Submitted:
28 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
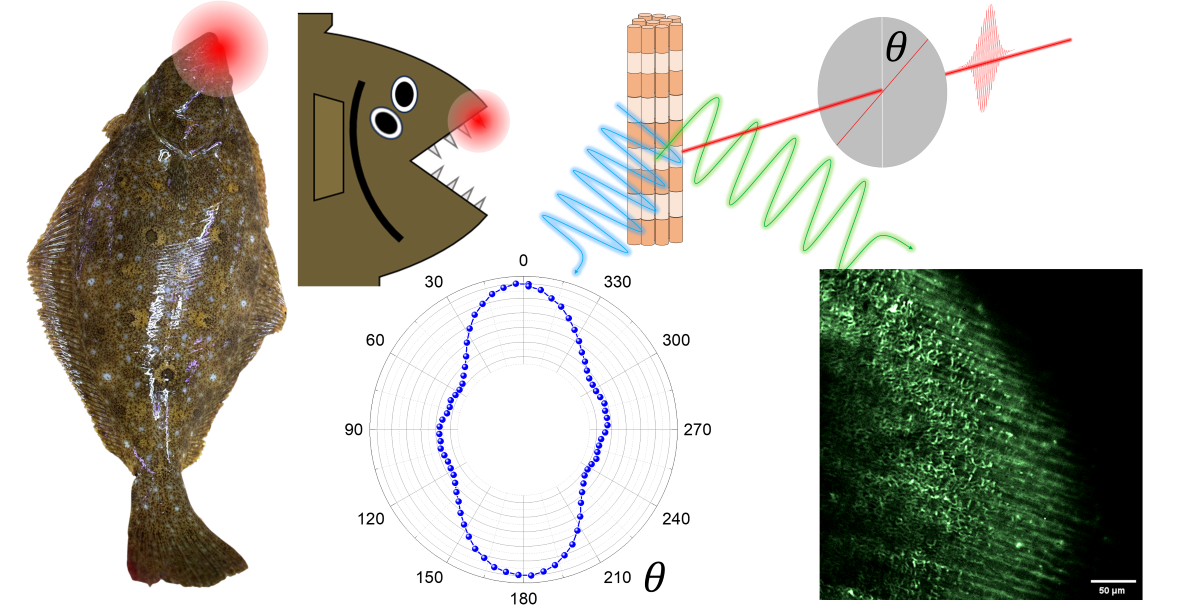
1. Introduction
2. Material and Method
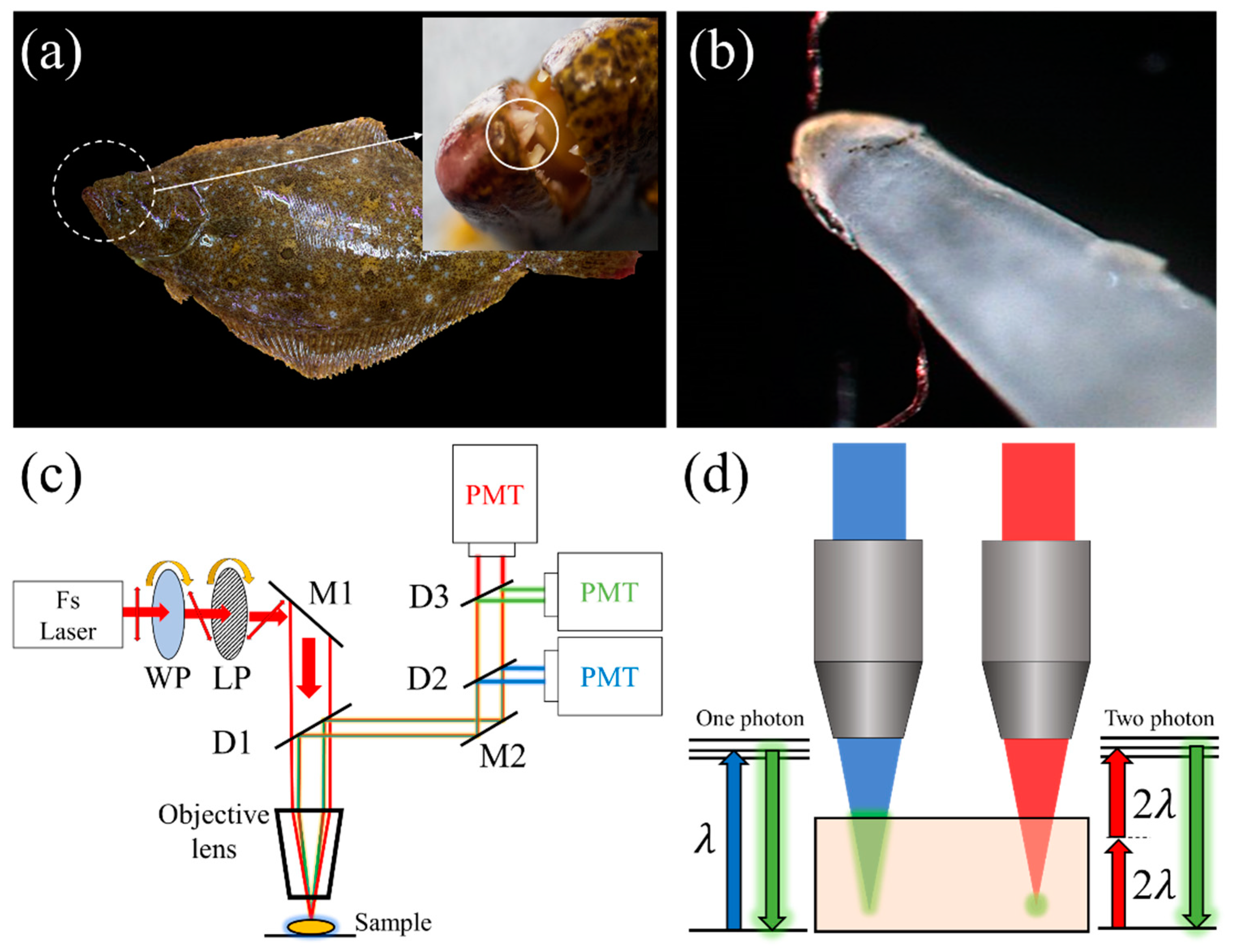
2.1. Sample Preparation
2.3. Nonlinear Optical Microscopy
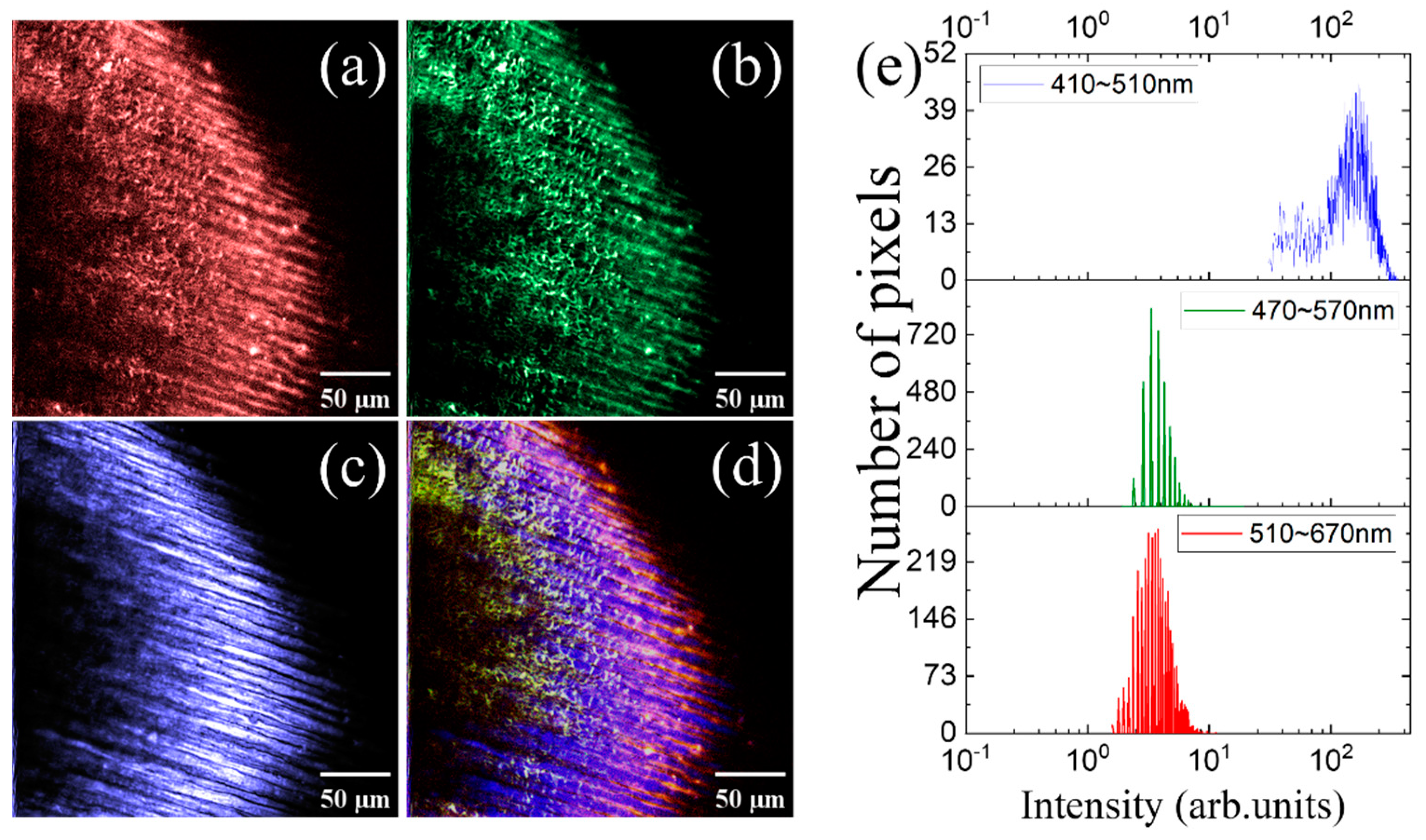
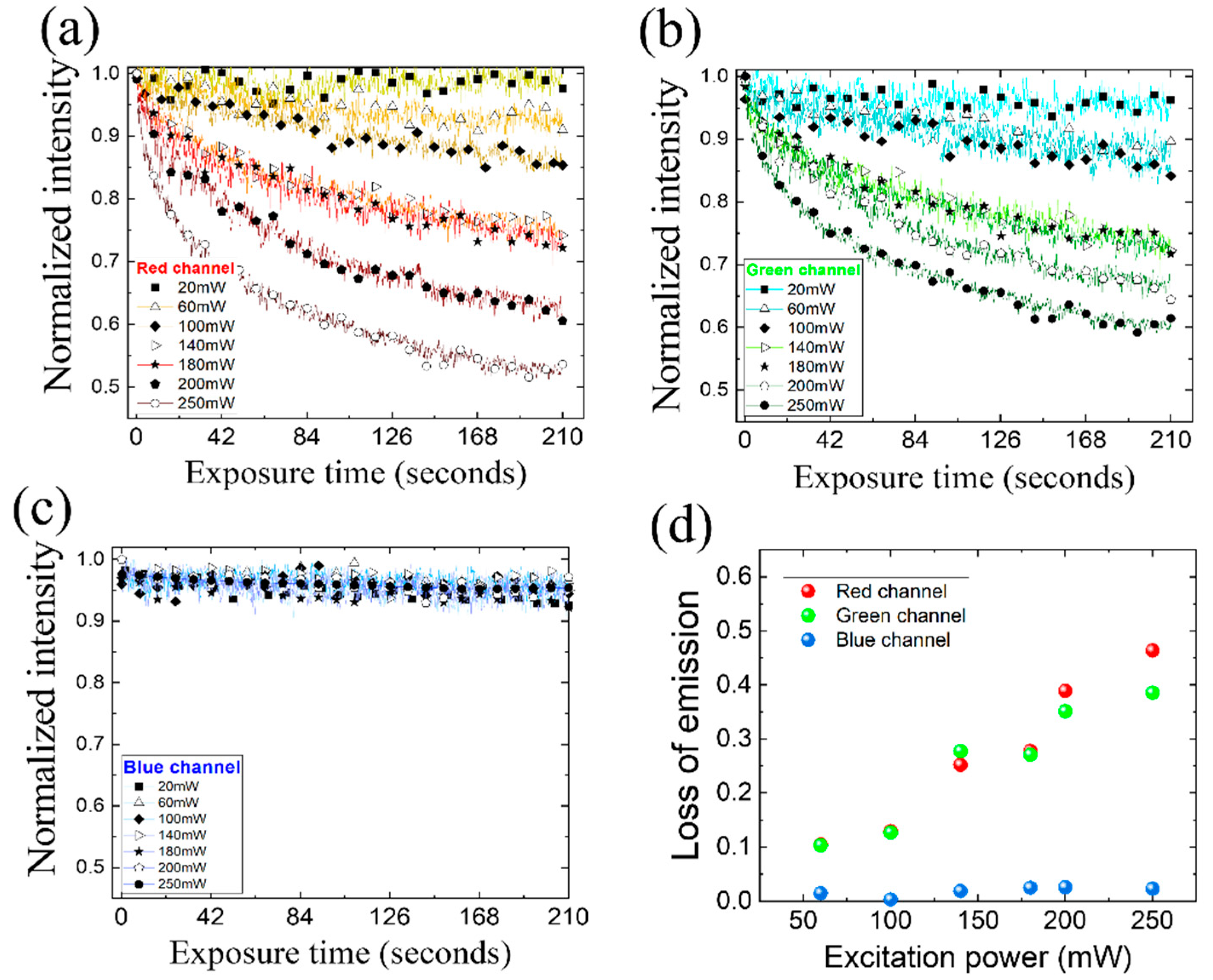
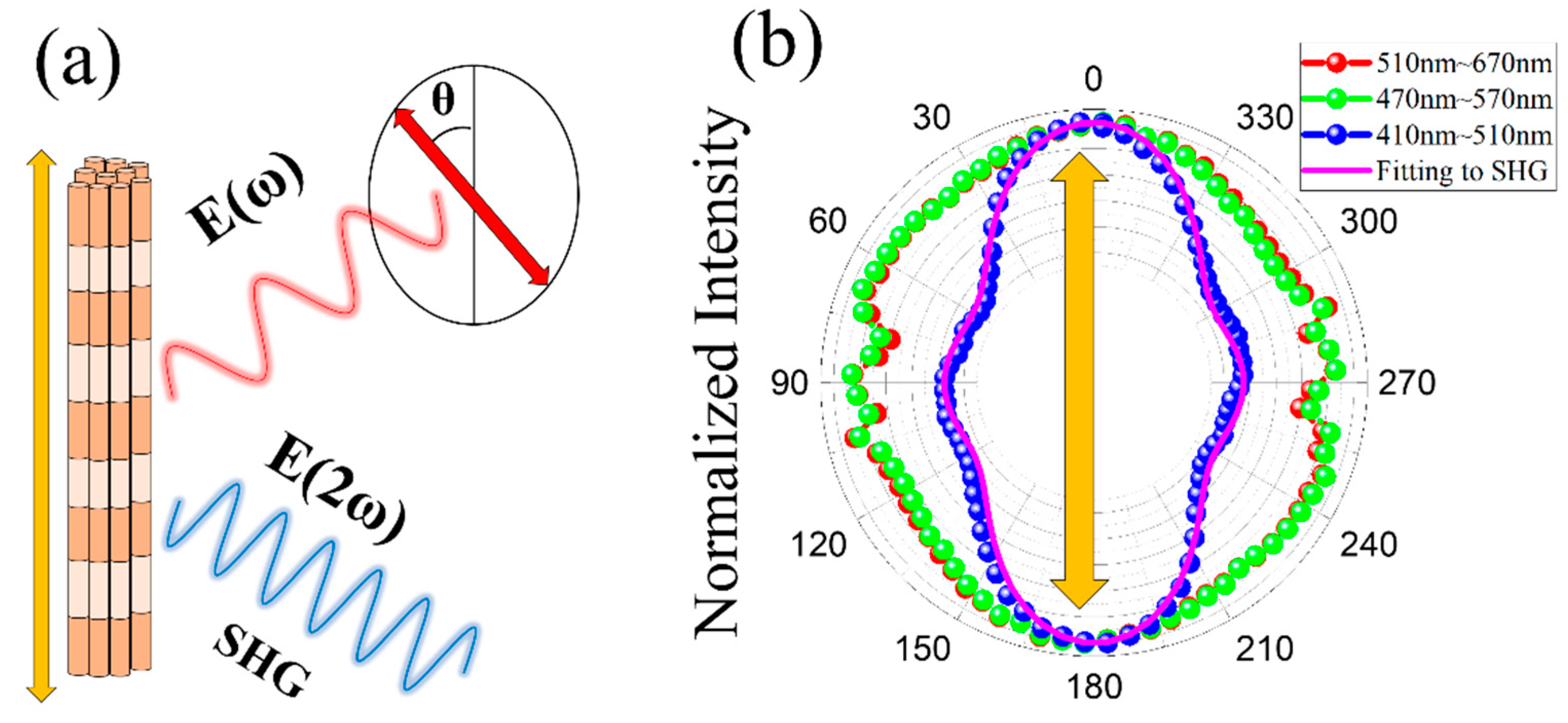
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Ethics Approval
References
- Pulin, M.; Stockhausen, K.E.; Masseck, O.A.; Kubitschke, M.; Busse, B.; Wiegert, J.S.; Oertner, T.G. Orthogonally-Polarized Excitation for Improved Two-Photon and Second-Harmonic-Generation Microscopy, Applied to Neurotransmitter Imaging with GPCR-Based Sensors. Biomed Opt Express 2022, 13, 777. [Google Scholar] [CrossRef]
- Chen, W.-L. Multiphoton Autofluorescence and Second-Harmonic Generation Imaging of the Tooth. J Biomed Opt 2007, 12, 064018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qu, J.Y. Two-Photon Autofluorescence Spectroscopy and Second-Harmonic Generation of Epithelial Tissue. Opt Lett 2005, 30, 3045. [Google Scholar] [CrossRef]
- Rubart, M. Two-Photon Microscopy of Cells and Tissue. Circ Res 2004, 95, 1154–1166. [Google Scholar] [CrossRef]
- Cisek, R.; Joseph, A.; Harvey, M.; Tokarz, D. Polarization-Sensitive Second Harmonic Generation Microscopy for Investigations of Diseased Collagenous Tissues. Front Phys 2021, 9. [Google Scholar] [CrossRef]
- Chu, S.-W.; Chen, S.-Y.; Chern, G.-W.; Tsai, T.-H.; Chen, Y.-C.; Lin, B.-L.; Sun, C.-K. Studies of χ(2)/χ(3) Tensors in Submicron-Scaled Bio-Tissues by Polarization Harmonics Optical Microscopy. Biophys J 2004, 86, 3914–3922. [Google Scholar] [CrossRef]
- Roth, S.; Freund, I. Second Harmonic Generation in Collagen. J Chem Phys 1979, 70, 1637–1643. [Google Scholar] [CrossRef]
- Thomas, B.; McIntosh, D.; Fildes, T.; Smith, L.; Hargrave, F.; Islam, M.; Thompson, T.; Layfield, R.; Scott, D.; Shaw, B.; et al. Second-Harmonic Generation Imaging of Collagen in Ancient Bone. Bone Rep 2017, 7, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Stoller, P.; Reiser, K.M.; Celliers, P.M.; Rubenchik, A.M. Polarization-Modulated Second Harmonic Generation in Collagen. Biophys J 2002, 82, 3330–3342. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Millard, A.C.; Terasaki, M.; Hoppe, P.E.; Malone, C.J.; Mohler, W.A. Three-Dimensional High-Resolution Second-Harmonic Generation Imaging of Endogenous Structural Proteins in Biological Tissues. Biophys J 2002, 82, 493–508. [Google Scholar] [CrossRef]
- Ugryumova, N.; Jacobs, J.; Bonesi, M.; Matcher, S.J. Novel Optical Imaging Technique to Determine the 3-D Orientation of Collagen Fibers in Cartilage: Variable-Incidence Angle Polarization-Sensitive Optical Coherence Tomography. Osteoarthritis Cartilage 2009, 17, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, M.; Grupe, G. Experimental Chemical Degradation Compared to Natural Diagenetic Alteration of Collagen: Implications for Collagen Quality Indicators for Stable Isotope Analysis. Archaeol Anthropol Sci 2009, 1, 43–57. [Google Scholar] [CrossRef]
- Schoeninger, M.J.; Moore, K.M.; Murray, M.L.; Kingston, J.D. Detection of Bone Preservation in Archaeological and Fossil Samples. Applied Geochemistry 1989, 4, 281–292. [Google Scholar] [CrossRef]
- Rubin, M.A.; Rubin, J.; Jasiuk, I. SEM and TEM Study of the Hierarchical Structure of C57BL/6J and C3H/HeJ Mice Trabecular Bone. Bone 2004, 35, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Szpak, P. Fish Bone Chemistry and Ultrastructure: Implications for Taphonomy and Stable Isotope Analysis. J Archaeol Sci 2011, 38, 3358–3372. [Google Scholar] [CrossRef]
- Marcu, L.; Grundfest, W.S.; Maarek, J.I. Photobleaching of Arterial Fluorescent Compounds: Characterization of Elastin, Collagen and Cholesterol Time-resolved Spectra during Prolonged Ultraviolet Irradiation. Photochem Photobiol 1999, 69, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Maggiano, C.; Dupras, T.; Schultz, M.; Biggerstaff, J. Spectral and Photobleaching Analysis Using Confocal Laser Scanning Microscopy: A Comparison of Modern and Archaeological Bone Fluorescence. Mol Cell Probes 2006, 20, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yova, D.; Hovhannisyan, V.; Theodossiou, T. Photochemical Effects and Hypericin Photosensitized Processes in Collagen. J Biomed Opt 2001, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, M.; Jeong, S.; Hwang, J.; Kim, J.; Gourrier, A.; Vial, J.C.; Kyhm, K. Autofluorescence Loss in Photobleaching for Human Dentin Ex Vivo. Current Optics and Photonics 2022, 6, 86–91. [Google Scholar] [CrossRef]
- Patterson, G.H.; Piston, D.W. Photobleaching in Two-Photon Excitation Microscopy. Biophys J 2000, 78, 2159–2162. [Google Scholar] [CrossRef]
- Stoller, P.; Kim, B.-M.; Rubenchik, A.M.; Reiser, K.M.; Da Silva, L.B. Polarization-Dependent Optical Second-Harmonic Imaging of a Rat-Tail Tendon. J Biomed Opt 2002, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Spiesz, E.M.; Kaminsky, W.; Zysset, P.K. A Quantitative Collagen Fibers Orientation Assessment Using Birefringence Measurements: Calibration and Application to Human Osteons. J Struct Biol 2011, 176, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Tiaho, F.; Recher, G.; Rouède, D. Estimation of Helical Angles of Myosin and Collagen by Second Harmonic Generation Imaging Microscopy. Opt Express 2007, 15, 12286. [Google Scholar] [CrossRef] [PubMed]
- Gusachenko, I.; Latour, G.; Schanne-Klein, M.-C. Polarization-Resolved Second Harmonic Microscopy in Anisotropic Thick Tissues. Opt Express 2010, 18, 19339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kemp, N.J.; Rylander, H.G.; Milner, T.E. Complex Polarization Ratio to Determine Polarization Properties of Anisotropic Tissue Using Polarization-Sensitive Optical Coherence Tomography. Opt Express 2009, 17, 13402. [Google Scholar] [CrossRef]
- Kemp, N.J.; Zaatari, H.N.; Park, J.; Rylander III, H.G.; Milner, T.E. Form-Biattenuance in Fibrous Tissues Measured with Polarization-Sensitive Optical Coherence Tomography (PS-OCT). Opt Express 2005, 13, 4611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




