Introduction
In the EU, the deliberate release of genetically modified organisms (GMOs) into the environment and the functioning of the market for the corresponding GMO-derived products are regulated by a framework centered around Directive 2001/18/EC and Regulation (EC) No 1829/2003. The EU GMO framework has established a prior authorization system that comprises a case-by-case assessment of the risks to human health and the environment associated with releasing GMOs in accordance with the precautionary principle. Amongst others, authorization is also linked to mandatory post-market monitoring requirements.
Since the Directive 2001/18/EC came into force, the development of new genomic techniques (NGTs), such as genome editing CRISPR-Cas, has advanced the genetic modification of plants. With the targeted genetic approach and the potential absence of transgenic DNA sequences in the final NGT product, there has been public debate whether NGTs qualify for an exemption from Directive 2001/18/EC. In July 2018, the Grand Chamber of the European Court of Justice (ECJ) ruled that the environmental and health risks associated with plants generated with NGTs are comparable to the risks associated with the production and distribution of GMOs generated by transgenesis (European Court of Justice, Confédération paysanne, 2018). Further, NGTs enable the creation of a variety of GMOs at a much greater pace and scale than random mutagenesis techniques, which, according to the ECJ, argues for a strict application of the precautionary principle. The ECJ therefore confirmed that Directive 2001/18/EC is applicable for NGTs without restrictions.
Recently, the European Commission (EC) published a regulatory proposal (EC proposal; European Parliament, Council of the European Union, 2023a, 2023b) to adapt the prevailing application procedure, exclusively for genetically modified (GM) plants, including non-crops, generated with NGTs. The EC proposal specifically concerns targeted mutagenesis and cisgenesis, including intragenesis, with NGTs in plants. Prerequisite for any NGT application is that no DNA from outside of the breeders’ gene pool (non-crossable species) is present in the final NGT plant, including genetic material that has been temporarily inserted during the technical development of the plant. According to the current EC proposal such NGT plants shall be further divided into two categories based on their genetic modifications. Category 1 NGT plants (NGT1) shall comprise a maximum number of 20 genetic modifications fulfilling the following specifications: (i) Deletions and inversions of any number of nucleotides as well as insertions or substitution of DNA sequences with up to 20 arbitrary nucleotides shall be possible anywhere in the genome, while (ii) insertions or substitutions of any-sized contiguous DNA sequences must originate from the breeders’ gene pool and shall not disrupt any endogenous genes. (iii) On the basis that the resulting DNA sequence already occurs in a species from the breeders’ gene pool, any other targeted modification of any size is allowed. All other non-transgenic NGT plants that exceed the criteria for NGT1 are defined as category 2 plants (NGT2). To simplify the application procedure, the new EC proposal considers NGT1 plants as equivalent to conventionally bred plants and suggests a technical confirmation process without a case-by-case risk assessment and the non-application of all European law on genetic engineering. In category 1, no approval procedure, no risk assessment, no provision of detection methods, insufficient labeling and no monitoring is envisaged. For NGT2 plant applications information on hazard identification shall be required if the specific traits and intended use give rise to a plausible risk hypothesis. In this category 2, reduced requirements for risk assessment, detection and monitoring would be imposed. Concerning plants, only classic transgenic plant applications generated using NGT would continue to fall under the current genetic engineering legislation.
With this proposal the EU is confronted with a fundamental path decision affecting European goals and standards in climate and nature protection, precaution, and freedom of choice. Currently two main lines of argumentation can be described: Either NGTs and their risk profile are (i) GMOs under European law as ruled by the Grand Chamber of the ECJ or (ii) they should be compared with conventional breeding as proposed by the recent EC proposal. The diverging reasoning results in different positions on (i) whether NGT plants should remain under Directive 2001/18/EC or (ii) whether they should be exempted from it in future. In view of this discussion, we investigate which NGT plant applications may be affected by the current EC proposal. We thereby focus on potential environmental impacts from our perspective as GMO risk assessors, while other aspects such as consumer protection, co-existence with organic farming and patent issues are not addressed in this article.
Here, we show that most of the NGT plant applications affected by the EC proposal would be regulated as NGT1 (94%). Those 94% would comprise a wide variety of crop species and affect many different traits, with the most prominent ones being consumer- and industry-oriented traits. Those NGT 1 plants would enter the market without risk assessment, even though our analysis suggests that they could bear environmental risks comparable to other GMOs, including potential insecticidal NGT1-RNAi plants.
94% of Affected NGT Plants Would Enter the EU Market Without Risk Assessment
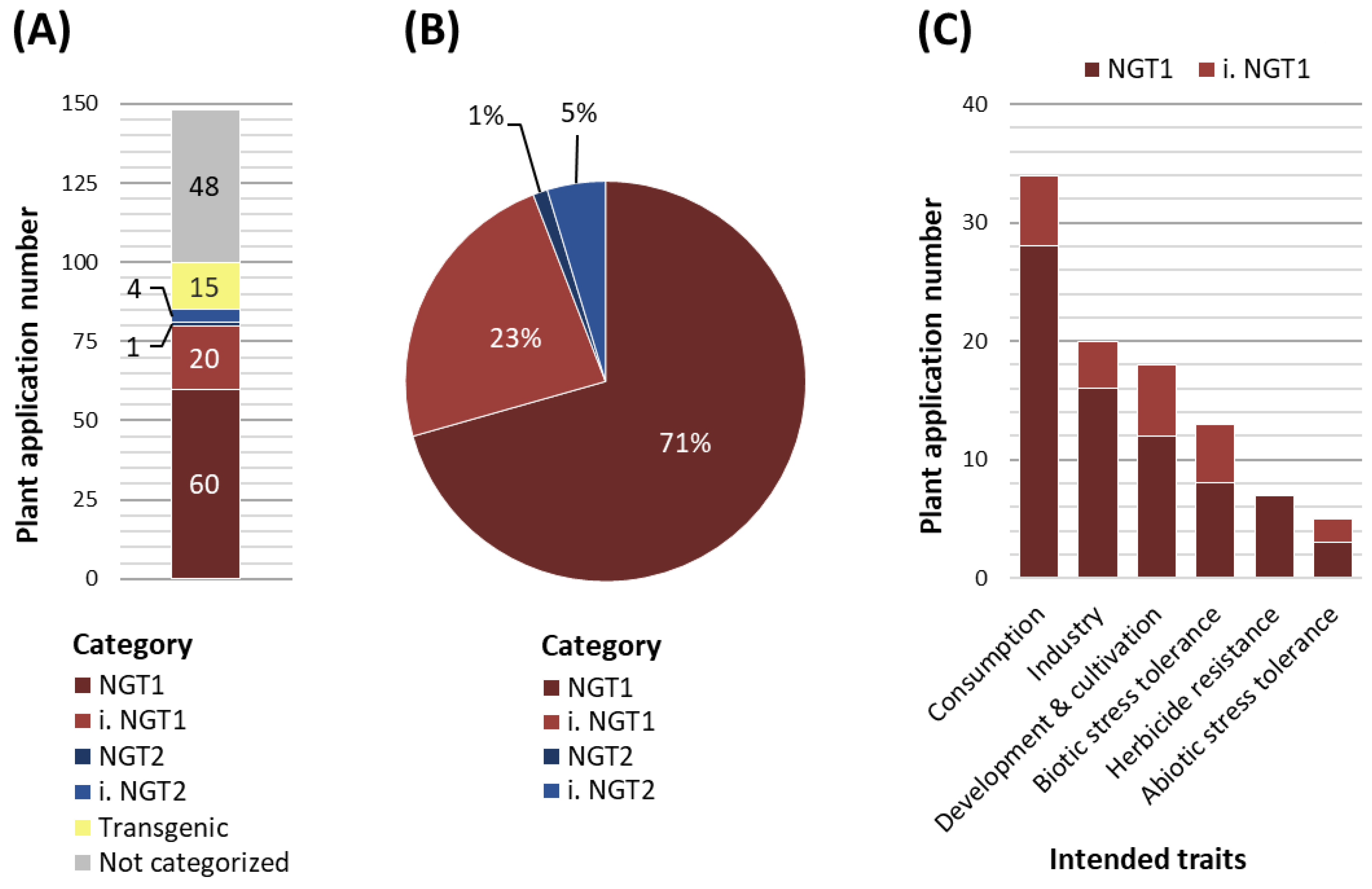
To examine where the EC’s proposed path leads us to and how the future regulation of NGT plant application might look like, we analyzed published data on plant applications that are currently in the commercialization pipeline. Our analysis is based on the ‘plant breeding commercialization pipeline and licensing agreements’ list, which was commissioned by the Swiss Federal Office for the Environment (Gelinsky, 2022). We categorized the 148 NGT plant applications according to the NGT1 and NGT2 specifications defined by the EC proposal (
Table S1). From these 148 NGT plant applications, 15 would be treated as transgenic NGTs falling under the current GMO legislation and 48 plant applications could not be categorized due to a lack of information or data inaccessibility related to confidential business information (
Figure 1A). Of the remaining 85 NGT plant applications, 60 could be clearly categorized as NGT1, while 20 plant applications were assigned to inferred NGT1 (i. NGT1) (
Figure 1A). Only one plant application was categorized as NGT2 and four plant applications as inferred NGT2 (i. NGT2) (
Figure 1A). Importantly, out of the 85 plant applications that would be affected by the EC proposal, 94% would be classified as NGT1 plants and 6% as NGT2 plants (
Figure 1B). When evaluated under the scope of the recent EC proposal, a similar distribution of NGT1 and NGT2 plant applications for cultivation and marketing can be observed in the U.S. (
Table S2), which was selected as a representative non-EU country.
With the development of NGTs, a wide range of plant species have become accessible for targeted mutagenesis. Accordingly, NGT1 categorized plant applications described in Gelinsky (2022) can be assigned to 26 different plant species (
Table S1). These include crops grown worldwide on a large scale, such as soybean (17 plant applications), corn (10), or potato (9), but also minor crops such as strawberries (2), raspberries (1), and physalis (1). However, not only crops will be affected by the current EC proposal, but also wild plant species including trees that can be categorized as NGT1, such as tall fescue, switchgrass or tree tobacco (
Table S2).
Plant applications categorized as NGT1 comprise a wide array of postulated traits (
Figure 1C,
Table S1). To visualize this broad range of postulated traits, we divided the NGT plant applications listed in Gelinsky (2022) into six trait groups (a-f), in the order of abundance:
- (a)
Consumption-oriented traits represent the largest group of intended traits (34 NGT1 plant applications), which covers for example traits affecting the nutrient content, visual and olfactory modifications or secondary metabolites of the crop. Examples for visual and olfactory modifications are non-browning fruits and vegetables.
- (b)
The second largest trait group concerns industry-oriented traits (20 NGT1 plant applications) such as modified ingredient compositions, storage and transportability qualities, or bioenergy usage. Here, camelina plant applications with adapted fatty acid biosynthesis to produce biofuels or dietary supplements represent common examples.
- (c)
The third group covers traits associated with plant development and cultivation (19 NGT1 plant applications), including plant growth, yield, reproduction and harvesting aspects. A plant application of this group is a “shatter-tolerant” NGT1 rapeseed developing more stable pods to prevent seed loss during harvesting.
- (d)
The fourth trait group contains traits aiming to confer tolerance against biotic stressors such as bacteria, fungi, nematodes or viruses (13 NGT1 plant applications). An example is a wheat plant with fungal resistance due to a mutation affecting the plant immune response.
- (e)
The fifth trait group includes herbicide resistances (7 NGT1 plant applications) that are mainly generated via point mutations in e.g. the ALS genes of soybean and rapeseed.
- (f)
The least represented trait group covers abiotic stress tolerances (5 NGT1 plant applications) of which drought tolerance is a favored trait, which is proposed to confer adaptation to climate changes (Sami et al., 2021; Eckardt et al., 2023).
Intended traits and unintended risks
The EC proposes to simplify the application process by the exemption of a case-by-case risk assessment for NGT1 plants. From our role as GMO risk assessors, we examine whether the potential environmental impact of NGT1 plant applications are comparable to those of GMOs. Therefore, we analyzed some examples mentioned above for the respective trait groups (a-f) and assessed these modified plants regarding the environmental risk areas that are defined in the respective Directive under which the EC proposal would act (Directive 2001/18/EC).
- (i)
Persistence and invasiveness: Stress tolerance, both biotic and abiotic, that alters plant fitness may impact the plants persistence and establishment in the environment, even for crop plants that are so far not invasive and especially in changing climate regimes. NGT1 plant applications listed in Gelinsky (2022) comprise a potentially invasive tree tobacco, which showed an increased fitness after drought stress (Negin et al. 2023). Such a drought stress-tolerant tobacco could potentially grow in areas that previously had been too dry and thus might lead to risks for biodiversity in the corresponding ecosystems. In general, the risk of generating plants with increased persistence and invasiveness might be enhanced when wide-spread plants such as wild grasses, trees and herbs become targets of genetic modification as listed in the U.S. APHIS plant applications (Blackburn et al., 2019;
Table S1). However, the EC proposal would not require any monitoring or detection concept for NGT1, which restricts risk management, including the capacity to remove invasive NGT plants to protect biodiversity.
- (ii)
Gene transfer and selective disadvantages: The transfer of traits from domesticated plants to wild plant species can result in an altered weed spread as well as the establishment of novel weeds, which can lead to an increased risk for the extinction of wild species (Ellstrand, 2003). We identified a NGT1 rapeseed, which grows more stable pods to prevent seed loss during the harvesting process ('shatter-tolerance',
Table S1). Unintended crossing of the ‘shatter-tolerant’ NGT1 rapeseed with wild plant populations could affect the fitness of wild plants and their natural reproduction due to possible restrictions in seed dispersal.
- (iii)
Altering cultivation, management or harvesting techniques: GMO’s need to be analyzed in regards to their impact on the cultivation, management and harvesting techniques compared to non-GMO plants. This includes a potential increase in insecticide, herbicide, or pesticide usage. We identified non-browning fruits and vegetables that potentially harbor an effect on the cultivation system. The trait ‘non-browning’ often comprises a mutation in at least one of the polyphenol oxidase genes. Polyphenol oxidases are known to play a role in the plant pathogen defense, while their loss is associated with an impairment in biotic stress response (Thipyapong et al., 2004). A modification of the plant’s pathogen defense mechanism might change the plants susceptibility to biotic stress and therefore might alter the plant pest management by a potential increase in pesticide usage.
- (iv)
Interactions with target and non-target organisms (NTOs): Environmental impacts resulting from direct and indirect interactions of GM plants with NTOs can also be identified for NGT plants. One example in which an interaction with NTOs cannot be excluded, is a patented NGT1 plant application. In this case a NGT1 plant would carry a genome edited microRNA (miRNA) conferring insecticidal activities in target (and potentially non-target) insects (see section “Within the realms of possibility: The NGT1-RNAi case”). In other cases, changes in metabolomics like in the protein or lipid content and composition as seen for many NGT1 plant applications in Gelinsky (2022), may also unintentionally affect the synthesis of byproducts and secondary metabolites potentially harmful to NTOs (Kawall, 2021).
As demonstrated by these examples, NGT1 plants can bear risks to human health and the environment as specified in Directive 2001/18/EC. Importantly, such NGT1 plants would not be risk assessed according to the EC proposal and potential hazards would thus not be recognized and evaluated in advance to a NGT1 plant release. We conclude that categorization according to molecular parameters, as suggested in the EC proposal would not exclude risks and can in consequence not per se define plants without risks.
Within the realms of possibility: The NGT1-RNAi case
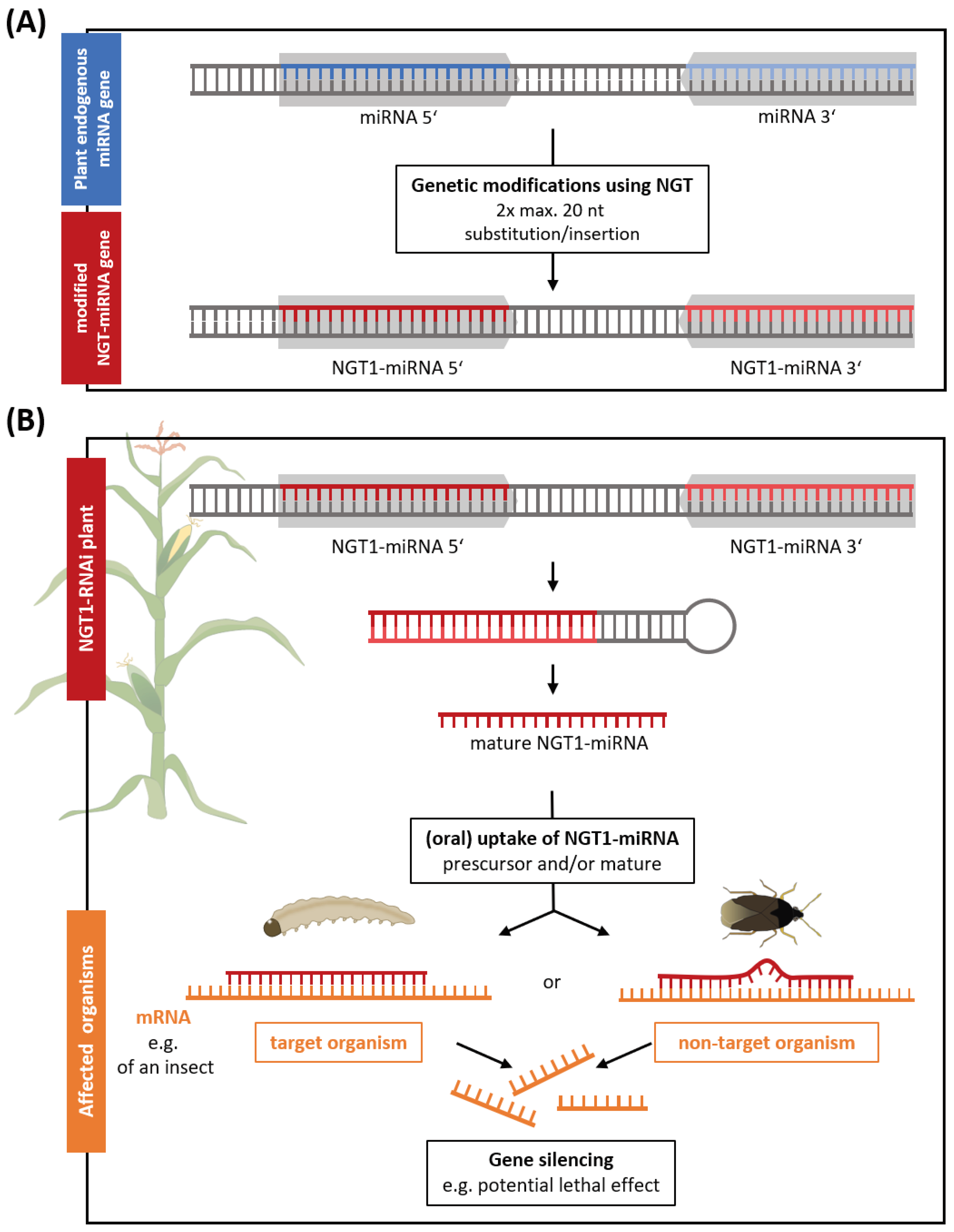
Within the analyzed plant applications, we identified a NGT1 plant application that utilizes the molecular mechanism of RNAi to generate non-browning potatoes (
Table S1). RNAi is an endogenous, cellular mechanism that internally regulates gene expression in most eukaryotes (Shabalina and Koonin, 2008). Among others in plants, small non-coding microRNAs (miRNAs) can be transcribed and act as templates to interfere with the plant gene expression, either by completely silencing complementary genes or by altering the genes’ expression level (Yu et al., 2017; Song et al., 2019). Here we describe for the first time the possibility to generate a fully functional miRNA application that would be classified as NGT1 according to the EC proposal. The recognition sequence (usually 20-24 nucleotides) of an endogenous plant miRNA gene would be edited to redirect the RNAi machinery against another distinct target gene of interest. For this, the complementary substitution/insertion of max. 20 nucleotides at two positions (5’ and 3’ miRNA) is sufficient (
Figure 2A). From this modified NGT1-miRNA gene an effective NGT1-miRNA is generated in a multistep cellular RNAi processing mechanism (
Figure 2B). This method has been patented as ‘Gene Editing induced Gene Silencing’ technology (GEiGS®) (Maori et al., 2019). Other RNAi applications in plants, such as dsRNA sprays or transgenic lines, have already been applied as insecticides to target genes of host species (Liu et al., 2020). Similarly, a potentially insecticidal maize is included in the patent from Maori et al. (2019). In this maize the endogenous miRNA (
zma-MIR166h) would be redirected against an essential gene transcript of chitinase of the European corn borer by modifying two times 20 nucleotides of the miRNA gene at the critical miRNA recognition sequence (Maori et al., 2019) (
Figure 2). Oral uptake of such a NGT1 miRNA expressing maize is assumed to have a lethal effect on the target species (Arakane and Muthukrishnan, 2010; Khajuria et al., 2010; Lu et al., 2023) with potential risks for NTOs. In this case, environmental risks would be comparable to transgenic plants expressing insecticidal RNAi constructs or toxins derived from
Bacillus thuringiensis (Bt), widely known as Bt crops. Although sharing the same principle mechanism as other RNAi applications, NGT1-RNAi applications would not be risk assessed under the current EC proposal, even though this is a strict requirement for transgenic RNAi plants (regulated by Directive 2001/18/EC) or RNAi spray applications (regulated by EC No. 1107/2009 and Directive 283/2013 and 284/2013).
Discussion
The EC proposal aims to deliberate the process of market authorization for plants modified with NGTs. NGT plant applications shall be categorized based on their molecular characteristics and regulated according to their respective category. On the basis of published data on NGT plants in development or in the commercialization pipeline (Gelinsky 2022) we show for the first time that the EC’s proposed path would in fact lead us to the deregulation of 94% of these affected NGT plants, as they would fall under category 1. They would therefore receive a market approval without risk assessment, monitoring provisions or the need for providing detection methods. When analyzing selected examples of NGT1 plant application in the commercialization pipeline, we identified possible risks to the environment and health, which are comparable to risks of classical GMOs. RNAi plant applications for example have the potential to severely impact NTOs, including protected species. RNAi plant applications are already approved GMO applications in the EU and other countries (European Commission, 2015a, 2015b, 2019, 2023) with a growing market perspective (Hernández-Soto and Chacón-Cerdas, 2021; Koch and Wassenegger, 2021). Therefore, the proposal would lead to the authorization of plants, that represent a justified concern in the sense of the precautionary principle. Importantly, the regulatory proposal does not foresee any instrument to retrieve any authorization for NGT1 plants, even in cases where a hazard might be shown after release. Our data show that already today, NGT2 plants would represent only a small fraction of NGT plant applications. However, an even broader spectrum of NGT plant applications may be expected in the future, as already observed in the U.S. (
Table S2). This is on the one hand owed to the rapid development in the field of genetic engineering. On the other hand, the EC proposal for NGT1 plants might additionally act as incentives to design new plant varieties fulfilling the criteria for NGT1. This incentive effect would lead to more NGT1 plant applications entering the market without environmental risk assessment.
We observe an increasing number of modified crop species, including crops that have not reached the EU-market as transgenics in the past (e.g. strawberries and physalis). For NGT plant applications, we also see a diversification of traits compared to the dominant traits for transgenic GMOs (insecticide resistance and herbicide tolerance). In contrast to the general expectations, we see quantitatively more NGT1 plant applications in the prevailing trait groups that are consumption- and industry-oriented, when compared to a minority of the expected traits that are supposed to enable e.g. an adaptation to climate change (abiotic stress).
As discussed above, we could show that most likely all future NGT applications would be deliberated from the current GMO framework that includes risk assessment and labeling, among others. Our analysis also reveals, that current NGT1 applications can clearly have potential risks in both relevant areas, environment and health according to Directive 2001/18/EC (Annex II D.2). It can therefore be concluded, that the analysis of a presumed equivalency with conventional plants is not a suitable criterion for assuming the safety of the NGT plant applications. In contrast a proof that these applications pose less risks than other products of genetic engineering (i.e. transgenics) would be inevitable. Here we demonstrate that already for current NGT1 cases this cannot be assumed. In addition, the specific criteria proposed in Annex I of the EC proposal are unsuitable for proving equivalence to conventionally bred plants: here we have shown concrete examples of NGT1 plant applications, which clearly cannot be produced with conventional breeding tools, as the NGT-RNAi case demonstrates particularly impressively. With respect to the consideration of safety and risk, we will not be able to avoid taking into account additional denominators for the profound assessment of NGT plant applications (Eckerstorfer et al., 2021).
With the current EC proposal and the associated political negotiations, the EU is now at a crossroad where the decision on NGTs will have a far-reaching impact – on the environment, land usage and biodiversity. Acknowledging the precautionary principle, the EU should decide responsibly which path it wants to take in the future.
Supplementary Material
TableS1_S2_NGT_case_studies.xlsx Categorization of NGT plant applications according to Annex I of the EC proposal and description of their intended traits. Methods for case study analysis: The first data sheet contains a detailed description of the NGT category definitions (NGT1, i. NGT1, NGT2, i. NGT2) and the respective traits. Table S1 Categorization of NGT plant applications listed in Gelinsky (2022). Tables S2 Categorization of plant applications listed by APHIS (from 2011-2020; 2021-2023).
Author Contributions
FB and RS contributed mainly to the analysis of plant applications and visualizing the results. JM and LZ contributed substantially to the analysis and visualization. All authors conceptualized the manuscript, contributed to writing and approved the submitted version.
Acknowledgments
We thank Mathias Otto and Karl Stracke for fruitful discussions and critical comments on the manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Arakane, Y., and Muthukrishnan, S. (2010). Insect chitinase and chitinase-like proteins. Cell Mol Life Sci 67, 201–216. [CrossRef]
- Blackburn, T. M., Bellard, C., and Ricciardi, A. (2019). Alien versus native species as drivers of recent extinctions. Frontiers in Ecol & Environ 17, 203–207. [CrossRef]
- Eckardt, N. A., Cutler, S., Juenger, T. E., Marshall-Colon, A., Udvardi, M., and Verslues, P. E. (2023). Focus on climate change and plant abiotic stress biology. Plant Cell 35, 1–3. [CrossRef]
- Eckerstorfer, M. F., Grabowski, M., Lener, M., Engelhard, M., Simon, S., Dolezel, M., et al. (2021). Biosafety of genome editing applications in plant breeding: Considerations for a focused case-specific risk assessment in the EU. BioTech 10. [CrossRef]
- Ellstrand, N. C. (2003). Dangerous liaisons? When cultivated plants mate with their wild relatives. Baltimore, Md. Johns Hopkins Univ. Press.
- European Commission (2013a). No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
- European Commission (2013b). No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
- European Court of Justice, Confédération paysanne. Judgement of the Court (Grand Chamber), 25 July 2018 in Case C-528/16. C-528-18.
- European Parliament, Council of the European Union (2001). Directive 2001/18/EC on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC: Directive 2001/18/EC.
- European Parliament, Council of the European Union (2009). No 1107/2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC.
- European Parliament, Council of the European Union (2023a). Annexes of the proposal on plants obtained by certain new genomic techniques and their food and feed, and amending regulation (EU) 2017/625: COM(2023) 411 Annexes 1 to 3.
- European Parliament, Council of the European Union (2023b). Proposal for a regulation on plants obtained by certain new genomic techniques and their food and feed, and amending regulation (EU) 2017/625: COM(2023)411.
- Gelinsky, E. (2022). Neue gentechnische Verfahren: Kommerzialisierungspipeline im Bereich Pflanzenzüchtung und Lizenzvereinbarungen: Im Auftrag des Bundesamtes für Umwelt (BAFU). Baldegg.
- Hernández-Soto, A., and Chacón-Cerdas, R. (2021). RNAi Crop protection advances. Int J Mol Sci 22. [CrossRef]
- Kawall, K. (2021). Genome-edited Camelina sativa with a unique fatty acid content and its potential impact on ecosystems. Environ Sci Eur 33. [CrossRef]
- Khajuria, C., Buschman, L. L., Chen, M.-S., Muthukrishnan, S., and Zhu, K. Y. (2010). A gut-specific chitinase gene essential for regulation of chitin content of peritrophic matrix and growth of Ostrinia nubilalis larvae. Insect biochemistry and molecular biology 40. [CrossRef]
- Koch, A., and Wassenegger, M. (2021). Host-induced gene silencing - mechanisms and applications. New Phytol 231, 54–59. [CrossRef]
- Liu, S., Jaouannet, M., Dempsey, D. A., Imani, J., Coustau, C., and Kogel, K.-H. (2020). RNA-based technologies for insect control in plant production. Biotechnol Adv 39, 107463. [CrossRef]
- Lu, Q., Xie, H., Qu, M., Liu, T., and Yang, Q. (2023). Group h chitinase: A molecular target for the development of lepidopteran-specific Insecticides. J Agric Food Chem. [CrossRef]
- Maori, E., Galanty, Y., Pignocchi, C., Chaparro Garcia, A., and Meir, O. (2019). Modifying the specificity of plant non-coding RNA molecules for silencing gene expression. EP3684930. European Union: Tropic Biosciences UK Limited.
- Sami, A., Xue, Z., Tazein, S., Arshad, A., He Zhu, Z., Ping Chen, Y., et al. (2021). CRISPR-Cas9-based genetic engineering for crop improvement under drought stress. Bioengineered 12, 5814–5829. [CrossRef]
- Shabalina, S. A., and Koonin, E. V. (2008). Origins and evolution of eukaryotic RNA interference. Trends Ecol Evol 23, 578–587. [CrossRef]
- Song, X., Li, Y., Cao, X., and Qi, Y. (2019). MicroRNAs and their regulatory roles in plant-environment interactions. Annu Rev Plant Biol 70, 489–525. [CrossRef]
- Thipyapong, P., Hunt, M. D., and Steffens, J. C. (2004). Antisense downregulation of polyphenol oxidase results in enhanced disease susceptibility. Planta 220, 105–117. [CrossRef]
- Yu, Y., Jia, T., and Chen, X. (2017). The 'how' and 'where' of plant microRNAs. New Phytol 216, 1002–1017. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).