1. Introduction
Hand-held echocardiography (HHE) systems are increasingly being used as an extension of the stethoscope for rapid bedside diagnoses in the emergency department and intensive care units. Studies have shown that trained, novice non-experts; including medical students, hospitalists, and medical residents can learn and become efficient in HHE with formal training [
1,
2]. Congestive heart failure (CHF) is the largest Diagnosis Related Group Medicare beneficiary discharge diagnosis in the US and is associated with recurrent hospital admissions and significant mortality imposing a significant economic burden on health care [
3]. Heart failure readmission occurs in 25% of CHF patients within 30 days of discharge [
4]. Left ventricular (LV) size, LV ejection fraction (LVEF) and right atrial pressure (RAP) by inferior vena cava (IVC) size and collapsibility can be readily assessed by HHE and can provide critical information to offer immediate and effective medical care to CHF patients. On post hoc analysis, one randomized study showed decreased length of stay by hospitalists using HHE to guide heart failure management [
5].
HHE has been used in clinical practice for many years by cardiologists, non-cardiologists (intensivists, emergency medicine physicians, hospitalists), and non-physician providers for quick assessment of cardiac function, pericardial effusion, and volume status. Training curriculum for HHE varies among different medical societies and organizations [
6], but most agree on the basic need for didactic lectures, hands-on training, and proper interpretation of the studies [
7,
8].
The role of advanced practice provider (APP) work force in cardiology is rapidly expanding in both outpatient and in-patient clinical settings. In a study by Gundersen et al, it was demonstrated that cardiac nurses who have received training in using HHE can assess the volume status of heart failure patients in a clinic setting. However, their evaluation focused on the pleural cavities and IVC without imaging of the heart [
9]. With formal training on focused cardiac images, APPs can facilitate efficient delivery of medical care and reduce length of hospital stay for CHF patients utilizing HHE at the bedside. Currently, there is no published research study on the use of HHE by APPs to evaluate cardiac size, function, and RAP in hospitalized CHF patients. Our aim in this study was to evaluate if hand-held echocardiography (HHE) performed and interpreted by trained Advanced Practice Providers (APPs) on hospitalized CHF patients has adequate image quality and interpretation, by comparing against expert echocardiographer and SE findings.
1.1. Sample Size
A one-year monthly analysis between 2020-2021 revealed that 25 to 71 in-patients, under all medical services, were discharged each month with the primary diagnosis of heart failure at our center. The average length of stay was 8.1 days with a range of 5.6-13.2 days. We targeted an average patient enrollment of up to 20 patients per month over a six-month period for the study.
The study was approved by the Institutional Review Board. The Institutional Review Board deemed APPs performing HHE as one research cohort and the hospitalized CHF patients as a second research cohort. Hence, patients’ consent was obtained by two study personnel APPs previously trained in HHE. All subjects provided a written consent to participate in the study.
2. Study Design
2.1. Methods
2.1.1. HHE Training for APPs:
In-patient general cardiology APPs, who were the first line medical providers for assessment and management of CHF patients, were trained to use HHE by the study echocardiographer (TZN) who had 23 years experiences in echocardiography including 14 years as a medical director of echocardiography laboratories. APPs’ training included didactic lectures, two simulation laboratory training sessions with both mannequin and live models and bedside rounds. Additionally, APPs participated in a 10-hour online course on HHE [
10]. Five APPs were recruited and provided informed consent to participate in this study. They were all required to perform and interpret 5 HHE studies with diagnostic image quality for LV size, LVEF and RAP before being certified by the study echocardiographer to perform HHE on study patients. Two study personnel
APPs who were also trained and certified on HHE were excluded from the study per Institutional Review Board protocol.
2.1.2. Patient Selection:
Inclusion Criteria: Patients who were 18 years or older admitted at Mayo Clinic Arizona Hospital by the in-patient general cardiology services from November 2021 to April 2022 with primary diagnosis of new onset heart failure or heart failure exacerbation either from our emergency department or from the outpatient clinic were included in the study.
Exclusion Criteria: Intensive care unit and hospice patients, inability to provide consent or declined study participation, end-stage renal failure who required renal replacement therapy and dyspneic disorder due to suspected pulmonary pathology (chronic obstructive pulmonary disease, pneumonia, pulmonary embolism).
2.1.3. APPs/Patient Enrollment and HHE Imaging:
A total of 80 hospitalized patients with primary diagnosis of CHF were enrolled after informed consent was obtained by APP study personnel. All patients had baseline data collected to include age, sex, body mass index, blood pressure, heart rate, and date of the first diuretic administration. HHEs were then performed and interpreted by the trained APPs who were recruited and consented to participate in the study as described above.
2.1.4. HHE Imaging Methods:
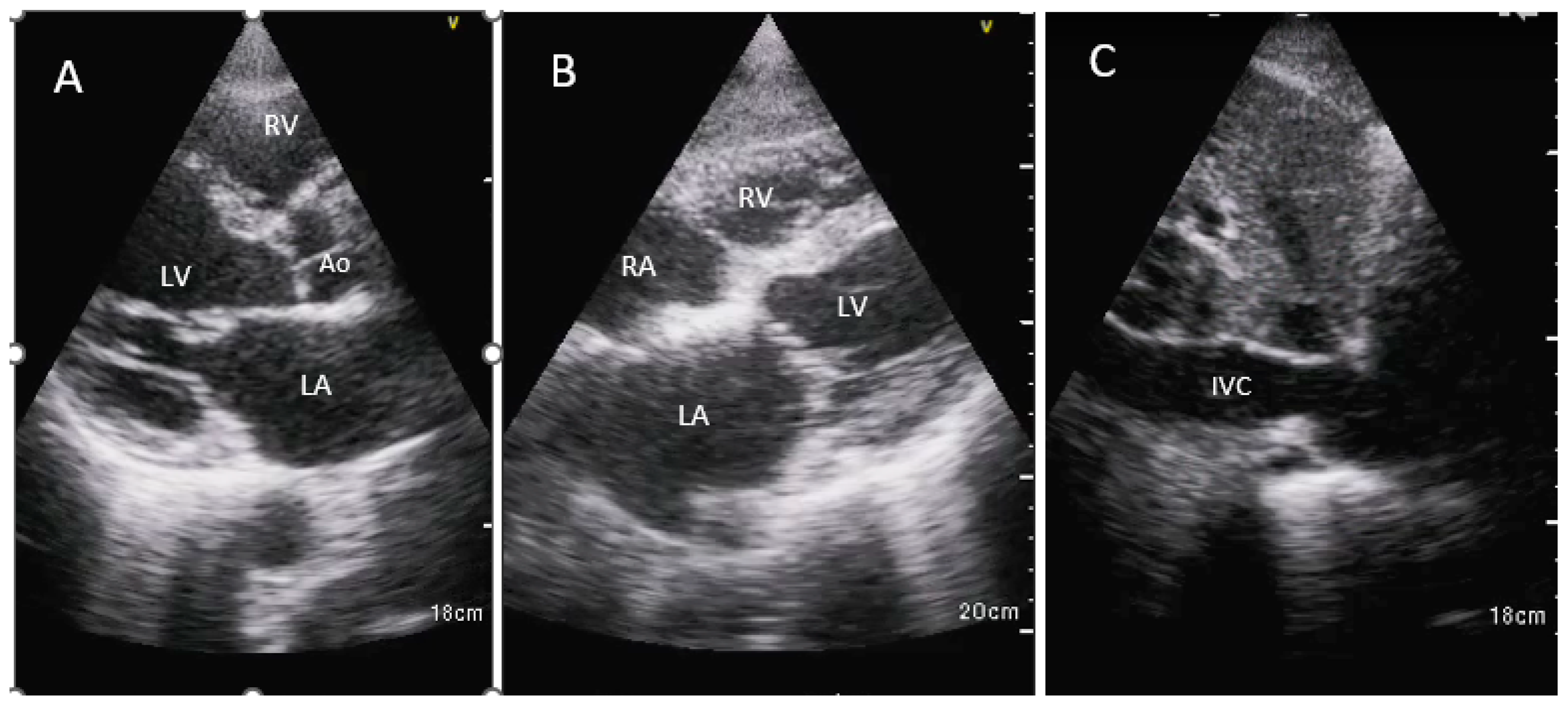
Three views were obtained using two imaging windows: parasternal long axis view, subcostal 4-chamber view and IVC long axis view (
Figure 1).
RAP was estimated based on IVC diameter and inspiratory collapse as per American Society of Echocardiography recommendations [
11].
APPs performed routine history and physical examination including review of ancillary investigations such as electrocardiogram, chest radiograph, complete blood count, basic metabolic panel, NT pro-BNP, troponin and other tests as needed for clinical care. After obtaining history and physical examination, laboratory blood work, and imaging assessment, CHF patients were consented and enrolled in the study. Trained APPs then obtained HHE images on CHF patients and recorded their assessment of study quality (poor, fair, good), LV size, LVEF (< or >40%) and estimated RAP on a pre-printed form. HHE images were shared with the cardiology team caring for the hospitalized CHF patients and management decisions were made independent of APP interpretation of HHE images. Diuretic dose, administered route, and other cardiac medications initiated as standard of care were recorded.
Images were saved on the HHE device and downloaded to a dedicated research computer. The study echocardiographer blindly evaluated these images and entered assessment of LV size, LVEF (both categorical cut off of 40% and as an estimated LVEF) and RAP based on those images. The study echocardiographer was also blinded to the patients’ clinical presentation upon admission. Each HHE imaging was graded as a) poor, b) fair, or c) good quality by the study echocardiographer.
Non-study related standard echocardiograms (SE) were ordered at the discretion of primary service and performed by the cardiac sonographers. The SE were interpreted by independent level 3-echocardiologists who were not aware of the research study. Comprehensive SE were performed as per clinical practice guidelines [
12].
2.1.5. Statistical Methods
Descriptive statistics were used to summarize demographic data along with SE, HHE results, and hospitalization details. Inter-rater agreement of the evaluation of HHE imaging on LV size, LVEF, and RAP was evaluated by kappa statistics. Statistical analysis was performed using R version 3.6.2.
2.1.5.5. Primary Analysis between the Study Echocardiographer and APPs Assessment:
Correlation of LV size, LVEF, and RAP were performed. Absolute difference in LV size, LV function, and RAP for each study were evaluated. Differences in study quality assessment was evaluated.
2.1.5.6. Exploratory Analysis between APPs, Study Echocardiographer and SE:
The evaluation of LV size, LVEF, and RAP between APPs and the study echocardiographer were compared against final evaluation by the SE with the expected effect of changes (especially in RAP) due to treatment by the time SE was performed.
3. Results
There were 80 CHF patients [age 73±14 yrs., 58% male; LVEF 45±19% by SE; 36% BMI ≥30 kg/m
2] who were enrolled in this study. More than half (53.8%) of the hospitalized CHF patients were noted to be in atrial fibrillation on hospital admission. There were 74 patients (92.5%) whose admission diagnosis was CHF exacerbation, and 37 patients (46.3%) were diagnosed as heart failure with preserved ejection fraction (HFpEF). Hypertension (80%) was the most common co-morbidity, followed by known history of diastolic heart failure (46.3%) and smoking history (46.3%) [
Table 1].
APPs recruited for this study were mostly female (80%). Highest level of education was Doctor of Nursing Practice (40%) followed by Master level (60%). The number of years in clinical practice as an APP varied from 2 years to 16 years [
Table 2].
3.1. Medical Therapies for CHF Patients
During hospitalization, 98.75% of the CHF patients received diuretic therapy. Only one patient did not receive a diuretic in the hospital. This patient was admitted to cardiology service based on findings of lower extremities edema and elevated NT-proBNP level from the emergency department. The patient did not appear to be clinically volume overloaded on presentation by APP assessment. HHE was performed at the bedside which revealed preserved LVEF and normal IVC with normal inspiratory collapse. These findings were subsequently confirmed with SE. She was later found to be markedly hypokalemic due to a thiazide diuretic she was taking for hypertension, which was discontinued. Potassium was repleted and the patient was discharged home the following day.
3.1.1. Guideline-Directed Medical Therapy Among Study Patients:
Guideline-directed medical therapy for heart failure was optimized at the time of discharge Beta-blockers; pre admission 49 (61.25%), at discharge 57 (73.08%), angiotensin converting enzyme inhibitor, angiotensin renin blocker or angiotensin receptor/neprilysin inhibitor; pre-admission 48.75%, at-discharge 55.13%, diuretic; pre-admission 65%, at-discharge 88.46%, MRA; pre-admission 23.75%, at-discharge 47.44%, SGLT2i; pre-admission 3.75%, at-discharge 23.08%)]. For patients who could not tolerate being on angiotensin converting enzyme inhibitor, angiotensin renin blocker or angiotensin receptor/neprilysin inhibitor due to renal dysfunction, allergies, or intolerance, hydralazine and nitrate combination were administered (pre-admission 1.25% and at-discharge 3.85%) [
Table 3].
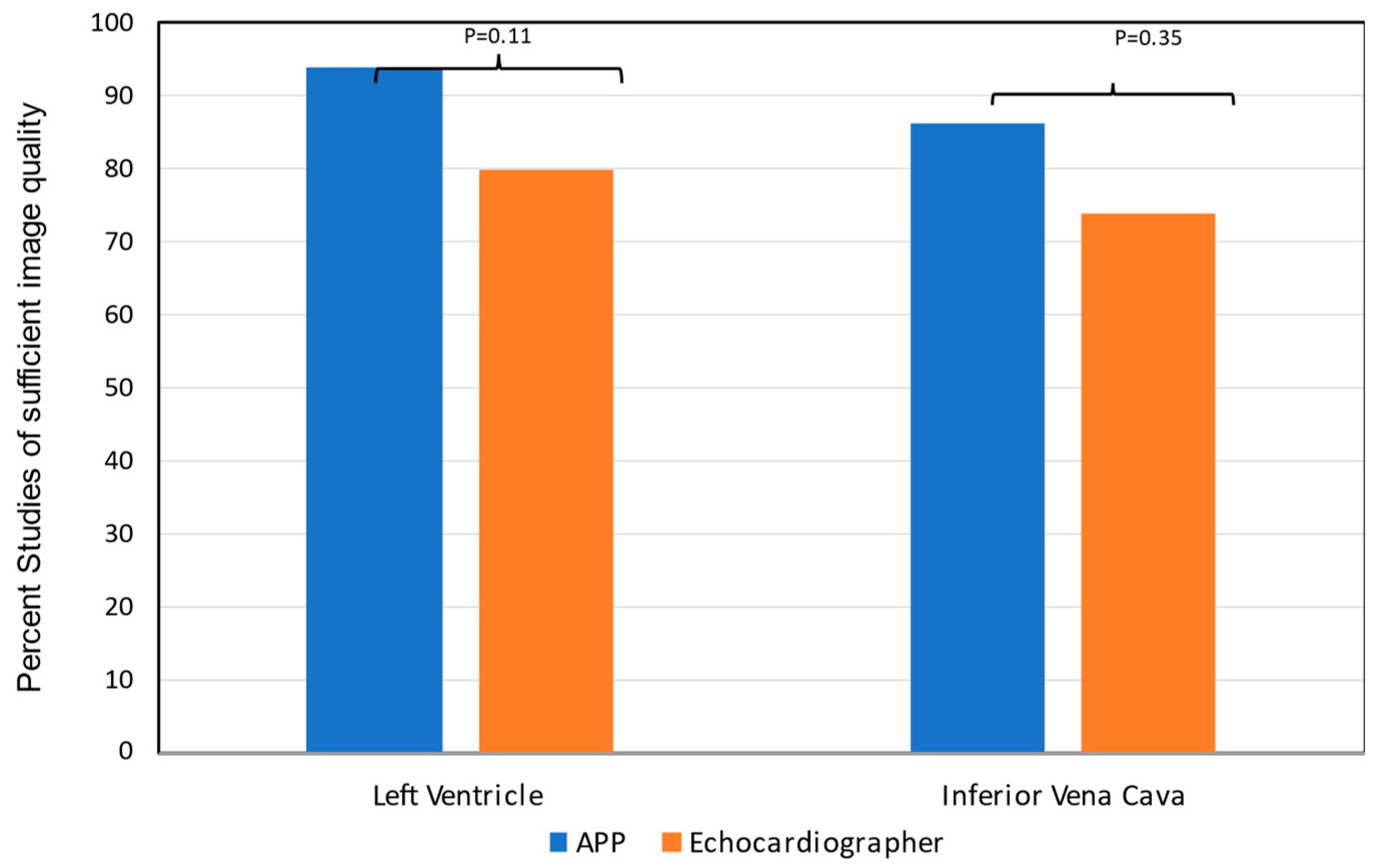
HHE image quality acquired by APPs was adequate to assess LV size (86%), LVEF (89%) and RAP (79%) as reviewed by the study echocardiographer (
Figure 2).
On HHE, 64% patients had LV enlargement, 51% had LVEF < 40%, and 71.4% had increased RAP as assessed by the study echocardiographer [
Table 4]. LVEF (r=0.79) and RAP (r=0.67) by APPs had good agreement with the study echocardiographer [
Table 4-top].
HHE exam was performed prior to SE in 79% of the patients [median 0.3 day]. LVEF assessment by APPs had good agreement (r=0.74) with SE but not for LV size and right atrial pressure (RAP) [
Table 4-bottom].
Absolute LVEF on HHE as assessed by the study echocardiographer correlated with LVEF by SE (r=0.88, p<0.0001) (
Figure 3).
The average length of stay for CHF patients enrolled in the study was 5.23 days compared to historical length of stay for patients with CHF of 8.1 days immediately prior to the initiation of the study.
4. Discussion
APPs without prior ultrasound experience, who received dedicated HHE didactic lectures and hands-on training were able to obtain diagnostic-quality images for evaluation of cardiac structure, cardiac function, and volume status assessment in hospitalized CHF patients. Additionally, trained APPs demonstrated the ability to interpret their HHE findings with good correlation to an expert study echocardiographer.
Our study demonstrates that with formal training, APPs can accurately utilize HHE to obtain and interpret LVEF and RAP. Furthermore, our findings suggest that the use of HHE by APPs upon admission for CHF patients can facilitate earlier decision making, optimization of medical therapy, and potentially reduce the length of hospital stay.
It should be noted that there was a variation in RAP between SE and HHE (K value 0.39). We suspect that this may be due to the lack of right ventricular and tricuspid regurgitation assessment with HHE, as well as SE images being obtained after initial treatment with a diuretic for CHF. However, there was a higher agreement between the study echocardiographer and SE for RAP (K value 0.67), indicating the effect of diuretic therapy on the differences in RAP between HHE and SE.
An important observation from the study is that the quality of images by HHE in the parasternal long axis and subcostal 4-chamber views allowed for visual assessment of numerical EF by an expert echocardiographer. This assessment showed excellent correlation of LVEF with SE. This suggests that even if APPs are not specifically trained to interpret LVEF, HHE image quality obtained by APPs may still be sufficient for expert interpretation of LVEF.
The study also showed an improvement in guideline-directed medical therapy for heart failure at the time of discharge, as well as a shorter average length of stay of 5.23 days compared to 8.1 days immediately prior to the initiation of the study. Our study supports previous finding by Lucas et al, who conducted a post hoc subgroup analysis that showed hospitalist care guided by HHE for cardiac assessment and management of CHF patients resulted in a reduced length of stay [
6,
13].
Trained medical residents have been shown to be able to distinguish LVEF at a 40% cut off range [
2]. This EF cut off range is important as it helps distinguish HFrEF from HFpEF and allows for early initiation of guideline-directed medical therapy. This is especially true for patients with presumed tachycardia-mediated cardiomyopathy due to atrial fibrillation. More than half of patients enrolled in the study were found to be in atrial fibrillation with rapid ventricular response. The presence of decreased LVEF with HHE in such patients allowed for the early restoration of sinus rhythm with chemical or electrical cardioversion.
Furthermore, evaluating cardiac function in atrial fibrillation with rapid ventricular response can pose challenges, as LVEF may appear reduced due to the fast heart rate during such episodes. However, the use of HHE for LVEF assessment, especially in patients who have undergone cardioversion (spontaneous, chemical, or electrical) can provide a relevant assessment of cardiac function in the immediate post cardioversion setting.
Serial cardiac assessment of LV function and volume status on HHE strongly predicts readmission for heart failure patients [
14]. While SE is the gold standard for cardiac assessment in such patients, it may not be readily available in the emergency department, at time of initial admission, in late hours, and on bedside rounds. HHE can be used at initial hospital admission with suspected CHF. Its use can be expanded for patient follow up during hospital stay as part of hospital rounds for daily assessment of diuretic dose along with other heart failure medications. This is particularly useful in patients who develop an increase in their serum creatinine despite significant volume overload, which may lead to discontinuation of guideline-directed medical therapy and prolonged hospital stay.
Conducting repeated assessments of cardiac size, function, and volume status through SE in hospitalized CHF patients may not be feasible and cost efficient while also increasing the workload of already overburdened echocardiography laboratories and cardiologists. It usually takes an average of 5 minutes to acquire images with HHE. These images provide vital information including LVEF, RAP, presence of pleural, and pericardial effusion, significant valvular heart disease, aortic pathologies to make treatment decision [15]. HHE is likely to decrease the use of SE for limited assessment of LVEF and RAP. It could potentially decrease the need for repeated radiological imaging as well as invasive hemodynamic monitoring in CHF patients. With increased use of HHE at the bedside and continued training, APPs may become more proficient with their HHE skills. Since the completion of this study, our trained APPs are routinely using HHE in hospitalized CHF patients.
APPs can also be trained on additional HHE views to identify pericardial and pleural effusions, as well as valvular abnormalities. Parasternal long axis and subcostal 4-chamber views may not only allow assessment of the presence and severity of pericardial effusion, but also provide 2D findings of cardiac tamponade, such as right ventricular and right atrial diastolic collapse.
There are indeed challenges that come with implementing new technology like HHE. Standardizing a training protocol for beginners and identifying models to practice on, are important components of training. Additionally, there are challenges in standardizing the documentation of HHE findings in the electronic medical record, as well as image storage and billing for the use of HHE. These are all areas that need to be addressed to fully integrate HHE into healthcare systems.
5. Limitations
There are several limitations to our study. Importantly, this study was undertaken during the COVID 19 pandemic. Alternating APP schedules with a busy hospital service limited our enrollment. Patients were also required to have the primary diagnosis of heart failure to enroll in the study. This excluded patients who were admitted with other primary diagnoses, but also had heart failure exacerbation. Another limitation was that the APPs performing the HHE were not permitted (per Institutional Review Board protocol) to consent patients - so additional time was needed to identify the patients and obtain consent by non-participating APPs.
Limited views were obtained, but despite this, a good agreement on LVEF compared with SE. We did not evaluate valvular heart disease in our study. While most HHE do not have spectral doppler capability, all devices allow visual assessment of valve regurgitation severity by color doppler, which is another parameter to follow in patients with significant valve regurgitation at time of admission. Diastolic function and pulmonary artery pressure were also not evaluated in the study due to the lack of pulsed wave or continuous wave doppler in the devices used. However, newer devices and software allow assessment of pulsed wave and continuous wave doppler.
5.1. Clinical Perspectives
This study validated the use of HHE by trained APPs in hospitalized CHF patients for assessment of LV function and RAP by IVC. Findings have implications on early recognition of cardiac function and volume status by first line care providers (APPs) and prompt initiation of treatment in hospitalized CHF patients which may lead to: a) early discharges and decrease length of hospital stay b) decrease workload burden on SE healthcare personnel, and c) reduce healthcare costs.
5.2. Clinical Competencies
Given increasing contribution of APPs to the cardiology work force, physician shortage and sky rocketing health care costs, our study findings suggest that ultrasound training and HHE use should be considered in the curriculum of APP providers via didactic lectures as well as hands on training. Scope of practice should be defined along with quality control to maintain competency to minimize patient harm.
5.3. Translational Outlook
Current enhancements with artificial intelligence technologies may allow guidance for proper image acquisition by transducer positioning, as well as artificial intelligence-guided interpretation of cardiac function, filling pressures and valve function. HHE imaging may be incorporated in the centered hospital care model.
Author Contributions
Maria-CecliaTagle-Cornell: Project administration, funding acquisition, writing study protocol, IRB submission and correspondence, obtained informed consent, supervision of study APPs, coordinating with TZN and SW for data collection and analysis, writing – original draft Preparation and revisions. Barbara Novais: Obtained informed consent, supervision of study APPs, writing – original draft Preparation and revisions, coordinating data analysis. Songnan Wen: Image processing and organization, data curation for HHE and SE images, formal data analysis. Justin Shipman: Assisted with hands on training of the study APPs. Deepa Mandale: Image processing and organization, training of APPs. Study APPs: Drew Flom: Sandeep Sahnan, Lindsey Kriz, Michelle Alland, Christen Bird. Methodology: Obtained training in performing HHE by didactic lectures, attending virtual course on HHE, performed 5 scans of adequate quality on random patients before study participation, performed HHE on study subjects. Tasneem Z Naqvi: Conceptualization and development of study protocol, assistance with obtaining grant funding, arranging equipment and software, APP training and approval for study participation, blind review of all HHE images for study quality and assessment of LV size, LVEF and RA pressure, supervision of the project and data analysis, review and editing of the manuscript versions. All authors have reviewed and approved the final version of the paper.
Funding
Mayo Clinic Department of Cardiovascular Medicine Advanced Practice Provider Grant.
Acknowledgments
We would like to acknowledge the Mayo Clinic Cardiovascular Research Center for selecting our pilot study and funding the Advance Practice Providers Grant.
Disclosures
No financial disclosures for any of the authors.
Abbreviations
| APPs |
Advanced practice providers |
| CHF |
Congestive heart failure |
| HFpEF |
Heart failure with preserve ejection fraction |
| HFrEF |
Heart failure with reduced ejection fraction |
| HHE |
Hand-held echocardiography |
| IVC |
Inferior vena cava |
| LV |
Left ventricular |
| LVEF |
LV ejection fraction |
| RAP |
Right atrial pressure |
| SE |
Standard Echocardiogram |
References
- Liebo, MJ, Israel RL, Lillie EO, Smith MR, Rubenson DS, Topol EJ. Is pocket mobile echocardiography the nest-generation stethoscope? A cross-section comparison of rapidly acquired images with standard transthoracic echocardiography. Ann Intern Med. 2011, 155, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patents admitted with decompensated heart failure. J Am Soc Echocardior. 2011, 24, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Hines AL, Barrett MS, Jiang J, Steiner C. Conditions with the largest number of adult hospital readmission by payer, 2011.
- Lucas BP, Candotti C, Margetta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011, 123, 766–774. [Google Scholar]
- Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J AM Soc Echocardiogr. 2013, 26, 567–581. [Google Scholar] [CrossRef] [PubMed]
- American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009, 53, 550–570. [Google Scholar] [CrossRef]
- Expert Round Table on Echocardiography in ICU. International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med. 2014, 40, 654–666. [Google Scholar] [CrossRef]
- Gundersen GH, Norekval TM, Haug HH, et al. Adding point of care ultrasound to assess volume status in heart failure patients in a nurse-led outpatient clinic. A randomised study. Heart 2016, 102, 29–34. [Google Scholar] [CrossRef]
- Available online: https://ce.mayo.edu/online-education/content/point-care-echocardiography-how-use-clinical-practice-online-cme-course.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J Am Soc Echocardiogr 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Lucas BP, Candotti C, Margetta B, at al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011, 123, 766–774. [Google Scholar]
- Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008, 1, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z. Point of Care Echocardiography – A case based visual guide; Elsevier, 2021; ISBN 9780323612845. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).