Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungal material
2.2. Polyamine analysis
2.3. Carbon and Nitrogen Elemental Analysis
2.4. Measurement of Total Phenolic, Total Flavonoid Contents and Assessment of Antioxidant Capacity
2.4.1. The total phenolic content
2.4.2. The total flavonoid content
2.4.3. Antioxidant capacity
- DPPH assay: The ability of the extracts to neutralize the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was assessed following the procedure outlined by Espín et al. [29] with slight modification. In summary, 10 µL of the sample was combined with 100 µL of 90 µM DPPH solution in MeOH and 190 µL of MeOH. The absorbance was recorded following a 30-minute incubation period in a dark place at 515 nm.
- ABTS assay: The assay involves spectrophotometric monitoring of the conversion of the blue-green colored cation radical ABTS•+ into its neutral, colorless form, was conducted following the method described by Arnao et al. [30]. ABTS•+ was generated by directly reacting a 7 mM ABTS solution with 2.45 mM K2S2O8. Subsequently, 10 µL of the fungal extracts were added to 290 µL of ABTS solution and mixed. The absorbance of the sample was read at 734 nm after 5-minute incubation at room temperature.
- NO assay: The inhibition of the nitric oxide radical (NO•) was evaluated using the Griess diazotization process, as outlined in the methodology developed by Green et al. [31]. The reaction mixture consisted of 15 µL of the extract, 250 µL of 10 mmol/L sodium nitroprusside, and 250 µL of phosphate buffer (pH 7.4). After incubation for 90 minutes at room temperature under constant light, 500 µL of Griess reagent (a combination of a 0.2% solution of N-(1-naphthyl)-ethylenediamine dihydrochloride and a 2% solution of sulfanilamide in 4% phosphoric acid) was added. The degree of inhibition was gauged by quantifying the absorbance of the resultant chromophore at 546 nm.
- FRAP assay: This assay was carried out according to Benzie and Strain [32]. The freshly prepared FRAP (Ferric Reducing Antioxidant Power) reagent comprises a solution containing 10 mmol/L TPTZ in 40 mmol/L HCl, 0.02 mmol/L FeCl3 x 6H2O, and acetate buffer (pH 3.6) in a ratio of 10:1:1. Briefly, 10 µL of each extract was combined with 225 µL of the FRAP reagent and 22.5 µL of distilled water (dH2O). Absorbance was measured after 6 min at 593 nm.
2.5. Neuroprotective activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Mycochemical analysis
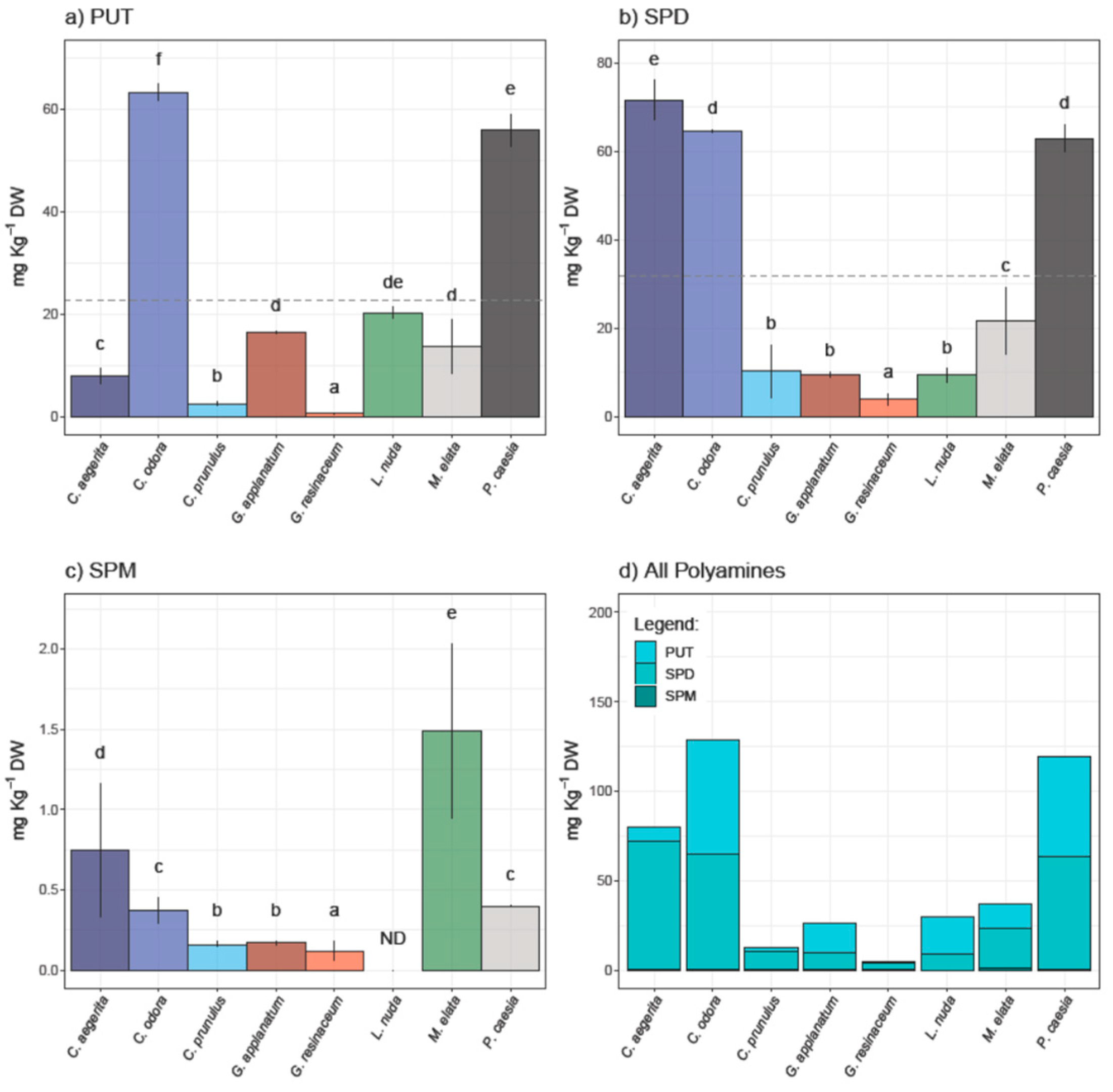
3.1.1. Polyamine content
3.1.2. Total Protein Content
3.1.3. Total Phenolic and Total Flavonoid Contents
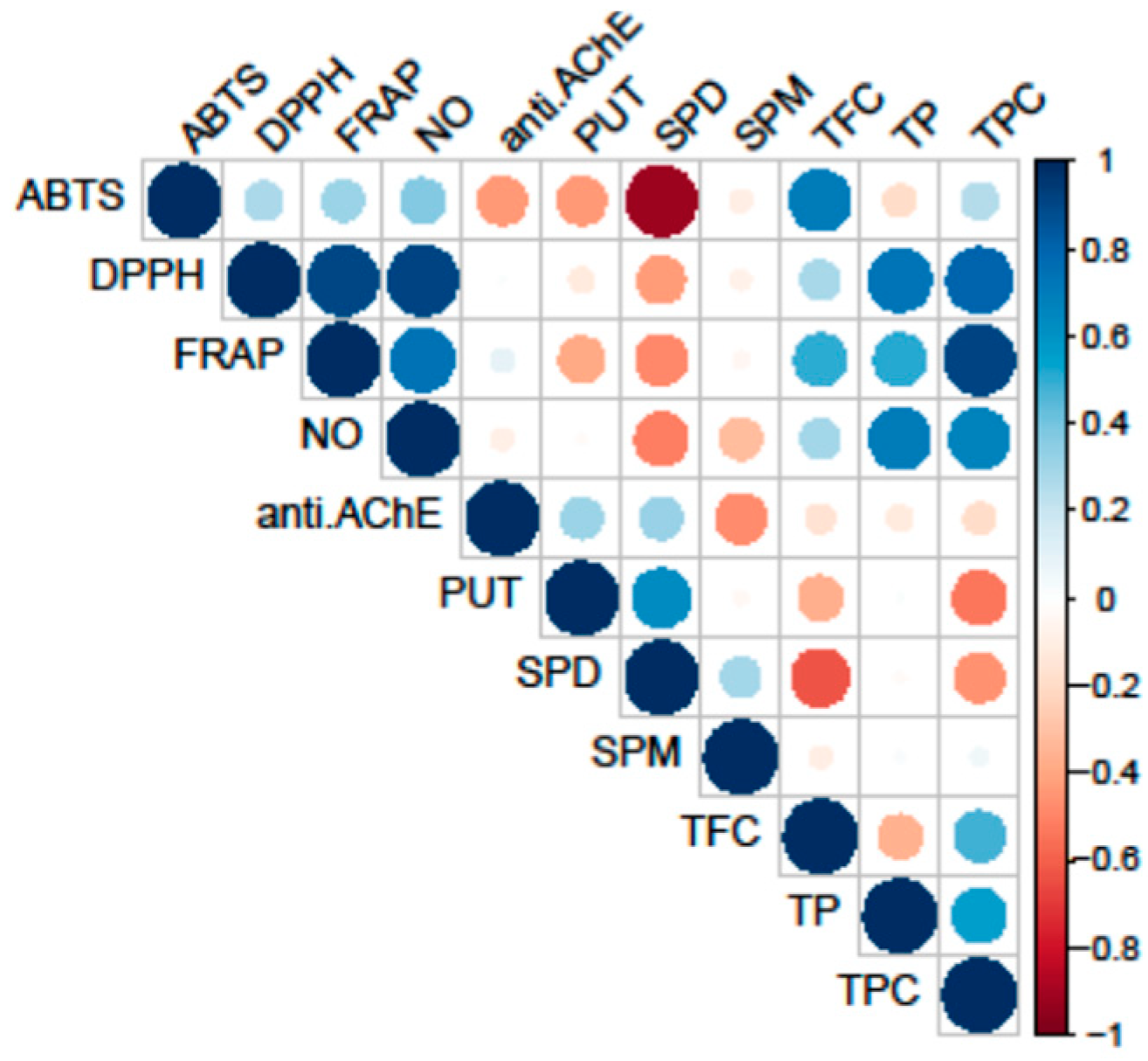
3.2. Antioxidant Potential
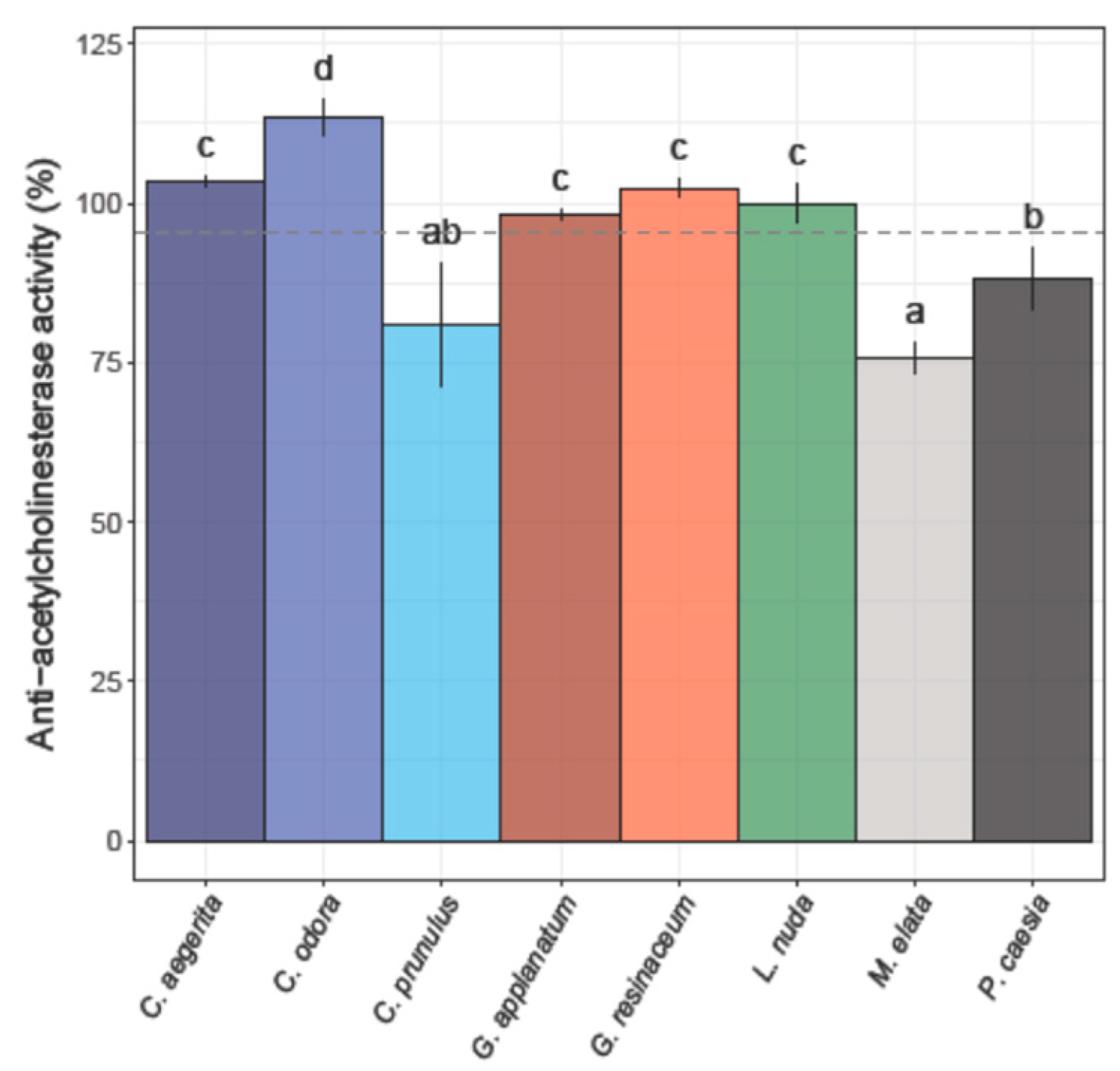
3.3. AChE inhibitory potential
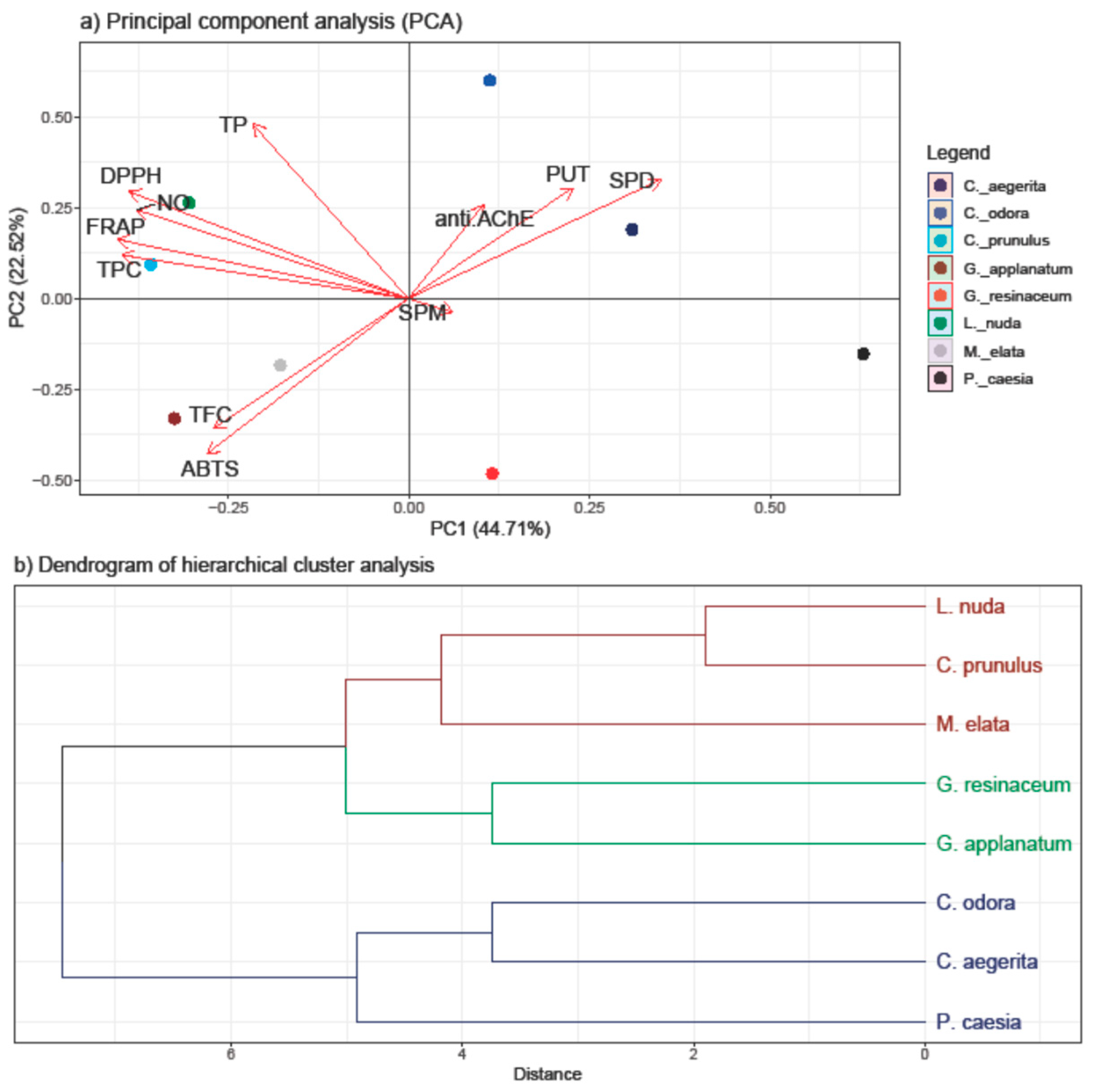
3.4. PCA analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Makletsova, M.G.; Syatkin, S.P.; Poleshchuk, V.V.; Urazgildeeva, G.R.; Chigaleychik, L.A.; Sungrapova, C.Y.; Illarioshkin, S.N. Polyamines in Parkinson’s disease: their role in oxidative stress induction and protein aggregation. J. Neurol. Res. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond antioxidant effects: nature-based templates unveil new strategies for neurodegenerative diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef]
- Mišković, J.; Karaman, M.; Rašeta, M.; Krsmanović, N.; Berežni, S.; Jakovljević, D.; Piattoni, F.; Zambonelli, A.; Gargano, M.L.; Venturella, G. Comparison of two Schizophyllum commune strains in production of acetylcholinesterase inhibitors and antioxidants from submerged cultivation. J. Fungi 2021, 7, 115. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Rockwood, K.; Martin, E.; Darvesh, S. Cholinesterase inhibition in Alzheimer's disease: is specificity the answer? J. Alzheimer's Dis. 2014, 42, 379–384. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B.; Oyedola, E.T.; Olasehinde, T.A.; Oyeleye, S.I. In vitro anticholinesterase, antimonoamine oxidase and antioxidant properties of alkaloid extracts from kola nuts (Cola acuminata and Cola nitida). J. Complement. Integr. Med. 2018, 16, 20160155. [Google Scholar] [CrossRef]
- Reis, G.C.; Guidi, L.R.; Fernandes, C.; Godoy, H.T.; Glória, M.B. UPLC-UV method for the quantification of free amino acids, bioactive amines, and ammonia in fresh, cooked, and canned mushrooms. Food Anal. Methods 13, 1613–1626. [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes versicolor: medicinal mushroom with important health benefits. Not. Bot. Horti. Agrobot. Cluj Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Beara, I.; Šibul, F.; Zengin, G.; Krstić, S.; Karaman, M. Anti-inflammatory, antioxidant and enzyme inhibition activities in correlation with mycochemical profile of selected indigenous Ganoderma spp. from Balkan region (Serbia). Chem. Biodivers 2000, 18, e2000828. [Google Scholar]
- Rašeta, M.; Popović, M.; Čapo, I.; Stilinović, N.; Vukmirović, S.; Milošević, B.; Karaman, M. Antidiabetic effect of two different Ganoderma species tested in alloxan diabetic rats. RSC Advances, 2020, 10, 10382–10393. [Google Scholar]
- Krsmanović, N.; Rašeta, M.; Mišković, J.; Bekvalac, K.; Bogavac, M.; Karaman, M.; Isikhuemhen, O.S. Effects of UV stress in promoting antioxidant activities in fungal species Тrametes versicolor (L.) Lloyd and Flammulina velutipes (Curtis) Singer. Antioxidants 2023, 12, 302. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. In vitro antioxidant activity of dietary polyamines. Food Res. Int. 2013, 51, 141–147. [Google Scholar] [CrossRef]
- Reis, G.C.; Custódio, F.B.; Botelho, B.G.; Guidi, L.R.; Gloria, M.B.A. Investigation of biologically active amines in some selected edible mushrooms. J. Food Compost. Anal. 86, 103375. [CrossRef]
- Bae, D.H.; Lane, D.J.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj. BBA-GEN SUBJECTS 2018, 1862, 2053–2068. [Google Scholar] [CrossRef]
- Rocha, R. O.; Wilson, R.A. Essential, deadly, enigmatic: polyamine metabolism and roles in fungal cells. Fungal Biol. Rev. 2019, 33, 47–57. [Google Scholar] [CrossRef]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson's disease: balancing between neurotoxicity and neuroprotection. Annu. Rev. Biochem. 2023, 92. [Google Scholar] [CrossRef]
- Đorđievski, S.; Vukašinović, E.L.; Čelić, T.V.; Pihler, I.; Kebert, M.; Kojić, D.; Purać, J. Spermidine dietary supplementation and polyamines level in reference to survival and lifespan of honey bees. Sci. Rep. 2023, 13, 4329. [Google Scholar] [CrossRef]
- Minois, N. Molecular basis of the ‘anti-aging' effect of spermidine and other natural polyamines-a mini-review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef]
- Peng, D.; Wang, X.; Li, Z.; Zhang, Y.; Peng, Y.; Li, Y.; He, X.; Zhang, X.; Ma, X.; Huang, L.; Yan, Y. NO is involved in spermidine-induced drought tolerance in white clover via activation of antioxidant enzymes and genes. Protoplasma 2016, 253, 1243–1254. [Google Scholar] [CrossRef]
- Park, S.J.; Kwak, M.K.; Kang, S.O. Schiff bases of putrescine with methylglyoxal protect from cellular damage caused by accumulation of methylglyoxal and reactive oxygen species in Dictyostelium discoideum. Int. J. Biochem. Cell Biol. 2017, 86, 54–66. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: a review. Bioorg. Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
- Sun, X.Z.; Liao, Y.; Li, W.; Guo, L.M. Neuroprotective effects of Ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis. Neural Regen. Res. 2017, 12, 953–958. [Google Scholar]
- Sułkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyżak, A.; Popiół, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszyńska, B. Bioactivity and mycochemical profile of extracts from mycelial cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A review of chemical composition and bioactivity studies of the most promising species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Dadáková, E.; Pelikánová, T.; Kalač, P. Content of biogenic amines and polyamines in some species of European wild-growing edible mushrooms. Eur. Food Res. Technol. 2009, 230, 163–171. [Google Scholar] [CrossRef]
- Scaramagli, S.; Biondi, S.; Capitani, F.; Gerola, P.; Altamura, M.M.; Torrigiani, P. Polyamine conjugate levels and ethylene biosynthesis: inverse relationship with vegetative bud formation in tobacco Thin layers. Physiol. Plant. 1999, 105, 366–375. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumaravel, S. Study on phenolic content, antioxidant activity and CHNS elemental analysis of Amorphophallus sylvaticus. Int. J. Agric. Life Sci. 2016, 2, 12–17. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis 2002, 10, 178–182. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Green, L.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid and concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe-friendly framework for basic statistical tests. [R package rstatix version 0.6.0]. 2020. [Google Scholar]
- Galili, T. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Wiley interdisciplinary reviews: computational statistics. 2011, 3, 180–185. [Google Scholar]
- Reis, G.L.; Custódio, F.B.; Botelho, B.G.; Guidi, L.R.; Glória, M.B. Investigation of biologically active amines in some selected edible mushrooms. J. Food Compost. Anal. 2020, 86, 103375. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Jakovljevic, D.; Jadranin, M.B.; Nikšić, M.P. Health impact of the commercially cultivated mushroom Agaricus bisporus and wild-growing mushroom Ganoderma resinaceum - a comparative overview. J. Serb. Chem. Soc. 2020, 85, 721–735. [Google Scholar] [CrossRef]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Pinto, S.; Barros, L.; Sousa, M.J.; Ferreira, I.C. Chemical characterization and antioxidant properties of Lepista nuda fruiting bodies and mycelia obtained by in vitro culture: effects of collection habitat and culture media. Food Res. Int. 2013, 51, 496–502. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Ćirić, A.; Barros, L.; Ferreira, I.C.; Soković, M.D. Nutritional value, chemical composition, antioxidant activity and enrichment of cream cheese with chestnut mushroom Agrocybe aegerita (Brig.) Sing. J. Food Sci. Technol 2015, 52, 6711–6718. [Google Scholar]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Ouzouni, P. K.; Petridis, D.; Koller, W.D.; Riganakos, K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Karaman, M.; Stahl, M.; Vulić, J.; Vesić, M.; Čanadanović-Brunet, J. Wild-growing lignicolous mushroom species as sources of novel agents with antioxidative and antibacterial potentials. Int. J. Food Sci. Nutr. 2014, 65, 311–319. [Google Scholar] [CrossRef]
- Zheng, X.P.; Suwandi, J.F.; Fuller, J.; Doronila, A.I.; Ng, K.S. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 2012, 18, 367–379. [Google Scholar] [CrossRef]
- Sevindik, M. Total phenolic, total flavonoid contents and antioxidant potential of the wild edible mushroom Clitocybe odora. KSU J. Agric Nat. 2024, 27, 75–81. [Google Scholar] [CrossRef]
- Dimitrijević, M.V.; Jovanović, V.S.; Cvetkovic, J.S.; Mihajilov-Krstev, T.M.; Stojanović, G.S.; Mitić, V.D. Screening of antioxidant, antimicrobial and antiradical activities of twelve selected Serbian wild mushrooms. Anal. Methods 2015, 7, 4181–4191. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer's disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Rašeta, M.; Karaman, M.; Jakšić, M.; Šibul, F.; Kebert, M.; Novaković, A.; Popović, M. Mineral composition, antioxidant and cytotoxic biopotentials of wild-growing Ganoderma species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.) Pat. Int. J. Food Sci. Technol 2016, 51, 2583–2590. [Google Scholar] [CrossRef]
- Rajoriya, A.; Tripathy, S.S.; Gupta, N. In vitro antioxidant activity of selected Ganoderma species found in Odisha, India. Trop. Plant Res. 2015, 2, 72–77. [Google Scholar]
- Siangu, B.N.; Sauda, S.; John, M.K.; Njue, W. Antioxidant activity, total phenolic and flavonoid content of selected Kenyan medicinal plants, sea algae and medicinal wild mushrooms. Afr. J. Pure Appl. 2019, 13, 43–48. [Google Scholar]
- Ćilerdžić, J.; Stajić, M.; Vukojevic, J. Potential of submergedly cultivated mycelia of Ganoderma spp. as antioxidant and antimicrobial agents. Curr. Pharm. Biotechnol. 2016, 17, 275–282. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Yin, F.; Chen, H.; Yang, D.; Liu, X.; Jin, Q.; Lv, X.; Mans, D.R.; Zhang, X.; Liang, Z. Antioxidative and cytoprotective effects of Ganoderma applanatum and Fomitopsis pinicola in PC12 adrenal phaeochromocytoma cells. Int. J. Med. Mushrooms 2022, 24, 15–29. [Google Scholar] [CrossRef]
- Cör, D.; Botić, T.; Gregori, A.; Pohleven, F.; Knez, Ž. The effects of different solvents on bioactive metabolites and “in vitro” antioxidant and anti-acetylcholinesterase activity of Ganoderma lucidum fruiting body and primordia extracts. Maced. J. Chem. Chem. 2017, 36, 129–141. [Google Scholar] [CrossRef]
- Bal, C.; Sevindik, M.; Akgul, H.; Selamoglu, Z. Oxidative stress index and antioxidant capacity of Lepista nuda collected from Gaziantep/Turkey. Sigma J Eng. Nat. Sci. 2019, 37, 1–5. [Google Scholar]
- Ramya, H.; Ravikumar, K.S.; Fathimathu, Z.; Janardhanan, K.K.; Ajith, T.A.; Shah, M.A.; Reshi, Z.A. Morel mushroom, Morchella from Kashmir Himalaya: a potential source of therapeutically useful bioactives that possess free radical scavenging, anti-inflammatory, and arthritic edema-inhibiting activities. Drug Chem Toxicol. 2022, 45, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncu, F.; Oskay, M.; Sağlam, H.; Erdoğan, T.F.; Tamer, A.Ü. Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J. Med. Food 2010, 13, 415–419. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P.; Inbaraj, B.S. Evaluation of in vitro antimicrobial, antioxidant, and anti-quorum sensing activity of edible mushroom (Agrocybe aegerita). Foods 2023, 12, 3562. [Google Scholar] [CrossRef]
- Cör, D.; Botić, T.; Knez, Ž.; Batista, U.; Gregori, A.; Pohleven, F.; Bončina, T. Two-stage extraction of antitumor, antioxidant and antiacetylcholinesterase compounds from Ganoderma lucidum fruiting body. J. Supercrit. Fluids 2014, 91, 53–60. [Google Scholar] [CrossRef]
- Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Rehman, M.U.; Adhikari, A. Türkoğlu, A., Choudhary, M.I. Phytochemical investigation, antioxidant and anticholinesterase activities of Ganoderma adspersum. Ind. Crops Prod. 2015, 76, 749–754. [Google Scholar] [CrossRef]
- Akata, I.; Zengin, G.; Picot, C.M.; Mahomoodally, M.F. Enzyme inhibitory and antioxidant properties of six mushroom species from the Agaricaceae family. S. Afr. J. Bot. 2019, 120, 95–99. [Google Scholar] [CrossRef]
- Dündar, A.; Okumuş, V.; Özdemir, S.; Çelik, K.S.; Boğa, M.S.; Ozcagli, E.; Özhan, G.; Yildiz, A. Antioxidant, antimicrobial, cytotoxic and anticholinesterase activities of seven mushroom species with their phenolic acid composition. J. Hortic. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Lim, B.O. Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of Ganoderma lucidum grown on germinated brown rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef]
- Karaman, M.; Tesanovic, K.; Novakovic, A.; Jakovljevic, D.; Janjusevic, L.; Sibul, F.; Pejin, B. Coprinus comatus filtrate extract, a novel neuroprotective agent of natural origin. Nat. Prod. Res. 2020, 34, 2346–2350. [Google Scholar] [CrossRef]
- Orhan, I.E.; Üstün, O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. J. Food Compost. Anal. 2011, 24, 386–390. [Google Scholar] [CrossRef]
- Zawadzka, A.; Kobus-Cisowska, J.; Szwajgier, D.; Szczepaniak, O.M.; Szulc, P.; Siwulski, M. Dual functional cholinesterase inhibitors and complexing of aluminum ions of five species of fungi family depended of drying conditions and extraction process - in vitro study. LWT. 2021, 154, 112712. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2022, 229, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, L.; Tong, A.; Zhen, H.; Han, D.; Yuan, H.; Li, F.; Wang, C.; Fan, G. Anti-aging effect of Agrocybe aegerita polysaccharide through regulation of oxidative stress and gut microbiota. Foods 2022, 11, 3783. [Google Scholar] [CrossRef]
- Makletsova, M.G.; Rikhireva, G.T.; Kirichenko, E.Y.; Trinitatsky, I.Y.; Vakulenko, M.Y.; Ermakov, A.M. The role of polyamines in the mechanisms of cognitive impairment. Neurochem. J. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Zhong, R.; Ye, C.; Chen, J. Structure characterization and biological activities evaluation of two hetero-polysaccharides from Lepista nuda: cell antioxidant, anticancer and immune-modulatory activities. Int. J. Biol. Macromol. 2023, 125204. [Google Scholar] [CrossRef] [PubMed]
- Dhara, M.; Matta, J.A.; Lei, M.; Knowland, D.; Yu, H.; Gu, S.; Bredt, D.S. Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: functions, metabolism, and role in human disease management. Med. Sci. (Basel). 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Savarin, P.; Pastré, D. Polyamine signal through gap junctions: A key regulator of proliferation and gap-junction organization in mammalian tissues? BioEssays 2016, 38, 498–507. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M.J. The role of glia in stress: polyamines and brain disorders. Psychiatr. Clin. North. Am. 2014, 37, 653–678. [Google Scholar] [CrossRef]
- Kabir, A.F.; Jash, C.; Payghan, P.V.; Ghoshal, N.; Kumar, G.S. Polyamines and its analogue modulates amyloid fibrillation in lysozyme: a comparative investigation. Biochim. Biophys. Acta Gen. Subj. 2020, 129557. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of learning and memory by natural polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Kossorotow, A.; Wolf, H.U.; Seiler, N. Regulatory effects of polyamines on membrane-bound acetylcholinesterase. Biochem. J. 1974, 144, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kibe, S.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; Kakeyama, M.; Benno, Y.; Matsumoto, M. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef]
| Species name | Locality1 | Sampling date | Voucher number |
| Clitocybe odora | Tara Mountain | 29.10.2021. | 12-00906 |
| Clitopilus prunulus | Tara Mountain | 29.10.2021. | 12-00907 |
| Lepista nuda | Tara Mountain | 29.10.2021. | 12-01046 |
| Postia caesia | Tara Mountain | 29.10.2021. | 12-01047 |
| Morchella elata | Petrovaradin Hill | 8.4.2023 | 12-01048 |
| Cyclocybe aegerita | Novi Sad town | 5.11.2019. | 12-01049 |
| Ganoderma applanatum | Morović´s forest | 17.5.2023. | 12-00714 |
| Ganoderma resinaceum | Novi Sad town | 21.5.2023. | 12-00722 |
| Species | ABTS | DPPH | FRAP | NO | TFC | TP | TPC |
| C. aegerita | 28.08±4.00a | 5.33±0.94b | 14.31±1.42bc | 9.22±1.15a | ND | 30.27±1.24c | 35.72±1.65c |
| C. odora | 39.04±10.88b | 9.01±0.73d | 16.61±2.43c | 10.44±1.19b | 0.21±0.01a | 39.70±0.95d | 29.69±2.04bc |
| C. prunulus | 58.84±5.46d | 8.89±0.78cd | 17.27±1.59cd | 10.80±1.94bc | 1.76±0.01bc | 46.70±1.74e | 49.02±0.59d |
| G. applanatum | 70.42±2.60e | 7.98±0.30c | 19.24±1.77d | 10.41±0.56b | 7.02±0.89d | 17.24±0.49b | 45.75±5.36d |
| G. resinaceum | 69.70±2.54e | 5.16±0.44b | 12.68±1.47b | 9.48±0.56ab | 2.11±0.51c | 13.64±1.09a | 20.20±2.56b |
| L. nuda | 60.93±4.72d | 9.28±0.31de | 18.32±2.89d | 10.96±0.44bc | 1.09±0.00b | 47.40±1.28e | 39.60±3.69c |
| M. elata | 68.88±1.13e | 8.09±0.11c | 16.62±1.06c | 10.10±0.74b | 2.60±0.44c | 34.62±c0.89d | 39.75±2.77c |
| P. caesia | 46.92±7.03c | 3.03±0.29a | 5.40±0.91a | 9.35±0.42a | ND | 18.62±.042b | 5.56±0.47a |
| One way ANOVA | |||||||
| F test | 23.85 | 50.01 | 18.08 | 1.34 | 26.84 | 24.88 | 77.34 |
| p | 2.48 E-07 | 1.06 E-09 | 1.71 E-06 | 2.94 E-01 | 1.07 E-07 | 1.01 E-07 | 3.84 E-11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





