Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals, reagents, and culture media
2.2. Microorganisms
2.3. In vitro biocontrol assay of diffusible organic compounds
2.4. In vitro biocontrol assay of volatile organic compounds
2.5. In vivo biocontrol assay
2.6. Statistical analysis
3. Results
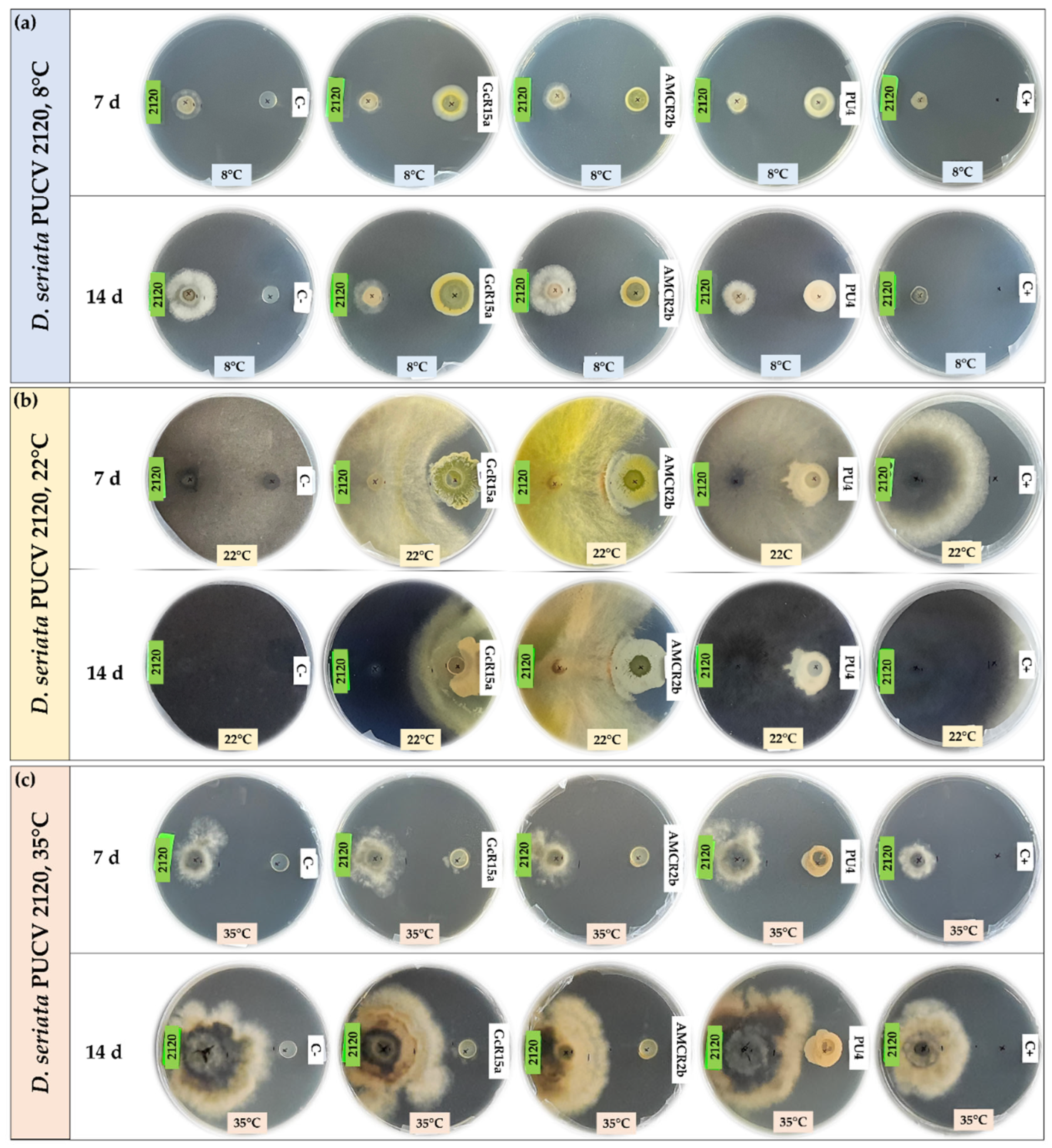
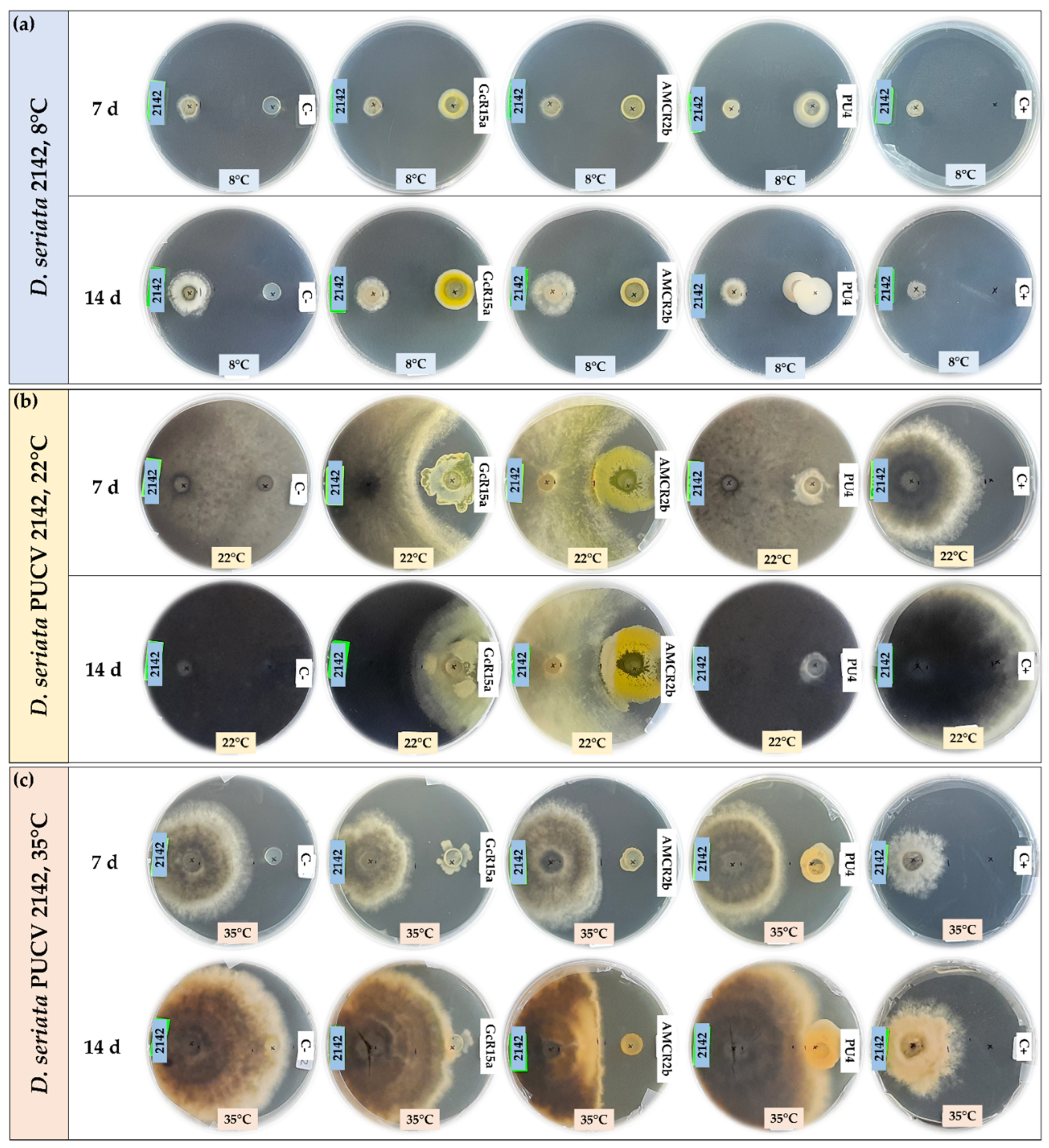
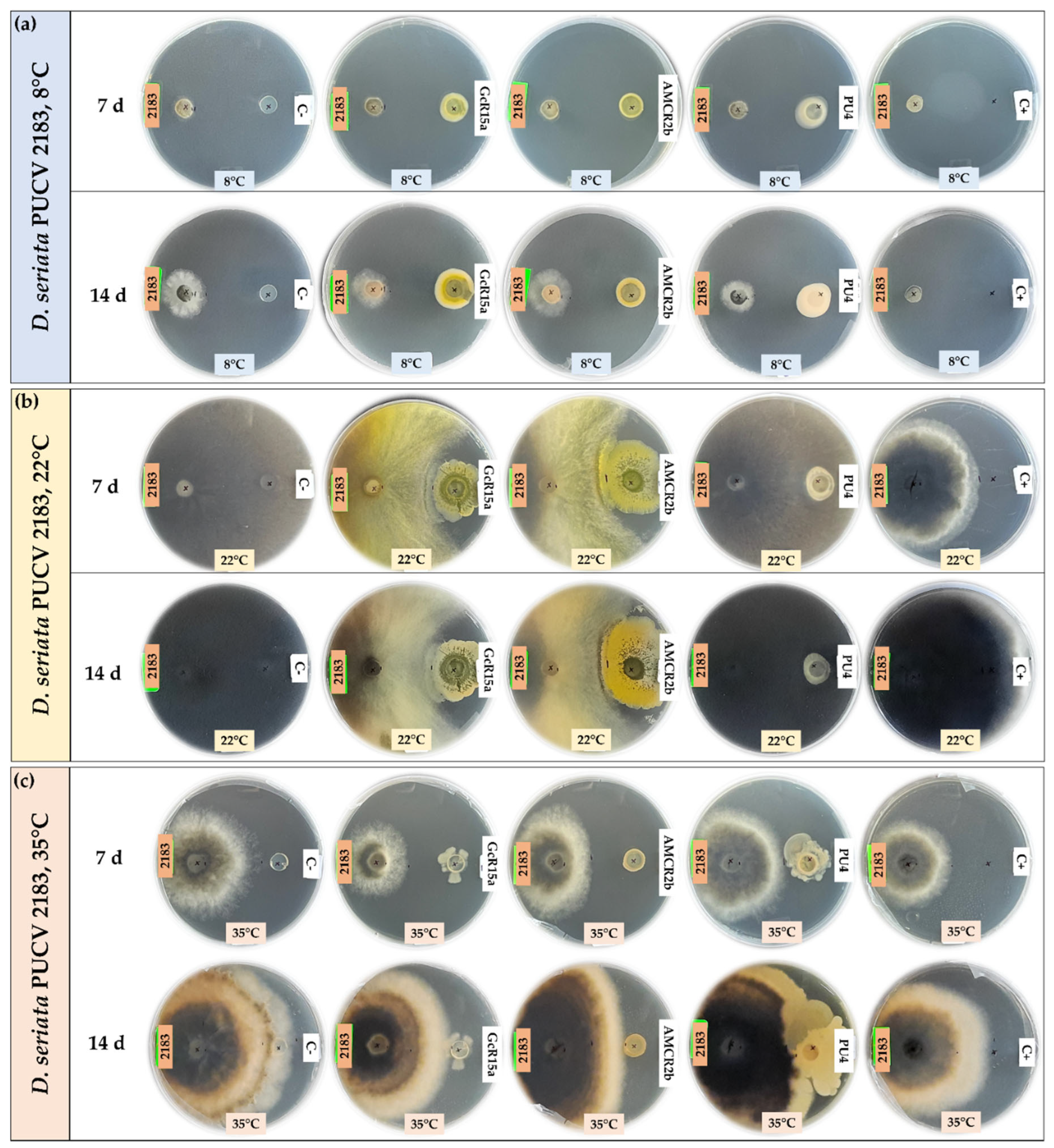
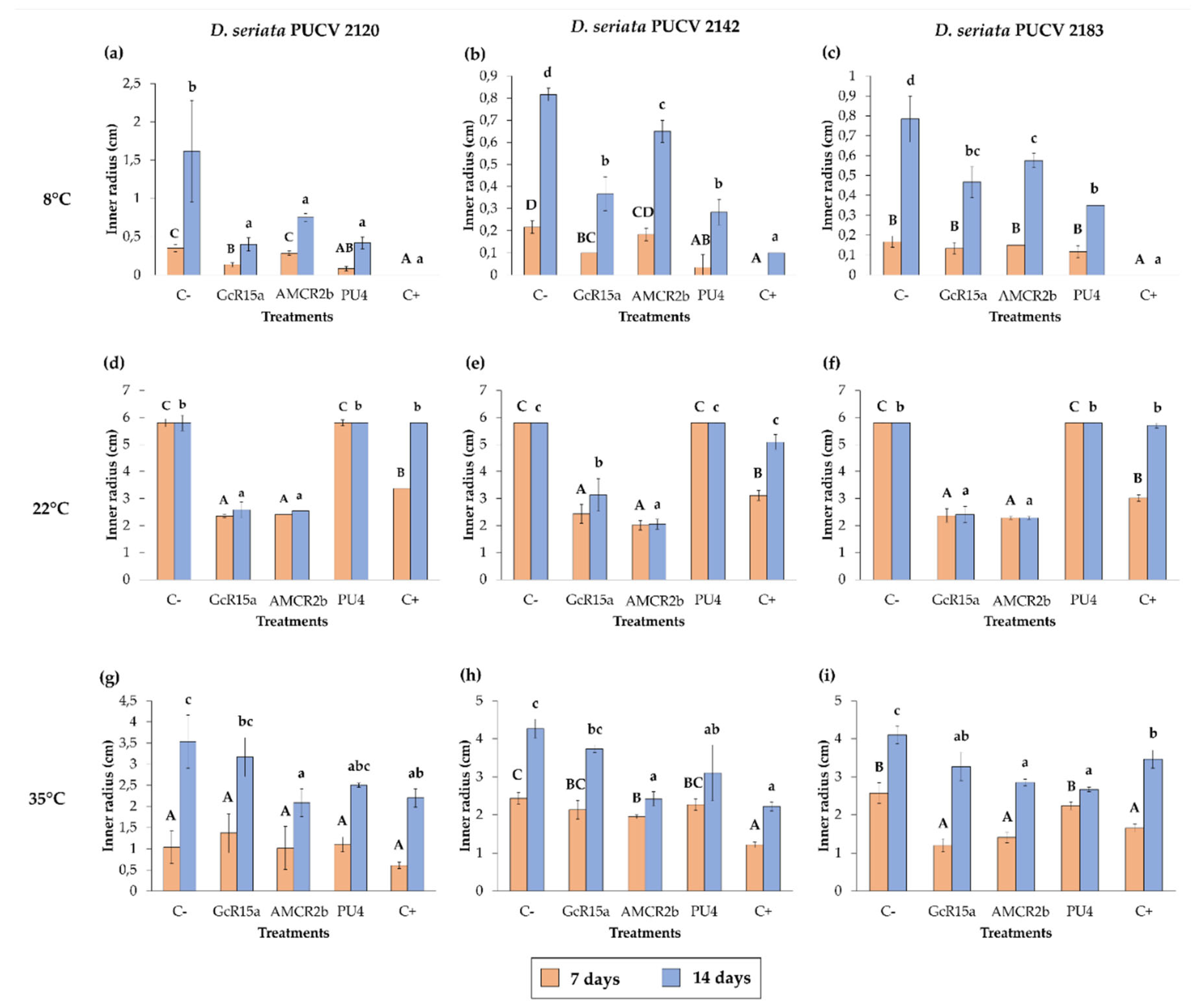
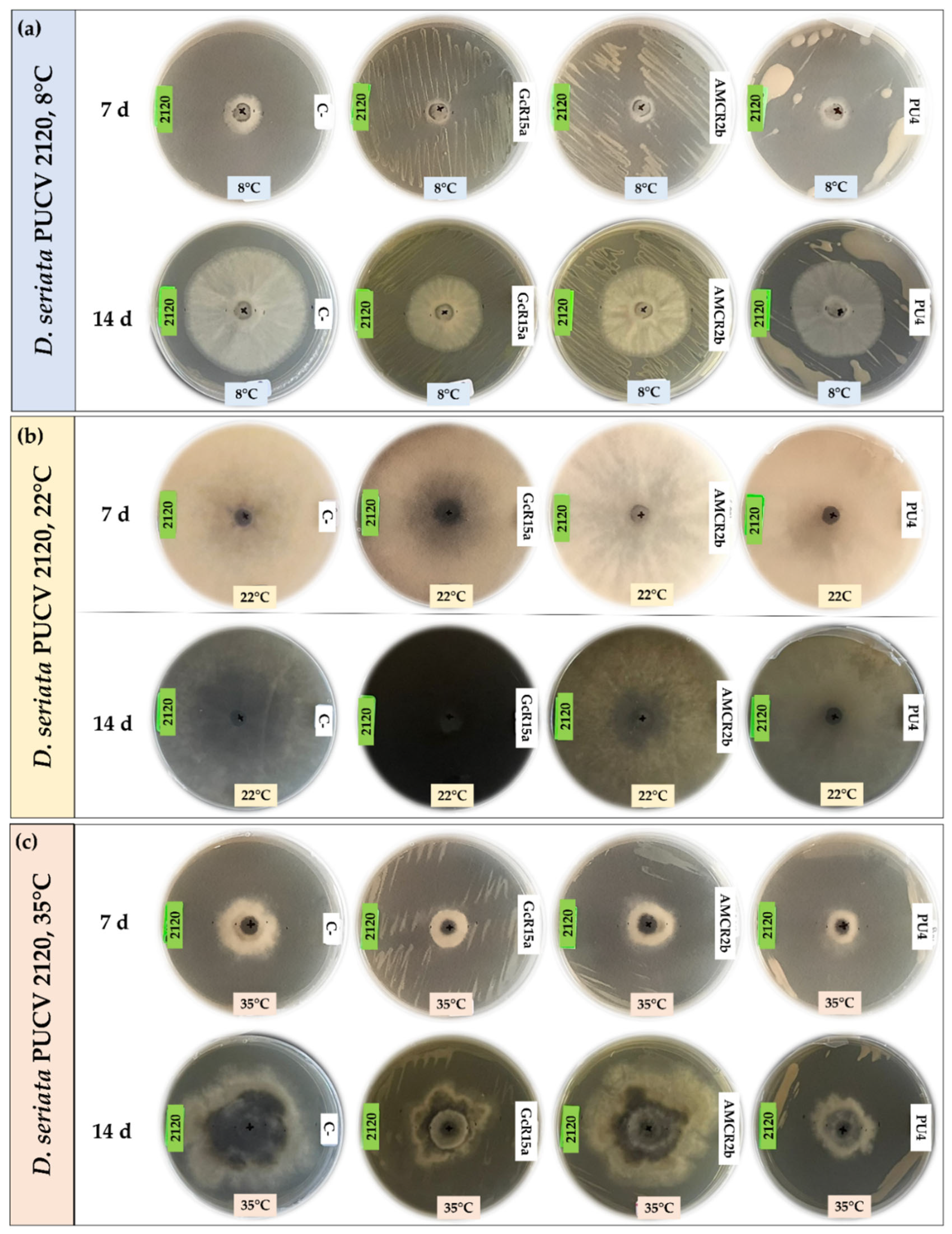
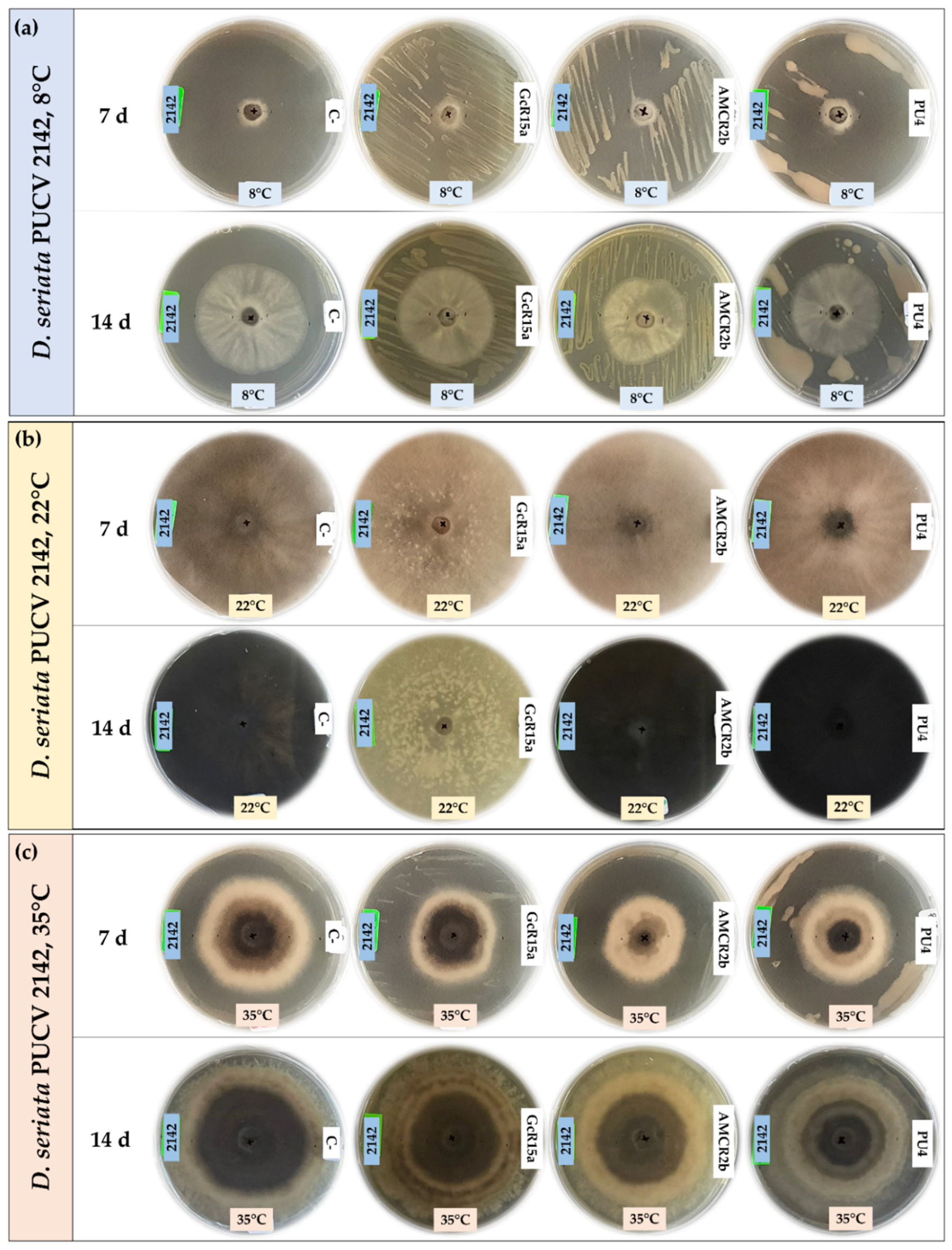
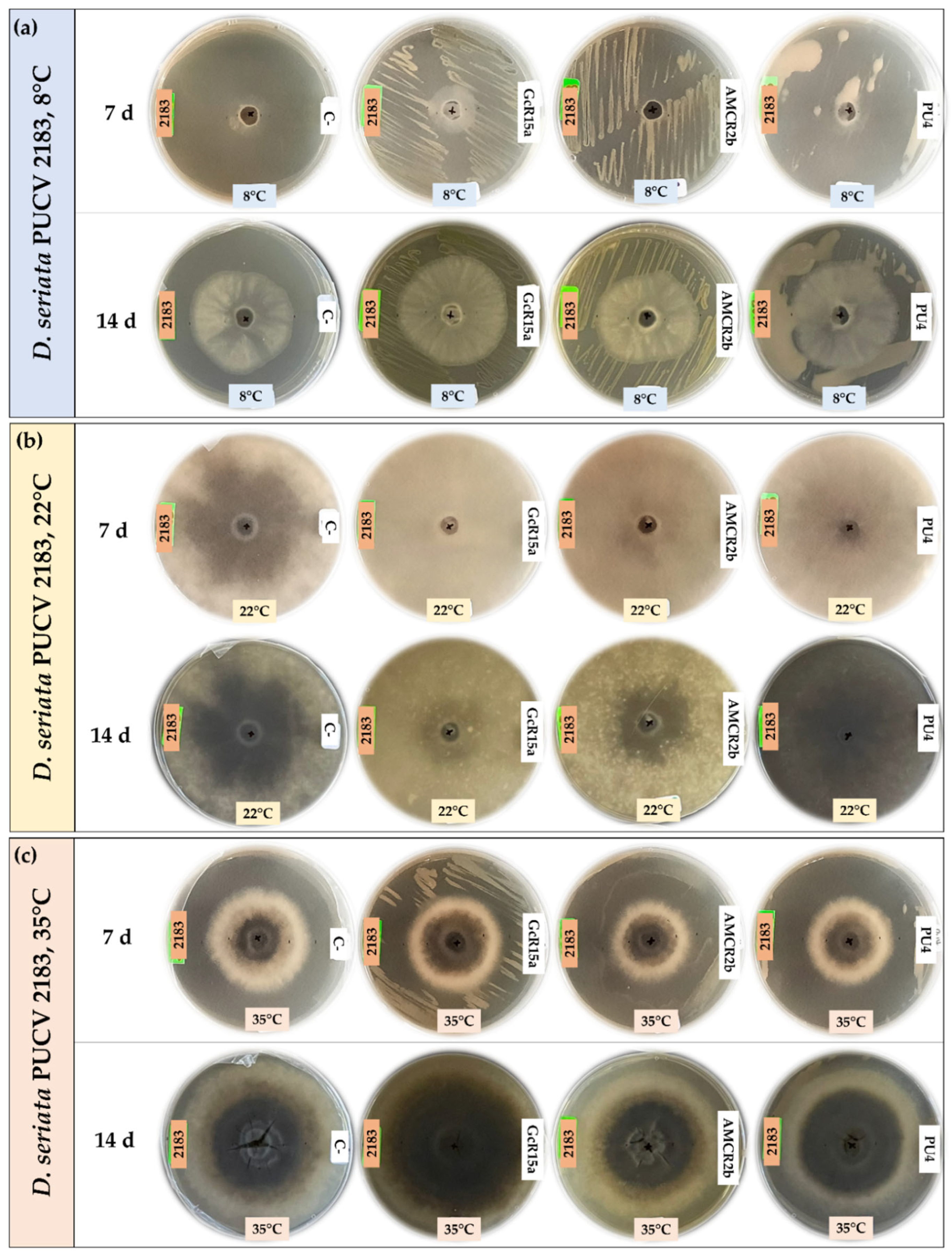
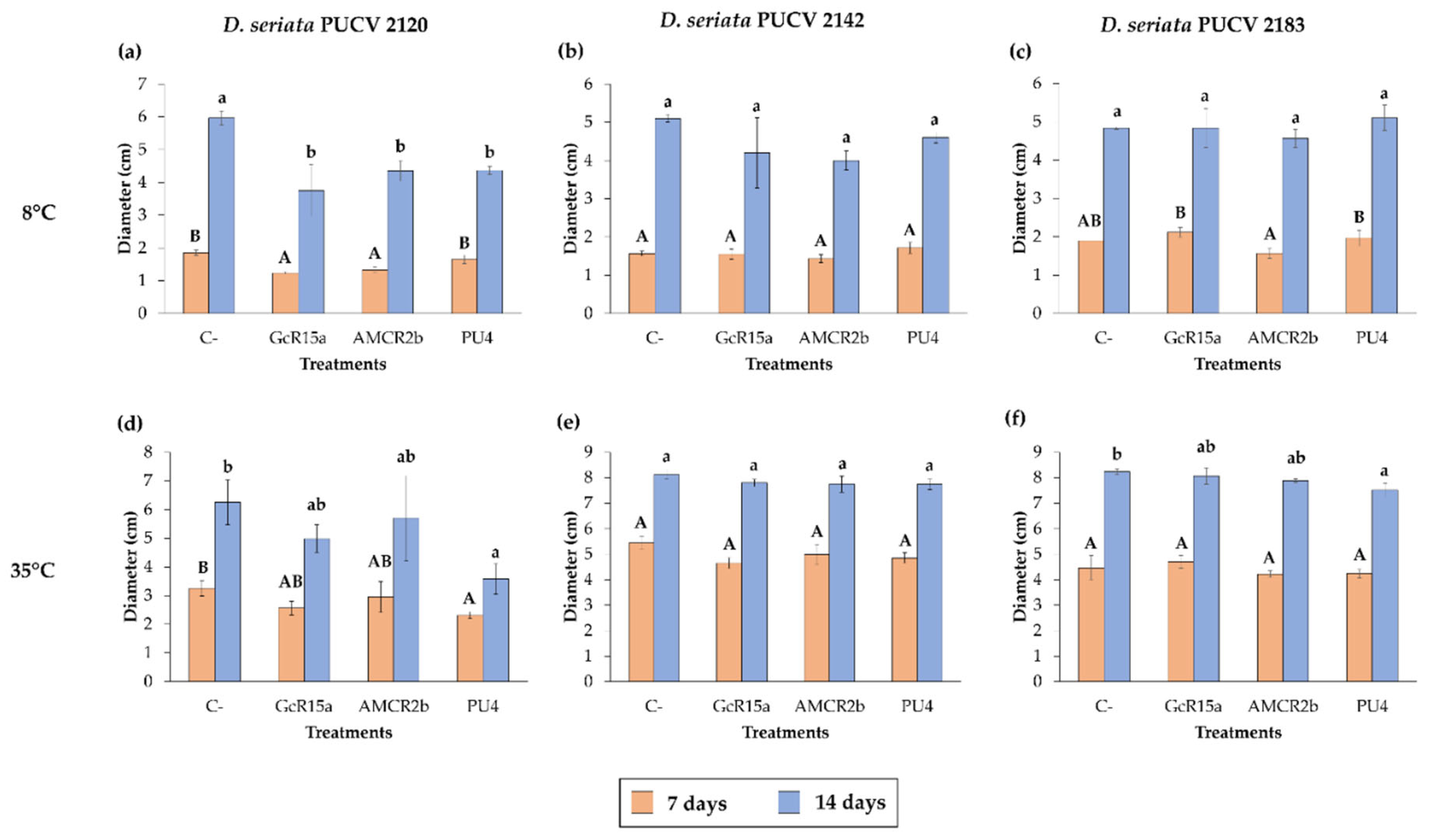
3.1. In vitro biocontrol by diffusible organic compounds
3.2. In vitro biocontrol by volatile organic compounds
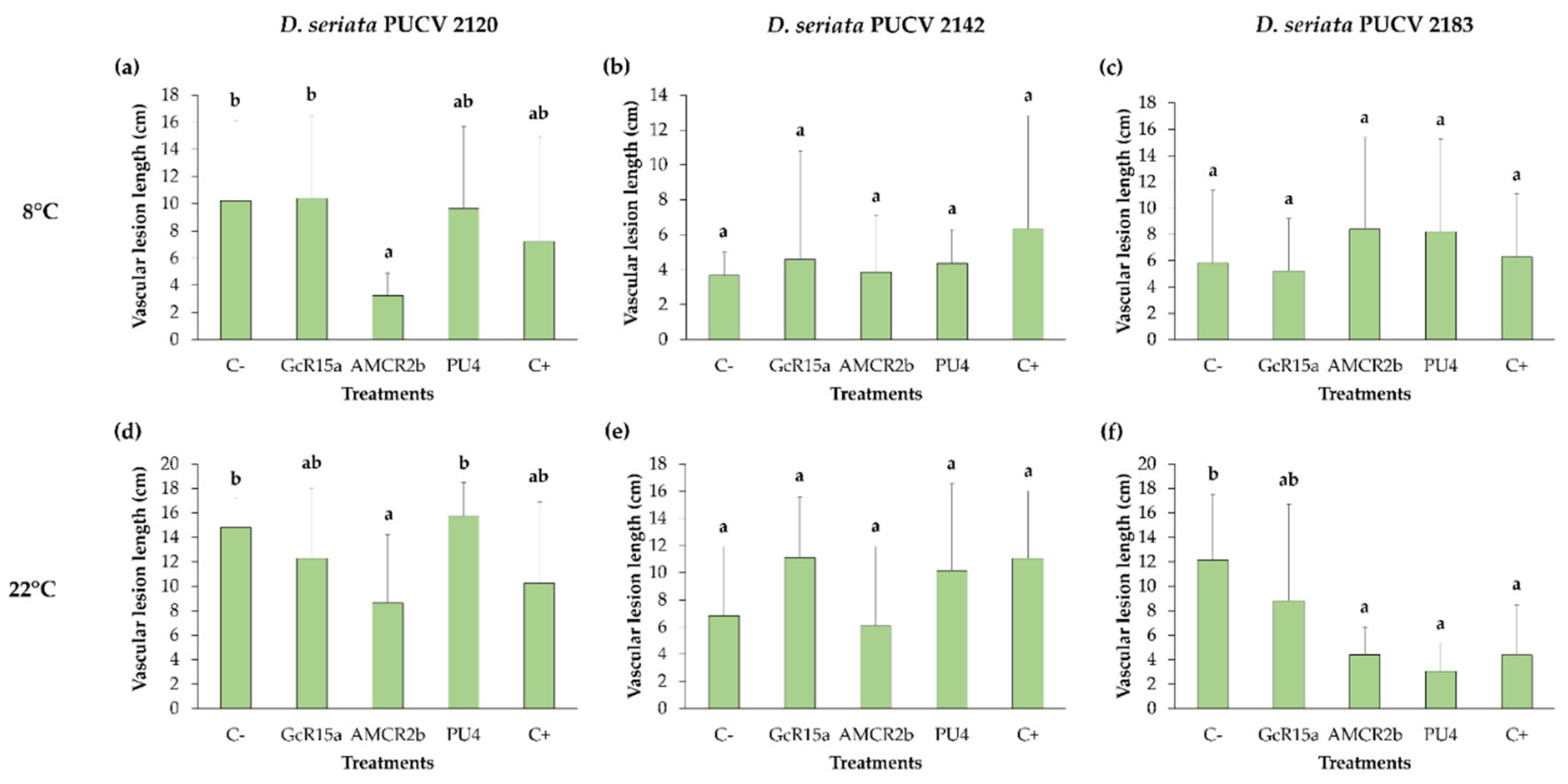
3.3. Biocontrol on grapevine cuttings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sargolzaei, M.; Rustioni, L.; Cola, G.; Ricciardi, V.; Bianco, P. A.; Maghradze, D.; Failla, O.; Quaglino, F.; Toffolatti, S. L.; de Lorenzis, G. Georgian grapevine cultivars: Ancient biodiversity for future viticulture. Front. Plant Sci. 2021, 12, 630122. [CrossRef]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; El Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlali, R.; Barka, E. A. A panoramic view on Grapevine Trunk Diseases threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. J. Fungi 2022, 8(6), 595. [CrossRef]
- Kovács, C.; Sándor, E. The increasing importance of grapevine trunk diseases. Int. J. Hortic. Sci. 2016, 22(3-4), 21–30. [CrossRef]
- Larach, A.; Torres, C.; Riquelme, N.; Valenzuela, M.; Salgado, E.; Seeger, M.; Besoain, X. Yield loss estimation and pathogen identification from Botryosphaeria dieback in vineyards of Central Chile over two growing seasons. Phytopathol. Mediterr. 2020, 59(3), 537–548.
- Lade, S. B.; Štraus, D.; Oliva, J. Variation in fungal community in grapevine (Vitis vinifera) nursery stock depends on nursery, variety and rootstock. J. Fungi 2022, 8(1), 47.
- Larach, A.; Vega-Celedón, P.; Salgado, E.; Salinas, A.; Riquelme, N.; Castillo-Novales, D.; Sanhueza, P.; Seeger, M.; Besoain, X. Higher virulence of Diplodia seriata isolates on vines of cv. Cabernet Sauvignon associated with 10-Year-old wood compared to young tissue. Plants 2023, 12, 2984. [CrossRef]
- Kaplan J.; Travadon R.; Cooper M.; Hillis V.; Lubell M.; Baumgartner K. Identifying economic hurdles to early adoption of preventative practices: The case of trunk diseases in California winegrape vineyards. Wine Econ. Policy 2016, 5, 127–141. [CrossRef]
- Besoain, X. Grapevine Trunk Diseases (GTDs): Impact in table grapes and wine vineyards in Chile. In: Grapes and Wines- Advances in production, processing, analysis and valorization (A.M. Jordao, F. Cosme, ed.). Intechopen, Londres, 2018, 43–58.
- Gramaje, D.; Urbez-Torres, J. R.; Sosnowski, M. R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102(1), 12–39. [CrossRef]
- Úrbez-Torres, J. R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50(4), 5–45.
- ODEPA. Oficina de Estudios y Políticas Agrarias. Rubros. Vinos. 2022. Available on: https://www.odepa.gob.cl/rubros/vinos-y-alcoholes (accessed on 06 September 2023).
- SAG. Catastro Vitícola Nacional. 2020. Available on: https://www.sag.gob.cl/noticias/sag-presenta-catastro-viticola-nacional-2020 (accessed on 06 September 2023).
- Morales, A.; Latorre, B. A.; Piontelli, E.; Besoain, X. Botryosphaeriaceae species affecting table grape vineyards in Chile and cultivar susceptibility. Cienc. Inv. Agr. 2012, 39(3), 445–458. [CrossRef]
- Díaz, G. A.; Auger, J.; Besoain, X.; Bordeu, E.; Latorre, B. A. Prevalence and pathogenicity of fungi associated with grapevine trunk diseases in Chilean vineyards. Cien. Inv. Agr. 2013, 40(2), 327–339. [CrossRef]
- Valencia, D.; Torres, C.; Camps, R.; López, E.; Celis-Diez, J. L.; Besoain, X. Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaíso Region of Chile. Phytopathol. Mediterr. 2015, 54(2), 394–402.
- Úrbez-Torres, J. R.; Battany, M.; Bettiga, L. J.; Gispert, C.; McGourty, G.; Roncoroni, J.; Smith, R. J.; Verdegaal, P.; Gubler, W. D. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010a, 94(6), 717–724. [CrossRef]
- Úrbez-Torres, J. R.; Leavitt, G. M.; Guerrero, J. C.; Guevara, J.; Gubler, W. D. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in Mexico. Plant Dis. 2008, 92(4), 519–529.
- Martín, M. T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol. Mediterr. 2007, 46, 18–25.
- Luque, J.; Elena, G.; Garcia-Figueres, F.; Reyes, J.; Barrios, G.; Legorburu, F. J. Natural infections of pruning wounds by fungal trunk pathogens in mature grapevines in Catalonia (Northeast Spain). Aust. J. Grape Wine Res. 2014, 20(1), 134–143. [CrossRef]
- Reis, P.; Gaspar, A.; Alves, A.; Fontaine, F.; Lourenço, I.; Saramago, J.; Mota, M.; Rego, C. Early season symptoms on stem, inflorescences and flowers of grapevine associated with Botryosphaeriaceae species. Plants 2020, 9(11), 1427.
- Kuntzmann, P.; Villaume, S.; Larignon, P.; Bertsch, C. Esca, BDA and Eutypiosis: foliar symptoms, trunk lesions and fungi observed in diseased vinestocks in two vineyards in Alsace. Vitis 2010, 49, 71–76.
- Mohammadi, H.; Gramaje, D.; Banihashemi, Z.; Armengol, J. Characterization of Diplodia seriata and Neofusicoccum parvum associated with grapevine decline in Iran. J. Agr. Sci. Tech. 2013, 15, 603–616.
- Choueiri, E.; Jreijiri, F.; Chlela, P.; Louvet, G.; Lecomte, P. Occurrence of grapevine declines and first report of black dead arm associated with Botryosphaeria obtusa in Lebanon. Plant Dis. 2006, 90(1):115. [CrossRef]
- Ammad, F.; Benchabane, M.; Toumi, M.; Belkacem, N.; Guesmi, A.; Ameur, C.; Lecomte, P.; Merah, O. (2014). Occurrence of Botryosphaeriaceae species associated with grapevine dieback in Algeria. Turk. J. Agric. For. 2014, 38(6), 865–876. [CrossRef]
- Chebil, S.; Fersi, R.; Bouzid, M.; Quaglino, F.; Chenenaoui, S.; Melki, I.; Durante, G.; Zacchi, E.; Bahri, B. A.; Bianco, P. A.; Rhouma, A. Fungi from the Diaporthaceae and Botryosphaeriaceae families associated with grapevine decline in Tunisia. Cienc. Investig. Agrar. 2017, 44(2), 127–138.
- Savocchia, S.; Steel, C. C.; Stodart, B. J. Pathogenicity of Botryosphaeria species isolated from declining grapevines in sub tropical regions of eastern Australia Grapevine Trunk Disease. Vitis 2007, 46(1), 27–32.
- Úrbez-Torres, J. R.; Bruez, E.; Hurtado, J.; Gubler, W. D. Effect of temperature on conidial germination of Botryosphaeriaceae species infecting grapevines. Plant Dis. 2010b, 94(12), 1476–1484. [CrossRef]
- Slippers, B.; Wingfield, M. J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21(2–3), 90–106. [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102(7), 1189–1217. [CrossRef]
- Niem, J. M.; Billones-Baaijens, R.; Stodart, B.; Savocchia, S. Diversity profiling of grapevine microbial endosphere and antagonistic potential of endophytic Pseudomonas against Grapevine Trunk Diseases. Front. Microbiol. 2020, 11, 477. [CrossRef]
- Silva-Valderrama, I.; Toapanta, D.; Miccono, M. de los A.; Lolas, M.; Díaz, G. A.; Cantu, D.; Castro, A. Biocontrol potential of grapevine endophytic and rhizospheric fungi against trunk pathogens. Front. Microbiol. 2021, 11, 614620. [CrossRef]
- Álvarez-Pérez, J. M.; González-García, S.; Cobos, R.; Olego, M. Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J. J. R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83(24). [CrossRef]
- Laassami, A.; Yekkour, A.; Meklat, A.; Djemouai, N.; Zitouni, A.; Mokrane, S.; Lecomte, P.; Rey, P.; Berraf-Tebbal, A. Actinobacteria associated with vineyard soils of Algeria: Classification, antifungal potential against grapevine trunk pathogens and plant growth-promoting features. Curr. Microbiol. 2020, 77(10), 2831–2840. [CrossRef]
- Vega-Celedón, P.; Bravo, G.; Velásquez, A.; Cid, F. P.; Valenzuela, M.; Ramírez, I.; Vasconez, I. N.; Álvarez, I.; Jorquera, M. A.; Seeger, M. Microbial diversity of psychrotolerant bacteria isolated from wild flora of Andes Mountains and Patagonia of Chile towards the selection of plant growth-promoting bacterial consortia to alleviate cold stress in plants. Microorganisms 2021, 9(3), 1–28. [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6(2), 71–79. [CrossRef]
- Torres, C.; Latorre, B. A.; Undurraga, P.; Besoain, X. Evaluation of DMI fungicides against species of Diplodia and Neofusicoccum associated with Botryosphaeria canker of grapevine. Cienc. Inv. Agr. 2013, 40(1), 131–138. [CrossRef]
- Delgado, N.; Olivera, M.; Cádiz, F.; Bravo, G.; Montenegro, I.; Madrid, A.; Fuentealba, C.; Pedreschi, R.; Salgado, E.; Besoain, X. Volatile Organic Compounds (VOCs) produced by Gluconobacter cerinus and Hanseniaspora osmophila displaying control effect against table grape-rot pathogens. Antibiotics 2021, 10, 663. [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [CrossRef]
- Haidar, R.; Deschamps, A.; Roudet, J.; Calvo-Garrido, C.; Bruez, E.; Rey, P.; Fermaud, M. Multi-organ screening of efficient bacterial control agents against two major pathogens of grapevine. Biol. Control 2016, 92, 55–65. [CrossRef]
- Kotze, C.; van Niekerk, J.; Halleen, F.; Mostert, L.; Pourie, P. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 2011, 50, 247–263.
- Larach, A.; Riquelme, N.; Salinas, A.; Rolshausen, P. E.; Seeger, M.; Besoian, X. First report of Diaporthe ambigua associated with dead arm disease on grapevine in Chile. Plant Dis. 2021, 106(7). [CrossRef]
- Elena, G.; Garcia-Figueres, F.; Reigada, S.; Luque, J. Intraspecific variation in Diplodia seriata isolates occurring on grapevines in Spain. Plant Pathol. 2015, 64(3), 680–689. [CrossRef]
- Mukherjee, P. K.; Raghu, K. Effect of temperature on antagonistic and biocontrol potential of Trichoderma sp. on Sclerotium rolfsii. Mycopathologia 1997, 139(3), 151–155.
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining biocontrol agents to reduce the variability of biological control. Phytopathology 2001, 91(7), 621–627. [CrossRef]
- Landa, B. B.; Navas-Cortés, J. A.; Jiménez-Díaz, R. M. Influence of temperature on plant-rhizobacteria interactions related to biocontrol potential for suppression of fusarium wilt of chickpea. Plant Pathol. 2004, 53, 341–352. [CrossRef]
- Mannaa, M.; Kim, K. D. Effect of temperature and relative humidity on growth of Aspergillus and Penicillium spp. and biocontrol activity of Pseudomonas protegens AS15 against aflatoxigenic Aspergillus flavus in stored rice grains. Mycobiology 2018, 46(3), 287–295.
- Rolshausen, P. E., Úrbez-Torres, J. R.; Rooney-Latham, S.; Eskalen, A.; Smith, R. J. Evaluation of pruning wound susceptibility and protection against fungi associated whith grapevine trunk diseases. Am. J. Enol. Vitic. 2009, 60, 113–119.
- Fujiyoshi, P. T.; Lawrence, D. P.; Travadon, R.; Cooper, M.; Verdegaal, P.; Schwebs, S.; Baumgartner, K. Detection of spores of causal fungi of dieback-type trunk diseases in young, asymptomatic vineyards and mature, symptomatic vineyards. Crop Prot. 2021, 150:105798. [CrossRef]
- Naamala, J.; Smith, D. L. Relevance of plant growth promoting microorganisms and their derived compounds, in the face of climate change. Agronomy 2020, 10, 1179. [CrossRef]
- Fadiji, A. E.; Babalola, O. O.; Santoyo, G.; Perazzolli, M. The potential role of microbial biostimulants in the amelioration of climate change-associated abiotic stresses on crops. Front. Microbiol. 2022, 12:829099. [CrossRef]
- van Niekerk J. M.; Fourie; P. H.; Halleen, F.; Crous, P. Botryosphaeria spp. as grapevine trunk disease pathogens. Phytopathol. Mediterr. 2006, 45, 43–54.
- Úrbez-Torres, J. R.; Gubler, W. B. Susceptibility of grapevine pruning wounds to infection by Lasiodiplodia theobromae and Neofusicoccum parvum. Plant Pathol. 2011, 60, 261–270. [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; Orozco-Mosqueda, M. del C.; Macías-Rodríguez, L. I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [CrossRef]
- Simionato, A. S.; Navarro, M. O. P.; de Jesus, M. L. A.; Barazetti, A. R.; da Silva, C. S.; Simões, G. C.; Balbi-Peña, M. I.; de Mello, J. C. P.; Panagio, L. A.; de Almeida, R. S. C.; Andrade, G.; de Oliveira, A. G. The effect of phenazine-1-carboxylic acid on mycelial growth of Botrytis cinerea produced by Pseudomonas aeruginosa LV strain. Front. Microbiol. 2017, 8, 1102.
- Kong, W. L.; Li, P. S.; Wu, X. Q.; Wu, T. Y.; Sun, X. R. Forest tree associated bacterial diffusible and volatile organic compounds against various phytopathogenic fungi. Microorganisms 2020, 8(4), 590. [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [CrossRef]
- Win, K. T.; Kobayashi, M.; Tanaka, F.; Takeuchi, K.; Oo, A. Z.; Jiang, C. J. Identification of Pseudomonas strains for the biological control of soybean red crown root rot. Sci. Rep. 2022, 12, 14510. [CrossRef]
- Chane, A.; Barbey, C.; Robert, M.; Merieau, A.; Konto-Ghiorghi, Y.; Beury-Cirou, A.; Feuilloley, M.; Pátek, M.; Gobert, V.; Latour, X. Biocontrol of soft rot: Confocal microscopy highlights virulent pectobacterial communication and its jamming by rhodococcal quorum-quenching. Mol. Plant Microbe Interact. 2019, 32(7), 802–812. [CrossRef]
- Zhou, Z.; Wu, X.; Li, J.; Zhang, Y.; Huang, Y.; Zhang, W.; Shi, Y.; Wang, J.; Chen, S. A novel quorum quencher, Rhodococcus pyridinivorans XN-36, is a powerful agent for the biocontrol of soft rot disease in various host plants. Biol. Control 2022, 169, 104889. [CrossRef]
- Rangel-Montoya, E. A.; Paolinelli, M.; Rolshausen, P.; Hernandez-Martinez, R. The role of melanin in the grapevine trunk disease pathogen Lasiodiplodia gilanensis. Phytopathol. Mediterr. 2020, 59(3), 549–563.
- Eisenman, H. C.; Greer, E. M.; McGrail, C. W. The role of melanins in melanotic fungi for pathogenesis and environmental survival. Appl. Microbiol. Biotechnol. 2020, 104(19), 4247–4257. [CrossRef]
- Heleno, S. A.; Ferreira, I. C. F. R.; Esteves, A. P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M. J. R. P. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




