Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. HCV-Host relationship: a mutual endurance
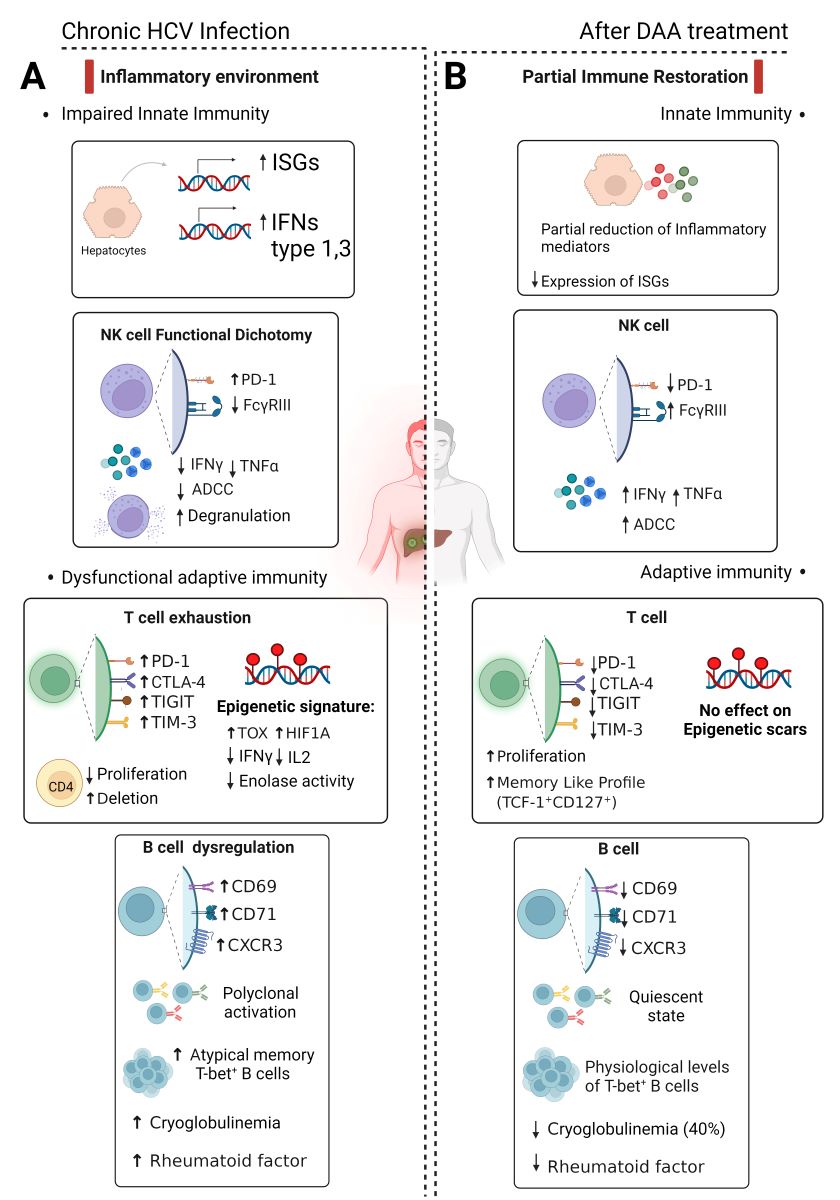
2.1. Innate immunity: friend or foe?
2.2. T-cell exhaustion disrupts adaptive immunity and sets the stage for HCV persistence
2.3. B-cell responses are not protective but play a role in lymphoproliferative disorders
3. Can direct-acting antivirals (DAAs) reverse HCV-altered immune responses?
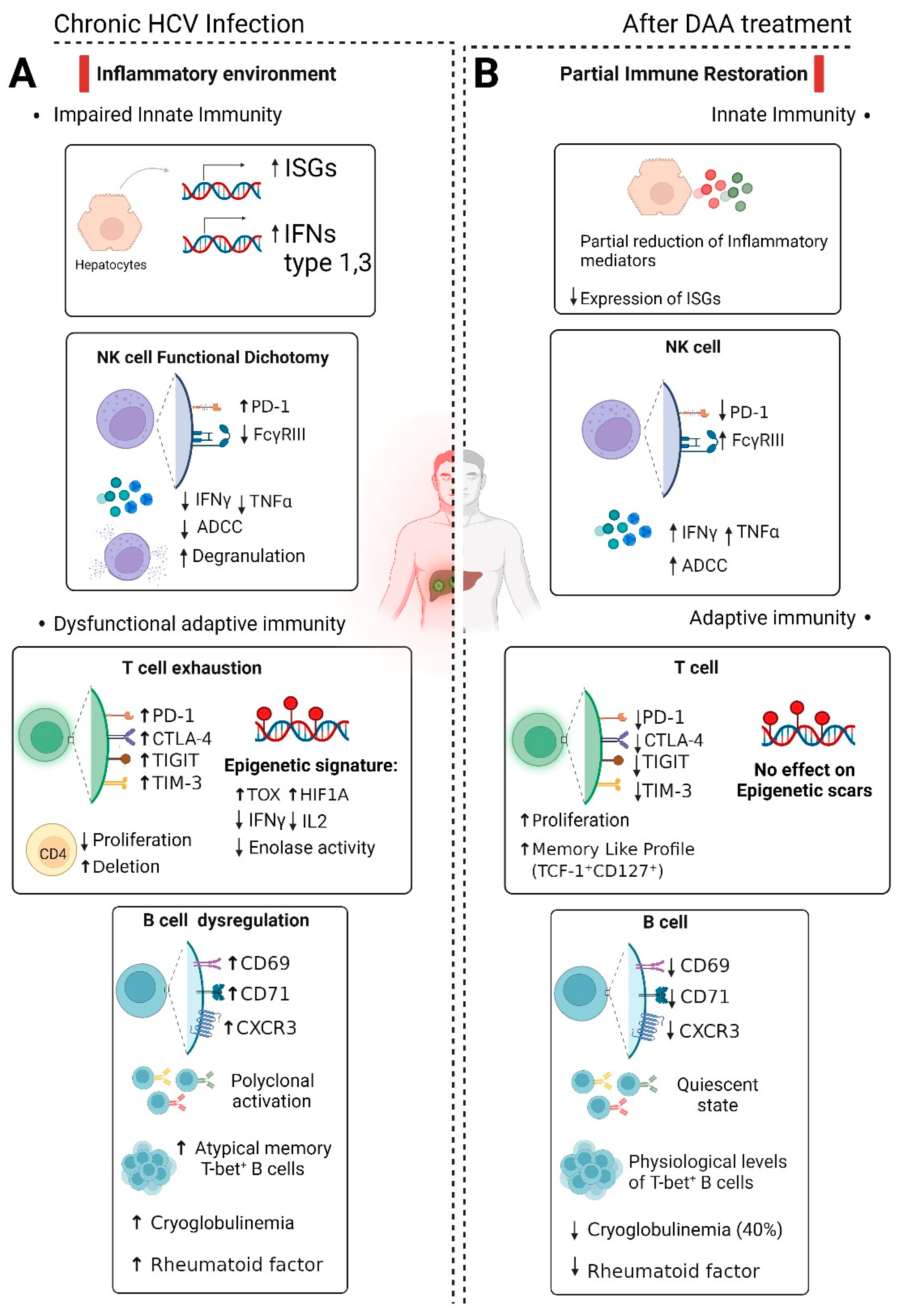
3.1. Effect of DAA treatment on liver inflammation and fibrosis
3.2. Controversial recovery of NK cell function after HCV cure
3.3. T-cell exhaustion is only partially reversed after HCV cure and leaves indelible epigenetic scars
3.4. B cell activation may be reversed by DAAs even though cryoglobulins may persist for years after recovery
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Martinello, M.; Naggie, S.; Rockstroh, J.; Matthews, G. V. Direct-Acting Antiviral Therapy for Treatment of Acute and Recent Hepatitis C Virus Infection: A Narrative Review. Clinical Infectious Diseases 2023, 77 (Supplement_3), S238–S244. [CrossRef]
- Ferrari, C.; Barili, V.; Varchetta, S.; Mondelli, M.U. Immune mechanisms of viral clearance and disease pathogenesis during viral hepatitis. In Liver Biology and Pathobiology, 6th ed.; Arias IM, Alter HJ, Boyer JL, Cohen DE, Shafritz DA, Thorgeirsson SS, Wolkoff AW, Eds.; Wiley Blackwell, Oxford UK, 2020; pp. 821-850.
- Thimme, R.; Oldach, D.; Chang, K.M.; Steiger, C.; Ray, S.C.; Chisari, F.V. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001, 19;194(10):1395-406. [CrossRef]
- Penna, A.; Pilli, M.; Zerbini, A.; Orlandini, A.; Mezzadri, S.; Sacchelli, L.; Missale, G.; Ferrari, C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007, 45(3):588-601. [CrossRef]
- Barili, V.; Fisicaro, P.; Montanini, B.; Acerbi, G.; Filippi, A.; Forleo, G.; Romualdi, C.; Ferracin, M.; Guerrieri, F.; Pedrazzi, G.; Boni, C.; Rossi, M.; Vecchi, A.; Penna, A.; Zecca, A.; Mori, C.; Orlandini, A.; Negri, E.; Pesci, M.; Massari, M.; Missale, G.; Levrero, M.; Ottonello, S.; Ferrari, C. Targeting P53 and Histone Methyltransferases Restores Exhausted CD8+ T Cells in HCV Infection. Nature Communications 2020, 11 (1). [CrossRef]
- Osburn, W.O.; Fisher, B.E.; Dowd, K.A.; Urban, G.; Liu, L.; Ray, S.C.; Thomas, D.L.; Cox, A.L. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010, 138(1):315-24. [CrossRef]
- Ono, A.; Goossens, N.; Finn, R.S.; Schmidt, W.N.; Thung, S.N.; Im, G.; Hoshida, Y. Precision Liver Cancer Prevention Consortium. Persisting risk of hepatocellular carcinoma after hepatitis C virus cure monitored by a liver transcriptome signature. Hepatology. 2017 66(4):1344-1346. [CrossRef]
- Romano, A.; Angeli, P.; Piovesan, S.; Noventa, F.; Anastassopoulos, G.; Chemello, L.; Cavalletto, L.; Gambato, M.; Russo, F. P.; Burra, P.; Vincenzi, V.; Scotton, P. G.; Panese, S.; Tempesta, D.; Bertin, T.; Carrara, M.; Carlotto, A.; Capra, F.; Carolo, G.; Scroccaro, G.; Alberti, A. Newly Diagnosed Hepatocellular Carcinoma in Patients with Advanced Hepatitis C Treated with DAAs: A Prospective Population Study. Journal of Hepatology 2018, 69 (2), 345–352. [CrossRef]
- Missale, G.; Pilli, M.; Zerbini, A.; Penna, A.; Ravanetti, L.; Barili, V.; Orlandini, A.; Molinari, A.; Fasano, M.; Santantonio, T.; Ferrari, C. Lack of Full CD8 Functional Restoration after Antiviral Treatment for Acute and Chronic Hepatitis C Virus Infection. Gut 2012, 61 (7), 1076–1084. [CrossRef]
- Abdel-Hakeem, M. S.; Bédard, N.; Badr, G.; Ostrowski, M.; Sékaly, R. P.; Bruneau, J.; Willems, B.; Heathcote, E. J.; Shoukry, N. H. Comparison of Immune Restoration in Early versus Late Alpha Interferon Therapy against Hepatitis C Virus. Journal of Virology 2010, 84 (19), 10429–10435. [CrossRef]
- Heim, M. H.; Thimme, R. Innate and Adaptive Immune Responses in HCV Infections. Journal of Hepatology 2014, 61 (1), S14–S25. [CrossRef]
- Kawai, T.; Akira, S. The Roles of TLRs, RLRs and NLRs in Pathogen Recognition. International Immunology 2009, 21 (4), 317–337. [CrossRef]
- Su, A. I.; Pezacki, J. P.; Wodicka, L.; Brideau, A. D.; Supeková, L.; Thimme, R.; Wieland, S.; Bukh, J.; Purcell, R. H.; Schultz, P. G.; Chisari, F. V. Genomic Analysis of the Host Response to Hepatitis C Virus Infection. Proceedings of the National Academy of Sciences of the United States of America 2002, 99 (24), 15669–15674. [CrossRef]
- Loo, Y.; Owen, D. M.; Li, K.; Erickson, A. K.; Johnson, C.; Fish, P. M.; Carney, D. S.; Wang, T.; Ishida, H.; Yoneyama, M.; Fujita, T.; Saito, T.; Lee, W. M.; Hagedorn, C. H.; Lau, D. T. Y.; Weinman, S. A.; Lemon, S. M.; Gale, M. Viral and Therapeutic Control of IFN-β Promoter Stimulator 1 during Hepatitis C Virus Infection. Proceedings of the National Academy of Sciences of the United States of America 2006, 103 (15), 6001-6006. [CrossRef]
- Horner, S. M.; Liu, H. M.; Park, H. S.; Briley, J.; Gale, M. Mitochondrial-Associated Endoplasmic Reticulum Membranes (MAM) Form Innate Immune Synapses and Are Targeted by Hepatitis C Virus. Proceedings of the National Academy of Sciences of the United States of America 2011, 108 (35), 14590- 14595. [CrossRef]
- Oshiumi, H.; Miyashita, M.; Matsumoto, M.; Seya, T. A Distinct Role of Riplet-Mediated K63-Linked Polyubiquitination of the RIG-I Repressor Domain in Human Antiviral Innate Immune Responses. PLOS Pathogens 2013, 9 (8), e1003533. [CrossRef]
- Vazquez, C.; Tan, C. Y.; Horner, S. M. Hepatitis C Virus Infection Is Inhibited by a Noncanonical Antiviral Signaling Pathway Targeted by NS3-NS4A. Journal of Virology 2019, 93 (23). [CrossRef]
- Otsuka, M.; Kato, N.; Moriyama, M.; Taniguchi, H.; Wang, Y.; Dharel, N.; Kawabe, T.; Omata, M. Interaction between the HCV NS3 Protein and the Host TBK1 Protein Leads to Inhibition of Cellular Antiviral Responses. Hepatology 2005, 41 (5), 1004–1012. [CrossRef]
- Nitta, S.; Sakamoto, N.; Nakagawa, M.; Kakinuma, S.; Mishima, K.; Kusano-Kitazume, A.; Kiyohashi, K.; Murakawa, M.; Nishimura-Sakurai, Y.; Azuma, S.; Tasaka-Fujita, M.; Asahina, Y.; Yoneyama, M.; Fujita, T.; Watanabe, M. Hepatitis C Virus NS4B Protein Targets STING and Abrogates RIG-I-Mediated Type I Interferon-Dependent Innate Immunity. Hepatology 2013, 57 (1), 46–58. [CrossRef]
- Ding, Q.; Cao, X.; Lu, J.; Huang, B.; Liu, Y. J.; Kato, N.; Shu, H.; Zhong, J. Hepatitis C Virus NS4B Blocks the Interaction of STING and TBK1 to Evade Host Innate Immunity. Journal of Hepatology 2013, 59 (1), 52–58. [CrossRef]
- Liang, Y.; Cao, X.; Ding, Q.; Zhao, Y.; He, Z.; Zhong, J. Hepatitis C Virus NS4B Induces the Degradation of TRIF to Inhibit TLR3-Mediated Interferon Signaling Pathway. PLOS Pathogens 2018, 14 (5), e1007075. [CrossRef]
- Hiet, M.; Bauhofer, O.; Zayas, M.; Roth, H.; Tanaka, Y.; Schirmacher, P.; Willemsen, J.; Grünvogel, O.; Bender, S.; Binder, M.; Lohmann, V.; Lotteau, V.; Ruggieri, A.; Bartenschlager, R. Control of Temporal Activation of Hepatitis C Virus-Induced Interferon Response by Domain 2 of Nonstructural Protein 5A. Journal of Hepatology 2015, 63 (4), 829–837. [CrossRef]
- Çevik, R. E.; Cesarec, M.; Da Silva Filipe, A.; Licastro, D.; McLauchlan, J.; Marcello, A. Hepatitis C Virus NS5A Targets Nucleosome Assembly Protein NAP1L1 To Control the Innate Cellular Response. Journal of Viro ogy 2017, 91 (18). [CrossRef]
- Refolo, G.; Ciccosanti, F.; Di Rienzo, M.; Perdomo, A. B.; Romani, M.; Alonzi, T.; Tripodi, M.; Ippolito, G.; Piacentini, M.; Fimia, G. M. Negative Regulation of Mitochondrial Antiviral Signaling Protein–Mediated Antiviral Signaling by the Mitochondrial Protein LRPPRC during Hepatitis C Virus Infection. Hepatology 2018, 69 (1), 34–50. [CrossRef]
- Heim, M. H.; Moradpour, D.; Blum, H. E. Expression of Hepatitis C Virus Proteins Inhibits Signal Transduction through the Jak-STAT Pathway. Journal of Virology 1999, 73 (10), 8469–8475. [CrossRef]
- Lin, W.; Kim, S. S.; Yeung, E.; Kamegaya, Y.; Blackard, J. T.; Kim, K.-A.; Holtzman, M. J.; Chung, R. T. Hepatitis C Virus Core Protein Blocks Interferon Signaling by Interaction with the STAT1 SH2 Domain. Journal of Virology 2006, 80 (18), 9226–9235. [CrossRef]
- Lin, W.; Choe, W. H.; Hiasa, Y.; Kamegaya, Y.; Blackard, J. T.; Schmidt, E. V.; Chung, R. T. Hepatitis C Virus Expression Suppresses Interferon Signaling by Degrading STAT1. Gastroenterology 2005, 128 (4), 1034–1041. [CrossRef]
- Taylor, D. J.; Shi, S. T.; Romano, P. R.; Barber, G. N.; Lai, M. M. C. Inhibition of the Interferon- Inducible Protein Kinase PKR by HCV E2 Protein. Science 1999, 285 (5424), 107–110. [CrossRef]
- Qi, H.; Chu, V.; Wu, N. C.; Chen, Z.; Truong, S.; Brar, G.; Su, S.; Du, Y.; Arumugaswami, V.; Olson, C. A.; Chen, S. H.; Lin, C. Y.; Wu, T.; Sun, R. Systematic Identification of Anti-Interferon Function on Hepatitis C Virus Genome Reveals P7 as an Immune Evasion Protein. Proceedings of the National Academy of Sciences of the United States of America 2017, 114 (8), 2018–2023. [CrossRef]
- Rehermann, B.; Nascimbeni, M. Immunology of Hepatitis B Virus and Hepatitis C Virus Infection. Nature Reviews Immunology 2005, 5 (3), 215–229. [CrossRef]
- Gale, M.; Foy, E. Evasion of Intracellular Host Defence by Hepatitis C Virus. Nature 2005, 436 (7053), 939–945. [CrossRef]
- Neumann-Haefelin, C.; Thimme, R. Success and Failure of Virus-Specific T Cell Responses in Hepatitis C Virus Infection. Digestive Diseases 2011, 29 (4), 416–422. [CrossRef]
- Hartnell, F.; Esposito, I.; Swadling, L.; Brown, A.; Phetsouphanh, C.; De Lara, C.; Gentile, C.; Turner, B. L.; Dorrell, L.; Capone, S.; Folgori, A.; Barnes, E.; Klenerman, P. Characterizing Hepatitis C Virus–Specific CD4+ T Cells Following Viral-Vectored Vaccination, Directly Acting Antivirals, and Spontaneous Viral Cure. Hepatology 2020, 72 (5), 1541–1555. [CrossRef]
- Ramalingam, R.; Meyer-Olson, D.; Shoukry, N. H.; Bowen, D. G.; Walker, C. M.; Kalams, S. A. Kinetic Analysis by Real-Time PCR of Hepatitis C Virus (HCV)-Specific T Cells in Peripheral Blood and Liver after Challenge with HCV. Journal of Virology 2008, 82 (21), 10487–10492. [CrossRef]
- Wolski, D.; Foote, P. K.; Chen, D. Y.; Lewis-Ximenez, L. L.; Fauvelle, C.; Aneja, J.; Walker, A.; Tonnerre, P.; Torres-Cornejo, A.; Kvistad, D.; Imam, S.; Waring, M. T.; Tully, D. C.; Allen, T. M.; Chung, R. T.; Timm, J.; Haining, W. N.; Kim, A. Y.; Baumert, T. F.; Lauer, G. M. Early Transcriptional Divergence Marks Virus-Specific Primary Human CD8+ T Cells in Chronic versus Acute Infection. Immunity 2017, 47 (4), 648-663.e8. [CrossRef]
- Wiesch, J. S. Z.; Ciuffreda, D.; Lewis-Ximenez, L. L.; Kasprowicz, V.; Nolan, B. E.; Streeck, H.; Aneja, J.; Reyor, L. L.; Allen, T. M.; Lohse, A. W.; McGovern, B.; Chung, R. T.; Kwok, W. W.; Kim, A. Y.-S.; Lauer, G. M. Broadly Directed Virus-Specific CD4+ T Cell Responses Are Primed during Acute Hepatitis C Infection, but Rapidly Disappear from Human Blood with Viral Persistence. Journal of Experimental Medicine 2012, 209 (1), 61–75. [CrossRef]
- Wildner, N. H.; Walker, A.; Brauneck, F.; Ditt, V.; Peine, S.; Huber, S.; Haag, F.; Beisel, C.; Timm, J.; Wiesch, J. S. Z. Transcriptional Pattern Analysis of Virus-Specific CD8+ T Cells in Hepatitis C Infection: Increased Expression of TOX and Eomesodermin during and after Persistent Antigen Recognition. Frontiers in Immunology 2022, 13. [CrossRef]
- Jenkins, E.; Whitehead, T.; Fellermeyer, M.; Davis, S. J.; Sharma, S. The Current State and Future of T-Cell Exhaustion Research. Oxford Open Immunology 2023, 4 (1). [CrossRef]
- Gardiner, D. F.; Lalezari, J.; Lawitz, E.; DiMicco, M. A.; Ghalib, R.; Reddy, K. R.; Chang, K.; Sulkowski, M. S.; Marro, S. O.; Anderson, J.; He, B.; Kansra, V.; McPhee, F.; Wind-Rotolo, M.; Grasela, D. M.; Selby, M.; Korman, A. J.; Lowy, I. A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients with Chronic Hepatitis C Virus Infection. PLOS ONE 2013, 8 (5), e63818. [CrossRef]
- Sangro, B.; Gómez-Martín, C.; De La Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J. I.; Larrea, E.; Alfaro, C.; Sarobe, P.; Lasarte, J. J.; Pérez-Gracia, J. L.; Melero, I.; Prieto, J. A Clinical Trial of CTLA-4 Blockade with Tremelimumab in Patients with Hepatocellular Carcinoma and Chronic Hepatitis C. Journal of Hepatology 2013, 59 (1), 81–88. [CrossRef]
- Takaki, A.; Wiese, M.; Maertens, G.; Depla, E.; Seifert, U.; Liebetrau, A.; Miller, J. L.; Manns, M. P.; Rehermann, B. Cellular Immune Responses Persist and Humoral Responses Decrease Two Decades after Recovery from a Single-Source Outbreak of Hepatitis C. Nature Medicine 2000, 6 (5), 578–582. [CrossRef]
- Cashman, S. B.; Marsden, B. D.; Dustin, L. B. The Humoral Immune Response to HCV: Understanding Is Key to Vaccine Development. Frontiers in Immunology 2014, 5. [CrossRef]
- Page, K.; Melia, M. T.; Veenhuis, R. T.; Winter, M. E.; Rousseau, K. E.; Massaccesi, G.; Osburn, W. O.; Forman, M.; Thomas, E. D.; Thornton, K.; Wagner, K.; Vassilev, V.; Lin, L.; Lum, P. J.; Giudice, L. C.; Stein, E.; Asher, A.; Chang, S.; Gorman, R. L.; Ghany, M. G.; Liang, T. J.; Wierzbicki, M. R.; Scarselli, E.; Nicosia, A.; Folgori, A.; Capone, S.; Cox, A. L. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. The New England Journal of Medicine 2021, 384 (6), 541–549. [CrossRef]
- Rosa, D.; Saletti, G.; De Gregorio, E.; Zorat, F.; Comar, C.; D’Oro, U.; Nuti, S.; Houghton, M.; Barnaba, V.; Pozzato, G.; Abrignani, S. Activation of Naïve B Lymphocytes via CD81, a Pathogenetic Mechanism for Hepatitis C Virus-Associated B Lymphocyte Disorders. Proceedings of the National Academy of Sciences of the United States of America 2005, 102 (51), 18544–18549. [CrossRef]
- Oliviero, B.; Cerino, A.; Varchetta, S.; Paudice, E.; Pai, S. S.; Ludovisi, S.; Zaramella, M.; Michelone, G.; Pugnale, P.; Negro, F.; Barnaba, V.; Mondelli, M. U. Enhanced B-Cell Differentiation and Reduced Proliferative Capacity in Chronic Hepatitis C and Chronic Hepatitis B Virus Infections. Journal of Hepatology 2011, 55 (1), 53–60. [CrossRef]
- Oliviero, B.; Mantovani, S.; Ludovisi, S.; Varchetta, S.; Mele, D.; Paolucci, S.; Baldanti, F.; Mondelli, M. U. Skewed B Cells in Chronic Hepatitis C Virus Infection Maintain Their Ability to Respond to Virus-induced Activation. Journal of Viral Hepatitis 2014, 22 (4), 391–398. [CrossRef]
- Sugalski, J. M.; Rodrı́Guez, B.; Moir, S.; Anthony, D. D. Peripheral Blood B Cell Subset Skewing Is Associated with Altered Cell Cycling and Intrinsic Resistance to Apoptosis and Reflects a State of Immune Activation in Chronic Hepatitis C Virus Infection. Journal of Immunology 2010, 185 (5), 3019–3027. [CrossRef]
- Friedman, S. L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134 (6), 1655 1669. [CrossRef]
- Holmes, J. A.; Carlton-Smith, C.; Kim, A. Y.; Dumas, E. O.; Brown, J.; Gustafson, J.; Lauer, G. M.; Silva, S. T.; Robidoux, M.; Kvistad, D.; Alatrakchi, N.; Tonnerre, P.; Cohen, D. E.; Zhang, H.; Shulman, N. S.; Chung, R. T. Dynamic Changes in Innate Immune Responses during Direct-Acting Antiviral Therapy for HCV Infection. Journal of Viral Hepatitis 2019, 26 (3), 362–372. [CrossRef]
- Owusu Sekyere S, Suneetha PV, Hardtke S, Falk CS, Hengst J, Manns MP, Cornberg M, Wedemeyer H, Schlaphoff V. Type I Interferon Elevates Co-Regulatory Receptor Expression on CMV- and EBV-Specific CD8 T Cells in Chronic Hepatitis C. Front Immunol. 2015 Jun 10;6:270. [CrossRef]
- Hengst, J.; Falk, C. S.; Schlaphoff, V.; Deterding, K.; Manns, M. P.; Cornberg, M.; Wedemeyer, H. Direct-Acting Antiviral–Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients with Chronic Hepatitis C. The Journal of Infectious Diseases 2016, 214 (12), 1965–1974. [CrossRef]
- Khera, T.; Du, Y.; Todt, D.; Deterding, K.; Strunz, B.; Hardtke, S.; Aregay, A.; Port, K.; Hardtke-Wolenski, M.; Steinmann, E.; Björkström, N. K.; Manns, M. P.; Hengst, J.; Cornberg, M.; Wedemeyer, H. Long-Lasting Imprint in the Soluble Inflammatory Milieu despite Early Treatment of Acute Symptomatic Hepatitis C. The Journal of Infectious Diseases 2021, 226 (3), 441–452. [CrossRef]
- Aregay, A.; Engel, B.; Port, K.; Vondran, F. W. R.; Bremer, B.; Niehaus, C.; Khera, T.; Richter, N.; Jaeckel, E.; Cornberg, M.; Taubert, R.; Wedemeyer, H. Distinct Immune Imprints of Post–Liver Transplantation Hepatitis C Persist despite Viral Clearance. Liver Transplantation 2021, 27 (6), 887–899. [CrossRef]
- Sekyere, S. O.; Port, K.; Deterding, K.; Cornberg, M.; Wedemeyer, H. Inflammatory Patterns in Plasma Associate with Hepatocellular Carcinoma Development in Cured Hepatitis C Cirrhotic Patients. United European Gastroenterology Journal 2021, 9 (4), 486–496. [CrossRef]
- Debes, J. D.; Van Tilborg, M.; Groothuismink, Z. M. A.; Hansen, B. E.; Wiesch, J. S. Z.; Von Felden, J.; De Knegt, R. J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154 (3), 515-517.e3. [CrossRef]
- Knop, V.; Hoppe, D.; Welzel, T. M.; Vermehren, J.; Herrmann, E.; Vermehren, A.; Friedrich-Rust, M.; Sarrazin, C.; Zeuzem, S.; Welker, M.-W. Regression of Fibrosis and Portal Hypertension in HCV-Associated Cirrhosis and Sustained Virologic Response after Interferon-Free Antiviral Therapy. Journal of Viral Hepatitis 2016, 23 (12), 994–1002. [CrossRef]
- Bachofner, J. A.; Valli, P. V.; Kröger, A.; Bergamin, I.; Künzler, P.; Baserga, A.; Braun, D. L.; Seifert, B.; Moncsek, A.; Fehr, J.; Semela, D.; Magenta, L.; Müllhaupt, B.; Beretta-Piccoli, B. T.; Mertens, J. C. Direct Antiviral Agent Treatment of Chronic Hepatitis C Results in Rapid Regression of Transient Elastography and Fibrosis Markers Fibrosis-4 Score and Aspartate Aminotransferase-platelet Ratio Index. Liver International 2016, 37 (3), 369–376. [CrossRef]
- Rosato, V.; Ascione, A.; Nevola, R.; Fracanzani, A. L.; Piai, G.; Messina, V.; Claar, E.; Coppola, C.; Fontanella, L.; Lombardi, R.; Staiano, L.; Valente, G.; Fascione, M. C.; Giorgione, C.; Mazzocca, A.; Galiero, R.; Perillo, P.; Marrone, A.; Sasso, F. C.; Adinolfi, L. E.; Rinaldi, L. Factors Affecting Long-term Changes of Liver Stiffness in Direct-acting Anti-hepatitis C Virus Therapy: A Multicentre Prospective Study. Journal of Viral Hepatitis 2021, 29 (1), 26–34. [CrossRef]
- Yoo, H. W.; Park, J. Y.; Kim, S. G.; Jung, Y. K.; Lee, S. H.; Kim, M. Y.; Jun, D. W.; Jang, J. Y.; Lee, J. W.; Kwon, O. S. Regression of Liver Fibrosis and Hepatocellular Carcinoma Development after HCV Eradication with Oral Antiviral Agents. Scientific Reports 2022, 12 (1). [CrossRef]
- Salomone, F.; Petta, S.; Micek, A.; Pipitone, R. M.; Distefano, A.; Castracani, C. C.; Rini, F.; Di Rosa, M.; Gardi, C.; Calvaruso, V.; Di Marco, V.; Volti, G. L.; Grimaudo, S. Hepatitis C Virus Eradication by Direct Antiviral Agents Abates Oxidative Stress in Patients with Advanced Liver Fibrosis. Liver International 2020, 40 (11), 2820–2827. [CrossRef]
- Suzuki, T.; Matsuura, K.; Nagura, Y.; Ogawa, S.; Fujiwara, K.; Nojiri, S.; Watanabe, T.; Kataoka, H.; Tanaka, Y. Serum Angiopoietin-2 Levels Predict Regression of Mac-2 Binding Protein Glycosylation Isomer-based Liver Fibrosis after Hepatitis C Virus Eradication by Direct-acting Antiviral Agents. Hepatology Research 2022, 52 (11), 919–927. [CrossRef]
- Nagura, Y.; Suzuki, T.; Matsuura, K.; Ogawa, S.; Kawamura, H.; Kuno, K.; Fujiwara, K.; Nojiri, S.; Nonomura, K.; Iio, E.; Watanabe, T.; Kataoka, H.; Tanaka, Y. Serum Inducible Protein 10 kDa/C-X-C Motif Chemokine 10 Levels Predict Regression of M2BPGi-based Liver Fibrosis after Hepatitis C Virus Eradication by Direct-acting Antiviral Agents. Hepatology Research 2023. [CrossRef]
- Ferreira, J.; Oliveira, M. J. S.; Bicho, M.; Serejo, F. Role of Inflammatory/Immune Response and Cytokine Polymorphisms in the Severity of Chronic Hepatitis C (CHC) before and after Direct Acting Antiviral (DAAs) Treatment. International Journal of Molecular Sciences 2023, 24 (2), 1380. [CrossRef]
- Amadei, B.; Urbani, S.; Cazaly, A.; Fisicaro, P.; Zerbini, A.; Ahmed, P. S.; Missale, G.; Ferrari, C.; Khakoo, S. I. Activation of Natural Killer Cells during Acute Infection with Hepatitis C Virus. Gastroenterology 2010, 138 (4), 1536–1545. [CrossRef]
- Oliviero, B.; Varchetta, S.; Paudice, E.; Michelone, G.; Zaramella, M.; Mavilio, D.; Filippi, F.; Bruno, S.; Mondelli, M. U. Natural Killer Cell Functional Dichotomy in Chronic Hepatitis B and Chronic Hepatitis C Virus Infections. Gastroenterology 2009, 137 (3), 1151-1160.e7. [CrossRef]
- Miyagi, T.; Takehara, T.; Nishio, K.; Shimizu, S.; Kohga, K.; Li, W.; Tatsumi, T.; Hiramatsu, N.; Kanto, T.; Hayashi, N. Altered Interferon-α-Signaling in Natural Killer Cells from Patients with Chronic Hepatitis C Virus Infection. Journal of Hepatology 2010, 53 (3), 424–430. [CrossRef]
- Ahlenstiel, G.; Titerence, R.; Koh, C.; Edlich, B.; Feld, J. J.; Rotman, Y.; Ghany, M. G.; Hoofnagle, J. H.; Liang, T. J.; Heller, T.; Rehermann, B. Natural Killer Cells Are Polarized toward Cytotoxicity in Chronic Hepatitis C in an Interferon-Alfa–Dependent Manner. Gastroenterology 2010, 138 (1), 325-335.e2. [CrossRef]
- Spaan, M.; Van Oord, G.; Kreefft, K.; Hou, J.; Hansen, B. E.; Janssen, H. L. A.; De Knegt, R. J.; Boonstra, A. Immunological Analysis during Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. The Journal of Infectious Diseases 2015, 213 (2), 216–223. [CrossRef]
- Wang, X.; Luo, B.; Jiang, H.-J.; Xu, C.; Qian, J.; Ma, D.-L.; Wei, L.; Feng, B. Recovery of Natural Killer Cells Is Mainly in Post-Treatment Period in Chronic Hepatitis C Patients Treated with Sofosbuvir plus Ledipasvir. World Journal of Gastroenterology 2018, 24 (40), 4554–4564. [CrossRef]
- Serti, E.; Chepa-Lotrea, X.; Kim, Y. J.; Keane, M.; Fryzek, N.; Liang, T. J.; Ghany, M. G.; Rehermann, B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015, 149 (1), 190-200.e2. [CrossRef]
- Bozzano, F.; Picciotto, A.; Costa, P.; Marras, F.; Fazio, V.; Hirsch, I.; Olive, D.; Moretta, A.; De Maria, A. Activating NK Cell Receptor Expression/Function (NKp30, NKp46, DNAM-1) during Chronic Viraemic HCV Infection Is Associated with the Outcome of Combined Treatment. European Journal of Immunology 2011, 41 (10), 2905–2914. [CrossRef]
- Jiang, H.-J.; Wang, X.; Luo, B.; Xu, C.; Qian, J.; Qin, H.; Zhang, H.; Kong, X.; Wei, L.; Feng, B. Direct Antiviral Agents Upregulate Natural Killer Cell Potential Activity in Chronic Hepatitis C Patients. Clinical and Experimental Medicine 2019, 19 (3), 299–308. [CrossRef]
- Golden–Mason, L.; McMahan, R. H.; Kriss, M.; Kilgore, A.; Cheng, L.; Dran, R. J.; Wieland, A.; Rosen, H. R. Early and Late Changes in Natural Killer Cells in Response to Ledipasvir/Sofosbuvir Treatment. Hepatology Communications 2018, 2 (4), 364–375. [CrossRef]
- Perpiñán, E.; Pérez-del-Pulgar, S.; Londoño, M.-C.; Mariñó, Z.; Bartrés, C.; González, P.; García-López, M.; Pose, E.; Lens, S.; Maini, M. K.; Forns, X.; Koutsoudakis, G. Cirrhosis Hampers Early and Rapid Normalization of Natural Killer Cell Phenotype and Function in Hepatitis C Patients Undergoing Interferon-Free Therapy. Frontiers in Immunology 2020, 11. [CrossRef]
- Wijaya, R. S.; Read, S. A.; Selvamani, S. P.; Schibeci, S. D.; Azardaryany, M. K.; Ong, A.; Van Der Poorten, D.; Lin, R.; Douglas, M. W.; George, J.; Ahlenstiel, G. Hepatitis C Virus (HCV) Eradication with Interferon-Free Direct-Acting Antiviral-Based Therapy Results in KLRG1+ HCV-Specific Memory Natural Killer Cells. The Journal of Infectious Diseases 2020, 223 (7), 1183–1195. [CrossRef]
- Mele, D.; Oliviero, B.; Mantovani, S.; Ludovisi, S.; Lombardi, A.; Genco, F.; Gulminetti, R.; Novati, S.; Mondelli, M. U.; Varchetta, S. Adaptive Natural Killer Cell Functional Recovery in Hepatitis C Virus Cured Patients. Hepatology 2020, 73 (1), 79–90. [CrossRef]
- Strunz, B.; Hengst, J.; Deterding, K.; Manns, M. P.; Cornberg, M.; Ljunggren, H.; Wedemeyer, H.; Björkström, N. K. Chronic Hepatitis C Virus Infection Irreversibly Impacts Human Natural Killer Cell Repertoire Diversity. Nature Communications 2018, 9 (1). [CrossRef]
- Oliviero, B.; Mantovani, S.; Varchetta, S.; Mele, D.; Grossi, G.; Ludovisi, S.; Nuti, E.; Rossello, A.; Mondelli, M. U. Hepatitis C Virus-Induced NK Cell Activation Causes Metzincin-Mediated CD16 Cleavage and Impaired Antibody-Dependent Cytotoxicity. Journal of Hepatology 2017, 66 (6), 1130–1137. [CrossRef]
- Martin, B.; Hennecke, N.; Lohmann, V.; Kayser, A.; Neumann-Haefelin, C.; Kukolj, G.; Böcher, W. O.; Thimme, R. Restoration of HCV-Specific CD8+ T Cell Function by Interferon-Free Therapy. Journal of Hepatology 2014, 61 (3), 538–543. [CrossRef]
- Burchill, M. A.; Golden-Mason, L.; Wind-Rotolo, M.; Rosen, H. R. Memory Re-Differentiation and Reduced Lymphocyte Activation in Chronic HCV-Infected Patients Receiving Direct-Acting Antivirals. Journal of Viral Hepatitis 2015, 22 (12), 983–991. [CrossRef]
- Romani, S.; Stafford, K. A.; Nelson, A.; Bagchi, S.; Kottilil, S.; Poonia, B. Peripheral PD-1+ T Cells Co-Expressing Inhibitory Receptors Predict SVR with Ultra Short Duration DAA Therapy in HCV Infection. Frontiers in Immunoogy 2019, 10. [CrossRef]
- Han, J. W.; Sung, P. S.; Kim, K. H.; Hong, S.-H.; Shin, E.; Song, M. J.; Park, S. Dynamic Changes in Ex Vivo T-Cell Function after Viral Clearance in Chronic HCV Infection. The Journal of Infectious Diseases 2019, 220 (8), 1290–1301. [CrossRef]
- Wieland, D.; Kemming, J.; Schuch, A.; Emmerich, F.; Knolle, P. A.; Neumann-Haefelin, C.; Held, W.; Zehn, D.; Hofmann, M.; Thimme, R. TCF1+ Hepatitis C Virus-Specific CD8+ T Cells Are Maintained after Cessation of Chronic Antigen Stimulation. Nature Communications 2017, 8 (1). [CrossRef]
- Llorens-Revull, M.; Costafreda, M. I.; Rico, A.; Guerrero-Murillo, M.; Soria, M. E.; Píriz-Ruzo, S.; Vargas-Accarino, E.; Gabriel-Medina, P.; Rodríguez-Frías, F.; Riveiro-Barciela, M.; Perales, C.; Quer, J.; Sauleda, S.; Esteban, J. I.; Bes, M. Partial Restoration of Immune Response in Hepatitis C Patients after Viral Clearance by Direct-Acting Antiviral Therapy. PLOS ONE 2021, 16 (7), e0254243. [CrossRef]
- Casey, J. R.; Dore, G. J.; Grebely, J.; Matthews, G.; Cherepanov, V.; Martinello, M.; Marks, P.; Janssen, H. L. A.; Hansen, B. E.; Kaul, R.; MacParland, S. A.; Gehring, A. J.; Feld, J. J. Hepatitis C Virus-specific Immune Responses Following Direct-acting Antivirals Administered during Recent Hepatitis C Virus Infection. Journal of Viral Hepatitis 2023, 30 (1), 64–72. [CrossRef]
- Tonnerre, P.; Wolski, D.; Subudhi, S.; Aljabban, J.; Hoogeveen, R. C.; Damasio, M.; Drescher, H. K.; Bartsch, L. M.; Tully, D. C.; Sen, D. R.; Bean, D. J.; Brown, J.; Torres-Cornejo, A.; Robidoux, M.; Kvistad, D.; Alatrakchi, N.; Cui, A.; Lieb, D.; Cheney, J. A.; Gustafson, J.; Lewis-Ximenez, L. L.; Massenet-Regad, L.; Eisenhaure, T.; Aneja, J.; Haining, W. N.; Chung, R. T.; Hacohen, N.; Allen, T. M.; Kim, A. Y.; Lauer, G. M. Differentiation of Exhausted CD8+ T Cells after Termination of Chronic Antigen Stimulation Stops Short of Achieving Functional T Cell Memory. Nature Immunology 2021, 22 (8), 1030–1041. [CrossRef]
- Hensel, N.; Gu, Z.; Sagar; Wieland, D.; Jechow, K.; Kemming, J.; Llewellyn-Lacey, S.; Gostick, E.; Sogukpinar, O.; Emmerich, F.; Price, D. A.; Bengsch, B.; Böettler, T.; Neumann-Haefelin, C.; Eils, R.; Conrad, C.; Bartenschlager, R.; Grün, D.; Ishaque, N.; Thimme, R.; Hofmann, M. Memory-like HCV-Specific CD8+ T Cells Retain a Molecular Scar after Cure of Chronic HCV Infection. Nature Immunology 2021, 22 (2), 229–239. [CrossRef]
- Yates, K. B.; Tonnerre, P.; Martin, G.; Gerdemann, U.; Abosy, R. A.; Comstock, D. E.; Weiss, S. A.; Wolski, D.; Tully, D. C.; Chung, R. T.; Allen, T. M.; Kim, A. Y.; Fidler, S.; Fox, J.; Frater, J.; Lauer, G. M.; Haining, W. N.; Sen, D. R. Epigenetic Scars of CD8+ T Cell Exhaustion Persist after Cure of Chronic Infection in Humans. Nature Immunology 2021, 22 (8), 1020–1029. [CrossRef]
- Pauken, K. E.; Sammons, M. A.; Odorizzi, P. M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.; Chen, Z.; Sen, D. R.; Kurachi, M.; Barnitz, R. A.; Bartman, C.; Bengsch, B.; Huang, A. C.; Schenkel, J. M.; Vahedi, G.; Haining, W. N.; Berger, S. L.; Wherry, E. J. Epigenetic Stability of Exhausted T Cells Limits Durability of Reinvigoration by PD-1 Blockade. Science 2016, 354 (6316), 1160–1165. [CrossRef]
- Miller, B. C.; Sen, D. R.; Abosy, R. A.; Bi, K.; Virkud, Y.; LaFleur, M. W.; Yates, K. B.; Lako, A.; Felt, K. D.; Naik, G. S.; Manos, M. P.; Gjini, E.; Kuchroo, J. R.; Ishizuka, J. J.; Collier, J. L.; Griffin, G. K.; Maleri, S.; Comstock, D. E.; Weiss, S. A.; Brown, F. D.; Panda, A.; Zimmer, M. D.; Manguso, R. T.; Hodi, F. S.; Rodig, S. J.; Sharpe, A. H.; Haining, W. N. Subsets of Exhausted CD8+ T Cells Differentially Mediate Tumor Control and Respond to Checkpoint Blockade. Nature Imunology 2019, 20 (3),326336. [CrossRef]
- Callendret, B.; Eccleston, H. B.; Hall, S.; Satterfield, W. C.; Capone, S.; Folgori, A.; Cortese, R.; Nicosia, A.; Walker, C. M. T-Cell Immunity and Hepatitis C Virus Reinfection after Cure of Chronic Hepatitis C with an Interferon-Free Antiviral Regimen in a Chimpanzee. Hepatology 2014, 60 (5), 1531–1540. [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. GenomeBiology.com (London. Print) 2013, 14 (10), R115. [CrossRef]
- Oltmanns, C.; Liu, Z.; Mischke, J.; Tauwaldt, J.; Mekonnen, Y. A.; Urbanek-Quaing, M.; Debarry, J.; Maasoumy, B.; Wedemeyer, H.; Kraft, A.; Xu, C.; Cornberg, M. Reverse Inflammaging: Long-Term Effects of HCV Cure on Biological Age. Journal of Hepatology 2023, 78 (1), 90–98. [CrossRef]
- Winkler, F.; Hipp, A. M.; Ramı́Rez, C.; Martin, B.; Villa, M.; Neuwirt, E.; Gorka, O.; Aerssens, J.; Johansson, S. E.; Rana, N.; Llewellyn-Lacey, S.; Price, D. A.; Panning, M.; Groß, O.; Pearce, E. L.; Hermann, C.; Schumann, K.; Hannibal, L.; Neumann-Haefelin, C.; Böettler, T.; Knolle, P.; Hofmann, M.; Wohlleber, D.; Thimme, R.; Bengsch, B. Enolase Represents a Metabolic Checkpoint Controlling the Differential Exhaustion Programmes of Hepatitis Virus-Specific CD8+T Cells. Gut 2023, 72 (10), 1971–1984. [CrossRef]
- Aregay, A.; Sekyere, S. O.; Deterding, K.; Port, K.; Dietz, J.; Berkowski, C.; Sarrazin, C.; Manns, M. P.; Cornberg, M.; Wedemeyer, H. Elimination of Hepatitis C Virus Has Limited Impact on the Functional and Mitochondrial Impairment of HCV-Specific CD8+ T Cell Responses. Journal of Hepatology 2019, 71 (5), 889–899. [CrossRef]
- Smits, M. J.; Zoldan, K.; Ishaque, N.; Gu, Z.; Jechow, K.; Wieland, D.; Conrad, C.; Eils, R.; Fauvelle, C.; Baumert, T. F.; Emmerich, F.; Bengsch, B.; Neumann-Haefelin, C.; Hofmann, M.; Thimme, R.; Böettler, T. Follicular T Helper Cells Shape the HCV-Specific CD4+ T Cell Repertoire after Virus Elimination. Journal of Clinical Investigation 2020, 130 (2), 998–1009. [CrossRef]
- Osuch, S.; Laskus, T.; Berak, H.; Perlejewski, K.; Metzner, K. J.; Paciorek, M.; Radkowski, M.; Cortés, K. C. Decrease of T-Cells Exhaustion Markers Programmed Cell Death-1 and T-Cell Immunoglobulin and Mucin Domain-Containing Protein 3 and Plasma IL-10 Levels after Successful Treatment of Chronic Hepatitis C. Scientific Reports 2020, 10 (1). [CrossRef]
- Kondili, L. A.; Monti, M.; Quaranta, M. G.; Gragnani, L.; Panetta, V.; Brancaccio, G.; Mazzaro, C.; Persico, M.; Masarone, M.; Gentile, I.; Andreone, P.; Madonia, S.; Biliotti, E.; Filomia, R.; Puoti, M.; Fracanzani, A. L.; Laccabue, D.; Ieluzzi, D.; Coppola, C.; Rumi, M. G.; Benedetti, A.; Verucchi, G.; Coco, B.; Chemello, L.; Iannone, A.; Ciancio, A.; Russo, F. P.; Barbaro, F.; Morisco, F.; Chessa, L.; Massari, M.; Blanc, P.; Zignego, A. L. A Prospective Study of Direct-acting Antiviral Effectiveness and Relapse Risk in HCV Cryoglobulinemic Vasculitis by the Italian PITER Cohort. Hepatology 2022, 76 (1), 220–232. [CrossRef]
- Gragnani, L.; Lorini, S.; Marri, S.; Vacchi, C.; Madia, F.; Monti, M.; Ferri, C.; Zignego, A. L. Predictors of Long-Term Cryoglobulinemic Vasculitis Outcomes after HCV Eradication with Direct-Acting Antivirals in the Real-Life. Autoimmunity Reviews 2022, 21 (1), 102923. [CrossRef]
- Visentini, M.; Del Padre, M.; Colantuono, S.; Yang, B.; Minafò, Y. A.; Antonini, S.; Carnovale, M.; De Santis, A.; Pulsoni, A.; De Sanctis, G. M.; Gragnani, L.; Zignego, A. L.; Fiorilli, M.; Casato, M. Long-lasting Persistence of Large B-cell Clones in Hepatitis C Virus-cured Patients with Complete Response of Mixed Cryoglobulinaemia Vasculitis. Liver International 2019, 39 (4), 628–632. [CrossRef]
- Del Padre, M.; Marrapodi, R.; Minafò, Y. A.; Mortari, E. P.; Radicchio, G.; Bocci, C.; Gragnani, L.; Camponeschi, A.; Colantuono, S.; Stefanini, L.; Basili, S.; Carsetti, R.; Fiorilli, M.; Casato, M.; Visentini, M. Dual Stimulation by Autoantigen and CpG Fosters the Proliferation of Exhausted Rheumatoid Factor-Specific CD21low B Cells in Hepatitis C Virus-Cured Mixed Cryoglobulinemia. Frontiers in Immunology 2023, 14. [CrossRef]
- Chang, L.-Y.; Li, Y.; Kaplan, D. E. Hepatitis C Viraemia Reversibly Maintains Subset of Antigen-Specific T-Bet+ Tissue-like Memory B Cells. Journal of Viral Hepatitis 2016, 24 (5), 389–396. [CrossRef]
- Nishio, A.; Hasan, S.; Park, H.; Park, N.; Salas, J.; Salinas, E.; Kardava, L.; Juneau, P.; Frumento, N.; Massaccesi, G.; Moir, S.; Bailey, J. R.; Grakoui, A.; Ghany, M. G.; Rehermann, B. Serum Neutralization Activity Declines but Memory B Cells Persist after Cure of Chronic Hepatitis C. Nature Communications 2022, 13 (1). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




