the production of immunosuppressive cytokines, preventing inappropriate responses from effector Th lymphocytes [
6]. Gluten consumption by individuals with CD triggers a sequence of inflammatory reactions that result in the characteristic small intestine lesions, leading to partial or total atrophy of the intestinal villi and decreased nutrient absorption [
7,
8]. Additionally, it activates CD4+ T cells and increases intraepithelial lymphocytes in the intestinal mucosa. Gluten-derived epitopes typically contain multiple proline and glutamine residues that are selectively deamidated by tissue transglutaminase (TG2) [
9,
10,
11]. These modified peptides can bind to HLA-DQ2/DQ8, stimulating CD4+ T-helper 1 (Th1) cells in the lamina propria, which, upon recognizing gluten peptides, produce proinflammatory cytokines, primarily interferon gamma (IFN-γ) [
12]. Hence, the pathogenic model in CD involves factors acting at both the epithelial and lamina propria levels, including: i) incomplete digestion and trans-epithelial peptide transport, ii) the impact of gluten on the epithelium, iii) proliferation and activation of intraepithelial lymphocytes (IELs), and iv) recognition of gluten peptides by specific T lymphocytes with HLA-DQ2 restriction after modification by tissue transglutaminase (TG2) [
13,
14].
Celiac disease, like other autoimmune diseases, is a polygenic disorder strongly associated with HLA genes, particularly the CELIAC1 locus located on chromosome 6p21. In the majority of celiac patients, it is present a variant of the HLA-DQ2 molecule, which is encoded by the DQA105 and DQB102 alleles. Other patients carry DQ8 (DQA103, DQB10302) alleles, or some isolated alleles of DQ2 [
15,
16]. These genes exhibit a dose effect mediated by peptide presentation, which is more pronounced in HLA-DQ2 homozygotes. Interestingly, while approximately 25% of the population carries DQ2, only 1% develops CD. These HLA-DQ molecules present deamidated gluten peptides to CD4+ T cells [
16]. Whole-genome studies have identified other regions containing susceptibility genes, many of which are related to immune function and potentially shared with other chronic immune-based diseases [
17,
18,
19].
The immune system includes mechanisms that maintain homeostasis, including both activator and inhibitory mechanisms. Immune checkpoints are pivotal in modulating the signaling pathways responsible for immunological tolerance. In our research, we have identified alterations in signaling related to the co-inhibitory PD-L1 and the immunosuppressive IDO in celiac disease patients [
20,
21,
22]. We have provided the first evidence of elevated PD-L1 and IDO expression levels in celiac patients on the surface of intestinal epithelial and lamina propria cells, resulting in increased kynurenine levels in the serum of these patients [
21,
22].
Numerous studies have emphasized the importance of CD200 in controlling autoimmunity and inflammation in diseases associated with heightened immune system activity [
23]. The CD200 molecule and its receptor CD200R exert immunomodulatory effects, induce immune tolerance, and regulate the differentiation, adhesion, and chemotaxis of various cell populations [
24]. They also play a crucial role in the release of mediators and cytokines. Through its receptor, CD200 regulates macrophage function and controls alloimmune and autoimmune responses [
25]. While CD200 and CD200R share identical extracellular portions, they differ in their cytosolic COOH tails. CD200 lacks the ability to transmit activation or inhibition signals in its minimal intracellular region, whereas CD200R, upon binding, can downregulate the exaggerated activity of the immune system to protect the organism from damage due to hyperactivity [
26].
Elafin (peptidase inhibitor 3, PI3, and its biologically active precursor, pre-elafin) is a neutrophil serine proteinase inhibitor with a crucial role in preventing excessive tissue injury during inflammatory events [
27]. Elafin is constitutively produced by the epithelium, with its expression regulated by inflammatory stimuli such as proinflammatory cytokines (IL-1b, TNF-a), bacterial lipopolysaccharides, and elastase [
27]. Specifically, Elafin modulates colon inflammation and possesses antiprotease, immunomodulatory, and antiproliferative activities. Elafin is a member of the "trappin" gene family and features an amino-terminal transglutaminase substrate domain, identified as a substrate for tissue transglutaminase TG-2 cross-linking [
28,
29]. TG2 plays a critical role in CD pathogenesis by deamidating gluten peptides and increasing the binding affinity of HLA-DQ2 (DQ8) molecules to these peptides [
12].
Galipeau et al. [
30] explored the role of Elafin in CD and its interaction with factors involved in its pathogenesis using human small intestinal tissue and in vitro gliadin deamidation assays. They observed a lower presence of the Elafin in patients with active CD, which reflects inflammation and damage to the small intestinal epithelium. Furthermore, they reported an improvement in inflammation and the restoration of the small intestinal epithelial barrier in a gluten-sensitive mouse model treated with Elafin, describing Elafin as a molecule capable of interacting with gliadin peptides and affecting TG-2, which is involved in their deamidation [
30].
Our study thoroughly evaluated the expression of targetable immune checkpoint molecules in CD. Existing data on their expression or function in CD are limited. Our objective was to characterize the expression and function of the CD200/CD200R axis and PI3 in CD, along with their correlation with other immune checkpoints, such as IDO and PD1/PDL1. Our previous research has associated these checkpoints with CD development. Additionally, our aim was to investigate whether the interaction of CD200/CD200R reduces activation, proliferation, and Th17 cytokine production. We hypothesized that specific coding regions of the PI3 gene splice variants might predispose the Elafin protein to modify its expression and function, resulting in reduced functional protein levels in patients with active CD compared to healthy individuals. These findings could significantly contribute to understanding the clinical manifestation of CD.
2. Results
2.1. Diagnosis: serological, genetic, and histological analysis
Serological analyses revealed elevated levels of endomysial antibody (AEMA) and anti-transglutaminase antibody (ATGA) in all included patients diagnosed with CD. None of the patients exhibited IgA deficiency. All patients were confirmed to have active CD. Additionally, peripheral blood samples were collected at the time of diagnosis confirmation, along with small-intestinal mucosa biopsies.
Histological analysis was conducted based on the Marsh criteria (types I-IV). Intestinal biopsies from CD patients exhibited typical indications of the disease, including villi with total or partial atrophy and increased lymphocyte infiltration. Among the patients, 16% have Marsh III lesions (partial or complete villi atrophy and crypt hypertrophy), 76% have Marsh IV hypoplasia (completely flattened atrophied villi), and 8% have Marsh I lesions (only increased intraepithelial lymphocytes).
HLA genotype frequencies analysis showed that the most common allele among patients was HLA-DQ2 (DQA10501, DQB10201) (76%). The DQ8 genotype (DQA1*03:01, 03:03–DQB103:02) was present in 24% of CD patients.
2.2. CD200, CD200R and Elafin distribution in intestinal mucosa tissue from CD patients by Immunohistochemistry
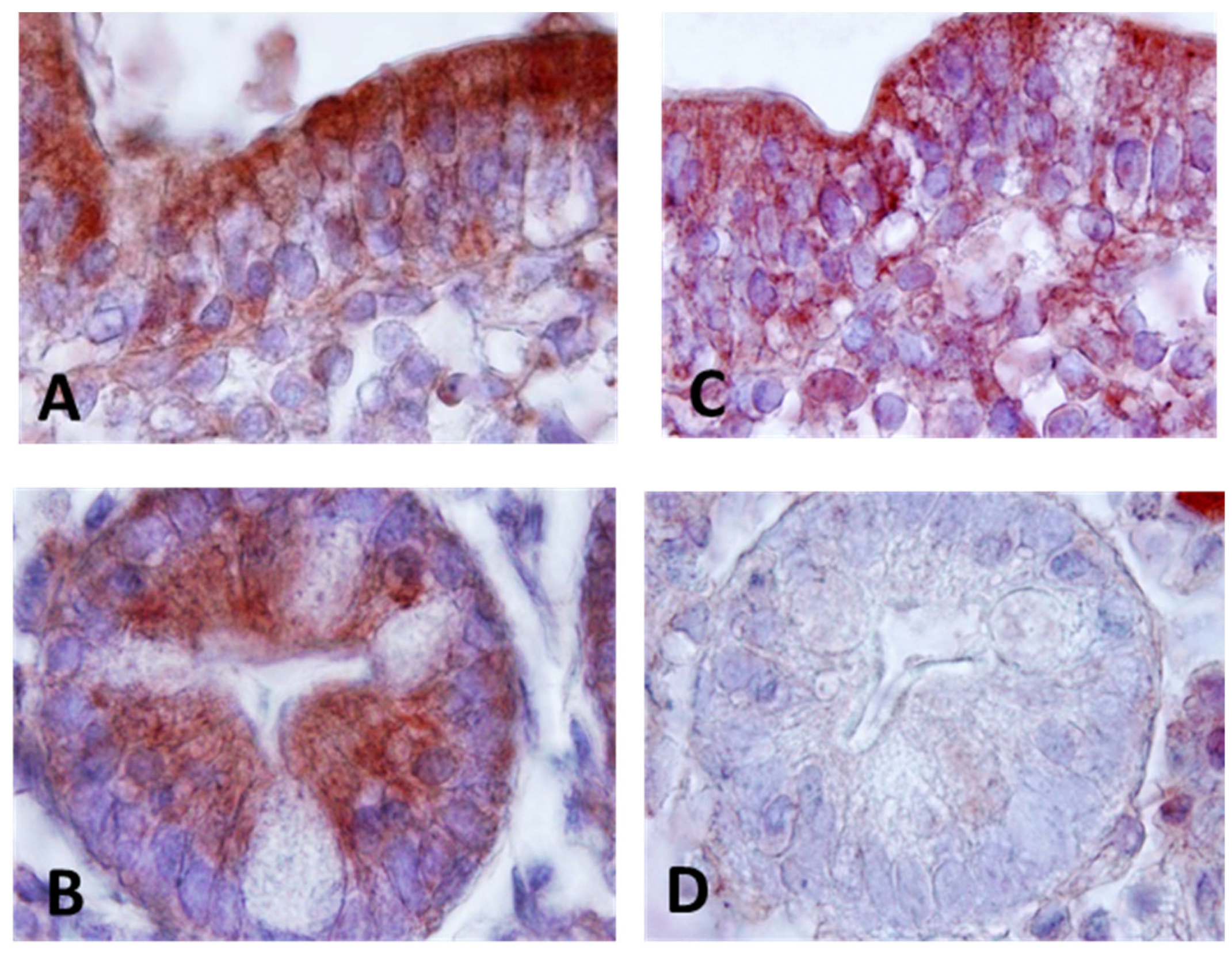
The diversity of cell types presents in intestinal samples makes it necessary to develop immunohistochemical methods for of CD200, CD200R and Elafin detection to evaluate the cellular source, which might provide an adequate interpretation of the results. To achieve this, an immunohistochemistry technique was used. CD200 and CD200R were expressed in epithelial cells in CD, with CD200 showing more intense reactivity compared to the receptor expression (
Figure 1A and
Figure 1C). In addition to epithelial expression, CD200, CD200R positive cells were also identified in the lamina propria, resembling cells morphologically similar to lymphocytes. Within the Lieberkühn crypts, we found an intense CD200 expression located in the basal part facing the lumen of the crypts, visible both on the cell membrane and as a secreted form within the cytoplasm of the cells (
Figure 1B). Also, we found expression on the apical surface of the crypts.
No expression of CD200R was detected at the Lieberkühn crypt level (
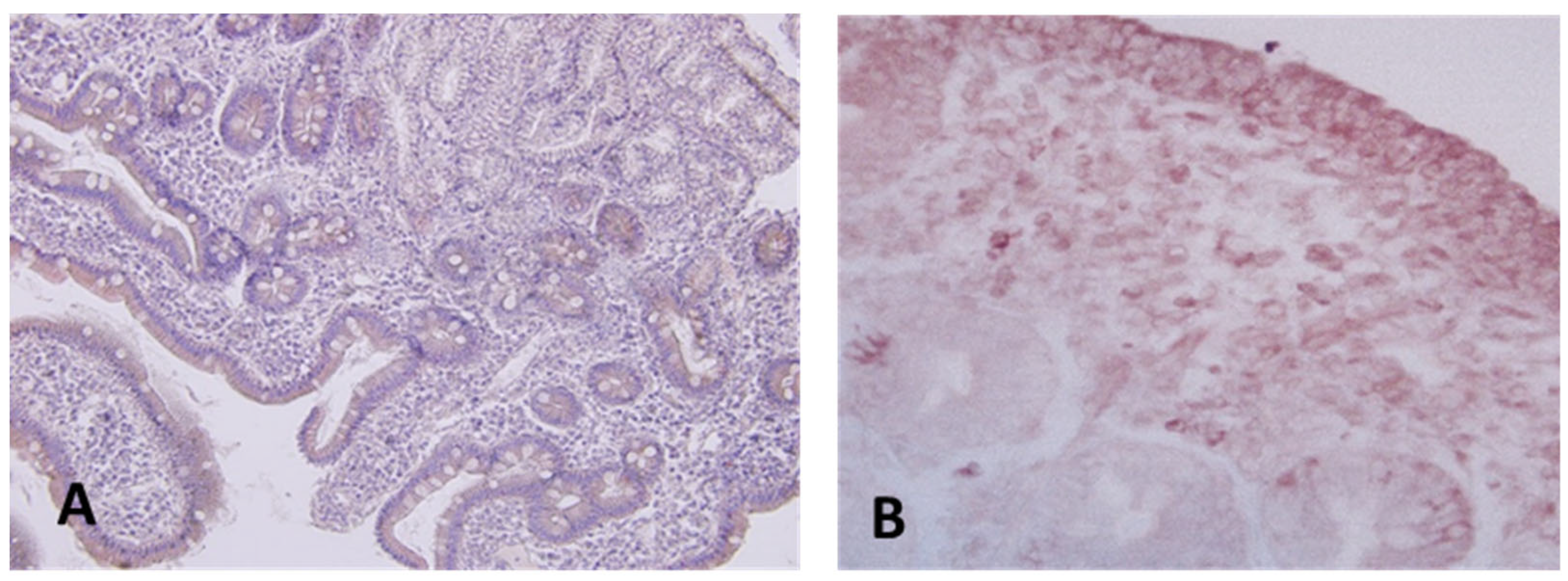
Figure 1D). However, strong Elafin expression was found in cells of the lamina propria resembling lymphocytes, as well as in epithelial cells in patients with CD. The expression pattern showed expression both in the surface epithelium as well as in crypt cells (
Figure 2A and
Figure 2B). When isotype controls were used, there was an absence of immune reactivity patterns (data not shown).
2.3. Detection of Cd200, Cd200R and Elafin protein in serum from CD patients by ELISA assays
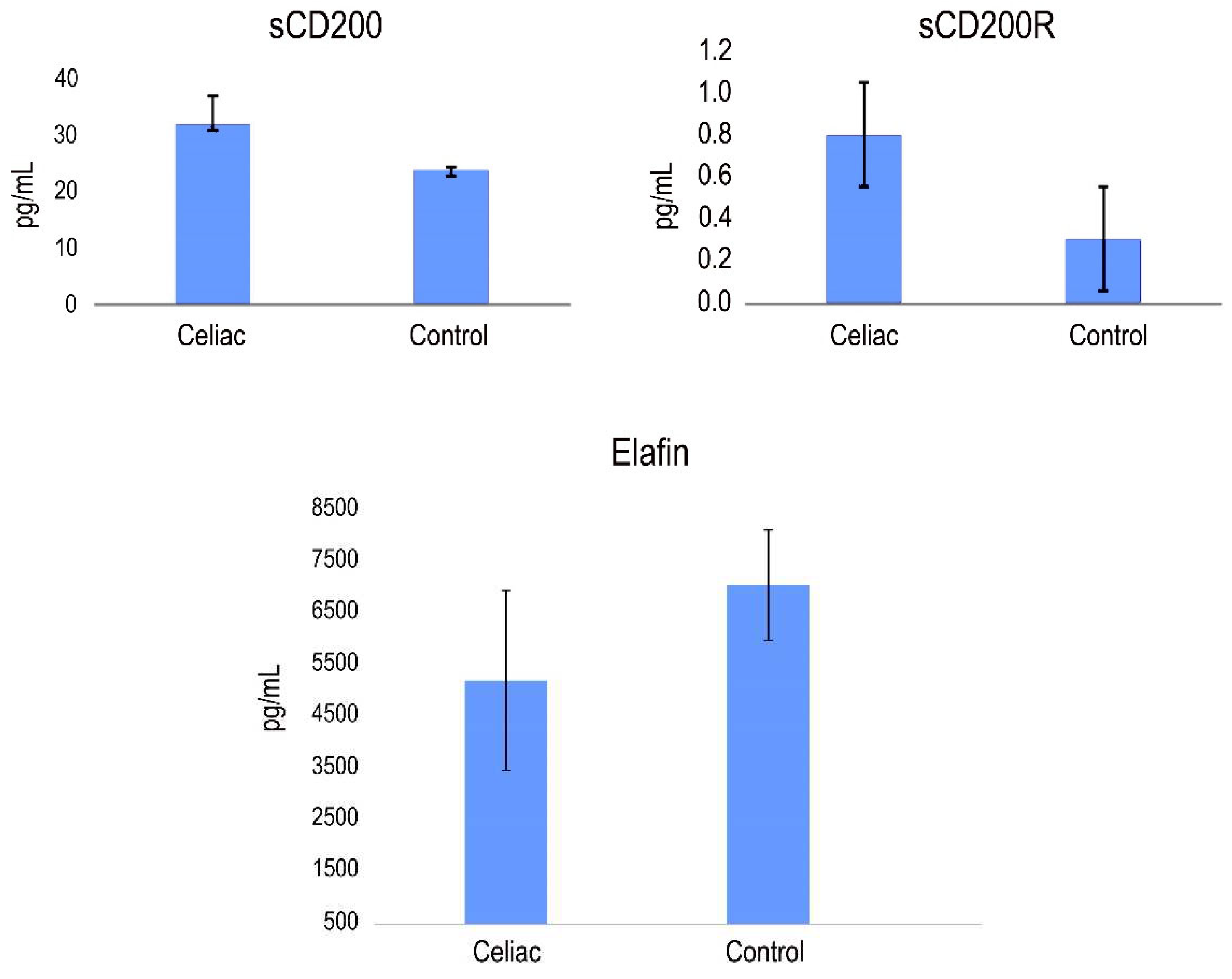
The potential biomarkers of CD identified in our study, PI3, CD200 and CD200R, were further validated using quantitative ELISA using the same cohort of samples. Our findings revealed higher expression levels of CD200 and CD200R, while PI3 exhibited lower expression levels compared to the healthy control group. As shown in
Figure 3, the mean serum levels of CD200 were 32.91±8.73 pg/mL in CD patients and 6.92±3.35 pg/mL in control patients. The mean levels of CD200R were 864±101 pg/mL, 21.98±6,54 pg/mL and 377±66 pg/mL in sera from CD patients and healthy patients, respectively. The mean levels of Elafin were 5217±1543 pg/mL, 7062±1066 pg/mL, in sera from CD patients and healthy patients, respectively (
Figure 3).
No consistent association was observed between the expression levels of Elafin, CD200 and CD200R with sex, age, clinical stage, or histology. However, we assessed the potential correlations between the levels of expression of Elafin, CD200 and CD200R (
Figure 3). Our analysis revealed that the levels of CD200 and Elafin exhibited significant correlations different from zero, with a confidence level of 95.0% (p < 0.05).
2.4. CD4 y CD8 IFN-gamma expresión in CD patients. Gamma interferon responses of CD4 and CD8 T-cell subsets
CD4 microbeads were designed for the isolation of human cells based on the CD4 antigen expression. CD8 microbeads was used to isolate highly pure cytotoxic T cells. The relative contributions of CD4 and CD8 T-cell subsets to IFN-γ production in CD were examined. Our findings revealed that both CD4+ T cells and CD8+ T cells showed increased IFN-γ secretion in CD compared to control patients (
Figure 4).
2.5. Analysis of Th1 and Th17 cytokines
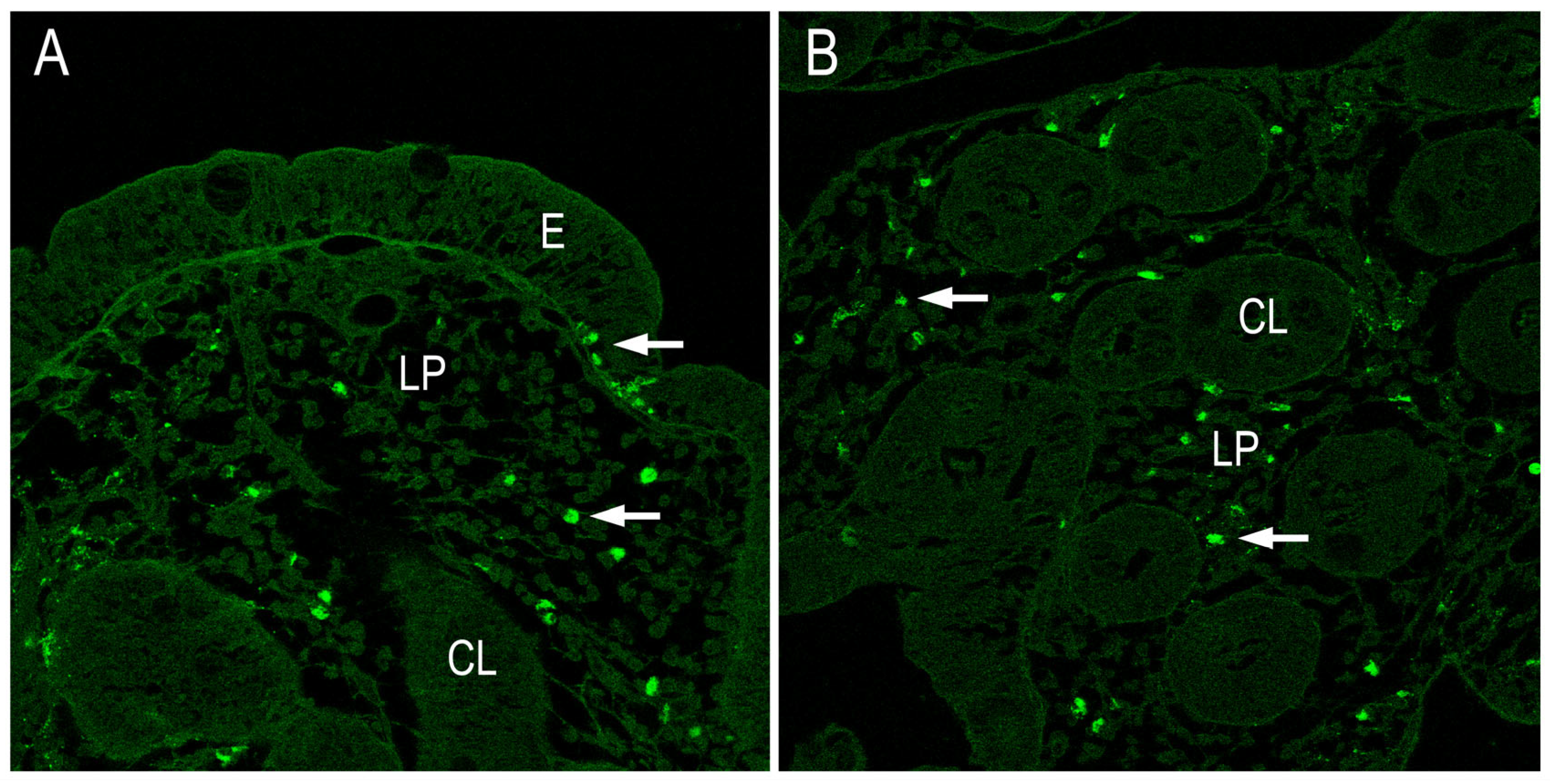
Using immunohistochemistry analysis, we examined the presence of IL-17 and lL-21 in intestinal biopsies obtained from patients with active disease and healthy individuals. The results suggest that IL-21 secretion is likely associated with lamina propria lymphocytes, as there is no evidence indicating that IELs might be also a source of this cytokine. In response to gluten stimulus, IL-21 is produced by lamina propria lymphocytes and it mediates induction of IFN-γ. The distribution of intestinal IL-17+ and IL-22+ cells in the biopsy tissues were immunohistochemically processed and evaluated by confocal microscopy. The results indicate an impaired secretion of both IL-17+ and IL-22+ cells in CD (
Figure 5). Also, a significative number of both IL-17 and IL-22 producing cells were found in the small intestinal mucosa.
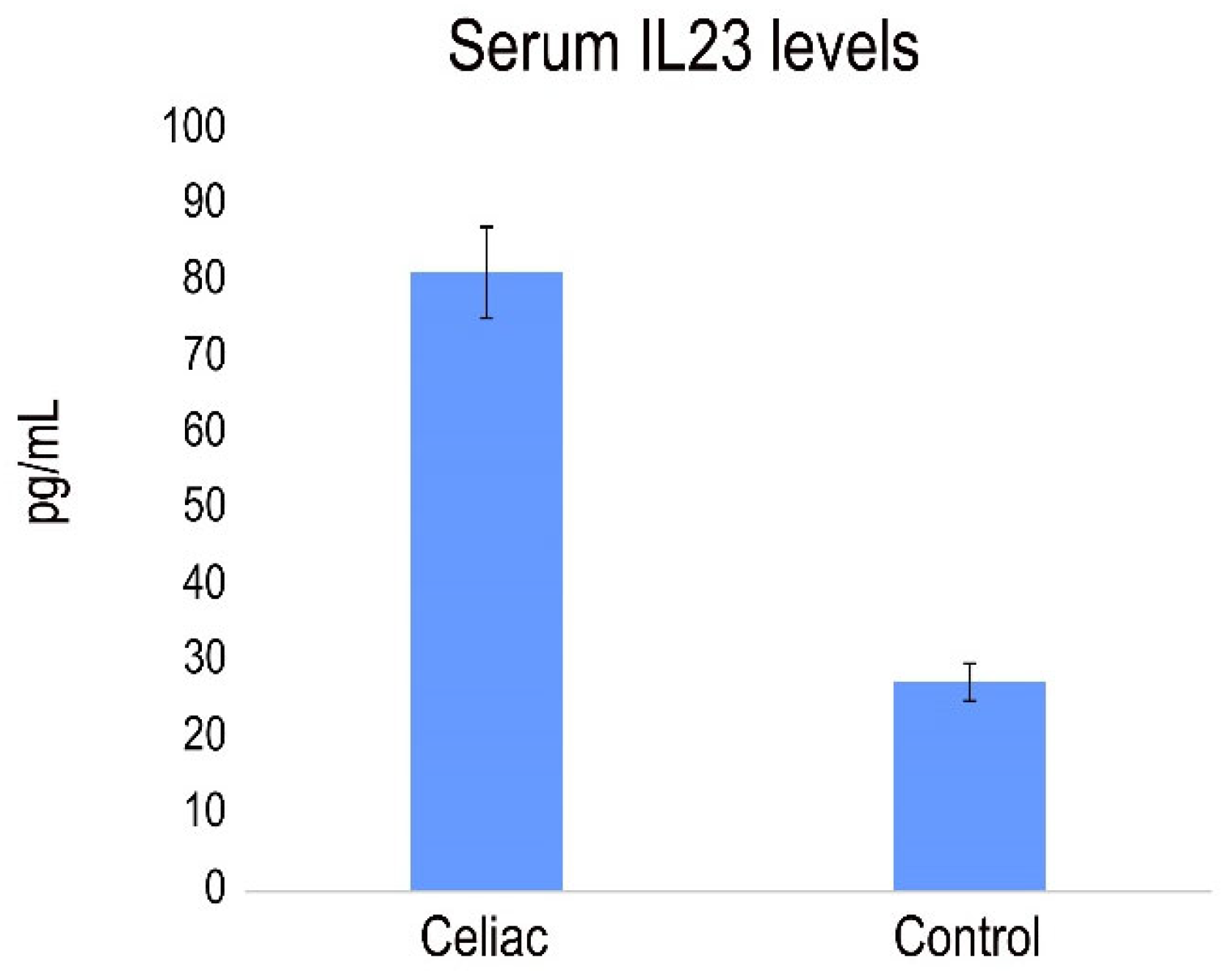
Previously, a role for IL-23-mediated inflammation in the pathogenesis of CD was demonstrated, showing that pepsin-A/trypsin digest of gliadin (PTG) stimulates the production of IL-23 induces in PBMC from CD patients [
31] We analyze the presence of IL-23 in serum from CD patients with active disease. The results revealed a pronounced and statistically significant increase in serum levels of IL-23 in celiac patients compared to compared to healthy individuals (
Figure 6).
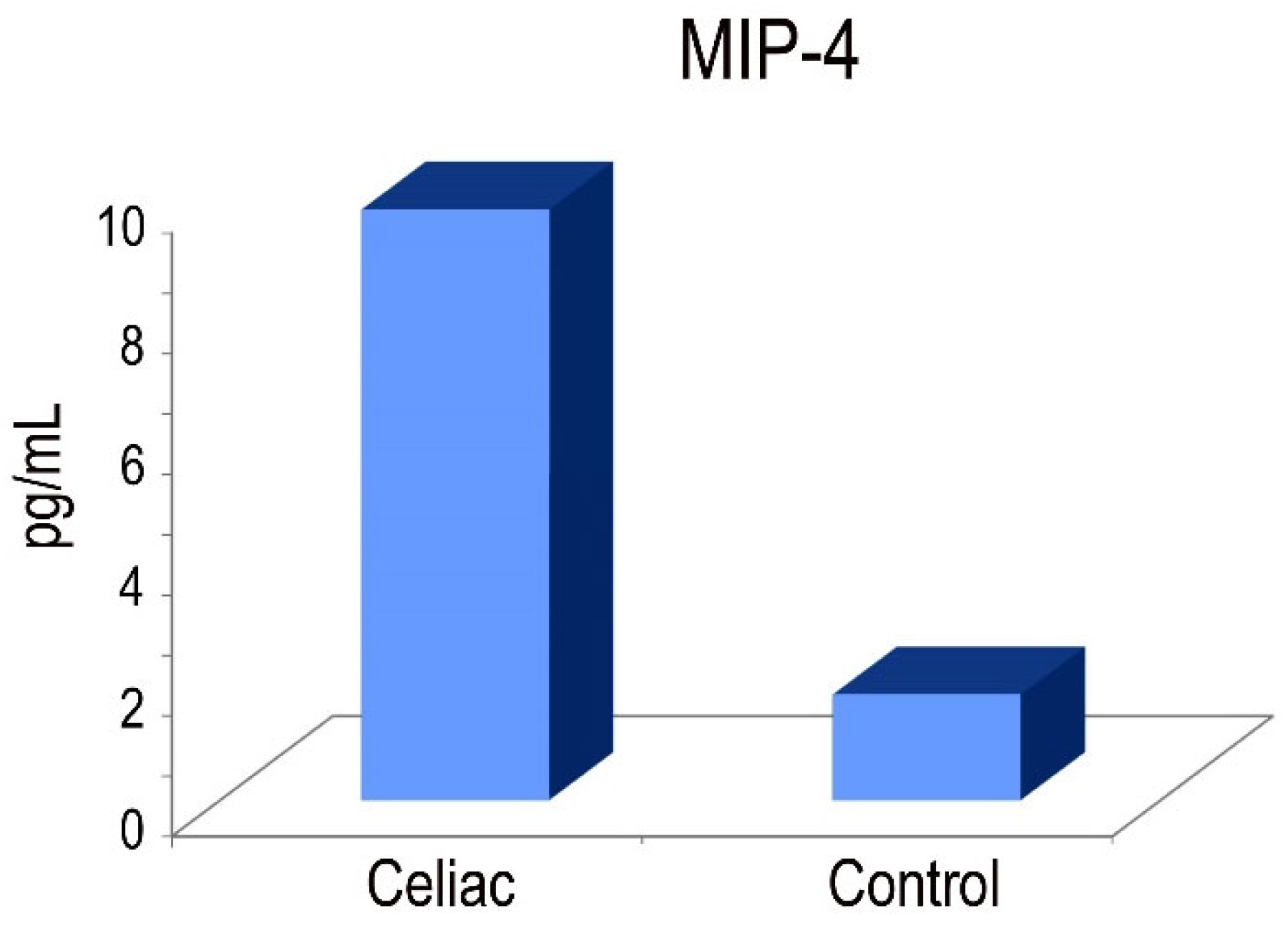
2.6. MIP-4 protein in CD
An in vitro enzyme-linked immunosorbent assay for the quantitative measurement of human MIP4 levels in serum was used. The MIP-4 protein is involved in adaptive immunity due to its capability to attract both CD4+ and CD8+ T lymphocytes [
32]. Our results demonstrated that macrophages derived from peripheral blood monocytes exhibited elevated levels of MIP-4 protein expression in CD patients (
Figure 7).
2.1. PI3 Genotype and Frequencies
The allele and genotype frequencies of SNPs rs1733103, rs41282752, and rs2664581 are shown in
Table 1. Notably, in this study, the observed genotype frequencies in the control and CD groups were consistent with the Hardy-Weinberg equilibrium.
For the SNP rs1733103, the CC genotype frequency was notably higher in healthy patients compared to celiac patients, where the CT genotype proportion was more prevalent. Statistical analysis using the Pearson's chi-square analysis revealed a significant association between the SNP variables and the study group (p < 0.05). The strength of this association, measured by Cramer's V, was moderate (0.274). Regarding the genotype association model with the disease risk compared to the reference genotype (CC) between the control and celiac groups, the codominant CT model represented a 2.71-fold increased risk of developing the disease compared to individuals without this genotype, with a statistically significant value (p < 0.05) and a goodness of fit (AIC) of 126.6 (
Table 2). The dominant CT/TT model represented a 2.00-fold increased risk compared to the reference genotype, with a statistically significant value (p < 0.05) and an AIC of 127.3, while the overdominant C/T model represented a 2.60-fold increased risk compared to the reference genotype, with a statistically significant value (p < 0.05) and an AIC of 125.4.
For the SNP rs41282752, the GG genotype was more prevalent in the celiac patient group, while the GA genotype was predominant in healthy patients (
Table 2). The chi-square analysis indicated no significant relationship between the polymorphism variables and the patient type (p > 0.05). Similarly, the Fisher's exact test also did not yield statistical significance (0.297). Furthermore, no significant associations were found between the patients' gender and predisposition to CD. Additionally, in the disease development association study, no genotypically representative risk models were identified.
Lastly, regarding the SNP rs2664581, Pearson's chi-square test revealed no significant relationship between the polymorphism variables and the patient type (p > 0.05) (
Table 2). Cramer's V statistic also did not demonstrate a statistically significant degree of association (0.160). In the analysis of the polymorphism's association with the disease, although models with the polymorphic allele demonstrated an increased risk—particularly the codominant C/A model, which showed a marginal susceptibility to disease risk with an odds ratio (OR) of 1.03 compared to the reference AA genotype—this susceptibility wasn't substantial enough to be considered a definitive risk model (p > 0.05). Furthermore, within the sample size selected for this study, no significant association was observed between the patients' gender and specific genotypes.
3. Discussion
Celiac disease is an autoimmune condition triggered by both genetic and environmental factors. It manifests through an abnormal immune response in the small intestine, resulting in chronic inflammation and several gastrointestinal and extra-intestinal symptoms [
1,
4]. Recent research has shed light on the role of immune checkpoints in the pathogenesis of CD, offering new perspectives on potential therapeutic strategies. The disruption of immune checkpoints in this disease leads to an abnormal immune response to gluten and chronic inflammation in the small intestine [
28].
Our previous research demonstrated altered signaling pathways involving the co-inhibitory PD-L1 and immunosuppressive IDO in CD patients compared to healthy individuals [
20,
21,
22]. So, elevated expression levels of PD-L1 and IDO on the surface of intestinal epithelial and lamina propria cells in patients with CD, contributing to the creation of an immunosuppressive environment.
The CD200/CD200R axis is considered an important immunological checkpoint, playing a pivotal role in maintaining immune tolerance and regulating cytokine release [
29]. In this study, we characterized, for the first time, the expression of the CD200/CD200R axis in CD using ELISA assay and immunohistochemistry techniques. Abnormalities were found in the expression of elements of the CD200/CD200R pathway in CD patients revealing an overexpression of both the ligand and the receptor when compared to healthy controls. Our findings demonstrated a significantly higher level of soluble CD200 and CD200R in the serum of CD patients as compared to healthy controls. We propose that the interaction between sCD200 and CD200R is critical for the downstream consequences of the CD200/CD200R axis of immunoregulation. Consistent with our results, CD200 expression is regulated by IFN-gamma in CD and is capable to induce CD200 expression, which, in turn, attempts to neutralize the inflammatory response.
The soluble forms of CD200 and CD200R are believed to play a significant role in CD by modulating the immune response and inflammation. The presence of soluble CD200 in the microenvironment is thought to assist in preserving immunological tolerance and restricting autoimmune damage at the intestinal level [
24]. Conversely, soluble CD200R forms, on the other hand, have the potential to compete with the membrane-bound CD200R in binding to its ligand CD200. This competition may lead to the reduction of inhibitory signaling, potentially prompting a heightened immune response and contributing to disease exacerbation by disrupting normal regulatory mechanism [
24].
Several studies have highlighted the significant role of macrophages in the development and/or progression of CD [
30]. Macrophages play a key role in the immune system, acting as a bridge between innate and adaptive immunity, and they are essential participants in the early events that drive CD pathogenesis [
30]. During intestinal inflammation, macrophages induce the secretion of pro-inflammatory cytokines such as IFN-γ from activated T cells. Chemokines play a central role in regulating hemopoietic cell movement both during the establishment of inflammation and immune responses [
33]. The MIP-4 protein is active on both CD4+ and CD8+ T cells in CD, suggesting that MIP-4 is involved in adaptive rather than innate immunity [
32]. The MIP-4 production could be up-regulated by environmental stimuli, such as gluten peptides, thereby prompting distinct functional phenotypes of alternatively activated macrophages.
Macrophages are considered as a primary source of IL23 in the intestine, potentially playing a crucial role in molecular communication with subsets of T cells and innate lymphoid cells within the intestinal environment. This study shows cells producing IL17 predominantly accumulate in the submucosa and muscularis propria of CD patients, and as a consequence elevated levels of IFN-γ and IL17 were observed in comparison to those in healthy controls.
In CD, IFN-γ, primarily secreted by T cells, has the capability to activate tissue transglutaminase 2 (TG2) via the phosphatidylinositol-3-kinase pathway. Conversely, IL-17 and IL-22 serve as regulators maintaining the integrity of the intestinal mucosa. In CD, the introduction of dietary gluten and the presence of positive TG2 autoantibodies can alter the capacity of intestinal CD4+ and CD8+ T lymphocytes to secrete these cytokines, thereby inducing structural changes in the intestinal tissue. Consequently, both IL-17 and IL-22 may contribute to the development of autoimmune diseases, such as rheumatoid arthritis [
34], inflammatory bowel disease [
35] and as observed in our study, in CD.
Elafin, an inducible and multifunctional peptide expressed by mucosal surfaces, including the intestinal epithelium, displays anti-bacterial and anti-inflammatory properties, playing a role in the innate defense during inflammatory events [
27]. Acting as an inhibitory protein of serine proteases in epithelial cells, Elafin serves a protective function by mitigating the effects of inflammatory process in immune responses. This includes neutralizing neutrophilic proteases and reducing uncontrolled tissue damage [
2].
Galipeau et al. [37] found lower levels of Elafin in patients with active CD, indicative of inflammation and damage to the small intestinal epithelium. Additionally, they described the Elafin´s capability to interact with gliadin peptides, impacting TG-2 involved in their deamidation. In our study, we observed that the expression of Elafin was restricted to the apical surface of the intestinal mucosa, in the lamina propria and on the surface of the Lieberkühn crypts.
We determined Elafin expression in serum of CD and healthy patients. The levels of serum Elafin did not exhibit positive correlations with clinical disease activity (Marsh). Soluble forms of Elafin can act as protease inhibitors in the extracellular environment, thereby limiting tissue degradation and reducing chronic inflammation. In CD, where the balance between proteases and their inhibitors is altered, soluble Elafin forms may help to counteract excessive inflammation and tissue damage.
In celiac patients, during the course of the disease, a robust anti-inflammatory response to gluten may occur. This response often leads to an uncontrolled production of Elafin in an attempt to mitigate inflammation. Paradoxically, this excessive production of Elafin can result in the recruitment of intraepithelial lymphocytes, contributing to the persistence of intestinal lesions. Elafin is primarily detected under inflammation conditions, suggesting its inducible nature. Consequently, numerous studies have shown that Elafin expression can be positively regulated in response to several proinflammatory stimuli such as lipopolysaccharide and several proinflammatory cytokines like IL-1β or TNF-α [38].
In this study, we determined the contribution of the PI3 polymorphism in a cohort of patients diagnosed with active CD and a control group without CD. Various SNPs were selected based on their location in critical regions responsible for the translation of the regulatory protein and its inhibitory function. These regions were chosen due to their usual resistance to alternative splicing. Given their presence in exons, significant coding regions, these SNPs underwent genotyping and subsequent analysis to determine their association with CD. The study revealed a notable correlation between the SNP rs1733103 and the risk of CD. The genotype containing the minor polymorphic allele T of rs1733103 is associated with an increased risk, with the overdominant model as the most suitable representation of this association, displaying superior goodness of fit and a lower AIC value. Thus, the presence of the polymorphic allele tentatively indicates an increased risk of altered or diminished activity of PI3, contributing to the excessive inflammatory activity in CD.
However, no significant consistency was observed in either of the other two studied polymorphisms. Nevertheless, we could maintain the hypothesis that variations in PI3 splicing might increase susceptibility to CD, considering the increased expression observed in celiac patients. Further expansion of the sample size is necessary to assert with greater confidence the non-association of these polymorphisms with the alteration of protein activity.
We acknowledge some limitations in our study. While we were able to delineate a series of association models involving the rs1733103 polymorphism significantly with CD, we did not observe a significant association between the rs2664581 and rs41282752 polymorphisms and their influence on CD pathogenesis. However, this lack of replication could be explained by the small sample size within both the cohort of CD patients and the healthy controls, which would require larger sample sizes to either exclude or confirm their contribution. The modification of function associated with alternative splicing variations in association with regulatory genes in autoimmune diseases have already been described [39]. Based on our preliminary results, it is plausible that the post-translational activity of PI3 could also be influenced by polymorphic activity in important coding regions of the gene.
4. Materials and Methods
4.1. Study Samples
A total of 96 participants took part in this study, comprising 58 celiac patients and 38 healthy individuals after excluding those with CD. Diagnosis was confirmed via serological tests for tissue transglutaminase (tTG) antibodies and specific HLA typing for CD. Additionally, biopsy of the small intestine was performed after gastrointestinal endoscopy to validate the diagnosis. Approval from the Ethics Committee was obtained before conducting the study.
4.2. Immunohistochemical staining
Paraffin sections were deparaffinized in xylene, followed by hydration through graded alcohols. Antibodies used in this study included: anti human receptor for the CD200/OX2 cell surface glycoprotein (Abcam, UK), anti-human CD200 (Abcam, UK), anti-human Elafin/ESI antibody contains 1 WAP domain, anti-human IL17 and anti-human IL-21. For antigen retrieval, sections were microwaved in 10 mM citrate buffer (pH 6.0) and subsequently cooled in phosphate-buffered saline (PBS). The section was then incubated with ab232941 at 0.1µg/mL for 15 mins at room temperature and then detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The sections were then counterstained with haematoxylin and mounted with DPX. To ensure specificity, a negative control section was included, in which the primary antibody was substituted by the corresponding isotype control antibody.
4.3. ELISA assays in serum from patients
The levels of soluble CD200, CD200R, and Elafin in serum of active celiac and non-celiac patients were determined using a commercial ELISA kit in accordance with the manufacturer’s instructions (Abcam). For each assay, the standards as well as the samples were tested in duplicate. The cytokine concentrations (pg/mL) were estimated for each assay. The sensitivity of the assays was determined to be >13 pg/mL for sCD200, 12 pg/mL for sCD200R, and 1 pg/mL for sElafin, respectively. The concentrations of sCD200, sCD200R, and sElafin were quantified from the optical density readings based on standard curves.
4.4. Analysis and expression of Th1 (IFNγ), Th17 (IL23) cytokine production
A commercial ELISA kit was used following the manufacturer's instructions (Thermo Scientific), with standards included on each plate. The sensitivity of the assay was <2 pg/mL. Antibodies employed in this study included anti human IL17 and anti-human IL22, both conjugate with FITC (FabGennix International, LSBio).
4.5. Elafin Genotype and Allele Frequencies
Genomic DNA was isolated from whole blood samples of the study subjects using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN). The genomic DNA was used as a template to generate two PCR products within the coding region of the PI3 gene. One product was 870 bp in length and contained two SNPs of interest (rs1733103 and rs41282752), while the other product was 463 bp and included the last SNP of interest for this study (rs2664581), corresponding to exons 1 and 2 of PI3, respectively. The used primers are listed in the
Table 1. PCR was performed using DreamTaq Green PCR Master Mix 2× (Thermo Fisher Scientific) with 50 ng of genomic DNA and 10 pmol of each primer in a total volume of 25 μL.
Table 3.
Primer sequences and fragments generated by PCR. The restriction endonuclease used for the analysis of each SNP is also indicated.
Table 3.
Primer sequences and fragments generated by PCR. The restriction endonuclease used for the analysis of each SNP is also indicated.
| Region |
Primer code |
Sequence |
PCR product
(bp) |
SNP |
Restriction
endonuclease |
| Exon 1 |
PI3-119-F |
CCCAGGTCCCTCCCAGAA |
850 |
rs1733103 |
BtsCI |
| PI3-969-R |
CCTTCCTCCACTCCAAGTCT |
rs41282752 |
BsrI |
| Exon 2 |
PI3-1080-F |
CTTCCCTACTCAGGCCATGG |
463 |
rs2664581 |
HpyCH4III |
| PI3-1523-R |
CGCTCAGCCTTCTTTTGTGT |
Figure 8.
(A–C) Genotyping of the polymorphisms (C/T), (G/A), (C/A) in the PI3 gene. (A) Schematic representation of the PCR-amplified fragment of 870 bp containing the SNP rs1733103. The presence of T in the SNP creates a recognition site for the BtsCI restriction endonuclease, resulting in the generation of three fragments of 130, 206, and 528 bp respectively. (B) Schematic representation of the PCR-amplified fragment of 870 bp containing the SNP rs41282752. The presence of A in the SNP creates an additional recognition site for the BsrI restriction endonuclease, resulting in the generation of four fragments of 137, 202, 250, and 281 bp. (C) Schematic representation of the PCR-amplified fragment of 463 bp containing the SNP rs2664581. The presence of A in the SNP creates an additional recognition site for the HpyCH4III restriction endonuclease, resulting in the generation of three fragments of 24, 130, and 309 bp. (D-F) Gel electrophoresis patterns of the PCR products digested with BtsCI, BsrI, and HpyCH4III, respectively, showing the different banding patterns obtained for rs1733103 (C/C, C/T, T/T), rs41282752 (G/G, G/A, A/A), rs2664581 (C/C, C/A, A/A).
Figure 8.
(A–C) Genotyping of the polymorphisms (C/T), (G/A), (C/A) in the PI3 gene. (A) Schematic representation of the PCR-amplified fragment of 870 bp containing the SNP rs1733103. The presence of T in the SNP creates a recognition site for the BtsCI restriction endonuclease, resulting in the generation of three fragments of 130, 206, and 528 bp respectively. (B) Schematic representation of the PCR-amplified fragment of 870 bp containing the SNP rs41282752. The presence of A in the SNP creates an additional recognition site for the BsrI restriction endonuclease, resulting in the generation of four fragments of 137, 202, 250, and 281 bp. (C) Schematic representation of the PCR-amplified fragment of 463 bp containing the SNP rs2664581. The presence of A in the SNP creates an additional recognition site for the HpyCH4III restriction endonuclease, resulting in the generation of three fragments of 24, 130, and 309 bp. (D-F) Gel electrophoresis patterns of the PCR products digested with BtsCI, BsrI, and HpyCH4III, respectively, showing the different banding patterns obtained for rs1733103 (C/C, C/T, T/T), rs41282752 (G/G, G/A, A/A), rs2664581 (C/C, C/A, A/A).
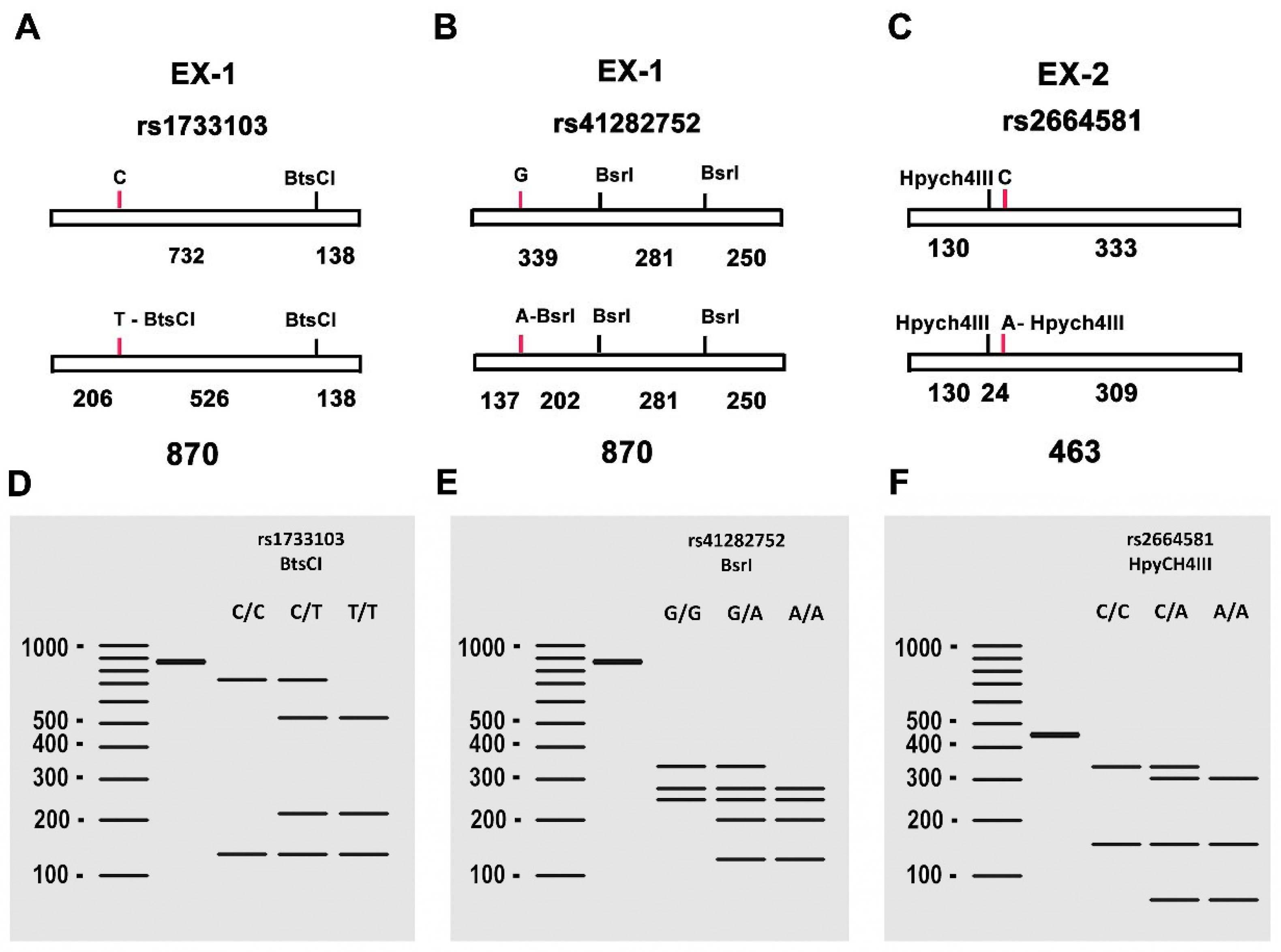
The PCR cycling profile consisted of an initial denaturation at 94°C (2 min), followed by 40 cycles of denaturation at 92°C (30 s), annealing at 68°C (30 s), and extension at 72°C (1 min). A final extension step was performed at 72°C for 5 min. Aliquots of 5 μL of the PCR product were analyzed by agarose gel electrophoresis (1%) to confirm the correct DNA amplification.
genotyping Different aliquots of 20 μL from the PCR reaction product were used for RFLP analysis to genotype the SNPs. For rs1733103 genotyping, 5 units of
BtsCI (New England Biolabs, UK) were used in a 40 μL reaction mixture, with incubation performed at 37°C. For rs41282752 genotyping, 5 units of
BsrI (New England Biolabs, UK) were used in a 40 μL reaction mixture, with incubation at 65°C. Finally, for SNP rs2664581genotyping, 20 μL of the PCR product fragment, along with 5 units of
HpyCH4III (New England Biolabs, UK), were incorporated into a 40 μl for digestion, followed by incubation at 37°C. The results of the digestions (
Figure 1) were visualized by electrophoresis on a 4% agarose gel in 0.5x TBE. Genotyping was performed based on the distinct molecular weight banding pattern obtained in each sample.
4.6. Statistical Analysis
Statistical analysis of the data was performed using Statgraphics software (StatPoint Technologies, Warrenton, VA, USA). The chi-square test was used for the comparison of qualitative data. The risk association analysis between the polymorphisms and CD was carried out using the SNPstats web tool (28), where the Hardy-Weinberg equilibrium was tested.
5. Conclusions
In CD, the dysregulation or compromise of the immune checkpoints contributes to inflammation and the autoimmunity process. CD200 inhibits the immune response by limiting the immune cells activation. While IDO regulates immune tolerance by catabolizing tryptophan. PD1 is a key protein that prevents excessive activation of T lymphocytes. Meanwhile, Elafin modulates the inflammatory response. The study of these checkpoints will led to the development of targeted therapies aimed at restoring immunological balance in CD. High level of soluble isoforms, such as PD1, Elafin, CD200 are observed in CD and their levels will indicate disease activity and they might be useful in disease monitoring. Soluble isoforms can also be natural competitive inhibitors to membrane-bound isoforms. Therefore, the former block downstream signaling pathways and are potential therapeutic targets.
Our study suggests that alternative splicing of PI3 might represent another piece in the intricate pathophysiology present CD patients. We hypothesize a potential differential regulation of PI3 splicing variants in CD. We hypothesize a potential differential regulation of PI3 splicing variants in CD. Aberrant splicing might result in elevated levels of defective PI3 protein, which fails to adequately counteract the detrimental effects of cytokines, thus triggering tissue damage associated with autoimmunity. The association study of genotypes derived from the allelic variants of rs1733103 revealed a significant association with increased CD risk. Conversely, we did not observe a notable risk association between the allelic variants rs41282752 SNPs and CD. Although the allelic variants of rs2664581 (C) showed an increased CD risk, statistical significance was not reached. Due to the sample size, the distribution of different genotypes presented in both CD patients and healthy controls did not provide the significant evidences to conclude that these polymorphisms were associated with CD and posed a risk for the disease. Although our findings were inconclusive, there appears to be a trend indicating active polymorphic activity in specific gene regions associated with CD, warranting further investigation in cohorts with larger samples.
In summary, our results corroborate the association between SNPs rs173310, rs2664581 and an elevated risk of CD, thereby supporting the role of PI3 in CD development. SNPs in the PI3 locus may potentially act synergistically, regulating PI3 gene expression and influencing pre-elafin biological functions.
Author Contributions
Conceptualization: PL, MALC, TP, and MIT. Methodology: CPL, PL, MALC, PM, TP, and MIT. For-mal analysis: CPL, PL, MALC, PM, TP, and MIT. Resources: MIT, and PL. Writing—original draft preparation: MIT and PL. Writing—review and editing: CPL, PL, MALC, PM, TP, and MIT. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Universidad de Jaén (through the program “Plan de Apoyo a la Investigación 2021-2022, Acción 1”).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved. The local Ethics Committee of the Hospital “Virgen de las Nieves” (Granada, Spain) approved the study protocol.
Informed Consent Statement
Written consent was obtained from parents or legal guardians of children involved.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- López-Casado, M.Á.; Lorite, P.; Ponce de León, C.; Palomeque, T.; Torres, M.I. Celiac Disease Autoimmunity. Arch. Immunol. Ther. Exp. (Warsz) 2018, 66, 423–430. [CrossRef]
- Sollid, L.M; Jabri, B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat. Rev. Immunol. 2013, 13:294–302. https://doi.org/10.1038/nri3407. 3 Torres MI, Palomeque T, Lorite P. Celiac disease and other autoimmune disorders. In: Chatzidionysiou K (ed) Autoimmunity-pathogenesis, clinical aspects and therapy of specific autoimmune diseases. Intech, Croatia, 2015; pp 131–151.
- Torres, M.I.; Palomeque, T.; Lorite, P. Celiac disease and other autoimmune disorders. In: Chatzidionysiou K (ed) Autoimmunity-pathogenesis, clinical aspects and therapy of specific autoimmune diseases. Intech. 2015, 131–151. [CrossRef]
- Fasano, A.; Catassi, C. Clinical practice. Celiac disease. N. Engl. J. Med. 2012, 367, 2419-2426. [CrossRef]
- Sollid, L.M. Molecular basis of celiac disease. Annu. Rev. Immunol. 2000, 18, 53–81. [CrossRef]
- Alaedini, A.; Green, P.H. Autoantibodies in celiac disease. Autoimmunity 2008, 41, 19–26. [CrossRef]
- Kagnoff, M.F. Celiac disease: pathogenesis of a model immunogenetic disease. J. Clin. Invest. 2007, 117, 41–49. https://doi.org /10.1172/JCI30253.
- Abadie, V.; Sollid, L.M.; Barreiro, L.B.; Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011, 29, 493–525. [CrossRef]
- Di Sabatino, A.; Vanoli, A.; Giuffrida, P.; Luinetti, O.; Solcia, E.; Corazza, GR. The function of tissue transglutaminase in celiac disease. Autoimmun. Rev. 2012, 11, 746–753. [CrossRef]
- Lindfors, K.; Mäki, M.; Kaukinen, K. Transglutaminase 2-targeted autoantibodies in celiac disease: pathogenetic players in addition to diagnostic tools? Autoimmun. Rev. 2010, 9, 744–749. [CrossRef]
- Fleckenstein, B.; Molberg, Ø.; Qiao, S. W.; Schmid, D. G.; Von der Mülbe, F.; Elgstøen, K.; Jung, G.; Sollid, L. M. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J. Biol. Chem. 2002, 277, 34109–34116. [CrossRef]
- Arentz-Hansen, H.; Körner, R.; Molberg, O.; Quarsten, H.; Vader, W.; Kooy, Y. M.; Lundin, K. E.; Koning, F.; Roepstorff, P.; Sollid, L. M.; McAdam, S. N. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 2000, 191, 603–612. [CrossRef]
- Bodd, M.; Kim, C. Y.; Lundin, K. E.; Sollid, L. M. T-cell response to gluten in patients with HLA-DQ2.2 reveals requirement of peptide-MHC stability in celiac disease. Gastroenterology. 2012, 142, 552–561. [CrossRef]
- Lundin, K.E.; Scott, H.; Hansen, T.; Paulsen, G.; Halstensen, T.S., Fausa, O.; Thorsby, E.; Sollid, L.M. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 1993, 178, 187–196. [CrossRef]
- Megiorni, F.; Pizzuti, A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J. Biomed. Sci. 2012, 19, 88-94. [CrossRef]
- Fallang, L. E.; Bergseng, E.; Hotta, K.; Berg-Larsen, A.; Kim, C. Y.; Sollid, L. M. Differences in the risk of celiac disease associated with HLA-DQ2.5 or HLA-DQ2.2 are related to sustained gluten antigen presentation. Nat. Immunol. 2009, 10, 1096–1101. [CrossRef]
- Kumar, V.; Wijmenga, C.; Withoff, S. From genome-wide association studies to disease mechanisms: celiac disease as a model for autoimmune diseases. Semin. Immunopathol. 2012, 34, 567–580. [CrossRef]
- Zhernakova, A.; Stahl, E. A.; Trynka, G.; Raychaudhuri, S.; Festen, E. A.; Franke, L.; Westra, H. J.; Fehrmann, R. S.; Kurreeman, F. A., Thomson, B.; Gupta, N.; Romanos, J.; McManus, R.; Ryan, A. W.; Turner, G.; Brouwer, E.; Posthumus, M. D.; Remmers, E. F.; Tucci, F.; Toes, R.; … Plenge, R. M. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011, 7, e1002004. [CrossRef]
- Hunt, K. A.; Zhernakova, A.; Turner, G.; Heap, G. A.; Franke, L.; Bruinenberg, M.; Romanos, J.; Dinesen, L. C.; Ryan, A. W.; Panesar, D.; Gwilliam, R.; Takeuchi, F.; McLaren, W. M.; Holmes, G. K.; Howdle, P. D.; Walters, J. R.; Sanders, D. S.; Playford, R. J.; Trynka, G.; Mulder, C. J.; … van Heel, D. A. Newly identified genetic risk variants for celiac disease related to the immune response. Nat. Genet. 2008, 40, 395–402. [CrossRef]
- Ponce de León, C.; Lorite, P.; López-Casado, M.Á.; Barro, F.; Palomeque, T.; Torres, M.I. Significance of PD1 Alternative Splicing in Celiac Disease as a Novel Source for Diagnostic and Therapeutic Target. Front. Immunol. 2021, 12, 678400. [CrossRef]
- Ponce de León, C.; López-Casado, M. A.; Lorite, P.; Palomeque, T.; Torres, M.I. Dysregulation of the PD-1/PD-L1 pathway contributes to the pathogenesis of celiac disease. Cell. Mol. Immunol. 2019,16, 777-779. [CrossRef]
- Torres, M.I.; López-Casado, M.A.; Lorite, P.; Ríos, A. Tryptophan metabolism and indoleamine 2,3-dioxygenase expression in coeliac disease. Clin. Exp. Immunol. 2007, 148, 419-24. [CrossRef]
- Gorczynski, R.M. CD200 and its receptors as targets for immunoregulation. Curr. Opin. Investig. Drugs. 2005, 65, 483–488.
- Kotwica-Mojzych, K.; Jodłowska-Jędrych, B.; Mojzych, M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int. J. Mol. Sci. 2021, 5, 1602. [CrossRef]
- Koning, N.; van Eijk, M.; Pouwels, W.; Brouwer, M. S.; Voehringer, D.; Huitinga, I.; Hoek, R. M.; Raes, G.; Hamann, J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J. Innat. Immunol. 2010, 2, 195–200. [CrossRef]
- Hatherley, D.; Barclay, A.N. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur J Immunol 2004, 34, 1688–1694.
- Shaw, L.; Wiedow, O. Therapeutic potential of human elafin. Biochem. Soc. Trans. 2011, 39, 1450-1454. [CrossRef]
- Torres, M.I.; López Casado, M.A.; Palomeque, T.; Lorite, P. Immune Checkpoints as a Novel Source for Diagnostic and Therapeutic Target in Celiac Disease. Celiac Disease. IntechOpen. 2021, 1-17. [CrossRef]
- Chen, D.X.; He, H.; Gorczynski, R.M. Synthetic peptides from the N terminal regions of CD200 and CD200R1 modulate immunosuppressive and anti-inflammatory effects of CD200-CD200R1 interaction. Int. Immunol. 2005, 17, 289–296. https://doi.org /10.1093 /intimm/dxh208.
- Molaaghaee-Rouzbahani, S.; Asri, N.; Jahani-Sherafat, S.; Amani, D.; Masotti, A.; Baghaei, K.; Yadegar, A.; Mirjalali, H.; Rostami-Nejad, M. The modulation of macrophage subsets in celiac disease pathogenesis. Immun. Inflamm. Dis. 2022, 10, e741.
- Harris, K. M.; Fasano, A.; Mann, D.L. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: Implications for celiac disease. Clinic Immunol, 2010, 135, 430-439. [CrossRef]
- Schutyser, E.; Richmond, A.; Van Damme, J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J. Leukoc. Biol. 2005,78,14-26. [CrossRef]
- Serena, G.; Huynh, D.; Lima, R. S.; Vise, L. M.; Freire, R.; Ingano, L.; Leonard, M. M.; Senger, S.; Fasano, A. Intestinal Epithelium Modulates Macrophage Response to Gliadin in Celiac Disease. Front. Nutr. 2019, 5, 167-173. [CrossRef]
- Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 11, 415–429. [CrossRef]
- Sarra, M.; Pallone, F.; Macdonald, T. T.; Monteleone, G. IL-23/IL-17 axis in IBD. Inflamm. Bowel. Dis. 2010, 16, 1808–1813. [CrossRef]
- Guyot, N.; Zani, M. L.; Maurel, M. C.; Dallet-Choisy, S.; Moreau, T. Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase. Biochemistry. 2005, 44,15610–15618. [CrossRef]
- Galipeau, H. J.; Wiepjes, M.; Motta, J. P.; Schulz, J. D.; Jury, J.; Natividad, J. M.; Pinto-Sanchez, I.; Sinclair, D.; Rousset, P.; Martin-Rosique, R.; Bermudez-Humaran, L.; Leroux, J. C.; Murray, J. A.; Smecuol, E.; Bai, J. C.; Vergnolle, N.;Langella, P.; Verdu, E. F. Novel role of the serine protease inhibitor elafin in gluten-related disorders. Am. J. Gastroenterol. 2014, 109, 748–756. [CrossRef]
- Zhang, W.; Teng, G.; Wu, T.; Tian, Y.; Wang, H. Expression and clinical significance of elafin in inflammatory bowel disease. Inflamm. Bowel. Dis. 2017, 23, 2134–2141. [CrossRef]
- Ren, P.; Lu, L.; Cai, S.; Chen, J.; Lin, W.; Han, F. Alternative Splicing: A New Cause and Potential Therapeutic Target in Autoimmune Disease. Front. Immunol. 2021, 12, 713540. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).